Abstract
A major consideration in the selection of new and improved iron chelators for clinical use is preferential interaction with the most toxic iron compartment. We describe the biologic properties of a new synthetic hexadentate iron chelator (IRC011) that is a substituted polyaza compound. Unlike deferoxamine (DF ), the polyaza structure of IRC011 does not contain any readily hydrolyzable covalent bonds and is anticipated to resist in vivo biotransformation. In the present studies, the ability of IRC011 to remove radioiron from iron-loaded heart cells in vitro was similar to DF, with a decrease to 20.0 ± 0.4% and 19.7 ± 0.5% of initial values after 24 hours of incubation with 0.3 mmol/L of DF or IRC011, respectively. The in vivo interaction of IRC011 with specific iron stores was studied in hypertransfused rats using selective labeling of reticuloendothelial (RE) iron stores with 59Fe-heat-denatured red blood cells (DRBCs) and of hepatocellular stores with 59Fe-ferritin. The pattern of radioiron excretion with IRC011 was quite different from that with DF. Although with both compounds, hepatocellular iron excretion was through the bile, whereas RE iron excretion was mainly in the urine, the magnitude of these effects was quite different. After the administration of a single parenteral dose of 200 mg/kg representing a 53% higher iron-binding capacity for IRC011 compared with DF, 48-hour urinary excretion of RE iron with IRC011 was 22.8% ± 1.1% (% of total body 59Fe), but only 6.0% ± 3.6% with DF. By contrast, the corresponding biliary excretion of hepatocellular radioiron was 14.2% ± 3.2% with DF, but only 0.7% ± 0.3% with IRC011. Thus, the new iron chelator IRC011 is distinguished from DF by the following features: (1) a higher affinity to Fe(III), (2) anticipated resistance to in vivo catabolism, (3) preferential interaction with RE iron derived from RBC breakdown, and (4) selective renal excretion. Because RBC breakdown is the most likely source of the toxic nontranferrin plasma iron, IRC011 may be a useful iron chelator for protecting vital organs from peroxidative damage.
AFTER THE INTRODUCTION of regular iron-chelating therapy with deferoxamine (DF ), a significant improvement in the life expectancy of patients with transfusional iron overload has been witnessed. This is largely attributed to the prevention of heart disease in well-treated patients and, in a few, to the reversal of existing heart disease by aggressive intravenous DF therapy.1,2 Despite the proven efficacy of DF in the treatment of iron overload, there is an obvious need for the development of alternative drugs that would be convenient for use and available for an increasing number of patients in need of long-term iron-chelating therapy. This need is underlined by the recognition that the use of deferiprone (L1), the synthetic oral iron chelator presently under evaluation for the management of thalassemia, is associated with a very high incidence of toxic complications, raising serious questions regarding its future use.3-6
In the present study, we undertook the evaluation of a new synthetic hexadentate iron chelator IRC011,7 a substituted polyaza compound. Because a major consideration in drug selection is preferential interaction with the most toxic iron compartment, we wished to define the source of iron chelated in vivo by IRC011 in hypertransfused rats labeled with selective radioiron probes, using heat-denatured red blood cells (59Fe-DRBC) to introduce radioiron to reticuloendothelial (RE) cells and 59Fe-ferritin to label hepatic parenchymal cells.8 In this report, we describe the results of these studies, indicating that the new synthetic iron chelator interacts preferentially with catabolic RBC iron, a feature that may be very useful in protecting vital organs from peroxidative damage in transfusional iron overload.
MATERIALS AND METHODS
In Vivo Studies
Female Wistar rats of the Hadassah strain weighing 170 to 200 g were used throughout. Hypertransfusion was performed by two intravenous injections of 2 mL packed cells per 100 g body weight on days 4 and 1 before storage iron labeling. The mean hematocrit level on the first day of study was 69% ± 1%, the serum iron level was 459 ± 18 mg/dL, and the unsaturated iron binding capacity was less than 10 μg/dL. The distribution and excretion of radioiron-labeled chelates (10 mg/animal) was assessed after intraperitoneal injection of the chelator mixed with a trace amount of 59FeCl3 . Prelabeling of iron stores was accomplished via intravenous injection of radioiron labels through a tail vein. Animals were killed under ether anesthesia by exsanguination through the abdominal aorta into heparinized syringes.
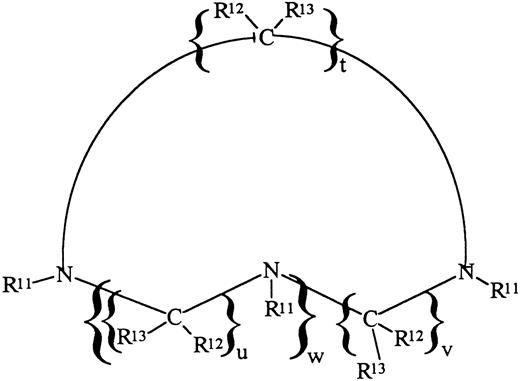
IRC011 (Ferofix A) was supplied by Israel Resources Corp Ltd (Haifa, Israel). The subject compound is a member of the family of substituted cyclic polyaza compounds having the formula shown below (Fig 1), in which R12 and R13 are H, t = u = v = 2 and w = 1 and R11 are methylene phosphonic acid groups (-CH2-PO3H2).7 The formula weight of the acid form is 411 Daltons, whereas the formula weight of the monohydrate of the acid form provided for the subject experiments is 429 Daltons. It can be appreciated that, by varying the nature of R11, R12, R13, t, u, and w, analogues can be obtained possessing a wide range of properties. DF was supplied as the methane-sulfonate salt (Desferal) by Ciba-Geigy Ltd (Basel, Switzerland).
Chemical description of IRC011 (Ferofix A). For explanation of symbols, see the Materials and Methods.
Chemical description of IRC011 (Ferofix A). For explanation of symbols, see the Materials and Methods.
Counting Methods
The radioactivity of spleen, kidney, and weighed portions of the liver and 1-mL samples of blood was determined in an automatic well-type scintillation counter (Auto-Gamma, Model 5360; Packard Instruments Co, Inc, Downer's Grove, IL). Whole body counts were performed in a small-animal counter (Packard Model 446, Armac liquid scintillation detector). To measure the excretion of radioactivity after 59Fe labeling, the animals were confined in solitary metabolic cages with stainless steel grid bottoms, and urine and stool samples were collected separately. Radioiron excretion was also determined by whole body counts on the first and last days of the study, and corrections were made for decay and differences in geometry. Recovery of radioactivity in excreta compared with the reduction in whole body radioactivity was greater than 90%.
In preliminary studies using 51Cr-labeled erythrocytes, the blood volume was found to be 6.4 mL/100 g body weight and the proportion of blood trapped in the liver was 2.4%. These factors were used in subsequent studies to calculate net hepatic and total blood radioactivity. Residual tissue radioactivity was calculated from total body radioactivity minus the combined radioactivity of liver, spleen, blood, and kidneys.
Preparation of Radioiron Labels
Heat-damaged erythrocytes (59Fe-DRBC). In vivo 59Fe-labeled erythrocytes were prepared in rats by injecting 100 to 200 μCi of 59Fe-citrate intravenously for 5 days or more before harvesting the cells. Repeated injections of 50 to 100 μCi 59Fe were administered as required to maintain a specific activity of 0.05 to 0.1 μCi of 59Fe per milligram of hemoglobin. Blood was removed in volumes of 0.5 to 1.0 mL, and after three washes of cool normal saline, cells were suspended in 5 vol of acid citrate dextrose formula B and incubated at 40°C for 15 minutes. After 2 more washings, the cells were resuspended in saline to a final hemoglobin concentration of 5 mg/mL and injected intravenously without delay in aliquots of 1 mL/animal.
Soluble ferritin (59Fe-ferritin). In vivo 59Fe-labeled ferritin was prepared by injecting 100 to 200 μCi of 59Fe-citrate into rats that had received 12 mg of iron dextran during the preceding week. The animals were killed 24 hours later, and purified radioactive ferritin was prepared by the method of Bjorklid and Helgeland.9 Acrylamide gel electrophoresis of the ferritin preparation at pH 8.5 showed a single protein band, and a single precipitin line was obtained on immunodiffusion against antiferritin serum. The specific activity of 59Fe-ferritin was 5 to 10 μCi/mg iron.
Initial processing of storage iron labels. This has been described in detail in previous communications.10-12 Briefly, 89% ± 1% of soluble ferritin and 81% ± 2% of DRBC were located to the liver and spleen within 1 hour of intravenous injection. After administration of DRBC, the cellular distribution of radioactivity in the liver determined by a quantitative autoradiographic method was 100% in RE cells and 0% in parenchymal cells. In contrast, soluble 59Fe-ferritin was located primarily to hepatic parenchymal cells (97% to 100%), with little labeling of RE cells (0% to 3%). Thus, with one or the other source of radioiron, it was possible to label selectively either RE or parenchymal iron stores. The T1/2 in plasma of intravenously injected soluble ferritin was 16 ± 1 minutes and that of DRBC was 2 ± 1 minutes. Within 1 day of injection, 72% of 59Fe-ferritin in the liver could be recovered in the ferritin fraction of tissue homogenates and the rest in nonferritin iron. Hemoglobin in 59Fe-DRBC was completely catabolized within 24 hours of injection.
Total nonheme iron in the liver was determined by the method of Torrance and Bothwell.13
Design of Animal Studies
This study has been approved by the institutional committe for animal experimentation of the Hebrew University Hadassah Medical School. All studies were performed in hypertransfused animals. Hypertransfused rats were housed in metabolic cages and stool and urine samples were collected for 7 consecutive days. One hour after intravenous injection of the radioiron label on day 0, a single 200 mg/kg dose of chelator was administered intraperitoneally. Because both IRC011 (molecular weight [MW] 429 Daltons) and DF (MW 657 Daltons) are hexadentate chelators binding iron at a 1:1 ratio, the identical milligrams per kilogram dose of chelators used in these in vivo studies represents a 53% higher iron binding capacity for IRC011 compared with DF. In all experiments, each subgroup of control or treated animals consisted of at least 4 (maximum, 11) subjects. All animals were killed on day 7 and the organ distribution of radioiron was determined as decribed above. Whole body radioactivity was determined on day 0 (representing 100% initial radioactivity) and day 7 to determine total radioiron excretion. This measurement was used as an independent confirmation of total radioiron excretion based on the measurement of cumulative fecal and urinary radioiron excretion.
For the statistical evaluation of differences between treatment groups, the Student's t-test has been used.
In Vitro Studies
Ham F-10 culture medium (GIBCO, Grand Island, NY) supplemented with 135 mg/L CaCl2⋅H2O, 200,000 U/L penicillin, 0.2 mg/L streptomycin, 10% horse serum, and 10% fetal bovine serum (Beth Ha'Emek, Beth Ha'Emek, Israel) was used as growth medium. For iron loading experiments, heterologous serum was replaced by serum-free medium. For mincing and washing the organs and for trypsinization, H solution (Ham F-10 medium without calcium and magnesium) was used. Trypsin (type III; Sigma Chemical Co, St Louis, MO) was dissolved in 0.1% wt/vol H solution.
Cell cultures. Cultures from 1-day-old rats (Hebrew University strain) were obtained using methods described in detail in our previous studies.14-17 Briefly, the ventricles were minced and trypsinized, and the combined fractions were resuspended in growth medium into a sterile 250-mL flask (Nunc; Nunclon Delta, Herlev, Denmark) through a sterile mesh to exclude explants. The pooled cells were diluted in growth medium to a density of 9 × 105 to 1 × 106 cells/mL and seeded into a 35-mm diameter Petri dish (Falcon 3001; Falcon Labware, Oxnard, CA). This concentration yielded after 24 to 36 hours an almost confluent layer of beating heart cells at a final density of about 2 × 106 cells. Cultures were kept at 37°C in an atmosphere of 5% CO2 and 95% air. Experiments were performed at 5 days of culture, when greater than 80% of the cells were beating myocardial cells. Continued viability of cultured iron-loaded cells was documented by supravital dye exclusion and by the absence of enzyme (lactate dehydrogenase) leakage into the culture medium after 24 hours of iron loading.
Radioactive iron labeling.59FeCl3 (specific activity, 10 to 15 μCi/μg; Amersham Radiochemical Centre, Amersham, UK) was diluted in 0.005 N HCl and mixed with sufficient sterile ferric ammonium citrate (BDH Fine Chemicals Ltd, Poole, UK) to provide a concentration of 100 μg/mL elemental iron. In all studies, a final concentration of 20 μg/mL (0.36 mmol/L) iron was used for iron-loading and radioiron labeling of heart cell cultures. To terminate incubations, the culture plates were washed twice with 1 mL cold culture medium. The cells were scraped and transferred into counting tubes by means of a rubber policeman and resuspended in 0.5 mL culture medium. 59Fe activity was determined in an automatic well scintillation counter (Auto-Gamma model 5360; Packard Instrument Co).
RESULTS
Studies in Normal Rats
The fate of chelator-bound radioiron after intravenous injection into normal rats of 59Fe-citrate preincubated in vitro with 10 mg of either DF or IRC011 is described in Table 1. Control animals received 59Fe-citrate without the iron chelator. Spontaneous radioiron excretion in normal controls was 4.6% ± 0.1% of the injected dose, restricted entirely to the stool. Iron retained in the body was distributed between circulating RBC (41.2%), the liver (13.1%), and residual tissues (41.1%). In contrast to normal animals showing no urinary iron excretion, 75.4% of iron bound to DF and 91.3% of iron bound to IRC011 were excreted in the urine. Fecal excretion of chelated iron was not appreciably greater than in normal controls. The organ distribution of iron retaining in the body was similar in all three groups, with most of the radioiron found in blood and residual tissues and about one tenth in the liver.
Fate of Chelator-Bound Radioiron
| . | Control . | DF . | IRC011 . |
|---|---|---|---|
| No. of animals | 4 | 4 | 4 |
| Whole body | 95.4 ± 1.0 | 20.8 ± 2.0 | 1.9 ± 0.1 |
| Urine | 0 | 75.4 ± 2.0 | 91.3 ± 0.1 |
| Stool | 4.6 ± 0.1 | 3.8 ± 0.2 | 6.8 ± 0.5 |
| Blood | 41.2 ± 0.8 | 8.7 ± 0.9 | 0.5 ± 0.1 |
| Liver | 13.1 ± 0.5 | 2.7 ± 0.4 | 0.2 ± 0.1 |
| Residual tissues | 46.1 ± 0.4 | 9.4 ± 0.9 | 1.2 ± 0.1 |
| . | Control . | DF . | IRC011 . |
|---|---|---|---|
| No. of animals | 4 | 4 | 4 |
| Whole body | 95.4 ± 1.0 | 20.8 ± 2.0 | 1.9 ± 0.1 |
| Urine | 0 | 75.4 ± 2.0 | 91.3 ± 0.1 |
| Stool | 4.6 ± 0.1 | 3.8 ± 0.2 | 6.8 ± 0.5 |
| Blood | 41.2 ± 0.8 | 8.7 ± 0.9 | 0.5 ± 0.1 |
| Liver | 13.1 ± 0.5 | 2.7 ± 0.4 | 0.2 ± 0.1 |
| Residual tissues | 46.1 ± 0.4 | 9.4 ± 0.9 | 1.2 ± 0.1 |
Studies in Hypertransfused Rats
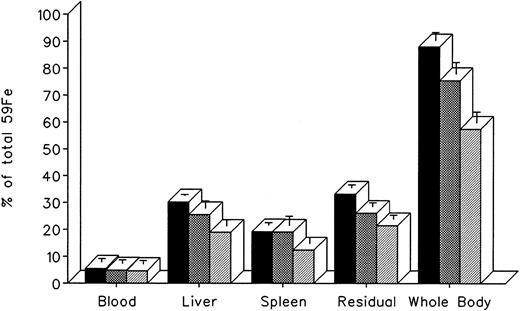
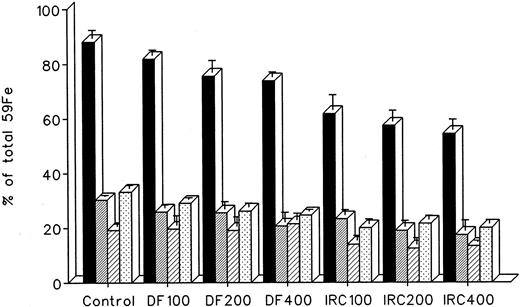
The effect of in vivo iron chelating treatment on hypertransfused rats tagged with the reticuloendothelial label 59Fe-DRBC (heat-damaged erythrocytes) is described in Fig 2. In untreated controls, 7 days after labeling, total body radioactivity was 88.1% ± 3.0% of the injected dose. Of this, 5.5% ± 1.3% was in circulating RBC, 30.2% ± 0.5% was in the liver, 19.2% ± 1.0% was in the spleen, and 33.2% ± 1.1% was in residual tissues (total body radioactivity minus blood, liver, and spleen). As shown in Fig 1, the injection of a single dose of 200 mg/kg DF or IRC011 resulted in a decrease in whole body radioactivity to 75.6% ± 4.4% and 57.5% ± 4.0%, respectively. This was accompanied by a parallel decrease in liver radioactivity from 30.2% ± 0.5% to 25.5% ± 2.7% and 18.9% ± 2.3% in the groups treated by DF and ICR011, respectively. Variations in blood radioactivity in the chelator-treated groups compared with untreated controls were minimal and insignificant. By contrast, the decrease in splenic and residual tissue radioactivity was similar to that in the liver and, as a rule, more profound after IRC011 than with DF treatment.
Radioiron distribution in hypertransfused rat 7 days after the intravenous injection of radioiron-tagged heat-damaged erythrocytes (59Fe-DRBC). Treatment with DF or IRC011 consisted of a single intraperitoneal injection of 200 mg/kg 1 hour after labeling. Residual radioactivity represents whole body radioactivity minus the combined radioactivity of blood, spleen, and liver. Each treatment group consisted of 4 to 11 animals. (▪) Control; () DF; (▨) IRC011.
Radioiron distribution in hypertransfused rat 7 days after the intravenous injection of radioiron-tagged heat-damaged erythrocytes (59Fe-DRBC). Treatment with DF or IRC011 consisted of a single intraperitoneal injection of 200 mg/kg 1 hour after labeling. Residual radioactivity represents whole body radioactivity minus the combined radioactivity of blood, spleen, and liver. Each treatment group consisted of 4 to 11 animals. (▪) Control; () DF; (▨) IRC011.
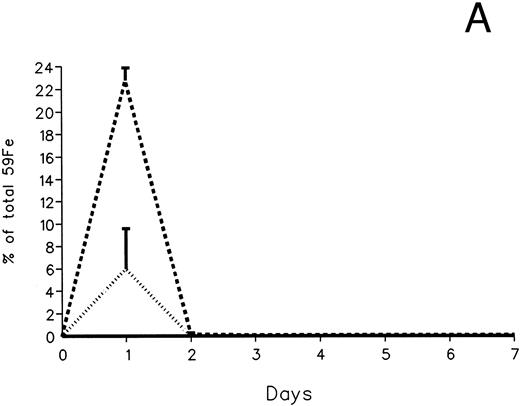
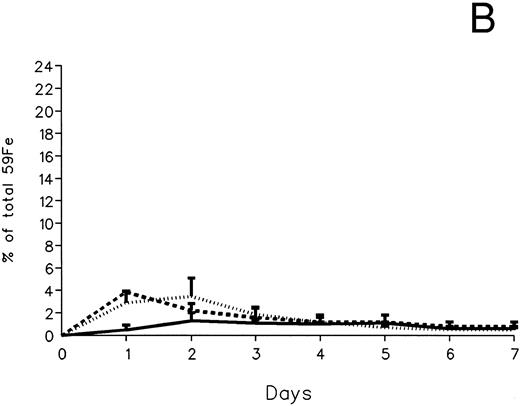
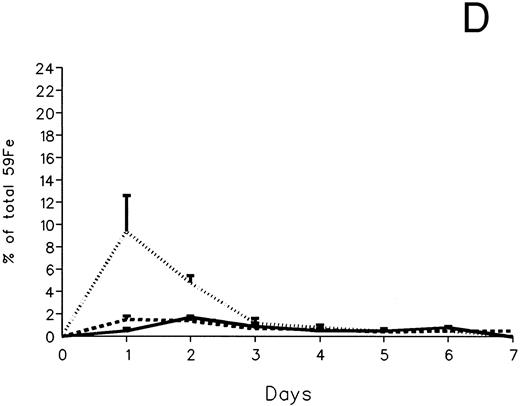
The fecal and urinary excretion of radioiron in the three experimental groups of 59Fe-DRBC–labeled animals is described in Fig 3A and B. In untreated controls, there was no urinary radioiron excretion and spontaneous fecal excretion amounted to roughly 1.5% of the total body radioactivity daily. In the chelator-treated animals, most of the urinary radioiron excretion was completed within the first day of the study, amounting to 6.0% ± 3.6% with DF and 22.8% ± 1.1% with IRC011. Fecal excretion of 59Fe-DRBC radioactivity was relatively modest, amounting to 2.9% ± 1.0% on day 1 with DF and 3.9% ± 0.1% on day 1 with IRC011. Thus, radioiron excretion of the reticuloendothelial label DRBC was predominantly urinary and roughly four times more effective after IRC011 than with DF.
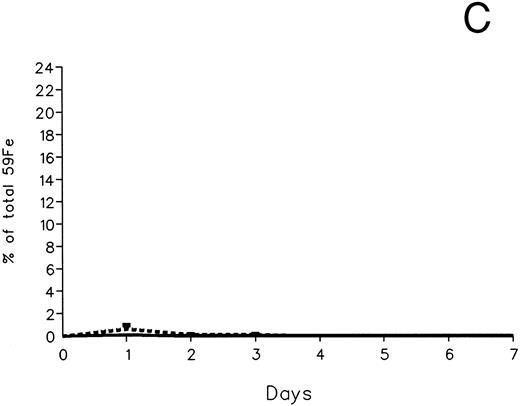
Radioiron excretion in urine and stool. (A) Urinary excretion in 59Fe-DRBC–labeled animals. (B) Fecal excretion in 59Fe-DRBC–labeled animals. (C) Urinary excretion in 59Fe-ferritin–labeled animals. (D) Fecal excretion in 59Fe-ferritin–labeled animals. (——) Control; (⋅⋅⋅⋅) DF; (- - - -) IRC011.
Radioiron excretion in urine and stool. (A) Urinary excretion in 59Fe-DRBC–labeled animals. (B) Fecal excretion in 59Fe-DRBC–labeled animals. (C) Urinary excretion in 59Fe-ferritin–labeled animals. (D) Fecal excretion in 59Fe-ferritin–labeled animals. (——) Control; (⋅⋅⋅⋅) DF; (- - - -) IRC011.
The effect of in vivo iron-chelating treatment in hypertransfused rats tagged with the hepatic parenchymal cell label 59Fe-ferritin is described in Fig 4. The excretion pattern was quite different from that of 59Fe-DRBC, representing almost a mirror image of the former. In untreated controls, total body radioactivity 7 days after labeling was 92.4% ± 1.2% of the injected dose. Of this, 0.8% ± 0.3% was in circulating RBC, 77.4% ± 3.3% was in the liver, 0.5% ± 0.1% was in the spleen, and 13.4% ± 0.2% was in residual tissues. As shown in Fig 4, the injection of a single dose of 200 mg/kg DF resulted in a decrease in whole body radioactivity to 81.5% ± 4.7%, but remained unchanged (93.5% ± 1.9%) after 200 mg/kg of IRC11. This was accompanied by a decrease in liver radioactivity from 77.4% ± 3.3% to 60.7% ± 3.8% after DF, but no change (77.7% ± 2.4%) after IRC011. Variations in blood, spleen, and residual radioactivity in the chelator-treated groups compared with untreated controls were minimal, with a slight increase in residual radioactivity from 13.4% ± 0.2% to 18.5% ± 1.1% in the DF group.
Radioiron distribution in hypertransfused rats 7 days after the intravenous of radiation-tagged ferritin. All experimental conditions are as in the legend to Fig 2. (▪) Control; () DF; (▨) IRC011.
Radioiron distribution in hypertransfused rats 7 days after the intravenous of radiation-tagged ferritin. All experimental conditions are as in the legend to Fig 2. (▪) Control; () DF; (▨) IRC011.
In contrast to DRBC, radioiron excretion after parenchymal cell labeling with 59Fe-ferritin was almost entirely fecal (Fig 3C and D). Again, in contrast to DRBC, DF-induced radioiron excretion was very effective, amounting to 9.4% ± 3.2% on day 1 and 4.8% ± 0.6% on day 2, compared with a very modest increase of less than 1% above control in the IRC011-treated group.
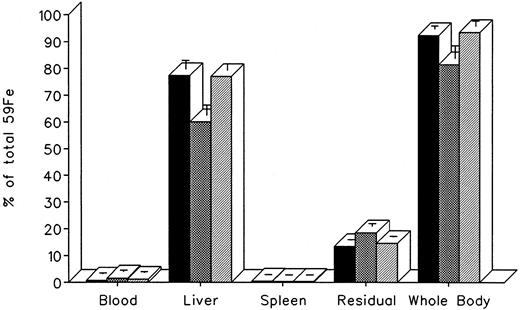
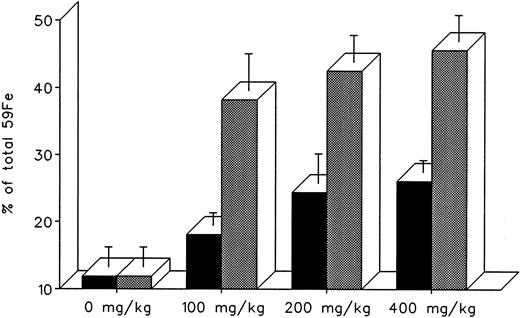
The effect of dose escalation on total 7-day DRBC radioiron excretion and residual DRBC radioiron distribution is described in Figs 5 and 6. At all doses tested, IRC011 was clearly more effective than DF in promoting radioiron excretion (Fig 4). Although with increasing drug dose there was a progressive increase in radioron excretion, the dose-response was quite modest, and excretion at 400 mg/kg IRC011 was only 7.4% higher than with 100 mg/kg. Comparison of residual radioiron distribution before and after chelating therapy (Fig 6) shows that all organs (liver, spleen, and residual tissues) contributed a roughly equal share of radioiron for excretion, with the exception of the spleen, which showed no apparent response to DF.
Total 7-day radioiron excretion in response to a single dose of 100 to 400 mg/kg DF or IRC011 administered 1 hour after 59Fe-DRBC tagging. All experimental conditions are as in the legend to Fig 2. (▪) DF; () IRC011.
Total 7-day radioiron excretion in response to a single dose of 100 to 400 mg/kg DF or IRC011 administered 1 hour after 59Fe-DRBC tagging. All experimental conditions are as in the legend to Fig 2. (▪) DF; () IRC011.
Whole body radioactivity and organ distribution of radioactivity after treatment with a single dose of 100 to 400 mg/kg DF or IRC011 administered 1 hour after 59Fe-DRBC tagging. All experimental conditions are as in the legend to Fig 2. (▪) Whole body; (▨) liver; (▨) spleen; (▧) residual.
Whole body radioactivity and organ distribution of radioactivity after treatment with a single dose of 100 to 400 mg/kg DF or IRC011 administered 1 hour after 59Fe-DRBC tagging. All experimental conditions are as in the legend to Fig 2. (▪) Whole body; (▨) liver; (▨) spleen; (▧) residual.
Studies in Cultured Heart Cells
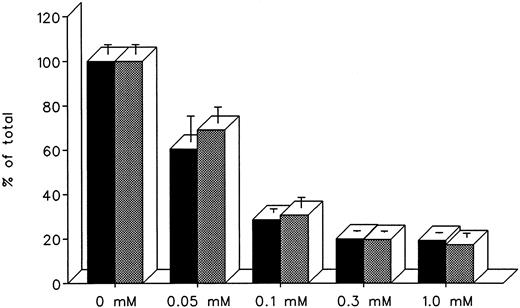
The ability of DF and IRC011 to remove iron directly from the heart was studied in cultured iron-loaded rat heart cells. Five-day-old rat heart cell cultures were exposed for 24 hours to 0.36 mmol/L iron supplied as ferric ammonium citrate, washed, and treated subsequently for an aditional 24 hours with 0.05 to 1.0 mmol/L DF or IRC011 (Fig 7). Washed, untreated control cells had a radioiron content of 29.6% ± 2.6% of the total radioactivity in the iron-loading culture medium. As shown in Fig 7, the ability of DF and IRC011 to remove heart cell radioiron was similar, with a decrease to 60.6% ± 11.6% and 69.1% ± 7.2% of initial values, respectively, after 24 hours of in vitro treatment with 0.05 mmol/L and with a decrease to 20.0% ± 0.4% and 19.7% ± 0.5%, respectively, after 24 hours of treatment with 0.3 mmol/L of each drug.
Effect of drug concentration on radioiron mobilization from cultured, iron-loaded heart cells. Five-day-old cultures were first incubated with 0.36 mmol/L 59Fe-labeled ferric ammonium citrate for 24 hours, washed, and then exposed for an additional 24 to 0.05 to 1.0 mmol/L DF or IRC011. (▪) DF; () IRC011.
Effect of drug concentration on radioiron mobilization from cultured, iron-loaded heart cells. Five-day-old cultures were first incubated with 0.36 mmol/L 59Fe-labeled ferric ammonium citrate for 24 hours, washed, and then exposed for an additional 24 to 0.05 to 1.0 mmol/L DF or IRC011. (▪) DF; () IRC011.
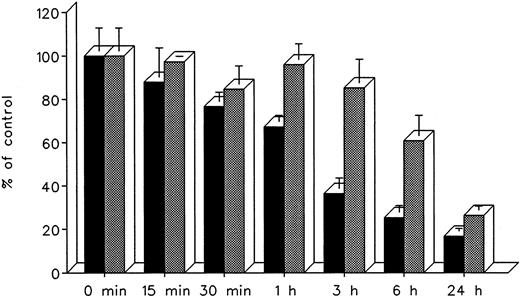
The effect of treatment duration on heart cell radioiron mobilization at a constant chelator concentration of 0.3 mmol/L and treatment times ranging from 15 minutes to 24 hours is described in Fig 8. At all times studied, DF appeared to remove radioiron faster than IRC011, with the most pronounced differences observed at 3 and 6 hours. However, by 24 hours, the final effect of both chelators was comparable, with 16.8% ± 1.3% residual radioactivity after DF and 26.4% ± 1.9% after IRC011 (Fig 8).
Effect of treatment duration on radioiron mobilization from cultured, iron-loaded heart cells. Five-day-old cultures were first incubated with 0.36 mmol/L 59Fe-labeled ferric ammoniun citrate for 24 hours, washed, and then exposed for 0.25 to 24 hours to 0.3 mmol/L of DF or IRC011. (▪) DF; () IRC011.
Effect of treatment duration on radioiron mobilization from cultured, iron-loaded heart cells. Five-day-old cultures were first incubated with 0.36 mmol/L 59Fe-labeled ferric ammoniun citrate for 24 hours, washed, and then exposed for 0.25 to 24 hours to 0.3 mmol/L of DF or IRC011. (▪) DF; () IRC011.
DISCUSSION
In the present studies, we undertook the evaluation of a new synthetic iron chelator IRC011, a substituted polyaza compound, with a number of promising features.7 (1) It is a hexadentate chelator binding Fe(III) at a 1:1 ratio and therefore avoiding the risk of possible toxic intermediate complexes, in contrast with L1, which is a bidentate chelator requiring 3 molecules of the drug for each Fe(III) ion.18 19 (2) IRC011 is water soluble and its stability constant with Fe(III) is more than 1,000 times that of DF. (3) Its acute LD50 in mice exceeds 4.0 mmol/L/kg, compared with 0.44 mmol/L/kg for DF. (4) Unlike DF, polyaza compounds with the structure of IRC011 do not contain any readily hydrolyzable covalent bonds and are anticipated to resist in vivo biotransformation.
In our preliminary experiments, we wished to examine the fate of circulating iron after its binding to the chelator, using radioiron preincubated with the drug before its intravenous injection. Only 1.9% of the iron bound to IRC011 was retained in the body, as compared with 20.8% of iron bound to DF. With both chelators, iron excretion was almost entirely through the urine. The organ distribution of iron retained in the body was identical with that of untreated controls and most likely represents exchange of chelated iron with plasma transferrin. These finding are in keeping with the superior affinity of IRC11 to iron and its resistance to in vivo catabolism.7
The objective of the next phase of our studies was to define the source of iron mobilized in vivo by IRC011. Because transferrin and hemoglobin iron are unavailable for chelation,20,21 the only two alternative sources of chelatable iron are iron stores in parenchymal cells acquired from plasma iron and in RE cells acquired mainly from RBC catabolism.11 The use of selective radioiron probes for labeling the major body iron stores in the hepatic parenchyma (59Fe-ferritin) and the RE system (59Fe-DRBC) has greatly improved the sensitivity and specificity of animal models for the study of in vivo iron chelation.8 In contrast to other studies in which radioiron was bound to transferrin or lactoferrin, all of the radioiron injected with the compounds used by us is located initially to parenchymal or RE stores, resulting in a striking increase in the availability of injected radioiron for interaction with the chelator. Furthermore, hypertransfusion simulates the clinical condition of hypertransfused patients and increases the specificity of the model by suppressing de novo RBC production, decreasing the rate of storage iron release and the recycling of radioiron from its initial site of deposition.
Using these selective radioiron probes, we have found a major difference in the in vivo activity of IRC011 compared with DF. As shown in our previous studies, DF is able to interact with hepatic parenchymal iron stores and to promote the biliary excretion of hepatocellular iron.11 In addition, and to a more limited extent, DF also mobilizes catabolic RBC iron from RE cells and such iron is excreted in the urine.21 22 By contrast, the major source of iron mobilized by IRC011 is iron derived from the catabolism of RBC hemoglobin in the RE system, and the weight per weight efficiency of IRC011 in mobilizing RE iron is about 4 times higher than that of DF. The observed differences in radioiron excretion should be interpreted with the recognition that the doses of chelators used in these studies provided a 53% higher iron-binding capacity for IRC011 compared with DF. As shown in our dose-response studies, the relative advantage of IRC011 compared with DF in RE iron mobilization was highest at the lowest dose used. These findings imply that, because of its higher affinity to iron and anticipated resistance to catabolism, IRC011 may be effective at doses lower than presently required for DF or deferiprone. Whether the fourfold greater efficacy of IRC011 compared with DF in RE iron mobilization may also represent a therapeutic advantage in humans is at present unproven. In view of the likelihood of significant interspecies differences in chelator pharmacokinetics, the present findings in a rodent model should not be extrapolated uncritically to the clinical setting. In addition, the present studies confined to radioiron measurements should be supplemented with iron balance studies to determine the net effect of long-term chelating treatment on body iron stores.
Unlike DF, which interacts readily with hepatocellular iron stores, the present studies indicate that IRC011 has a very limited effect on hepatocellular iron. In view of this limitation, we wished to see whether IRC011 is able to remove iron from the heart, the most critical target of iron toxicity in transfusional iron overload.23 24 As shown in our last experiments using iron-loaded rat heart cells in culture, although the rate of removal was faster with DF, the amount of iron removed from the heart after 24 hours of in vitro treatment was equal for both IRC011 and DF.
The beneficial effects of iron mobilization by phlebotomy in hereditary hemochromatosis show that there is considerable internal redistribution after iron depletion and that the primary form and site of iron subject to removal may not be critical for the long-term prevention of iron toxicity. However, in emergency situations such as symptomatic myocardial disease in which life expectancy is very short, immediate protection is required and direct interaction of a chelator with the critical toxic iron compartment may be life-saving. Thus, precise knowledge of the mechanism of in vivo iron chelation is pertinent for the design and development of improved synthetic iron chelators for use in well-defined clinical settings. Thalassemia major is marked not only by an inappropriately high rate of iron absorption, but also by a striking increase in plasma iron turnover caused by ineffective erythropoiesis and hemolysis.25 As shown in our own animal and clinical studies21,26 and in the elegant clinical studies of Pippard et al,27 reticuloendothelial RBC catabolism in uncontrolled hemolysis is the major source of urinary iron excretion in DF therapy. Because in thalassemia the contribution of RBC catabolism to the influx of iron to the plasma is incomparably higher than that of iron absorption, it is reasonable to assume that RBC catabolism is the predominant source of the toxic and readily chelatable nontransferrin bound plasma iron (NTBI) compartment.28-33 Thus, IRC011 with its superior ability to interact with catabolic RBC iron may not only improve the rate of urinary iron excretion in thalassemia, but possibly also afford better protection of vital tissues from peroxidative damage by preventing the formation or eliminating NTBI. This potential advantage of IRC011 is underlined by the recent observation of Porter et al34 that, despite the presence of excess plasma DF, part of the NTBI in thalassemic patients persists during continuous DF infusion.
The selective interaction of IRC011 with catabolic RBC iron is similar to that described previously with diethylene triamine penta-acetic acid (DTPA), a hydrophilic chelator of proven clinical efficacy.35-38 Although DTPA is unable to penetrate cells, the magnitude of DTPA-induced urinary iron excretion in thalassemic patients is similar to that with DF and sufficient to maintain patients in a negative iron balance.37 38 Thus, although IRC011 is unable to interact directly with hepatocellular iron stores, prior experience with DTPA supports the contention that this does not preclude the subsequent redistribution and depletion of hepatocellular iron stores.
The oral efficacy of IRC011 was not studied in the present set of experiments. Because preliminary studies performed by the manufacturers in mice indicate limited and variable intestinal absorption, additional studies are required to determine whether the degree of its intestinal absorption is or is not adequate for clinical purposes or whether certain of its analogues might show greater intestinal absorption. Previous experience with another family of synthetic chelators, the hydroxypyrid-4-ones, shows that lipophilicity is an important factor in determining the oral effectiveness of an iron chelator and its ability to penetrate cells.18 19 Because IRC011 is a water-soluble compound, its membrane permeability is limited, but it may be regarded as a useful parent compound to a wide spectrum of polyaza analogues. Substitution of its synthetic arms by more lipophilic ligands may result in improved interaction with hepatocellular iron stores and possibly also better oral activity. Such studies are presently under way.
Supported by the Israel Science Foundation administered by the Israel Academy of Sciences and Humanities.
Address reprint requests to Chaim Hershko, MD, Department of Medicine, Shaare Zedek Medical Center, PO Box 293, Jerusalem, Israel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal