Abstract
Donor leukocyte infusions (DLI) can induce sustained remissions in patients with acute and chronic myeloid leukemia who relapse after allogeneic bone marrow transplantation (allo-BMT). Also, in multiple myeloma (MM), incidental reports have indicated the existence of a graft-versus-myeloma effect (GVM) induced by allo-reactive T cells. We performed a retrospective study in a larger group of MM patients to characterize better the effect, prognostic factors, and toxicity of this new treatment modality. Thirteen patients with relapsed MM after allo-BMT were studied. Patients received a total of 29 DLI with T-cell doses ranging from 1 × 106/kg to 33 × 107/kg. Repetitive courses, sometimes with escalated cell doses, were undertaken in case of no response to or relapse after DLI. Eight of 13 patients responded: 4 patients achieved a partial remission and 4 patients achieved a complete remission. Dose escalation was effective in 3 patients. The time to response was median 6 weeks (range, 4 to 10 weeks). Major toxicities were secondary to acute and chronic graft-versus-host disease (GVHD), which occurred in 66% and 56% of all patients and in 87% and 85% of the responders, respectively. Two responding patients developed fatal BM aplasia. The only prognostic factors for response were a T-cell dose greater than 1 × 108/kg and the occurrence of GVHD. Seven of nine patients developing acute GVHD responded, as compared with only 1 response in the 4 patients without GVHD and 6 of 7 patients with chronic GVHD responded, whereas no response was observed in the 5 patients without chronic GVHD. DLI are effective in a high percentage of patients with relapsed MM after allo-BMT, although it is associated with a high treatment-related toxicity. The dose of T cells used may be important in determining the GVM effect, with the highest probability of response after infusion of more than 1 × 108 T cells. Because the optimal individual dose may vary, patient-adapted therapy consisting of repeated infusions with escalating dose of donor leukocytes until maximum response is achieved may therefore be preferable.
INTENSIVE TREATMENT, including high-dose chemotherapy/radiotherapy with either allogeneic or autologous stem cell transplantation has been widely used in multiple myeloma (MM) patients during the last decade and may improve long-term survival and induce long periods of unmaintained disease control.1-6 It is still controversial whether allogeneic bone marrow transplantation (allo-BMT) should be offered to younger patients with an HLA-identical sibling. In a recent retrospective analysis of the European Group for Blood and Marrow Transplantation (EBMT), no advantage could be shown for allo-BMT in general as compared with autologous stem cell transplantation (ASCT).7 A high treatment-related mortality (TRM) in the first year after allo-BMT was the major problem in MM. However, the fact that in patients alive at 1 year after transplantation the disease-free survival was significantly longer after allo-BMT as compared with ASCT suggests a favorable graft-versus-myeloma (GVM) effect. Recently, the existence of GVM was shown more directly by remissions occurring after donor leukocyte infusions (DLI) in patients with relapse MM after allo-BMT.8,9 We described 2 patients who achieved complete remission (CR) after DLI, which has persisted in 1 patient for already more than 3 years.9 Both patients who received 3.3 × 108 and 1.2 × 108 T cells/kg, respectively, developed moderate graft-versus-host disease (GVHD) that was responsive to mild immunosuppressive therapy.9
This study, which summarizes the results of DLI in a larger group of patients from BMT centers in the Netherlands, shows that this form of adoptive immunotherapy induces response in the majority of relapsed MM patients. This observation adds further proof for the existence and benefits of a GVM effect.
MATERIALS AND METHODS
Patient characteristics. Sixty-one patients with MM received an allo-BMT from HLA-identical siblings from 1987 until now at four BM centers in the Netherlands. Patients who were refractory to or relapsed after allo-BMT were candidates for DLI. Patients were ineligible if they had a performance status 4 (World Health Organization), active GVHD, severe infection, and abnormal liver or renal function. A total of 13 patients (median age at the time of first DLI, 48 years; range, 38 to 56 years) with relapsed MM after allo-BMT were included. Two patients (patients no. 1 and 5) have been described before.9 Their follow-up has been updated. Patient characteristics including pretransplant therapies, BMT procedures, and outcome are summarized in Table 1. Except for patient no. 9, who received a transplant from a haploidentical brother, all patients received BMT from HLA-identical siblings. All patients received a partial T-cell–depleted graft, generally containing 1 × 105 T cells/kg recipients' weight (Table 1). All patients received prophylactic immunosuppression consisting of cyclosporin A. Immunosuppressive treatment was stopped 3 months after BMT in case GVHD ≥grade 2 was absent. In 12 patients, immunosuppression was stopped at least 6 months before DLI. In patient no. 8, cyclosporin was stopped 2 months after allo-BMT and 1 month before DLI in the absence of GVHD.
Patient Characteristics
| Patient No. . | Age/Sex at Time of DLI . | MM Stage at Diagnosis . | Disease Status at BMT . | Lines of Previous Chemotherapy (n) . | Conditioning* . | Date of BMT (MM/DD/YY) . | Donor Sex . | TCD . | GVHD Post-BMT (acute/chronic) . | Response to BMT . | Relapse/Progression mo Post-BMT . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49/F | 2A | Resistant | 2 | a | 04/03/92 | F | +1 | 0/nil | PR | 15 |
| 2 | 45/M | 2A | Resistant | 3 | a | 09/04/91 | M | +1.1 | 1/limited | NR | 15 |
| 3 | 47/M | 1A | PR | 1 | d | 07/20/94 | M | +0.6 | 0/nil | CR | 8 |
| 4 | 37/M | 1A | Resistant | 1 | a | 06/18/87 | M | +1 | 0/nil | PR | 87 |
| 5 | 53/M | 3A | PR | 2 | a | 08/01/92 | M | +1 | 2/nil | PR | 25 |
| 6 | 37/F | 2A | PR | 1 | a | 05/09/90 | F | +0.8 | 1/limited | CR | 60 |
| 7 | 50/M | 3A | Resistant | 1 | b | 12/02/92 | M | +0.9 | 0/nil | NR | 25 |
| 8 | 47/M | 3A | Resistant | 2 | c | 06/28/95 | F | +1 | 0/nil | CR | 5 |
| 9† | 43/F | 2A | PR | 1 | a | 05/18/88 | M | +1.3 | 1/limited | CR | 63 |
| 10 | 55/M | 3A | Resistant | 1 | a | 07/21/95 | F | +1 | 1/nil | PR | 9 |
| 11 | 49/F | 2A | PR | 2 | a | 08/27/93 | M | +1 | 1/nil | PR | 27 |
| 12 | 44/M | 3A | Resistant | 2 | a | 06/16/95 | M | +1 | 2/limited | PR | 11 |
| 13 | 40/M | 3A | PR | 2 | a | 01/11/96 | M | +1 | 2/limited | PR | 8 |
| Patient No. . | Age/Sex at Time of DLI . | MM Stage at Diagnosis . | Disease Status at BMT . | Lines of Previous Chemotherapy (n) . | Conditioning* . | Date of BMT (MM/DD/YY) . | Donor Sex . | TCD . | GVHD Post-BMT (acute/chronic) . | Response to BMT . | Relapse/Progression mo Post-BMT . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49/F | 2A | Resistant | 2 | a | 04/03/92 | F | +1 | 0/nil | PR | 15 |
| 2 | 45/M | 2A | Resistant | 3 | a | 09/04/91 | M | +1.1 | 1/limited | NR | 15 |
| 3 | 47/M | 1A | PR | 1 | d | 07/20/94 | M | +0.6 | 0/nil | CR | 8 |
| 4 | 37/M | 1A | Resistant | 1 | a | 06/18/87 | M | +1 | 0/nil | PR | 87 |
| 5 | 53/M | 3A | PR | 2 | a | 08/01/92 | M | +1 | 2/nil | PR | 25 |
| 6 | 37/F | 2A | PR | 1 | a | 05/09/90 | F | +0.8 | 1/limited | CR | 60 |
| 7 | 50/M | 3A | Resistant | 1 | b | 12/02/92 | M | +0.9 | 0/nil | NR | 25 |
| 8 | 47/M | 3A | Resistant | 2 | c | 06/28/95 | F | +1 | 0/nil | CR | 5 |
| 9† | 43/F | 2A | PR | 1 | a | 05/18/88 | M | +1.3 | 1/limited | CR | 63 |
| 10 | 55/M | 3A | Resistant | 1 | a | 07/21/95 | F | +1 | 1/nil | PR | 9 |
| 11 | 49/F | 2A | PR | 2 | a | 08/27/93 | M | +1 | 1/nil | PR | 27 |
| 12 | 44/M | 3A | Resistant | 2 | a | 06/16/95 | M | +1 | 2/limited | PR | 11 |
| 13 | 40/M | 3A | PR | 2 | a | 01/11/96 | M | +1 | 2/limited | PR | 8 |
Abbreviation: TCD, T-cell dose ×105/kg.
a, 120 mg/m2 cyclophosphamide + TBI; b, ifosfamide + melphalan; c, busulfan + cyclophosphamide; d, idarubicine + cyclofosfamide + TBI.
Haploidentical sibling BMT, all other patients genotypically identical sibling.
Immunotherapy with DLI. Donor leukocytes from the original marrow donor were obtained by leukapheresis in 1 to 5 sessions. Cells were infused without further manipulation. Calculation of the number of T cells infused was performed by FACScan analysis of buffy coat cells using CD3-specific monoclonal antibodies. A total of 29 DLI procedures were performed in 13 patients. In patients who did not respond or relapsed after DLI, repetitive courses of DLI, sometimes with escalated T-cell doses, were undertaken. The cumulative number of T cells infused ranged from 1 × 106/kg to 33 × 107/kg. T-cell numbers per DLI course are listed in Table 2.
Details of T-Cell Doses Infused and Response to DLI
| Patient No. . | Time From Relapse or Progression to DLI (wk) . | No. of T Cells Infused (×107/kg) . | Time From Prior Infusion (wk) . | Response of Disease . | Time From DLI to Response (wk) . | Duration of Response (mo) . | GVHD (acute/chronic) . | T-Cell Chimerism Before DLI . | Survival After DLI (mo) . | Causes of Death . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 33 | — | CR | 6 | 38+ | 3/extensive | Donor | 38+ | |
| 2 | 16 | 16 | — | NR | NA | — | 0/nil | ND | 28 | Progressive disease |
| 3 | 32 | 7 | — | NR | NA | — | 1/nil | Donor | 17+ | |
| 4 | 24 | NR | NA | — | 0/nil | Donor | ||||
| 8 | 9 | NR | NA | — | 0/nil | Donor | ||||
| 8 | 20 | NR | NA | — | 0/nil | Donor | ||||
| 4 | 13 | 5 | — | NR | NA | — | 0/nil | ND | 26+ | |
| 10 | 12 | NR | NA | — | 2/nil | ND | ||||
| 25 | 22 | PR | 10 | 5* | 3/limited | |||||
| 14 | 44 | PR (NA) | 4 | 3+ | 0/nil | Donor | ||||
| 5 | 12 | 12 | — | CR | 7 | 14 | 2/limited | Donor | 25+ | |
| 24 | 1 | 82 | NR | NA | — | 0/nil | Donor | |||
| 10 | 9 | NE | NA | — | NE | |||||
| 6 | 10 | 9 | — | NR | NA | — | 0/nil | Donor | 20+ | |
| 8 | 9 | NR | NA | — | 0/nil | Donor | ||||
| 10 | 32 | NR | NA | — | 0/nil | Donor | ||||
| 20 | 12 | NR | NA | — | 0/nil | Donor | ||||
| 7 | 68 | 7 | — | CR | 8 | 18+ | 3/extensive | Mixed | 18+ | |
| 8 | 1 | 8 | — | PR | 5 | 2s | 3/NE | Mixed | 3 | Septicemia |
| 9 | 112 | 3 | — | NR | NA | — | 1/limited | Donor | 9 | Septicemia |
| 6 | 18 | PR | 6 | 3s | 1/limited | |||||
| 10 | 11 | 5 | — | PR | 5 | 2+ | 0/nil | Donor | 7+ | |
| +0.3 × 106 | CD34/kg | |||||||||
| 15 | 7 | CR | 5 | 7+ | 3/limited | |||||
| +0.6 × 106 | CD34/kg | |||||||||
| 11 | 28 | 5 | — | NR | NA | — | 0/nil | Donor | 6+ | |
| 10 | 10 | NR | NA | 0/nil | ||||||
| 12 | 12 | 0.1 | — | PR | 6 | 4+ | 0/nil | Donor | 8+ | |
| 1 | 14 | NE | NA | — | 0/nil | |||||
| 13 | 34 | 3 | NR | NA | — | 2/nil | ND | 5+ | ||
| 5 | 16 | NE | NA | NE |
| Patient No. . | Time From Relapse or Progression to DLI (wk) . | No. of T Cells Infused (×107/kg) . | Time From Prior Infusion (wk) . | Response of Disease . | Time From DLI to Response (wk) . | Duration of Response (mo) . | GVHD (acute/chronic) . | T-Cell Chimerism Before DLI . | Survival After DLI (mo) . | Causes of Death . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 33 | — | CR | 6 | 38+ | 3/extensive | Donor | 38+ | |
| 2 | 16 | 16 | — | NR | NA | — | 0/nil | ND | 28 | Progressive disease |
| 3 | 32 | 7 | — | NR | NA | — | 1/nil | Donor | 17+ | |
| 4 | 24 | NR | NA | — | 0/nil | Donor | ||||
| 8 | 9 | NR | NA | — | 0/nil | Donor | ||||
| 8 | 20 | NR | NA | — | 0/nil | Donor | ||||
| 4 | 13 | 5 | — | NR | NA | — | 0/nil | ND | 26+ | |
| 10 | 12 | NR | NA | — | 2/nil | ND | ||||
| 25 | 22 | PR | 10 | 5* | 3/limited | |||||
| 14 | 44 | PR (NA) | 4 | 3+ | 0/nil | Donor | ||||
| 5 | 12 | 12 | — | CR | 7 | 14 | 2/limited | Donor | 25+ | |
| 24 | 1 | 82 | NR | NA | — | 0/nil | Donor | |||
| 10 | 9 | NE | NA | — | NE | |||||
| 6 | 10 | 9 | — | NR | NA | — | 0/nil | Donor | 20+ | |
| 8 | 9 | NR | NA | — | 0/nil | Donor | ||||
| 10 | 32 | NR | NA | — | 0/nil | Donor | ||||
| 20 | 12 | NR | NA | — | 0/nil | Donor | ||||
| 7 | 68 | 7 | — | CR | 8 | 18+ | 3/extensive | Mixed | 18+ | |
| 8 | 1 | 8 | — | PR | 5 | 2s | 3/NE | Mixed | 3 | Septicemia |
| 9 | 112 | 3 | — | NR | NA | — | 1/limited | Donor | 9 | Septicemia |
| 6 | 18 | PR | 6 | 3s | 1/limited | |||||
| 10 | 11 | 5 | — | PR | 5 | 2+ | 0/nil | Donor | 7+ | |
| +0.3 × 106 | CD34/kg | |||||||||
| 15 | 7 | CR | 5 | 7+ | 3/limited | |||||
| +0.6 × 106 | CD34/kg | |||||||||
| 11 | 28 | 5 | — | NR | NA | — | 0/nil | Donor | 6+ | |
| 10 | 10 | NR | NA | 0/nil | ||||||
| 12 | 12 | 0.1 | — | PR | 6 | 4+ | 0/nil | Donor | 8+ | |
| 1 | 14 | NE | NA | — | 0/nil | |||||
| 13 | 34 | 3 | NR | NA | — | 2/nil | ND | 5+ | ||
| 5 | 16 | NE | NA | NE |
Abbreviations: NE, not evaluable; NA, not applicable; ND, not done; S, sensored; NR, no response; CR, complete response.
Relapse was treated with VAD (3×) and intermediate-dose melphalan (70 mg/m2 IV).
Definition of response and relapse. A partial remission (PR) was defined as at least 50% or more tumor reduction as measured by decrease in myeloma proteins in serum and/or urine measured at least twice and lasting for at least 2 months. In responding patients, BM evaluation had to be in agreement with myeloma protein measurement and had to be accompanied by improvement of clinical symptoms as well. A CR was defined as the complete disappearance of myeloma proteins and absence of bone marrow monoclonal plasma cells.10 Relapse was defined as the doubling of myeloma proteins measured at least twice and in patients with CR as the reappearance and subsequent increase of myeloma proteins.
Definition of GVHD. The clinical manifestations of acute GVHD were graded I to IV according to the criteria described by Thomas et al.11 Chronic GVHD was classified as limited or extensive as described by Shulman et al.12 If GVHD occurred ≤3 months after BMT or ≤3 months after donor leukocyte infusions, it was defined as acute. If the patient had GVHD beyond 3 months, it was defined as chronic.
Analysis of chimerism. Analysis of chimerism of peripheral blood T cells and non-T cells was performed by polymerase chain reaction of tandem-repeat sequences of hypervariable regions of human DNA as described before.13 In addition, in sex-mismatched transplants, chimerism was evaluated by fluorescence in-situ hybridization (FISH) using X-chromosome– and Y-chromosome–specific probes. These probes were used in conjunction with T-cell–specific monoclonal antibodies, eg, CD5 and CD3, coupled to alkaline phosphatase-antialkaline phosphatase (APAAP) and cells were analyzed on cytospin slides as described before.14
RESULTS
Response. Eight of 13 (62%) patients responded. Four patients achieved a PR and 4 patients a CR. Six patients responded after the first dose of leukocytes containing between 1 × 106 and 33 × 107 T cells/kg. Dose escalation did induce a partial remission in 2 initial nonresponding patients and a CR in 1 partially responding patient. Dose escalation had no effect in 3 nonresponding patients. It is still too early to evaluate the effect of dose escalations in 2 other patients (patients no. 12 and 13). The median time to response was 6 weeks (range, 4 to 10 weeks). Two patients relapsed biochemically and overt clinically 5 and 14 months after DLI, respectively. The first patient (no. 4) then received 3 courses of VAD chemotherapy (vincristine, adriamycin, dexamethasone)15 followed by intermediate-dose melphalan at 70 mg/m2 intravenously (IDM)4 followed by infusion of unmanipulated peripheral blood stem cells (PBSC; 4.6 × 106 CD34+/kg) containing 14 × 107 T cells/kg. The patient went into PR, but is not assessable for response to the last DLI due to the prior chemotherapy. The other patient (no. 5) received 1 × 107 donor T cells/kg without response, followed 9 weeks later by 1 × 108 donor T cells/kg, still without response evaluated 4 weeks after the last DLI. The median response duration was 5 months (range, 2+ to 38+ months). Details of T-cell doses administered, responses in correlation with toxicity profiles, and durations of survival are shown in Table 2.
Follow-up of nonresponding patients. Patient no. 2 received no further treatment and died from progressive disease 28 months after DLI.
Two patients were treated with chemotherapy. Patient no. 3, who had progressive symptomatic disease after the fourth DLI, showed a good clinical and partial biochemical response to 3 courses of cyclophosphamide, adriamycin, and prednisone combination lasting 6 months. It is too early to evaluate the response to the current treatment with conventionally dosed melphalan and prednisone. Patient no. 6, who had progressive symptomatic disease during and after DLI, was treated with melphalan and prednisone starting 8 months after the last DLI. The effect of chemotherapy is not yet evaluable. Patient no. 11 was rapidly progressive after the last DLI and has received palliative radiotherapy on the thoracic spine and left shoulder. Durations of survival are listed in Table 2.
Toxicity. Two patients developed BM aplasia (patients no. 8 and 9) and died of septicemia. In 1 patient (no. 8), BM aplasia was associated with acute GVHD grade 3. The other patient (no. 9) developed severe thrombopenia (<30 × 109/L) 6 weeks and severe neutropenia 8 weeks (<1 × 109/L) after DLI. In this patient, DLI was followed by acute GVHD grade 1 and limited chronic GVHD. BM aplasia did not occur in the other patients. Three patients developed acute GVHD grade I, 1 patient developed grade II, 5 patients developed grade III, and 6 patients developed chronic GVHD (4 limited and 2 extensive).
Factors correlating with response to DLI. Seven of the 9 patients with acute GVHD and 1 of the 4 patients without acute GVHD responded, suggesting a correlation of GVHD with response (P = .12; Fisher's exact test, two-sided). This correlation was statistically significant in the patients with chronic GVHD: 6 of 7 patients developing chronic GVHD responded, whereas none of the 5 patients without chronic GVHD showed a response (P = .015; Fisher's exact test, two-sided).
Response was induced after 4 of 6 T-cell doses with more than 1.0 × 108/kg, whereas it occurred in only 4 of 21 T-cell doses ≤1.0 × 108/kg (P = .02; Fisher's exact test, two-sided). One of the 2 nonresponding patients who received more than 1.2 × 108 T cells/kg did not develop GVHD. The relationship between T-cell dose, GVM, and GVHD is shown in Table 3.
Correlation Between T-Cell Dose, GVM, and GVHD
| T-Cell Dose/kg . | No. of DLI With This Cell Dose . | GVM Effect . | Acute GVHD . | Chronic GVHD . |
|---|---|---|---|---|
| <1 × 107 | 1 | 1 | 0 | 0 |
| 1 × 107 to ≤5 × 107 | 7 | 1 | 2 | 1 |
| 5 × 107 to ≤1 × 108 | 11 | 3 | 4 | 2 |
| >1 × 108 | 6 | 4 | 4 | 4 |
| T-Cell Dose/kg . | No. of DLI With This Cell Dose . | GVM Effect . | Acute GVHD . | Chronic GVHD . |
|---|---|---|---|---|
| <1 × 107 | 1 | 1 | 0 | 0 |
| 1 × 107 to ≤5 × 107 | 7 | 1 | 2 | 1 |
| 5 × 107 to ≤1 × 108 | 11 | 3 | 4 | 2 |
| >1 × 108 | 6 | 4 | 4 | 4 |
No definite conclusions could be drawn from the analysis of T-cell chimerism in relation to response to DLI. Two (responding) patients were mixed T-cell chimeras before DLI, whereas 9 other patients (3 CR, 3 PR, and 3 no responses [NR]) were complete donor T-cell chimeras.
Of the 8 responding patients, 4 (50%) had no GVHD with initial BMT, and in 4 of 8 responding patients, the time interval between BMT and DLI was less than 2 years.
DISCUSSION
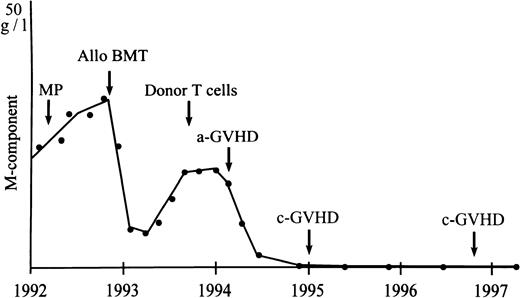
The antileukemic effect of GVHD and DLI is well documented in both acute leukemia and chronic myeloid leukemia (CML).16-28 Also, in myeloma, incidental reports of DLI in small numbers of patients have indicated the existence of a GVM effect.8,9 Our study in a larger group of patients confirms the potency of donor leukocytes to induce responses in relapsed MM after allo-BMT. Sixty-two percent (8 of 13) of our patients responded, including 4 with CR (30%). However, the ultimate response rate was 46%, because 2 responding patients died treatment-related early deaths due to BM aplasia. Five responding patients received systemic prednisone for treatment of GVHD. However, the influence of corticosteroids on response was unlikely, because 4 of these patients had received high-dose prednisone before without a response. Moreover, CR (in 4 patients) induced by prednisone alone is improbable. From the 2 patients with a PR receiving prednisone, in 1 patient (the patient with CR after dose escalation) myeloma protein declined significantly before the onset and treatment of GVHD. The other patient was progressive after CHOP chemotherapy, whereas a response was measurable at the onset of GVHD. CRs may be durable, because 1 patient is still free of disease (no signs of myeloma in blood and/or urine as determined by immunofixation and BM as determined by immunophenotyping of plasma cells) 3 years after DLI (Fig 1). Two other patients are still in CR, with a follow-up of 18 and 7 months, respectively, whereas 1 patient in CR relapsed after 14 months. However, longer times of observation in more patients will be necessary to determine more exactly the overall response and duration after DLI in MM. The median time to document a response was 6 weeks (range, 4 to 10 weeks), which is comparable to patients with acute leukemia and CML.16,19 21
Durable GVM effect as shown by response of serum M-protein to infusion of donor T cells in a patient with relapsed myeloma after allogeneic BMT. MP, melphalan and prednisone chemotherapy.
Durable GVM effect as shown by response of serum M-protein to infusion of donor T cells in a patient with relapsed myeloma after allogeneic BMT. MP, melphalan and prednisone chemotherapy.
The number of donor leukocytes infused may be important in mediating a GVM effect. A T-cell dose of more than 1 × 108 cells/kg induced a response in 4 of 6 patients (67%), compared with only 4 responses after 21 DLI containing ≤1 × 108 T cells/kg. The T-cell dose/response relation was quite evident in 3 patients who responded after dose escalation, although it cannot be excluded completely that repeated infusion with the lower dose would have provided the same results. Also, a late response to the previous lower dose seemed unlikely, because in 2 previous NR patients, the time from the prior infusion was 18 and 22 weeks, respectively (patients no. 4 and 9), whereas the third patient (no. 11) was progressive after initial PR to the lower T-cell dose.
Apart from the kind of malignancy, pre-DLI characteristics have been described that may predict response, including GVHD after initial BMT, time interval between BMT and DLI, and the occurrence of GVHD post-DLI.21 29 In our patients, only the occurrence of GVHD and T-cell dose of greater than 1 × 108/kg were correlated with response. Seven of 8 responding patients developed acute GVHD and 6 of 7 responders assessable for evaluation developed chronic GVHD. All 5 patients with GVHD grade 3 responded as compared with only 1 responder in 4 patients without GVHD. It is likely that the association between higher T-cell dose and response is due to a close relationship between GVHD and GVM. The high success rate of DLI in our group of patients may therefore be partially due to the fact that the initial BMT was performed with a T-cell–depleted graft containing 105/kg T cells with minimal or no GVHD posttransplantation. Although the presence or absence of GVHD at initial BMT did not relate with response to DLI in our series, it is possible that the outcome for non–T-cell–depleted grafts with more initial severe acute and for chronic GVHD may be less successful. In addition, responses may have occurred in some patients who would not have relapsed if they had received unmodified grafts. This may be shown by the fact that the only lasting CRs are present in 3 patients with no or very mild GVHD at initial BMT and grade III acute GVHD plus chronic GVHD after DLI.
A T-cell dose-response effect of DLI had already been shown in leukemia, especially in CML.18,19 However, T-cell doses required to induce responses in MM may be higher than in CML. Patients with CML may respond to doses as low as 1 × 106/kg. Although T-cell doses as low as 1 × 106/kg may induce GVM (patient no. 12 and the patient described by Tricot et al8 ), our data indicate that 5 × 107/kg T cells may be preferred as the starting dose. In case of no response within 10 weeks, the infusion can be repeated with a higher number of donor lymphocytes individually adapted on preceding GVHD.
The major toxicities were secondary to acute and chronic GVHD, which occurred in 66% and 56% of all patients and in 87% and 85% of the responders, respectively. The high incidence of GVHD in the responders may be related to the high number of donor T cells that were used to achieve remission. BM aplasia developed in only 2 responding patients, but was fatal in both patients. Marrow aplasia in up to 50% of patients receiving DLI for relapsed CML and acute leukemia has been reported.15,16 Kolb et al16 showed that DLI in CR of acute leukemia or in cytogenetic relapse of CML are less prone to severe myelosuppression. It was recently shown that persisting residual donor hematopoiesis in relapsed patients protects them from DLI-induced BM aplasia.30 In our group of patients, only 2 were mixed T-cell chimeras, but both still had residual donor hematopoiesis at the time of DLI, as indicated by analysis of peripheral blood non-T cells. GVHD grade III probably contributed to the fatal aplasia in patient no 8. In the other patients, complete donor T-cell chimerism was likely to be associated with residual normal donor hematopoiesis. This further indicates that donor hematopoiesis usually persists in relapsed MM after allo-BMT and that the incidence of DLI-induced myelosuppression may therefore be low as compared with acute leukemia and CML.
The recent introduction of techniques to abrogate excessive GVHD by using donor T lymphocytes transduced with the suicide gene viral thymidine kinase (TK) may be helpful to reduce this complication.29,31 However, the close relationhsip between response and GVHD suggests that GVM is mediated by alloreactive T cells directed against minor histocompatibility antigens present on both normal and myeloma cells. Elimination of the TK-transduced T cells by in vivo administration of ganciclovir might abrogate the GVM effect as well.32 Studies to determine the cell types responsible for mediating the GVM effect are therefore urgently warranted. Infusion of purified allogeneic immune cells or selective elimination of subsets of TK-transduced donor T cells may elucidate whether GVM is mediated by a specific effector population.
In conclusion, donor leukocyte infusions can induce remissions in the majority of patients with relapsed MM after allo-BMT. The dose of donor leukocytes used may be important for the achievement of GVM. Doses of more than 1 × 108 donor T cells gave the highest probability of response, although the optimal individual dose may vary. In MM, individually adapted therapy consisting of repeated infusions with escalating doses of donor leukocytes until maximal response is achieved may therefore be preferable. Efforts, including infusion of subsets of T cells,33 combination of DLI with cytokines such as interleukin-2,19 34 and selective in vivo elimination of TK-transduced donor effector cells may reduce the toxicity of GVHD while preserving the GVM effect of DLI.
Address reprint requests to H.M. Lokhorst, MD, PhD, University Hospital Utrecht, Department of Haematology (G03.647), PO Box 85.500/Heidelberglaan 100, 3508 GA Utrecht/3584 CX Utrecht, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal