SINCE CHEMOTHERAPY beginnings 50 years ago by Farber and his colleagues, childhood leukemia treatment has been one of the most dramatic cancer success stories.1 Currently more than 70% of children with acute lymphoblastic leukemia are alive and disease-free at 5 years, probably making it the most successfully treated of the disseminated human cancers. Certain forms of acute childhood leukemia have a 90% probability of cure, yet there is a group that is still very therapy resistant. Concurrently, in recent years, our knowledge of the molecular and cellular biology underlying these pediatric leukemias has significantly increased. We are at the point where we can reasonably answer these questions: What are the reasons for childhood leukemia treatment success compared with other cancers? Why are certain subgroups of patients therapy-resistant while most patients have very therapy-sensitive disease? I believe that our knowledge of the molecular genetic abnormalities will provide the key to understanding the treatment successes and failures in childhood leukemia.
CHEMOTHERAPY BEGINNINGS
The post World War II era witnessed the availability of chemical agents with potential value for cancer treatment. In the late 1940s, the thinking by investigators such as Sidney Farber, was that agents that antagonize important metabolites, eg, folic acid, could be useful. One such agent, aminopterin, was found to produce temporary remission in children with acute leukemia.1 By the early 1950s, an entirely different group of agents, ACTH and the glucocorticoids, became available and showed responses in childhood acute leukemia.2 It was apparent, even from these early studies with both folic acid antagonists and glucocorticoids, that childhood acute leukemias were among the most responsive of all cancers studied. Despite these responses, these early agents resulted in few cures. As the 1960s, 1970s, and 1980s progressed, other chemical agents, eg, methotrexate, L-asparginase, epipodophylotoxins, vincristine, anthracyclines, oxazaphosphorines, and more recently myeloablative therapy followed by bone marrow transplantation, have become important in childhood acute leukemia treatment success.
COMBINATION CHEMOTHERAPY AS A MAJOR ADVANCE IN CHILDHOOD ACUTE LEUKEMIA
Acute Lymphoblastic Leukemia (ALL)
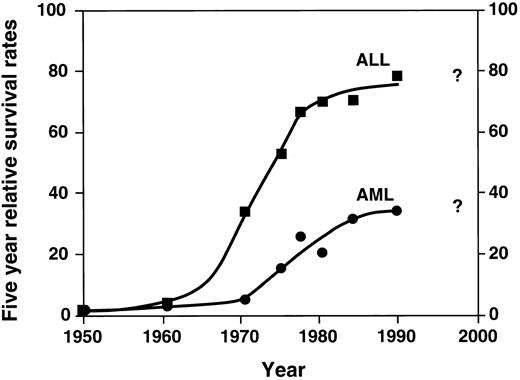
Probably the single most important advance in childhood acute leukemia treatment is the success with chemotherapy, using a combination of agents. While the late 1940s and 1950s were characterized by single agent chemotherapy development, the 1960s were characterized by the beginning of investigation into multiagent chemotherapy by several groups. These groups included the St. Jude Children's Research Hospital, led by Don Pinkel; the Children's Cancer Study Group “A” (the forerunner of the current Childrens Cancer Group); other investigators in Boston; and later the Pediatric Oncology Group and investigators elsewhere. The relatively rapid dissemination of results demonstrated the success of multiagent chemotherapy. This approach quickly became the accepted form of treatment with dramatic improvement in survival. These results are shown in Fig 1.3 Figure 1 demonstrates the change from the slow improvement of outcomes of single agent therapy in the 1950s to a second dramatic shift in the curve beginning in the mid 1960s, largely a result of combination chemotherapy. As shown in the figure, the results are most dramatic in ALL. Induction chemotherapy with vincristine, prednisone, and L-asparginase, followed by postremission therapy with mercaptopurine and methotrexate, quickly became the standard childhood ALL treatment.
Five-year relative survival rates for ALL and AML: End Results Group, National Cancer Institute Surveillance, Epidemiology and End Results Program, 1996. (▪: ALL) and (•: AML) represent defined time periods. The “best fit” curve was drawn by the author.
Five-year relative survival rates for ALL and AML: End Results Group, National Cancer Institute Surveillance, Epidemiology and End Results Program, 1996. (▪: ALL) and (•: AML) represent defined time periods. The “best fit” curve was drawn by the author.
There were at least two additional therapeutic advances responsible for the significant improvement in outcome during the 1970s and 1980s. One important advance was the introduction of presymptomatic therapy for central nervous system leukemia.4 A second advance was the introduction of alternative combinations and timings of chemotherapy combining multiple agents. Multiagent chemotherapy studies, while reported by several groups throughout the world, were particularly well organized by the Berlin-Frankfurt-Munster groups in Germany5 and the CCG, which demonstrated the value of delayed intensification.6 These multiagent, intensive therapy trials have continued to the present; their future probably will be tied to therapies specific for genetically defined patient subgroups.
After a dramatic improvement in the 1960s and 1970s, the survival curve for ALL began to plateau in the 1980s. Does the last point on the curve in Fig 1 indicate new advances? Time will answer this question and will determine whether the newer therapies will improve the outlook in the questionable period, ie, the 1990s and beyond.
Acute Myelocytic Leukemia (AML)
In childhood AML, combination chemotherapy also had a significant effect on outcomes, as illustrated in Fig 1, although the results have not been as dramatic as in ALL. Remission induction significantly improved with the introduction of cytarabine and anthracycline, resulting in successful remission induction in most patients.7,8 The role of postremission continuation chemotherapy in childhood AML remains uncertain, although recent evidence suggests that intensive postremission treatment with chemotherapy alone or in combination with bone marrow transplantation is often curative.9 Recently, intensive postremission therapies have been shown to result in 50% 5-year survival.10 However, as shown in the figure, there is evidence that newer therapies (and more widespread use of intensive therapies, eg, allogeneic transplantation as described in the next section) are very much needed in this group of diseases.
BLOOD AND BONE MARROW TRANSPLANTATION FOR CHILDHOOD ACUTE LEUKEMIA TREATMENT
One of the results of the early years of combination chemotherapy was the demonstration that many leukemias quickly develop drug resistance resulting in subsequent relapse. One solution to this problem was the development of more intensive chemotherapy combined with total body irradiation followed by bone marrow transplantation. Studies in the 1970s and early 1980s demonstrated both the feasibility of this approach and the idea that some patients with therapy-resistant leukemia could be cured with combination chemoradiotherapy followed by matched sibling transplantation.11-13
In childhood AML, chemotherapy results have always been inferior to those in ALL. Thus in AML, allogeneic bone marrow was proposed as a primary form of therapy. Transplantation demonstrated that intensive postremission chemoradiotherapy followed by allogeneic marrow transplantation resulted in a significant number of patients cured of their disease.11-14 In “biologically” randomized trials, which compared allogeneic matched sibling transplantation with intensive chemotherapy in patients in first remission, disease-free survival of transplanted patients was improved over those who received chemotherapy alone.13,14 In children with AML who have a matched sibling donor, allogeneic transplantation continues to be the treatment of choice. Also, in children with AML who relapse from chemotherapy, marrow transplantation provides an opportunity for disease cure. Patients who lack a matched sibling donor are candidates for autologous transplantation. While providing cures in some patients, the role of autologous transplantation in childhood AML is less clear than for allogeneic transplants. There is evidence that autologous transplants do not provide the immunologic graft-versus-leukemia effect of allogeneic transplants15 and that reinfused autologous marrow may contain leukemia cells.16
Blood and marrow transplantation in childhood ALL has, with several exceptions, generally been confined to patients who relapse from primary therapy. Because of the excellent outcomes of most children with chemotherapy, relapsed patients have been the focus of most studies. Analysis of results from a large number of patients demonstrate that allogeneic sibling transplant gives results that are superior to chemotherapy.17 In contrast, autologous transplantation in children with ALL gave results that are, in most cases, inferior to sibling transplants but provided alternatives to chemotherapy, especially in children who relapse following primary chemotherapy.18,19 In contrast to standard risk ALL, Philadelphia-chromosome positive ALL continues to have a very poor response to chemotherapy. Allogeneic bone marrow transplant is the preferred treatment, with transplant conducted soon after induction chemotherapy.20
One of the major difficulties with marrow transplant has been the lack of matched sibling donors for most patients. Alternative donor sources of stem cells represent an exciting development in the field of blood and marrow transplant for acute leukemias. The availability of matched unrelated donors or, more recently, matched, unrelated umbilical cord donors are becoming helpful in providing additional children with transplant options. Preliminary results suggest that children with acute leukemia who receive unrelated donor marrow have outcomes that come close to those with matched sibling donors.21 Unrelated umbilical cord blood has an additional advantage over marrow in that it is readily available.22 The development of large umbilical cord blood banks will allow study of additional patients who lack HLA-matched siblings.
CHRONIC MYELOGENOUS LEUKEMIA (CML): AN UNCOMMON FORM OF CHILDHOOD LEUKEMIA WITH BLOOD OR MARROW TRANSPLANT AS A CURATIVE THERAPY
CML is an uncommon leukemia in children, representing less than 4% of the total of approximately 2,600 cases of childhood leukemia in the United States. Childhood CML (not to be confused with juvenile myelomonocytic leukemia) is identical to adult CML with the t(9; 22)(q34; q11) translocation and 210-kD BCR-ABL fusion gene product. The fusion gene product differs from the 185-kD BCR-ABL fusion gene product in t(9; 22)(q34; q11) Philadelphia-chromosome positive ALL (see Table 1). Transplantation is frequently curative in this disease: CML results in children are generally superior to those in adults.23
Cellular Genotype Defines Major Forms of Childhood Leukemia: Leukemias Often Result from the Fusion of Genes Critical for Signal Transduction or Transcription
| Molecular Genetic Abnormality . | Translocation . | Biochemical Defect . | Associated Features . | Reference . |
|---|---|---|---|---|
| Chronic myelogenous leukemia | ||||
| BCR-ABL fusion (p210) | t(9; 22)(q34; q11) | Signal transduction | Myeloproliferation | 36 |
| B-cell lineage leukemia | ||||
| TEL-AML1 fusion | t(12; 21) cryptic | Transcription | Good prognosis | 50 |
| BCR-ABL fusion (p185) | t(9; 22)(q34; q11) | Signal transduction | Poor prognosis | 37 |
| E2A-PBX fusion | t(1; 19)(q23; p13) | Transcription | Pre-B phenotype, poor response to antimetabolites | 66 |
| MLL-AFX1 fusion | t(x; 11)(q13; q23) | Transcription | 67 | |
| MLL-AF4 fusion | t(4; 11)(q21; q23) | Transcription | Mixed lineage, infants, poor prognosis | 39, 40, 45 |
| MLL-ENL fusion | t(11; 19)(q23; p13) | Transcription | Hyperleukocytosis | 38 |
| IGH-MYC fusion | t(8; 14)(q24; q32) | Transcription | FAB L3, extramedullary disease | 68 |
| IGκ-MYC fusion | t(2; 8)(p12; q24) | Transcription | FAB L3, extramedullary disease | 68 |
| IGλ-MYC fusion | t(8; 22)(q24; q11) | Transcription | FAB L3, extramedullary disease | 68 |
| Hyperdiploidy | None | Unknown | Good prognosis | 49 |
| T-cell lineage leukemia | ||||
| TAL1 deletion | None | Transcription | Extramedullary disease, CD2+, CD10− | 69 |
| TCRδ-TAL1 fusion | t(1; 14)(p32; q11) | Transcription | Extramedullary disease, CD2+, CD10− | 69 |
| TCRβ-TAL1 fusion | t(1; 7)(p32; q35) | Transcription | Extramedullary disease, CD2+, CD10− | 69 |
| TCRα-MYC fusion | t(8; 14)(q24; q11) | Transcription | Extramedullary disease | 70 |
| TCRδ-RBTN1 fusion | t(11; 14)(p15; q11) | Transcription | Extramedullary disease | 71 |
| TCRδ-RBTN2 fusion | t(11; 14)(p13; q11) | Transcription | Extramedullary disease | 71 |
| TCRδ-HOX11 fusion | t(10; 14)(q24; q11) | Transcription | Extramedullary disease | 72 |
| TCRβ-LCK fusion | t(1; 7)(p34; q34) | Signal transduction | Extramedullary disease | 73 |
| Acute myelogenous leukemia | ||||
| AML 1-ETO fusion | t(8; 21)(q22; q22) | Transcription | FAB M2 | 41 |
| CBFβ-MYH11 fusion | inv(16)(p13; q22) | Transcription | FAB M4EO | 74 |
| DEK-CAN fusion | t(6; 9)(p23; q34) | Transcription | Basophilia | 75 |
| MLL-AF1p fusion | t(1; 11)(p32; q23) | Transcription | 76 | |
| MLL-AF1q fusion | t(1; 11)(q21; q23) | Transcription | FAB M4-M5, infants | 77 |
| MLL-AF6 fusion | t(6; 11)(q27; q23) | Transcription | 78 | |
| MLL-AF9 fusion | t(9; 11)(p22; q23) | Transcription | FAB M4, M5, infants | 79 |
| MLL-AF10 fusion | t(10; 11)(p12; q23) | Transcription | FAB M5 | 80 |
| MLL-AF17 fusion | t(11; 17)(q23; q21) | Transcription | 81 | |
| MLL-CBP fusion | t(11; 16)(q23; p13) | Transcription | FAB M4, M5, infants | 82 |
| MLL-EEN fusion | t(11; 19)(q23; p13) | Transcription | Infants | 83 |
| MLL-ENL fusion, MLL-ELL fusion | t(11; 19)(q23; p13) | Transcription | Myelodysplastic syndrome | 38, 84 |
| MLL-MLL fusion | None | Transcription | FAB M4, M5 | 85 |
| MOZ-CBP fusion | t(8; 16)(p11; p13) | Chromatin acetylation/Transcription | FAB M4, M5 | 86 |
| NUP98-HOXA9 fusion | t(7; 11)(p15; p15) | ? Activate HOXA9 | FAB M2, M4 | 87 |
| NPM-MLF 1 fusion | t(3; 5)(q25; q34) | Abnormal traffic | Myelodysplastic syndrome | 88 |
| PML-RARα fusion | t(15; 17)(q22; q21) | Retinoic acid response | FAB M3, coagulopathy | 42 |
| Molecular Genetic Abnormality . | Translocation . | Biochemical Defect . | Associated Features . | Reference . |
|---|---|---|---|---|
| Chronic myelogenous leukemia | ||||
| BCR-ABL fusion (p210) | t(9; 22)(q34; q11) | Signal transduction | Myeloproliferation | 36 |
| B-cell lineage leukemia | ||||
| TEL-AML1 fusion | t(12; 21) cryptic | Transcription | Good prognosis | 50 |
| BCR-ABL fusion (p185) | t(9; 22)(q34; q11) | Signal transduction | Poor prognosis | 37 |
| E2A-PBX fusion | t(1; 19)(q23; p13) | Transcription | Pre-B phenotype, poor response to antimetabolites | 66 |
| MLL-AFX1 fusion | t(x; 11)(q13; q23) | Transcription | 67 | |
| MLL-AF4 fusion | t(4; 11)(q21; q23) | Transcription | Mixed lineage, infants, poor prognosis | 39, 40, 45 |
| MLL-ENL fusion | t(11; 19)(q23; p13) | Transcription | Hyperleukocytosis | 38 |
| IGH-MYC fusion | t(8; 14)(q24; q32) | Transcription | FAB L3, extramedullary disease | 68 |
| IGκ-MYC fusion | t(2; 8)(p12; q24) | Transcription | FAB L3, extramedullary disease | 68 |
| IGλ-MYC fusion | t(8; 22)(q24; q11) | Transcription | FAB L3, extramedullary disease | 68 |
| Hyperdiploidy | None | Unknown | Good prognosis | 49 |
| T-cell lineage leukemia | ||||
| TAL1 deletion | None | Transcription | Extramedullary disease, CD2+, CD10− | 69 |
| TCRδ-TAL1 fusion | t(1; 14)(p32; q11) | Transcription | Extramedullary disease, CD2+, CD10− | 69 |
| TCRβ-TAL1 fusion | t(1; 7)(p32; q35) | Transcription | Extramedullary disease, CD2+, CD10− | 69 |
| TCRα-MYC fusion | t(8; 14)(q24; q11) | Transcription | Extramedullary disease | 70 |
| TCRδ-RBTN1 fusion | t(11; 14)(p15; q11) | Transcription | Extramedullary disease | 71 |
| TCRδ-RBTN2 fusion | t(11; 14)(p13; q11) | Transcription | Extramedullary disease | 71 |
| TCRδ-HOX11 fusion | t(10; 14)(q24; q11) | Transcription | Extramedullary disease | 72 |
| TCRβ-LCK fusion | t(1; 7)(p34; q34) | Signal transduction | Extramedullary disease | 73 |
| Acute myelogenous leukemia | ||||
| AML 1-ETO fusion | t(8; 21)(q22; q22) | Transcription | FAB M2 | 41 |
| CBFβ-MYH11 fusion | inv(16)(p13; q22) | Transcription | FAB M4EO | 74 |
| DEK-CAN fusion | t(6; 9)(p23; q34) | Transcription | Basophilia | 75 |
| MLL-AF1p fusion | t(1; 11)(p32; q23) | Transcription | 76 | |
| MLL-AF1q fusion | t(1; 11)(q21; q23) | Transcription | FAB M4-M5, infants | 77 |
| MLL-AF6 fusion | t(6; 11)(q27; q23) | Transcription | 78 | |
| MLL-AF9 fusion | t(9; 11)(p22; q23) | Transcription | FAB M4, M5, infants | 79 |
| MLL-AF10 fusion | t(10; 11)(p12; q23) | Transcription | FAB M5 | 80 |
| MLL-AF17 fusion | t(11; 17)(q23; q21) | Transcription | 81 | |
| MLL-CBP fusion | t(11; 16)(q23; p13) | Transcription | FAB M4, M5, infants | 82 |
| MLL-EEN fusion | t(11; 19)(q23; p13) | Transcription | Infants | 83 |
| MLL-ENL fusion, MLL-ELL fusion | t(11; 19)(q23; p13) | Transcription | Myelodysplastic syndrome | 38, 84 |
| MLL-MLL fusion | None | Transcription | FAB M4, M5 | 85 |
| MOZ-CBP fusion | t(8; 16)(p11; p13) | Chromatin acetylation/Transcription | FAB M4, M5 | 86 |
| NUP98-HOXA9 fusion | t(7; 11)(p15; p15) | ? Activate HOXA9 | FAB M2, M4 | 87 |
| NPM-MLF 1 fusion | t(3; 5)(q25; q34) | Abnormal traffic | Myelodysplastic syndrome | 88 |
| PML-RARα fusion | t(15; 17)(q22; q21) | Retinoic acid response | FAB M3, coagulopathy | 42 |
CHILDHOOD LEUKEMIA THERAPY LATE EFFECTS
One of the undesired outcomes following successful treatment of childhood leukemia is the development of late effects; these late effects include the development of second cancers, abnormality of growth, endocrine and cardiac dysfunction, and neuropsychological defects. Second cancers have been found with significant frequency following successful chemotherapy for childhood ALL. One very large CCG study of children followed almost 5 years, demonstrated a seven-fold excess for all cancers and a 22-fold excess for central nervous system (CNS) cancer;24 CNS neoplasms were seen in children who had undergone CNS irradiation and especially in those who were 5 years old or younger at the time of treatment.24 Second neoplasms have also been observed following bone marrow transplantation. One study demonstrated a 6.7-fold increase in second cancers, primarily non-Hodgkin's lymphoma, brain tumors, and melanoma.25 Another study demonstrated an overall incidence of second neoplasms at 9.9% at 13 years posttransplantation.26
Leukemias sometimes occur following treatment with the epipodophylotoxins, which are topoisomerase II enzyme inhibitors. These leukemias are generally myeloid in type and are the result of a fusion between a portion of the MLL gene (on chromosome 11q23) and one of a variety of other partners. The therapy-related leukemias are included in Table 1. The breakpoints in MLL and the partner genes in these secondary leukemias do not differ from those seen in the primary leukemias involving these same genes.27
DRAMATIC PROGRESS IN THE UNDERSTANDING OF CHILDHOOD LEUKEMIA CELLULAR AND MOLECULAR HETEROGENEITY
The past 20 years have resulted in an explosion in our knowledge of the phenotypic characteristics of leukemia. In more recent years, knowledge of the phenotype has extended to an understanding of the molecular genetic events that cause cells to become malignant.
IDENTIFICATION OF HETEROGENEITY OF ACUTE LEUKEMIA CELLULAR PHENOTYPES
As they became available, the application of newer laboratory analyses increased the understanding of acute leukemia biology. Morphologic evaluation over the last 50 years culminated in the widely used French-American-British (FAB) classification of childhood acute leukemia; these analyses demonstrated that approximately 80% of childhood acute leukemias group as ALL, including FAB LI-L3 types, and 20% group as AML, including FAB MO-M7 types.28 Studies in the 1970s and 1980s used antibodies to immunophenotype lymphoid leukemic blasts, which demonstrated that 85% of cases were B lineage, whereas 15% of cases of ALL were found to be of T lineage.29-31 Moreover, these B-cell lineage leukemias were found to have early B-lymphocyte development, before the maturation of surface immunoglobulin and, therefore, termed “B-precursor ALL.” More recent molecular analysis of ALL has demonstrated partial or incomplete rearrangement of both immunoglobulin (B-cell) and T-cell receptor genes in these leukemias; thus, these lineage relationships suggest that ALL results in aberrant phenotypes. In parallel studies in AML, myeloid cellular phenotype identification advanced significantly with monoclonal antibody development.32 The combination of antibody-based immunophenotyping and molecular techniques allow description of a vast array of cellular phenotypes. Mixed lineage phenotypes, including T + B, T + myeloid, T + B + myeloid, or B + monocyte have been observed.
Immunophenotyping studies suggest that malignant transformation sometimes results in both aberrant and unstable cellular phenotypes. A number of studies have demonstrated these unstable phenotypes but perhaps none more dramatically than one in which leukemia cells with a mature T-lymphocyte phenotype (and irreversible T-cell DNA rearrangements) changed to mature granulocytes in the appropriate environment.33 Such unstable phenotypes are most likely the result of aberrant gene transcription, which occurs as a result of the leukemogenic process.
Many studies over the past 20 years looked at the role of cellular phenotype in predicting therapy response. The associations generally have not been strong and are clearly less predictive than other biologic characteristics, such as molecular genetic abnormalities or total body leukemia burden.
HETEROGENEITY IN MOLECULAR GENETIC AND CYTOGENETIC ABNORMALITIES
The past 10 years have been an extremely productive period in understanding the molecular genetic abnormalities in childhood acute leukemia. Rapid advances have permitted analysis of the molecular basis for the abnormalities that cause leukemia cells to differ from their normal lymphoid or myeloid progenitor counterparts. A large number of chromosomal translocations associated with distinct molecular genetic abnormalities have been described in acute leukemia (Table 1, also reviewed in references 31, 34, 42). Occasionally, these abnormalities are a consequence of mistakes in the DNA rearrangement of normal lymphoid progenitor cells in the production of functionally diverse immunoglobulin and T-cell receptor molecules. At the time of DNA rearrangement, a cellular proto-oncogene gene, eg, MYC on chromosome 8, may form a fusion gene with the immunoglobulin gene (IGH) enhancer on chromosome 14 in B-lymphoid progenitors resulting in the t(8; 14)(q23; q32.) translocation. The functional outcome of this event is dysregulation of MYC transcription (Table 1, reviewed in ref 36).
In contrast to translocations involving an antigen receptor gene, most pediatric leukemias arise from recombinations that do not involve antigen receptor genes. These rearrangements are the result of breaks and subsequent fusion between portions of the two genes. The final result is a chimeric or fusion gene protein product that in turn produces malignant transformation. These oncogenic fusion proteins most often alter normal cell function through direct dysregulation of signal transduction pathways involved in controlling cellular differentiation and proliferation or, alternatively, transcription of genes critical to these pathways (Table 1). These genetic alterations distinguish the leukemias from solid tumors, which often demonstrate mutations and deletions of “gatekeeper” or “tumor suppressor” genes as opposed to the alteration of “caretaker” or “proto-oncogenes” seen in the pediatric leukemias.
Another interesting aspect of chromosomal translocations of pediatric leukemias is the frequency with which new genes are discovered by DNA breakpoint analysis. A number of new genes that are critical for normal cellular proliferation and differentiation have been discovered through childhood leukemia analysis. Examples of new genes that were discovered through analysis of chromosomal translocations include: the BCR gene in t(9:22) ALL37; the MLL/HRX/ALL-1 gene in t(4; 11) ALL38,39; the AF4/FEL40 genes in t(4; 11) ALL; the AML 1 gene in t(8; 21) AML41; and the PML gene in t(15; 17) acute promyelocytic leukemia.42
Many cases of childhood ALL do not have detectable chromosomal translocations but instead show hyperdiploidy (with >50 chromosomes) or hypodyloidy. Up to 50% of childhood leukemias do not yet have defined molecular genetic abnormalities. As noted in Table 1, there is a remarkable number of different molecularly defined types of childhood acute leukemias, but much work needs to be done to further define the various molecular genetic events that are critical to development of these leukemias.
CHEMOTHERAPY ACCELERATES DESTRUCTION OF CELLS THAT ARE “POISED TO DIE”
It is likely that chemotherapy success with ALL is partially a result of the ability of chemical agents to activate the apoptotic pathways in cells that are “poised to die.” In support of the importance of active programmed cell death, there are a number of studies that demonstrate that agents which are active in the treatment of ALL (eg, corticorsteroids, epipodophylotoxins, anthracyclines) kill cells in part by activation of apoptotic pathways.43 It is tempting to speculate that this killing of cells already poised for death is a very important factor for chemotherapy success in these leukemias. As stated earlier, the most frequent forms of childhood ALL involve cells that are early lymphoid progenitors. Normal cellular counterparts are actively rearranging DNA of the immunoglobulin and T-cell receptor genes in an attempt to produce a useful antigen receptor gene. Successful rearrangement of T-cell or immunoglobulin genes requires precise joining of V-D-J segments; this process has a high propensity for failure. For successful functional antigen-receptor lymphocyte development, these cells must also escape the negative selection processes that delete self-reactive lymphocytes, and finally, these cells must be positively selected by antigen. The entire process results in a very small percentage of lymphoid progenitor cells developing into mature T or B lymphocytes. The process of programmed cell death acts very effectively in preventing the accumulation of cells that have not matured into useful antigen-recognizing T or B lymphocytes.
A second prediction from the hypothesis that ALL arises in cells that are poised to die is that the normal progenitor cells from which these leukemias arise will also be easily killed by the same chemotherapeutic agents. In fact, the lympholytic nature of corticosteroids has long been known; and other potent chemotherapeutic agents, eg, the antimetabolites, have significant effects on the lymphoid system and are often used as immunosuppressive agents.
OTHER CYTOTOXIC AGENTS INDUCE APOPTOTIC CELL DEATH IN SUSCEPTIBLE LEUKEMIA CELLS
Radiation, like chemotherapy, induces programmed cell death in both normal progenitor cells and malignant counterparts in leukemia. Radiation therapy is generally used locally, primarily with the CNS and sometimes in treatment of sanctuary sites in childhood acute leukemias.4 Systemic use of irradiation is generally in the context of stem cell replacement from bone marrow or blood. As with the chemotherapeutic agents, cell death induced by radiation is predominately due to apoptosis.
Immune system cytotoxic cells (T lymphocytes and natural killer cells) are important components of childhood acute leukemia treatment with bone marrow/stem cell transplantation (as discussed below). Apoptosis also plays an important role in the immunologic cell death using foreign “killer” cells for therapy.
MOLECULAR GENETIC ANALYSIS PREDICT TREATMENT RESPONSES IN ACUTE LEUKEMIA
One of the major challenges in the next several years is continued definition of optimal childhood acute leukemia therapy. Accumulating evidence clearly indicates that molecular genetic characterization will provide the genotypic information to make this possible. A recent group of childhood ALL experts attempted to develop uniform risk criteria based on age, white count, immunophenotype, DNA index, cytogenetics, CNS status, and early response to therapy. Unfortunately, this attempt resulted only in consensus based on age and white count.44 While these features are useful, they lack the needed degree of predictability for the individual patient. An example is the case of infant ALL in which outcomes can be predicted by molecular genetic analysis (the presence or absence of MLL gene rearrangement) much more precisely than with any other feature.45
It is clear that acute leukemia response to chemotherapy is significantly affected by the presence of certain molecular genetic abnormalities. Some examples of these specific abnormalities and their influence on outcome are included in Table 1. In childhood leukemia, the prognostic importance of molecular genetics is probably most dramatically demonstrated with the abnormalities of the MLL-AF4 and BCR-ABL type. These molecular genetic abnormalities have a profound effect on cell proliferation, maturation, and metabolism. Moreover, these abnormalities define cases that respond extremely poorly to therapy, irrespective of age and other known prognostic features. In these leukemias, it is very likely that the ineffectiveness of chemotherapy in patients with these and other abnormalities is a direct reflection of the biological characteristics of the leukemia cells. The author's bias, shared by others,46 is that the understanding of cellular genotypes will be the most factor in deciding optimal therapy, and that leukemia genotype will soon replace age, white count, immunophenotype, and other surrogate markers for leukemia cell biology.
HYPERDIPLOIDY AND TEL GENE REARRANGEMENTS PREDICT A FAVORABLE RESPONSE OF ALL TO CHEMOTHERAPY
Studies in the 1980s demonstrated that ALL patients with increased blast cell DNA content had a more favorable response to chemotherapy.47 The increased blast cell DNA is the result of hyperdiploidy (>50 chromosome per cell). Of some interest are the observations that hyperdiploid ALL blasts tend to accumulate higher levels of methotrexate polyglutamates than ALL cells that are not hyperdiploid.48 Increased sensitivity to antimetabolities and other drugs may explain the more favorable response to chemotherapy among children who have hyperdiploid ALL.49
Recently, the prognostic importance of TEL gene rearrangement as an indicator of the TEL-AML1 fusion gene (associated with a cryptic translocation involving chromosomes 12 and 21) has emerged.50 Excellent prognosis in cases with TEL rearrangement is observed with the use of antimetabolite-based therapy, known to be relatively free of long-term side effects. Of interest will be future studies to determine why TEL-AML1 leukemia is so responsive to antimetabolites. Perhaps these studies will take us, full circle, back to the early work of Farber et al 50 years ago with antimetabolites. Finally, it should be noted that TEL rearrangements are generally not seen in cases with hyperdiploidy and therefore these two prognostic indicators are independent of one another.51
FUSION GENES AND GENE PRODUCTS AS THERAPY TARGETS
In addition to studying translocation-generated fusion genes and their encoded protein products for their ability to predict outcome, they are likely to become important as specific therapy targets. We already have one example in which the fusion product is used as a therapy target: the PML-RARα (retinoic acid receptor alpha) in acute promyelocytic leukemia (see Table 1). The fusion of PML (a putative transcription factor) with the retinoic acid receptor (RARα) results in a protein that inhibits differentiation and promotes myeloid precursor cell survival.52 Leukemia treatment with high doses of all trans-retinoic acid results in cellular differentiation and remission induction with reduced morbidity, lower treatment cost, and improved long-term outcome.53 This agent acts by directly binding to the PML-RARα protein and directly converting this molecule from an inhibitor to an activator of myeloid differentiation. Interestingly, this treatment alone is not curative, suggesting that additional genetic abnormalities are likely to be important in the disease.
As information develops as to the function of some of the newly defined genes that are altered in acute leukemia (eg, AML 1, MLL, AF4, AF9, PBX), it is likely that it will be possible to use the products of these genes as therapy targets. This type of specific targeting is likely to have advantages in reduced toxicity, including the ability to reduce the use of currently toxic chemotherapeutic agents.
In the future, additional molecular targeting approaches are likely to become important. One, at the DNA level, is to use specific oligonucleotides to induce DNA triplex formations.54 At the mRNA level, specific inhibition may be indicated by antisense oligonucleotide duplex formation55 or by ribozymes.56 At the protein level, a number of targeted approaches are potentially possible: Fusion gene products are generally leukemia specific and if they are expressed on the cell surface (complexed with MHC gene products), they could be recognized by specific cytotoxic T lymphocytes.57 Competitive antagonists or decoys could be used to inhibit several types of proteins, including antigen receptors58 and activated kinases.59 Many of the newly described fusion genes result in unique protein products that bind to DNA and alter transcription of important genes. These altered fusion genes could potentially be deactivated by a variety of techniques within the cell nucleus. Inhibition of the aberrant DNA binding domains of these leukemia-specific, fusion gene transcription factors may be useful for future therapy.
THE COMMITMENT OF PARENTS, CHILDREN, AND HEALTH CARE WORKERS TO RESEARCH HAS SIGNIFICANTLY CONTRIBUTED TO THE LAST 50 YEARS OF CHILDHOOD LEUKEMIA TREATMENT SUCCESS
Advances in childhood acute leukemia have, in the author's opinion, been dramatically aided by the willingness (even eagerness) of parents, children, physicians, nurses, and other health care workers to enroll patients on research studies that attempt to advance knowledge in the field. This degree of cooperation and collaboration among the various groups is almost unique in medical history. Children are treated on organized protocols, which, at a minimum, provide standardized treatments, and, frequently, conduct treatment studies that use randomization between one or more therapies. The cooperative spirit with which parents are willing to participate in these studies is remarkable, as most of United States children with acute leukemia are treated with one of these research protocol studies. The high compliance and cooperative spirit among all parties is remarkable and quite different from that in adult tumors. Is there a cause and effect between participation in clinical trials and better therapy outcome? These two factors could be causally unrelated but it is doubtful. In support of protocol-based treatment, there is one important study that indicates that protocol-treated patients have better outcomes than those treated off protocols using “the doctor knows what's best” therapy.60
WHY ARE CHILDHOOD LEUKEMIA TREATMENT RESULTS SUPERIOR TO THOSE OF OTHER CHILDHOOD AND ADULT TUMORS?
There are probably several reasons for the superior childhood leukemia treatment results when compared with other childhood and adult tumors. The protocol-based approach discussed above is important; however, it is the author's suspicion that bone marrow's vascular nature and leukemia cell biology are the most important reasons for these excellent results. Good treatment responses are probably related to the leukemia's highly vascular nature, resulting in good chemotherapeutic agent penetration and reduction of the likelihood of hypoxia which, in turn, reduces the efficacy of chemotherapy and irradiation.61 Interestingly, the leukemia cells may induce neovascularization.62 This neovascularization may be important in the pathogenesis of the leukemias.
The excellent childhood leukemia results are, undoubtedly, at least in part due to the nature of the malignant cells. As noted earlier, childhood leukemias arise in cells that are poised to die, resulting in a high likelihood of activation of pathways of programmed cell death (apotosis) induced by chemotherapy or radiotherapy. Moreover, leukemia cells that are hyperdiploid (as is the case frequently in ALL) have increased uptake of antimetabolites. What is the role of the frequently occurring fusion genes (and their protein products) in therapy response? The answer is probably specific for the particular leukemia, as some fusion gene leukemias have a poor prognosis (eg, BCR-ABL and MLL-AF4), while others have a good prognosis, eg, those involving TEL,51 and PML-RARA.63
WHAT IS CHILDHOOD LEUKEMIA'S FUTURE?
Prediction of the future is, of course, extremely hazardous and therefore it can only be speculated. Fifty years ago Farber et al used a folic acid antagonist as the first successful form of chemotherapy. It is of note that in these past 50 years we have learned that folic acid antagonists and other antimetabolites that inhibit DNA synthesis produce fewer secondary cancers than agents which directly interact with DNA (such as the topoisomerase II inhibitors and the anthracyclines). Incorporation of this information into planning for newer chemotherapy programs will be important.
An understanding of additional molecular genetic defects is clearly important, as the genetic defects shown in Table 1 are only a part of the story. The leukemia clone's evolution probably requires several genetic “hits” and, in late stages of the disease there are probably multiple genetic abnormalities. Examples of other additional genetic abnormalities in these leukemia cells include deletion of p53, the “cellular gatekeeper”64 or p16 INK4a, an important member of the CdK inhibitor family of genes.65 Rapid progress is underway in this field and will hopefully result in an understanding of the sequential and interdependent nature of the important molecular defects that influence tumor progression, therapy resistance, and prognosis.
Continuation of therapy improvements are likely with a better understanding of the genetic events resulting in leukemia. My expectation is that therapy will be individualized based on leukemia cell genotype. The genotype will be complex but therapy is likely to be based on the unique “leukemia genes,” which are central to the early steps in pathogenesis. These very specific “leukemia genes” are of the type shown in Table 1 and should be the basis of any future leukemia classification. Genotype-specific therapies with less systemic side effects (both early and late) will be important in reducing the currently high morbidity rate and improvement of quality of life. Finally, is prevention possible? As our understanding of environmental etiologic agents develops, the situation is hopeful.
ACKNOWLEDGMENT
I thank Drs Jim Downing, Don Pinkel, Norma Ramsay, Steve Sallan, and Bill Woods for their willingness to assist with this commentary. However, I should be held responsible for errors of omission or commission. I also apologize for my inability, because of space limitations, to properly credit the large number of physicians, scientists, and health care workers who have contributed to the dramatic progress in understanding childhood leukemia over the last 50 years. Who would have imagined this to be possible?
J.H.K. is a recipient of an Outstanding Investigator Grant Award (CA 49721) from the National Cancer Institute.
Address reprint requests to John H. Kersey, MD, University of Minnesota, Box 806 Mayo, 420 Delaware St SE, Minneapolis, MN 55455.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal