Abstract
Platelet activation and microthrombus formation are invariable features of xenograft rejection and the vascular injury observed when porcine organs are transplanted into primates. This pathological process could be mediated, at least in part, by aberrant interactions of von Willebrand Factor (vWF) associated with the donor vasculature with host platelets. Unlike human vWF, native porcine vWF (pvWF) interacts with human GPIb independently of shear stress or nonphysiological stimuli, eg, ristocetin. We therefore contrasted the potential of isolated human and porcine vWF–A1-domains to interact with human platelets in vitro. Both human and porcine vWF–A1-domains expressed as glycosyl phosphatidylinositol–linked FLAG fusion proteins on COS-7 cells induced GPIb-dependent aggregation and intracellular Ca++ uptake of platelets, independent of both the remainder of the vWF protein and additional modifying factors. Porcine A1-domains were more potent than human homologues, and in addition ristocetin could boost platelet aggregation only with the human A1-domain. Putative conformational changes in the porcine A1-domain could result in the heightened, ristocetin-independent interactions observed with human platelets and may be of importance for xenograft survival.
THE REJECTION OF discordant xenografts is invariably associated with platelet deposition and microvascular thrombosis that appear to progress independent of complement activation and xenoreactive natural antibodies.1 Evidence for the role of primary platelet activation in this situation has been derived from experiments, in which targeting those factors involved in platelet recruitment and activation has resulted in increased xenograft survival. Neutralizing the lipid mediator platelet activating factor (PAF) has been shown to prolong discordant xenograft survival times,2 and combinations of other PAF antagonists with antibodies directed at platelet adhesion proteins, such as P-selectin, result in further prolongation of graft survival.3 An antagonist of the platelet fibrinogen receptor GPIIbIIIa (GPI 562) has been shown to prolong graft survival in a discordant guinea pig-to-rat xenograft model.4 In other experiments, depletion of platelets in a working pig-heart model of hyperacute xenograft rejection has resulted in a significant increase in survival times and function ex vivo.5 This beneficial effect however could not be reproduced by the use of GPIIbIIIa antagonists in the same model.6 These observations may point to other important platelet adhesive interactions with the xenograft vasculature that may be initially independent of GPIIbIIIa.
At sites of vascular injury and endothelial damage, platelet activation will be initiated by interactions of von Willebrand Factor (vWF) and the specific receptor on platelets, termed glycoprotein GPIb. This effect is triggered by vWF in association with the subendothelial matrix7,8 and is modulated by shear stress provided by blood flow in the microvasculature.9,10 Platelet aggregation is then further promoted by GPIIbIIIa activation leading to binding of fibrinogen and vWF; the latter mediated at a different binding site of vWF to that of GPIb. These events result in platelet aggregation and associated intravascular microthrombus formation.11
vWF is synthesized in both endothelial cells (EC) and megakaryocytes and comprises a large, multimeric glycoprotein of up to 100 identical subunits. The protein is stored in the Weibel-Pallade bodies of EC and α-granules of platelets. Once released into the plasma, vWF is noncovalently bound to Factor VIII. The mature monomer consists of 2,050 amino acid residues, containing the A1-domain, which comprises 1 of 3 repeating domains with significant internal homology (classified as A-domains).12,13 The A1-domain is known to contain the GPIb-binding site of the vWF molecule, first assigned to a tryptic fragment spanning amino acids 449 to 728, which forms an intrachain loop (A1-loop) secondary to disulfide linkages (Cys509 to Cys695).14,15 The aminoglycoside ristocetin may trigger aggregation subsequent to modification of the A1-domain conformation resulting in “spontaneous” vWF interactions with GPIb.16
Recombinant soluble vWF-A1 binds to GPIb but, in contrast to the entire multimeric molecule, does not appear to initiate platelet aggregation.15,17,18 Therefore, to date, functional studies of isolated A1-domains have been dependent on assays showing intrinsic blocking effects on platelet aggregation in response to native, multimeric vWF.18-20
We questioned whether the extensive platelet recruitment, as observed during discordant xenograft rejection, could be potentiated by the documented aberrant interactions of human (or primate) platelet glycoprotein GPIb with xenogeneic vWF.21-23 This possibility has been validated in part by the observation of thrombocytopenia subsequent to administration of Factor VIII concentrates containing porcine vWF (pvWF) to hemophiliac patients.24,25 pvWF has been observed to both aggregate and activate human platelets, inducing intracellular Ca2+ uptake (Δ[Ca2+]i) in platelets via GPIb interactions in the absence of ristocetin or shear stress in vitro.22,26,27 This effect can be abolished by antibodies that result in GPIb blockade or by preincubation of platelets with recombinant human vWF-A1 fragments. This manner of spontaneous binding of pvWF and activation of human platelets is comparable with that previously described for asalio-vWF,28-30 as well as for the vWF protein purified from plasma from patients with type IIb von Willebrand disease (vWd).31,32 In several cases the vWF in type IIb-vWd has been found to contain single point mutations that may lead to conformational changes of the A1-domain partially comparable with those observed in the xenogeneic vWF.33
We therefore investigated whether cell-associated vWF-A1 in isolation had the capacity to aggregate and activate human and primate platelets. Human or porcine vWF-A1 were independently expressed as a glycosyl phosphatidylinositol (GPI)-linked FLAG-tag-fusion peptide on COS-7 cells. To provide a signal for covalent attachment of the glycophosphoinositol anchor to the peptide, the last 37 carboxyl-terminal amino acids of decay-accelerating factor (DAF), a GPI-anchored protein, were inserted. The GPI anchor, once fused to the carboxyl-terminus of a heterologous secreted protein, targeted the fusion protein to the plasma membrane.34 This technology had been previously used to express a truncated protein anchored to a mammalian cell line in a nonspecific manner.35 We were then able to study functional differences between interaction of human platelets with human and porcine A1 and could evaluate the influence of modifying stimuli, eg, ristocetin on this interaction.
Using this novel approach, we have shown both aggregatory responses and Δ [Ca2+]i of human platelets following on the binding of porcine or human vWF-A1 to GPIb. In both instances, activation was spontaneous and GPIb dependent. The effect seen with human vWF-A1 was less dramatic than with the porcine homologue. Ristocetin could only potentiate human A1-mediated platelet aggregation. Our data suggest that the A1-domain is capable of inducing spontaneous platelet aggregation and that the peptide sequence alteration noted in the porcine vWF-A1 dictates a variant structure that facilitates this increased platelet interaction.
MATERIALS AND METHODS
Reagents.Gel electrophoresis buffers and premixed polyacrylamide, cell culture media, trypsin, collagenase, lipofectamine, and the “Superscript preamplification system” including oligo dT and Taq polymerase were purchased from Life Technologies, Gaithersburg, MD. Plasmids, the TA-cloning kit, and INV-α competent cells (Escherichia coli strain, used for all bacterial amplification procedures) were from InvitroGen Co, San Diego, CA. Synthetic, custom designed oligonucleotides were provided by Midland Certified Reagent Co, Midland, TX. Plasmid maxi kit was acquired from Quiagen, Chartsworth, CA. Hybond-polyvinylidene difluoride (PVDF ) membrane and enhanced chemiluminescence (ECL) detection reagent were from Amersham Life Science, Inc, Buckinghamshire, England. Aequorin was from Friday Harbor Photoproteins, Friday Harbor, WA. Apyrase grade VI, dimethylsulphoxide, human fibrinogen, and EGTA were from Sigma, St Louis, MO. All restriction enzymes and buffers were purchased from New England Biolabs, Beverly, MA. COS-7 cells were purchased from ATCC, Rockville, MD. Baboon and cynomolgus monkey blood samples were kindly provided by Dr T. Kozlowski, MGH, Harvard Medical School, Boston, MA.
Thoracic aortas, a kind gift from Dr M. Cioffi, Children's Hospital, Boston, MA were taken from Yorkshire minipigs. Human umbilical vein endothelial cells (HUVEC) were provided by Dr B. Ewenstein, Brigham and Women's Hospital, Boston, MA.
Antibodies.Monoclonal mouse antibody 6D1 to human platelet GPIb was previously shown to completely block aggregation of human platelets induced by ristocetin.36 The monoclonal antibody (MoAb) 7E3 directed at human GPIIbIIIa can prevent aggregation of platelets by blockade of fibrinogen-GPIIbIIIa interactions.37 Monoclonal mouse antibody LJ-RG-46, which reacts with human vWF residues 474-488,38 was a generous gift of Dr Z. M. Ruggeri, Scripps Research Institute, La Jolla, CA. The monoclonal anti-c-myc antibody was purified from the hybridoma-cell line CRL-1729 MYC 1-9E10.2 from ATCC. The horseradish peroxidase (HRP)-labeled anti-mouse antibody was acquired from Pierce, Rockford, IL; the monoclonal anti-FLAG M2 antibody was from Eastman Kodak company, Rochester, NY. Fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibodies were purchased from Sigma.
Cell culture.COS-7 cells and HUVEC from fresh umbilical veins were cultured in M199 (BioWhittaker, Walkersville, MD) supplemented with L-glutamine (2 mmol/L), penicillin G (100 U/mL), streptomycin (100 μg/mL), and fetal calf serum (FCS; 10%). Pig aortic endothelial cells (PAEC) were prepared from fresh thoracic aortas derived from minipigs (Yorkshire strain), isolated after detachment with collagenase, and cultured on gelatine coated tissue culture plates in Dulbeco's modified Eagle's medium (DMEM). All cells were grown in culture dishes at 37°C in a humidified incubator with a 5% CO2 atmosphere. Cultured cells were obtained after detachment with trypsin, and endothelial cell (EC) passage number never exceeded 5.
Cloning of vWF–A1 domain cDNA.Human and porcine endothelial cells underwent lysis in a solution comprised of guanidium isothiocyanate (4 mol/L) sodium citrate (25 mmol/L, pH 7.0), sarcosyl (0.5%), and β-mercaptoethanol (100 mmol/L). RNA was extracted with phenol/CHCl3 , precipitated once with isopropanol then twice with sodium acetate plus 70% ethanol and was then used for reverse transcriptase-polymerase chain reaction (RT-PCR). PCR was performed to amplify the cDNA coding the human or the porcine vWF–A1-domain respectively (designated human and porcine A1: amino acids 473-713 in both cases). Oligonucleotide primers were designed on the base of the published sequences for human39 and porcine40 vWF–A1-domain introducing the BamHI restriction site for the 5′ end and EcoRI for the 3′ end. Sequences for these synthetic oligonucleotides were as follows:
•Porcine:
↓BamHI
5′ ATA ATA GGA TCC GGC CTG CGC GGA ACC GGT GCC
↓EcoRI
5′ ATA ATA GAA TTC GAC TTG GGC CAC TAG GGG GC
•Human:
↓BamHI
5′ ATA ATA GGA TCC GGC CTG CCA GGA GCC GGG AGG
↓EcoRI
5′ ATA ATA GAA TTC GAC TTG TGC CAT GTC GGG GG
To exclude dimer formation via the unpaired Cys474, we amplified a further truncated human A1-domain comprising amino acids 475-709 (human A1 475-709) by PCR for comparison with the standard human A1 construct (amino acids 473-713; Fig 1). Human A1 475-709 is known to be capable of binding to GPIb.18 Sequences for these synthetic oligonucleotides were as follows:
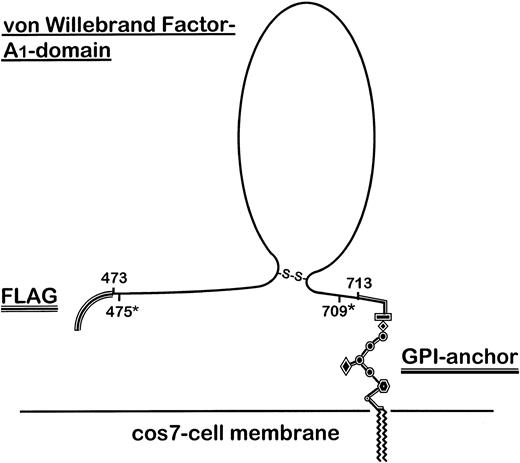
Outline structure of the FLAG-tagged GPI-anchored vWF–A1-domain fusion protein expressed on COS-7 cells. The secretion sequence of preprotrypsin, including the FLAG-epitope, was cloned as an oligonucleotide into the pcDNA3 vector. The human and porcine vWF sequence (aa 473-713) cloned from porcine and human endothelial cell RNA by RT-PCR and a further truncated fragment (aa 475*-709*) generated by PCR from human A1 (aa 473-713) were separately ligated into the vector. Finally, the C-terminal DAF-derived GPI-linker sequence was inserted into the construct.
Outline structure of the FLAG-tagged GPI-anchored vWF–A1-domain fusion protein expressed on COS-7 cells. The secretion sequence of preprotrypsin, including the FLAG-epitope, was cloned as an oligonucleotide into the pcDNA3 vector. The human and porcine vWF sequence (aa 473-713) cloned from porcine and human endothelial cell RNA by RT-PCR and a further truncated fragment (aa 475*-709*) generated by PCR from human A1 (aa 473-713) were separately ligated into the vector. Finally, the C-terminal DAF-derived GPI-linker sequence was inserted into the construct.
↓BamHI
5′ TAT TAG GGA TCC ACA AGA ACC GGG AGG CCT GGT GGT GC
↓EcoRI
5′ ATT AAT GAA TTC GTC GGG GGG CAG AGT AGG
PCR conditions were as follows: annealing temperature 57°C, MgCl2 1.5 mmol/L for human A1 ; 55°C, MgCl2 1.2 mmol/L for porcine A1-domain. In both cases 40 cycles were performed per PCR using Taq-polymerase. The PCR products were then cloned into the pcRII vector, according to the TA-cloning kit protocol. These constructs were sequenced or digested with restriction enzymes BamHI and EcoRI for subsequent cloning into the selected expression vector.
Assembly of the vWF–A1-domain expression vectors.Two vectors were designed to achieve GPI-anchored membranous expression of the human or porcine vWF–A1-domain in addition to the FLAG-tag fusion peptide in COS-7 cells. At the 5′ end of the constructs, synthetic oligonucleotides comprised of a published sequence41 were inserted. This insert used the mouse preprotrypsin leader as a secretory sequence and included the translational initiation signal. In addition, the sequence included the DNA sequence coding for the FLAG epitope, which included a HindIII restriction site at the 5′ end and a BamHI site at the 3′ end. This was then ligated into the multiple cloning region of the pcDNA3 vector. The sequence for this synthetic oligonucleotide was as follows:
HindIII ↓ start ↓
5′ AG CTT ATG TCT GCA CTT CTG ATC CTA
A TAC AGA CGT GAA GAC TAG GAT
5′ GCT CTT GTT GGA GCT GCA GTT GCT GAC
CGA GAA CAA CCT CGA CGT CAA CGA CTG
5′ TAC AAA GAC GAT GAC GAC AAG
ATG TTT CTG CTA CTG CTG TTC CTA G
↑ BamHI
In a second step, the human or porcine cDNA prepared as described above was ligated at the restriction sites BamHI (5′ end) and EcoRI (3′ end) into the remaining multiple cloning region of the vector. Finally, synthetic oligonucleotides were ligated into the two vectors, containing a sequence coding for the last 37 amino acids of DAF and providing a signal for covalent attachment of a GPI membrane anchor.34 The sequences of these synthetic oligonucleotides containing a stop codon, an EcoRI restriction site (5′ end), and an Not I restriction site (3′ end) were exactly as published.35 The resulting two vectors were amplified in transformed E coli and purified with the Qiagen plasmid maxi kit for sequencing and transfection. An outline structure of the expressed construct is shown in Fig 1.
Sequencing of the vector insert.Sequencing was performed by ABI 373 Fluorescent DNA sequencer (ABI, Foster City, CA) using SP6 and T7 primers as well as custom primers, designed for double stranded overlapping sequencing of this construct.
COS-7 cell transfection.DNA (85 ng of vector per cm2 culture plate surface area) was precipitated with 0.3 mol/L sodium acetate, pH 7.0, and 70% ethanol at −80°C for 1 hour before incubation with lipofectamine (3 μg/cm2 ) for 30 minutes at room temperature. COS-7 cells, at 80% confluency, were incubated with the DNA/lipofectamine mixture for 5 hours at 37°C in serum-free medium. The transfection was then terminated by adding FCS to a final concentration of 10%.
Cytofluorometric analysis.Transfected cells suspended in phosphate buffered saline (Dulbecco's PBS -Mg++, -Ca++; Sigma), supplemented with 5% FCS and 0.1% sodium azide, were stained in a two-step indirect procedure. Cells were first incubated with the monoclonal mouse antibodies LJ-RG-46, anti-FLAG, and anti-c-myc followed by incubation with the FITC-conjugated anti-mouse antibody for 20 minutes at 4°C. Cytofluorometric analysis was then performed on FACSort (Becton Dickinson, Mansfield, MA). Cell Quest software program (Becton Dickinson) was used for collecting and analyzing data. At least 20,000 cells were counted in each experiment.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).COS-7 cells were obtained 72 hours after transfection and underwent freeze-thawing on three occasions. After centrifugation at 10,000g, supernatants (SNF) were spun at 24,000g to enrich cell membranes. The pellet was resuspended and 15 μg of total protein was denatured in a 62.5-mmol/L Tris-buffer containing 20% Glycerol, 2% SDS, and 0.25 μg/mL bromophenol blue pH 6.8 (sample buffer). For reducing conditions, specimens were incubated in sample buffer with additional 5% β-mercaptoethanol for 5 minutes at 95°C. Following electrophoresis in a discontinuous 10% polyacrylamide gel using a miniprotean II-system (BioRad, Hercules, CA), proteins were trans-blotted to a PVDF membrane by a semidry technique on a TransBlot SD apparatus (BioRad). Membranes were blocked overnight with standard PBS, 0.1% Tween 20 (Fisher Scientific, Pittsburgh, PA) and 5% bovine nonfat milk (NÉSTLE food Co, Glendale, CA) at 4°C. In a two-step indirect immunostaining procedure, membranes were incubated with a primary antibody for 1 hour at room temperature followed by a 1-hour incubation with the HRP-labeled anti-mouse antibody. Antibody detected bands were visualized on autoradiographs with the ECL system (Amersham Life Science, Inc).
Preparation of human platelets and aequorin loading.Human blood was taken from apparently healthy volunteers, who gave their informed consent in accordance with the New England Deaconess Hospital Institutional Review Board approval. Human washed platelets (HWP) were prepared as described elsewhere using apyrase to prevent platelet aggregation during the preparation.42 HWP were adjusted to a final platelet count of 180,000 cells/μL. For GPIb-blocking experiments, platelet-Δ [Ca2+]i detection and direct comparison with baboon and cynamologous monkey platelets, HWP and primate WP were prepared as described by Cooper et al.43 Platelet-rich plasma was adjusted to pH 6.0 to inhibit binding of fibrinogen.44 Following centrifugation, platelets were resuspended in a wash solution containing HEPES pH 7.3 (10 mmol/L), NaCl (140 mmol/L), KCl (2.7 mmol/L), EGTA (5 mmol/L), glucose (5 mmol/L), and bovine serum albumin (BSA; 1 mg/mL). For the initial aggregation experiments, platelets were washed twice in this HEPES buffer containing EGTA. Then platelets were resuspended in suspension buffer comprising the above HEPES buffer without EGTA and adjusted to final concentration of 180,000 cells/μL. For Δ [Ca2+]i detection, platelets were resuspended in 50 μL of HEPES buffer (pH 7.3), containing 5 mmol/L EGTA, and then loaded with aequorin (3 mg/mL) in a dimethylsulphoxide permeabilization procedure.45 After washing three times with EGTA-lacking buffer (pH 7.3), platelet solutions were adjusted to a final 300,000/μL. All HWP solutions were allowed to stabilize for 20 minutes after CaCl2 and MgCl2 (1 mmol/L each) were added.
Platelet aggregation studies.Aggregation effects and platelet Δ[Ca2+]i in human platelets were evaluated in a dual-channel aggregometer (ChronoLog Corp, Harverton, PA) following incubation with transfected COS-7 cells. Samples of the washed platelet suspension (400 μL) were stirred in a mini-cuvette (ChronoLog Corp) at 1,000 rpm at 37°C with human fibrinogen to a final concentration of 800 μg/mL. In separate experiments HWP were preincubated with MoAB 6D1 (anti-GPIb) and 7E3 (anti-GPIIbIIIa). Platelet aggregation following the addition of transfected COS-7 cells was recorded as increases in light transmission.
Detection of platelet Δ[Ca2+]i.For the platelet Δ[Ca2+]i detection, the aggregometer simultaneously recorded alterations in luminescence while monitoring aggregation. Data were collected and analyzed using Aggro link software (ChronoLog Corp) and a Macintosh 6100 Power PC (Apple Computer Inc, Cupertino, CA), connected via an Aggrolink interface (ChronoLog Corp).
Statistical methods.Statistical analysis of data was performed with Sigma Stat software (Jandel Scientific, San Rafael, CA). Unless otherwise indicated, values in this study are represented by mean ± standard deviation.
RESULTS
Expression of the GPI-linked vWF–A1-domain FLAG-fusion peptides.The sequence of complete vector inserts was determined as described above and is shown in Table 1. The sequence of the derived human construct was confirmed to be identical to the published vWF–A1-domain.39 The porcine A1-domain from the Yorkshire mini-pig used in this study differed slightly from the published sequence for the pvWF40 at positions R589S, Q590E, Q596E, and F598L (Table 1). Repeated RT-PCR was performed to exclude artifacts using RNA purified from PAEC cultures taken from a different animal of the same species. These sequences were shown to be identical with our initial findings and showed closer homology to human A1 than previously published.40
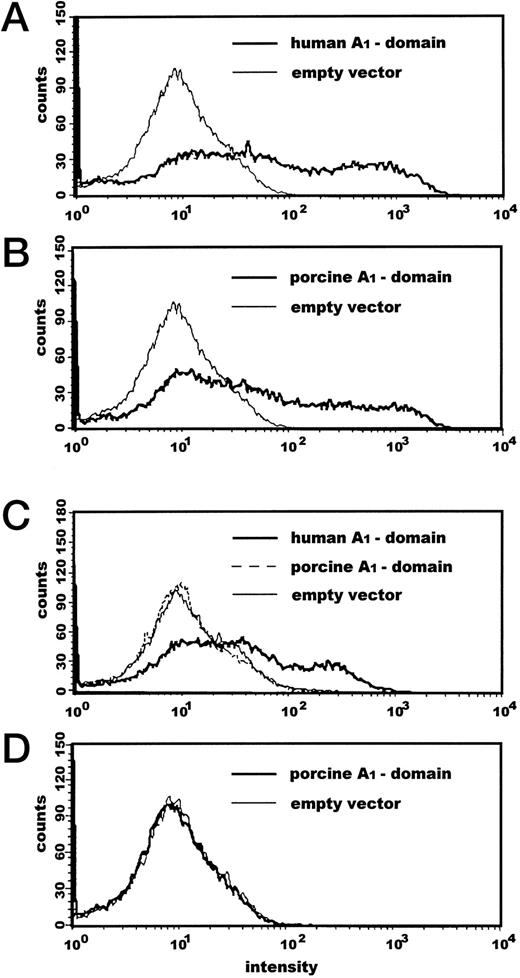
Transfection with the human vWF–A1-construct resulted in a expression rate of 33.7% ± 9.4% and the porcine A1 of 27.9% ± 7.2% as determined by cytofluorometric methods as detected with the FLAG MoAbs (Fig 2A and B); no significant differences in transfection rates were detected (P = 0.123). COS-7 cells transfected with empty pcDNA3 vector did not express detectable levels of A1-domain as determined with MoAb to human vWF-A1. The monoclonal antibody LJ-RG-46, which recognizes an epitope on human vWF AS 474-48838 could clearly discriminate between human and porcine A1 expression (Fig 2C).
Cytofluorometric analysis of vWF–A1-domain expressed on COS-7 cells. Transfected cells were first incubated with the monoclonal mouse antibodies anti-FLAG (A and B), LJ-RG-46, specifically detecting human A1-domain (C), and anti–c-myc (D) as a nonspecific control followed by incubation with a FITC-conjugated anti-mouse antibody for 20 minutes at 4°C. At least 20,000 cells were counted each experiment.
Cytofluorometric analysis of vWF–A1-domain expressed on COS-7 cells. Transfected cells were first incubated with the monoclonal mouse antibodies anti-FLAG (A and B), LJ-RG-46, specifically detecting human A1-domain (C), and anti–c-myc (D) as a nonspecific control followed by incubation with a FITC-conjugated anti-mouse antibody for 20 minutes at 4°C. At least 20,000 cells were counted each experiment.
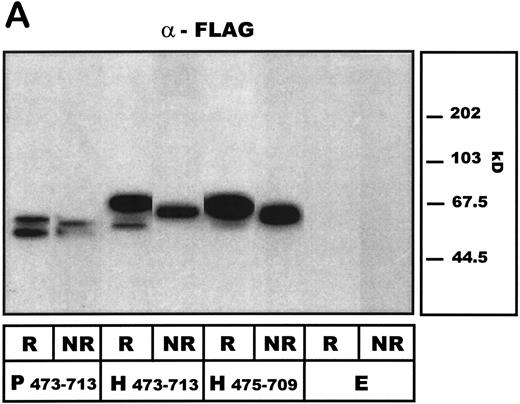
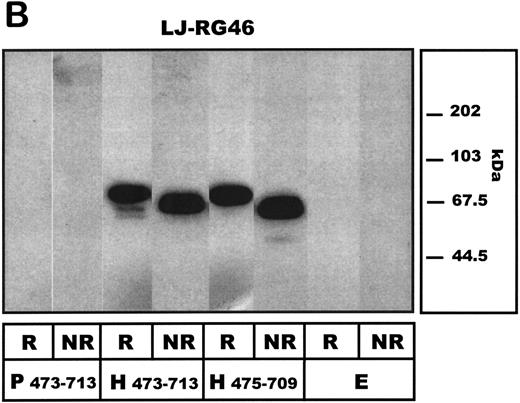
A1-domain FLAG-tagged fusion proteins, expressed by transfected COS-7 cells, were then subjected to SDS-PAGE analysis. Under reducing conditions, when probed with anti-FLAG antibodies, the porcine A1-construct isoform was detectable as a 57/50-kD double band, whereas the human A1-protein migrated at 52, 57, and predominantly 68 kD, respectively (Fig 3A). Such size heterogeneity has been noted by others for the mammalian-expressed human A1-domain20 and ascribed to different glycosylation patterns.16 The truncated human variant of A1, human A1 475-709 lacking the unpaired Cys474, migrated at 63 and 51 kD. When investigated under nonreducing conditions, all A1 constructs migrated as a single band and more rapidly than before, suggesting a disulfide bond between Cys509 and Cys695 maintaining the conformation of the A1-domain. Neither porcine, human A1, or human A1 475-709 showed detectable dimerization under nonreducing conditions. The antibody LJ-RG-46 reacted specifically with the human A1-domain, showing comparable band patterns to the anti-FLAG antibodies but did not identify the pvWF–A1-domain (Fig 3B). No vWF-A1 antigen was detected in the empty vector transfected COS-7 cells.
Western blot analysis of COS-7 cell–expressed human and porcine vWF–A1-domain constructs under reducing (R) and nonreducing (NR) conditions. Preparations of COS-7 cells transfected with standard porcine (P473-713) and human (H473-713) constructs or a further truncated human (aa 475-709: H475-709) vWF-A1 construct as well as with empty vector (E) are shown. (A) Detecting antibody LJ-RG46 (mouse anti-human A1-domain MoAb). (B) Detecting antibody α-FLAG (mouse anti-FLAG MoAb).
Western blot analysis of COS-7 cell–expressed human and porcine vWF–A1-domain constructs under reducing (R) and nonreducing (NR) conditions. Preparations of COS-7 cells transfected with standard porcine (P473-713) and human (H473-713) constructs or a further truncated human (aa 475-709: H475-709) vWF-A1 construct as well as with empty vector (E) are shown. (A) Detecting antibody LJ-RG46 (mouse anti-human A1-domain MoAb). (B) Detecting antibody α-FLAG (mouse anti-FLAG MoAb).
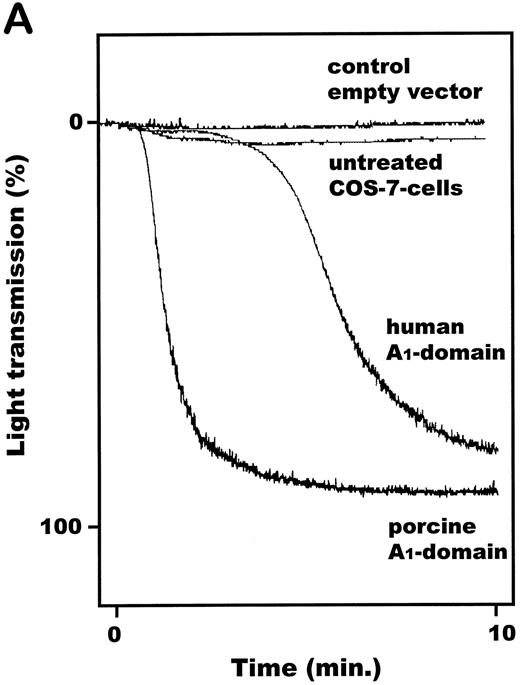
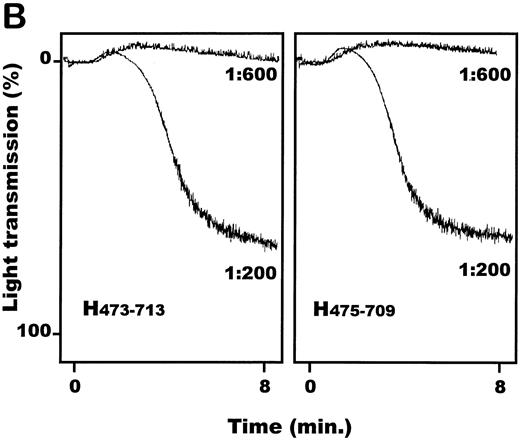
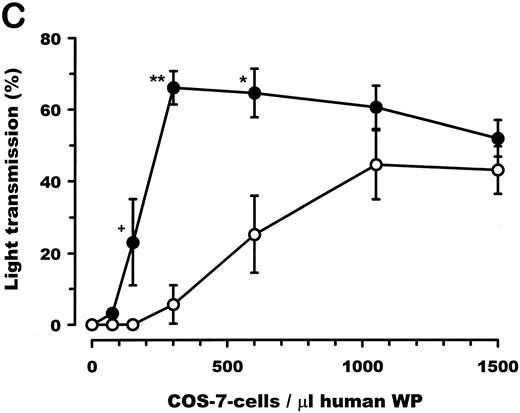
vWF–A1-domain expressed by COS-7 cells induces aggregation of human platelets.Transfected COS-7 cells were obtained as described and resuspended in PBS, pH 7.4. Both porcine and human vWF–A1-domain expressing COS-7 cells resulted in aggregation of HWP (Fig 4A) and platelet rich plasma (data not shown). Cells transfected with empty pcDNA3 vector or the nontransfected control COS-7 cells had no effect on human platelets. The aggregation response of human A1 (aa 473-713) expressing COS-7 cells compared with human A1 475-709 was identical (Fig 4B). Titrations of transfected COS-7 cells with human platelets showed the dose-dependency of this effect (Fig 4C). COS-7 cells expressing the porcine A1-domain produced maximal levels of aggregation at COS-7 cells to human platelet ratios of 1:600. A 3.5-fold increase in the concentration of human A1-domain–transfected COS-7 cells, relative to the porcine A1-variant, was required to achieve maximal aggregation. Porcine and human A1-domain yielded maximal aggregation levels of 66.0% ± 10.5% and 44.5% ± 23.6%, respectively; no significant difference was observed (P = .09).
Functional effect of vWF–A1-domain transfected COS-7 cells on human platelets. (A) Representative plot of recorded aggregation subsequent to incubation of COS-7 cells with platelets at a ratio of 1:200. (B) Comparison of aggregation subsequent to incubation of human A1-domains (aa 473-713: H473-713/aa 475-709: H475-709) transfected COS-7 cells with platelets at ratios of 1:200 and 1:600. (C) Dose-dependent effects of porcine (•) and human (○) A1-domain expressing cells incubated with human washed platelets, prepared as described in Materials and Methods (180,000 platelets/μL). All experiments were performed in the presence of MgCl2 (1.0 mmol/L), CaCl2 (1.0 mmol/L), and fibrinogen (final concentration: 800μg/mL). Aggregation was determined as percentage of maximal light transmission 10 minutes after addition of COS-7 cells. +P < .05 (Mann-Whitney Rank Sum test); **P < .001 and *P < .05 (Student's t-test) for the porcine versus human comparison (n = 5 or 6 separate transfections). Error bars indicate standard errors of the mean.
Functional effect of vWF–A1-domain transfected COS-7 cells on human platelets. (A) Representative plot of recorded aggregation subsequent to incubation of COS-7 cells with platelets at a ratio of 1:200. (B) Comparison of aggregation subsequent to incubation of human A1-domains (aa 473-713: H473-713/aa 475-709: H475-709) transfected COS-7 cells with platelets at ratios of 1:200 and 1:600. (C) Dose-dependent effects of porcine (•) and human (○) A1-domain expressing cells incubated with human washed platelets, prepared as described in Materials and Methods (180,000 platelets/μL). All experiments were performed in the presence of MgCl2 (1.0 mmol/L), CaCl2 (1.0 mmol/L), and fibrinogen (final concentration: 800μg/mL). Aggregation was determined as percentage of maximal light transmission 10 minutes after addition of COS-7 cells. +P < .05 (Mann-Whitney Rank Sum test); **P < .001 and *P < .05 (Student's t-test) for the porcine versus human comparison (n = 5 or 6 separate transfections). Error bars indicate standard errors of the mean.
Platelet Δ[Ca2+]i following incubation with human and porcine vWF–A1-domain.The demonstration of cytoplasmatic calcium mobilization (Δ[Ca2+]i) provided evidence for platelet activation in addition to platelet aggregation responses.46 To detect intracellular Δ[Ca2+]i , platelets were loaded with the photoprotein aequorin. The final cell counts of porcine and human A1-transfected cells coincubated with HWP were chosen to cause comparable extent of aggregation for both species of A1 . Following the incubation of COS-7 cells with HWP at ratios of 1:280, Δ[Ca2+]i was recorded at 15.3 seconds ± 2.2 seconds following the start of incubation with porcine A1-transfected cells versus 47.2 seconds ± 3.3 seconds with human A1 (Fig 5); differences determined to be significant (P < .001). Aggregation occured with a delay of 11.4 seconds ± 3.5 seconds (human A1) versus 8.4 seconds ± 3.2 seconds (porcine A1), respectively, to onset of cytoplasmatic calcium mobilization in both groups; differences not significant (P = 0.297). Peak changes of platelet Δ[Ca2+]i occurred at the steep phase of the aggregation curve. The time between addition of cells to HWP and peak Δ[Ca2+]i was significantly less (P < .001) in porcine (65.6 seconds ± 2.6 seconds) when compared with human (156.0 seconds ± 21.0 seconds). The nature of platelet Δ[Ca2+]i recorded in this study is in accord with previous reported platelet activation profiles using aequorin loaded collagen–stimulated platelets.43
Platelet aggregation and Ca++ influx. Washed platelets were prepared as described under Materials and Methods. For intracellular Ca++ uptake (Δ[Ca2+]i) detection, platelets were loaded with the photoprotein aequorin. MgCl2 (1.0 mmol/L), CaCl2 (1.0 mmol/L), and fibrinogen (final concentration: 800 μg/mL) were added before the experiments. Aggregation and luminescence following incubation with transfected COS-7 cells were recorded simultaneously. Each box displays one representative experiment for COS-7 to platelet ratios of 1:280. The upper (+)-tagged lines show human platelet aggregation as percentage of light transmission. The lower (×)-tagged lines give the simultaneously recorded change in luminescence due to platelet Δ[Ca2+]i .
Platelet aggregation and Ca++ influx. Washed platelets were prepared as described under Materials and Methods. For intracellular Ca++ uptake (Δ[Ca2+]i) detection, platelets were loaded with the photoprotein aequorin. MgCl2 (1.0 mmol/L), CaCl2 (1.0 mmol/L), and fibrinogen (final concentration: 800 μg/mL) were added before the experiments. Aggregation and luminescence following incubation with transfected COS-7 cells were recorded simultaneously. Each box displays one representative experiment for COS-7 to platelet ratios of 1:280. The upper (+)-tagged lines show human platelet aggregation as percentage of light transmission. The lower (×)-tagged lines give the simultaneously recorded change in luminescence due to platelet Δ[Ca2+]i .
Platelet aggregation and Δ[Ca2+]i is mediated by vWF-A1-GPIb interaction.The next series of experiments were designed to confirm that aggregation and platelet Δ[Ca2+]i , following incubation with human and porcine A1-domain transfected cells, were dependent on binding of A1 to GPIb receptor. HWP were preincubated with 6D1 (final concentration, 23 μg/mL) for 30 minutes. The aggregatory effect of porcine and human A1-expressing cells was observed to be blocked, using 6D1-pretreated HWP (Fig 6A). The platelet Δ[Ca2+]i subsequent to induction with A1-transfected cells was completely abolished (data not shown). The MoAb 6D1 also blocked aggregation, following ristocetin stimulation in the presence of low levels of COS-7 human A1 (Fig 5) as described above. These data show that the activating effect of transfected COS-7 cells on human platelets follows on the interaction with the human and porcine vWF–A1-domains and the human platelet receptor GPIb.
Inhibitory effect of anti GPIb (6D1) and anti-GPIIbIIIa (7E3) antibodies. (A) Representative plot of recorded platelet aggregation subsequent to addition vWF–A1–expressing COS-7 cells. The effect of anti-GPIb (αGPIb) on vWF–A1-domain expressing COS-7 cell–induced aggregation was determined at relatively high ratios (1:200 COS-7 cells/platelets). HWP were preincubated with the MoAb 6D1 (23.0 μg/mL), known to block GPIb-mediated aggregation by to vWF (see Materials and Methods). (B) Aggregation and simultaneously recorded intracellular Ca++ uptake (Δ [Ca2+]i) of human platelets due to porcine A1-domain (P473-713) transfected COS-7 cells after preincubation of human washed platelets with 7E3 (1.0 μg/mL). Washed platelets were prepared as described in Materials and Methods. The upper (+)-tagged lines show human platelet aggregation as percentage of light transmission. The lower (×)-tagged lines give the simultaneously recorded change in luminescence due to platelet Δ[Ca2+]i . The representative experiment for nonblocked platelets (left panel) is shown for COS-7 cell to platelet ratios of 1:280. The ratios had to be increased (1:140, right panel) to show platelet Δ[Ca2+]i in the presence of GPIIbIIIa antibodies.
Inhibitory effect of anti GPIb (6D1) and anti-GPIIbIIIa (7E3) antibodies. (A) Representative plot of recorded platelet aggregation subsequent to addition vWF–A1–expressing COS-7 cells. The effect of anti-GPIb (αGPIb) on vWF–A1-domain expressing COS-7 cell–induced aggregation was determined at relatively high ratios (1:200 COS-7 cells/platelets). HWP were preincubated with the MoAb 6D1 (23.0 μg/mL), known to block GPIb-mediated aggregation by to vWF (see Materials and Methods). (B) Aggregation and simultaneously recorded intracellular Ca++ uptake (Δ [Ca2+]i) of human platelets due to porcine A1-domain (P473-713) transfected COS-7 cells after preincubation of human washed platelets with 7E3 (1.0 μg/mL). Washed platelets were prepared as described in Materials and Methods. The upper (+)-tagged lines show human platelet aggregation as percentage of light transmission. The lower (×)-tagged lines give the simultaneously recorded change in luminescence due to platelet Δ[Ca2+]i . The representative experiment for nonblocked platelets (left panel) is shown for COS-7 cell to platelet ratios of 1:280. The ratios had to be increased (1:140, right panel) to show platelet Δ[Ca2+]i in the presence of GPIIbIIIa antibodies.
Platelet Δ[Ca2+]i mediated by vWF-A1-GPIb interaction does not require GPIIbIIIa.To show the effects of GPIIbIIIa blockade on the platelet aggregation and Δ[Ca2+]i mediated by platelet GPIb interaction with vWF-A1 , we investigated the effect of the anti-GPIIbIIIa antibody 7E3. Platelet aggregation in response to porcine A1 (Fig 6B) and human A1 (data not shown) was abrogated by blockade of GPIIbIIIa. This was in contrast to the simultaneously recorded platelet Δ [Ca2+]i that was maintained under these experimental conditions. However, to generate an equivalent Δ [Ca2+]i signal in the presence of 7E3, the ratios of transfected COS-7 cells to HWP were increased from 1:280 to 1:140.
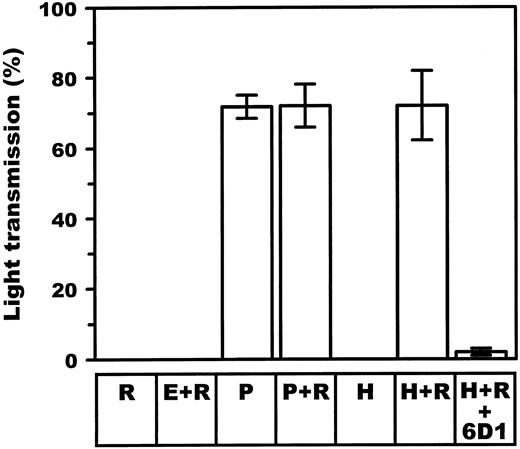
Ristocetin effect on aggregation responses.To determine whether COS-7 platelet aggregation following interaction with expressed human vWF-A1 could be further induced, the potentiating effect of ristocetin was investigated. Ristocetin was found to cause aggregation of HWP when incubated with COS-7 cells transfected with human A1-domain (0 v 72.0% ± 9.8%; P < .001) at relatively low COS-7 cell to platelet ratios (1:1,200). This aggregation response was comparable with the equivalent numbers of porcine transfected COS-7 cells in the absence of ristocetin (71.8% ± 3.3%; Fig 7). Ristocetin or human A1-domain transfectants alone had no proaggregatory effect on the platelets; neither had empty vector–transfected COS-7 cells when incubated with ristocetin. In addition, ristocetin also did not potentiate the aggregatory response secondary to porcine A1-expressing COS-7 cells.
Effect of ristocetin on aggregation of HWP after adding porcine and human vWF–A1-domain transfected COS-7 cells. HWP were prepared as described in Materials and Methods. The extent of aggregation is represented by the percentage of the maximal light transmission 10 minutes following addition of porcine (P) and human (H) vWF–A1-domain expressing COS-7 cells at low ratios (1:1,200 cells/platelets). Incubation of HWP with ristocetin (R) (0.32 mg/mL) alone or in combination with empty vector–transfected COS-7 cells (E) excluded significant human vWF contamination in platelet preparations. Specificity for A1-domain–GPIb interaction was further proven by incubating platelets with 6D1, a specific antibody known to block GPIb-mediated aggregation by vWF.
Effect of ristocetin on aggregation of HWP after adding porcine and human vWF–A1-domain transfected COS-7 cells. HWP were prepared as described in Materials and Methods. The extent of aggregation is represented by the percentage of the maximal light transmission 10 minutes following addition of porcine (P) and human (H) vWF–A1-domain expressing COS-7 cells at low ratios (1:1,200 cells/platelets). Incubation of HWP with ristocetin (R) (0.32 mg/mL) alone or in combination with empty vector–transfected COS-7 cells (E) excluded significant human vWF contamination in platelet preparations. Specificity for A1-domain–GPIb interaction was further proven by incubating platelets with 6D1, a specific antibody known to block GPIb-mediated aggregation by vWF.
Aggregation of baboon and cynomolgus monkey platelets by porcine A1.To determine whether the observed interactions with pvWF were specific for the pig to human combination, we next investigated interactions between porcine vWF–A1-domain with baboon and cynomolgus platelets. At ratios of 1:300 transfected COS-7 cells to WP, peak aggregation of 64% human, 58% baboon, and 76% cynomolgus platelet preparations, respectively, were recorded with comparable aggregation curve profiles (data not shown).
DISCUSSION
In this study, we show that both porcine and human monomeric vWF–A1-domains, when expressed on cell membranes, have the potential to initiate human platelet aggregation and activation independent of the remainder of the vWF molecule. The porcine vWF–A1-domain was determined to be far more effective on a equimolar basis with respect to the initiation of platelet aggregation. These effects were shown to be mediated by the GPIb receptor interacting with the expressed vWF-A1 . In addition, the Δ[Ca2+]i was shown to be largely independent of GPIIbIIIa functions.
Using RT-PCR, we isolated cDNA encoding the vWF–A1-domain residues 473-713. The sequence for human vWF-A1 was found to be as described before.39 The sequence coding for the pig A1-domain differed to that previously reported by Bahnak et al40 for amino acids 589R, 590Q, 596Q, and 598F. This observation may be related to strain differences between animals. When compared with the human vWF–A1-domain, the porcine sequences we determined were found to be more homologous, because amino acids 589S, 596E, and 598L were identical to those in the human sequence; 590E differed to the human sequence (Table 1). To prove that the human A1 construct we prepared was expressed as a monomer and did not undergo dimerization through the unpaired Cys474, further truncations of the human A1 fragment were performed to remove this residue. Similar truncated human A1 475-709 constructs, expressed as soluble monomeric recombinant proteins, have been shown to bind GPIb.18 19
Levels of A1-expression, determined after transfection on COS-7 cells by FACS analysis, appeared comparable in both groups. This ruled out the possibility that differences between the human and porcine vWF-A1 interaction with WP were concentration-dependent effects. Analysis of the FLAG-tagged fusion proteins by SDS-PAGE in membrane preparations under reducing conditions revealed antigen heterogeneity for the human and porcine peptides. The presence of two protein isoforms have been noted for mammalian cell expressed human A1-domains,20 and these are compatible with the presence of variable glycosylation patterns.16 Sequence analyses of these respective porcine and human A1-domains revealed differences in putative O-linked glycosylation sites47; residue 500 is changed from 500S (human) to 500P (pig) (Table 1). Additional structural changes in protein conformation are associated with this alteration. Western blot analysis under reducing and nonreducing conditions showed the presence of the disulfide bond (Cys509/Cys695). Despite the presence of Cys474 in the initial human A1 construct, no dimerization could be observed following PAGE and Western blot analysis.
Soluble vWF-A1 has been found to bind to platelet GPIb but does not have the ability to aggregate platelets ab initio.16-18 However, when expressed on a cell membrane, we observed that porcine and human vWF-A1 resulted in human platelet aggregation in a dose-dependent manner. Functional comparison of the human A1-domains (including or excluding Cys474) showed that interactions of the A1-domains with platelets were mediated by the monomeric A1-domain expressed on COS-7 cells. We were able to prove that platelet activation caused by vWF–A1-domain occurs in parallel with platelet aggregation by simultaneously monitoring Δ[Ca2+]i.37 48 Both aggregation and platelet Δ[Ca2+]i were shown to be GPIb-receptor mediated as they were both blocked by neutralizing antibodies (6D1). Irreversible platelet aggregation responses following interactions with vWF–A1-domains were shown to be GPIIbIIIa dependent. However, platelet Δ[Ca2+]i following interaction with membrane-expressed A1-domains did not involve GPIIbIIIa-mediated platelet interactions.
The platelet activating effect of the expressed vWF-A1 could be explained by a “pseudo-multimeric expression,” comparable to the biologically active vWF when present as a highly multimerized structure.49 Multimerization of vWF is considered necessary for optimal interaction with platelets leading to aggregation.50,51 The bleeding disorder vWd IIa, characterized by the absence of vWF high multimers and decreased interaction between vWF and platelets.32 52 indicates the importance of the vWF multimeric status for appropriate biological activity. It remains unclear for initiation of aggregation of platelets whether the number of A1-residues or their spatial association on the transfected COS-7 cells is the more important factor.
The intrinsic potential of the porcine vWF–A1-domain to stimulate GPIb-dependent platelet aggregation was shown to be substantially higher when contrasted with human A1 . These results confirm the importance of the isolated A1-domain in mediating platelet activation.23,53-55 Increased binding and aggregation capabilities of the cell membrane–expressed A1 constructs could mirror conformational alterations in native vWF structure that facilitate interaction with platelet GPIb under pathophysiological conditions.40 Such changes may be also apparent in the native vWF protein found in type IIb vWd-vWF. Indeed, many of the described point mutations in this bleeding disorder are found to be in a region of the vWF-A1 loop, in the so called “inhibitory segment” (aa 540-578).15 Mutations in this A1 region may cause conformational changes in both A1-domain and ultimately vWF protein with subsequent enhancement in GPIb interactions. Sequence differences in this region between porcine and human A1 , in part comparable to vWd IIb,40 could therefore explain some of the differences between porcine and human vWF-A1 interaction with platelet GPIb.
Differential effects between porcine and human vWF–A1-domain on human platelets, as shown in this study, may be of importance in determining mechanisms of platelet GPIb interactions with native vWF. The positive effect of ristocetin on aggregation secondary to human A1-domain–expressing COS-7 cells in low concentrations provided evidence for further inducibility of the human vWF–A1-domain. These data suggest that an increase in the affinity of the vWF–A1-domain for GPIb following its insertion in the cell membrane is less likely. Our results rather suggest that differences in human and porcine A1-domain dictate the properties of the native vWF-molecules.
Aggregation subsequent to low concentrations of human A1 in presence of ristocetin was increased to the levels observed with similar concentrations of porcine A1-domain in absence of ristocetin. These results suggest that the increased affinity of A1 is crucial for the functional status of vWF. Stimulating events (eg, shear stress, binding to subendothelial matrix, and presence of ristocetin, respectively) may be sufficient to change a nonaggregating status to the proaggregatory form by altering the A1-affinity for GPIb. The remainder of the human vWF may provide a “masking environment” for the A1-domain preventing binding to GPIb, which could be overcome by an increase of A1-affinity to the receptor. This hypothesis could explain the observed platelet aggregation following binding of human A1 in the absence of activating factors, which is necessary for entire native human vWF.56 In contrast, the A1-domain in porcine vWF could already provide an increased level of binding affinity to human GPIb necessary for the entire vWF molecule to mediate human platelet activation. However, unmasking of the A1-domain on vWF by conformational changes of the remaining molecule, described in association with shear stress,57 is also, at least in part, conceivable as a factor in changing the aggregating status of native vWF.
Differences in glycosylation patterns between human and porcine A1 as already discussed may also be relevant. Asialylated vWF-A1 has been described to have higher binding affinities for GPIb and to show increased blocking capability for ristocetin-induced platelet aggregation when compared with glycosylated vWF-A1 .18 Whether the glycosylation patterns or consequent conformational changes are more important for the activating capability of vWF-A1 remains a focus for further evaluation. Potentially, site directed mutagenesis at residue 500, switching the P to S, may neutralize the increased binding capacity for human platelet GPIb in vitro.
Aberrant interactions of platelet glycoprotein GPIb with vWF may contribute to the extensive platelet adhesion and recruitment observed during xenograft rejection.1,4 This hypothesis is supported by the laboratory findings of others.26 27 Our data suggest that heightened interactions between porcine vWF-A1 and human platelet GPIb, when compared with the human A1-domain, may be primarily involved in the in vivo process. Investigation of porcine A1-interactions with baboon and cynomolgus monkey platelets have shown that this effect is also seen with other nonhuman primate species.
Reduction of excessive vWF–A1-interaction in xenotransplant rejection could be achieved by administration of antibodies to GPIb or soluble vWF-A158 as a peptide. An ex vivo transplant model will be developed to address this point as VCL, a synthetic A1-domain analogue, has been shown in other small animal models to prevent restenosis of coronary vessels after balloon dilatation.59 Pigs with vWd may be suitable as xenograft donors, and these experiments may be helpful in the evaluation of the role of vWF in pig to primate xeno rejection.
The vWF–A1-domain in isolation has been shown to be capable of activating platelets if expressed by and anchored to COS-7 cells by GPI linkages. These observations provide a powerful tool for further investigation of vWF–A1-GPIb interactions. Alterations of the A1-domain structure and immediate consequences on the binding and activation of GPIb and platelets may now be studied directly. Further analysis of porcine A1-GPIb interactions could lead to therapeutic strategies for overcoming a potentially important molecular incompatibility in experimental xenotransplantation models.
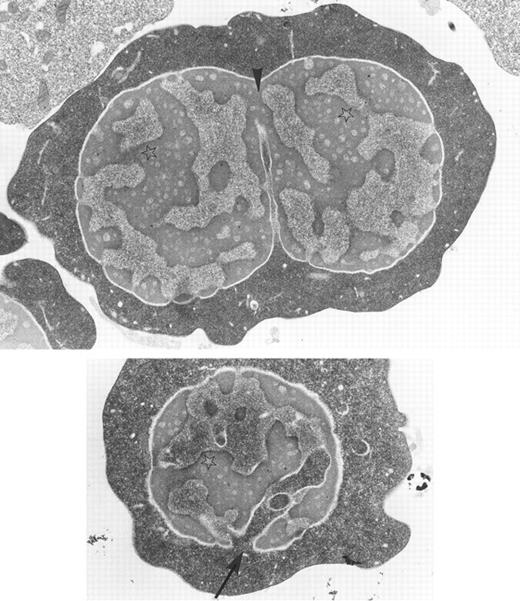
Bone marrow erythroblasts from a patient with congenital dyserythropoietic anemia type I: chromatin bridges between two adjacent nuclear lobes (arrow-head), spongy appearance of the chromatin (stars), and widening of nuclear pores with nucleus invasion by the cytoplasm (arrow) are characteristic ultrastructural features of the disease. Original magnification × 10,400. (Courtesy of Elisabeth M. Cramer and Josette Guichard, INSERM U.91, Hopital Henri Mondor, 94010 Creteil, France.)
Bone marrow erythroblasts from a patient with congenital dyserythropoietic anemia type I: chromatin bridges between two adjacent nuclear lobes (arrow-head), spongy appearance of the chromatin (stars), and widening of nuclear pores with nucleus invasion by the cytoplasm (arrow) are characteristic ultrastructural features of the disease. Original magnification × 10,400. (Courtesy of Elisabeth M. Cramer and Josette Guichard, INSERM U.91, Hopital Henri Mondor, 94010 Creteil, France.)
ACKNOWLEDGMENT
We thank E. Kaczmarek, K. Koziak, and F. H. Bach for their helpful advice and support. We also thank E. Czismadia for her skilled technical assistance.
This work was presented, in part, at the American Society of Hematology meeting in Orlando, FL, December 6-10, 1996 (abstract 1290). This is manuscript #712 from our laboratories.
Supported by Sandoz Pharma Inc, Basel, Switzerland.
J.S.A. was supported by a postdoctoral fellowship from the “Deutsche Forschungsgemeinschaft,” Germany. S.C.R. was supported from MRC UCT Liver Center, South Africa.
Address reprint requests to Jan Schulte am Esch II, MD, Sandoz Center for Immunobiology, Room 363, 99 Brookline Ave, Boston, MA 02215.


![Fig. 5. Platelet aggregation and Ca++ influx. Washed platelets were prepared as described under Materials and Methods. For intracellular Ca++ uptake (Δ[Ca2+]i) detection, platelets were loaded with the photoprotein aequorin. MgCl2 (1.0 mmol/L), CaCl2 (1.0 mmol/L), and fibrinogen (final concentration: 800 μg/mL) were added before the experiments. Aggregation and luminescence following incubation with transfected COS-7 cells were recorded simultaneously. Each box displays one representative experiment for COS-7 to platelet ratios of 1:280. The upper (+)-tagged lines show human platelet aggregation as percentage of light transmission. The lower (×)-tagged lines give the simultaneously recorded change in luminescence due to platelet Δ[Ca2+]i .](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4425/3/m_bl_0013f5.jpeg?Expires=1767950311&Signature=zw-DBDfgPXCCn7fXb0Gy0aGDDlc-OOI9Fr9UwE7UwVEym7kAxiiDNjS8hjXc7C-8mdzlCpgtsDIjD~DKNXLeP1dEnOz~0K8JAPAgjj5HcxiTnLJM9YWdfc1-KbFHIooA~YF3lyGkYwl35O2t7-rjuA~wQfNU9OFOmUOUrACWNEhD1Cgfpqi8dqTVCUArVrIOq-CPUzBiL~6TJxu0nB6WE9wAulX85IVJOcpOFVaUtkdMJymw3LTl3tqmDSJd9RAC8Cwh-diKYry2rNlv0c6Il-XuJ9ZUOPJde7OUGsZWIihmi6CtzvpFV2ZuGU2eHMdBtdwzqVIkiaY7nohquK5RaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Inhibitory effect of anti GPIb (6D1) and anti-GPIIbIIIa (7E3) antibodies. (A) Representative plot of recorded platelet aggregation subsequent to addition vWF–A1–expressing COS-7 cells. The effect of anti-GPIb (αGPIb) on vWF–A1-domain expressing COS-7 cell–induced aggregation was determined at relatively high ratios (1:200 COS-7 cells/platelets). HWP were preincubated with the MoAb 6D1 (23.0 μg/mL), known to block GPIb-mediated aggregation by to vWF (see Materials and Methods). (B) Aggregation and simultaneously recorded intracellular Ca++ uptake (Δ [Ca2+]i) of human platelets due to porcine A1-domain (P473-713) transfected COS-7 cells after preincubation of human washed platelets with 7E3 (1.0 μg/mL). Washed platelets were prepared as described in Materials and Methods. The upper (+)-tagged lines show human platelet aggregation as percentage of light transmission. The lower (×)-tagged lines give the simultaneously recorded change in luminescence due to platelet Δ[Ca2+]i . The representative experiment for nonblocked platelets (left panel) is shown for COS-7 cell to platelet ratios of 1:280. The ratios had to be increased (1:140, right panel) to show platelet Δ[Ca2+]i in the presence of GPIIbIIIa antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4425/3/m_bl_0013f6a.jpeg?Expires=1767950311&Signature=wGoWK78B~RGr4wOFawi3LCrxZXSUYxNxDKpn51bHxY4PSFXXK1a2S9~5VB3b~~r3Q5aPazExTp72twwO7ZzoXZVZUzdFFv~LPrf4frJd4EYHl2T6WO4RYfIs0ljus13Owdf6xbs1wd-KnYJOpKOoP192jd3oH1vkPjbLL3O5vB4XoWBATq23ESq83MyMZ5I2wiQ05ONalq39HTkvpnEvz8Bt-8VFKYLR2IraAfgau-tcoC6eyZpvLNG2TypcTCw0vWHDIzix1JlVlKSlXIskJKQU-cHV6mK-~2Eu~g-WrrvQ1OK0d4-1f~S26V99K37nTD28rIOzokNDokqYRu2LYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Inhibitory effect of anti GPIb (6D1) and anti-GPIIbIIIa (7E3) antibodies. (A) Representative plot of recorded platelet aggregation subsequent to addition vWF–A1–expressing COS-7 cells. The effect of anti-GPIb (αGPIb) on vWF–A1-domain expressing COS-7 cell–induced aggregation was determined at relatively high ratios (1:200 COS-7 cells/platelets). HWP were preincubated with the MoAb 6D1 (23.0 μg/mL), known to block GPIb-mediated aggregation by to vWF (see Materials and Methods). (B) Aggregation and simultaneously recorded intracellular Ca++ uptake (Δ [Ca2+]i) of human platelets due to porcine A1-domain (P473-713) transfected COS-7 cells after preincubation of human washed platelets with 7E3 (1.0 μg/mL). Washed platelets were prepared as described in Materials and Methods. The upper (+)-tagged lines show human platelet aggregation as percentage of light transmission. The lower (×)-tagged lines give the simultaneously recorded change in luminescence due to platelet Δ[Ca2+]i . The representative experiment for nonblocked platelets (left panel) is shown for COS-7 cell to platelet ratios of 1:280. The ratios had to be increased (1:140, right panel) to show platelet Δ[Ca2+]i in the presence of GPIIbIIIa antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4425/3/m_bl_0013f6b.jpeg?Expires=1767950311&Signature=mzNjDMD-7VNrsBtzpLdPxPg3oQFcNOahZ1zXNkNR4RqUE5kPsKshhXH1zjV3wWmeenJdWeQlo0x8zaCWC-4JhHoHbcMbYTrKhfvisOW2V8WaJaRARYiGx0OX2b~QWPPD36dQRtoOqOZotzsQ6D0PBfrgkbcFAAmvsvUmYCCzOs1F-vgt4n9VmOd2VfQ4E9pBNLfuWv1e2wBBO4c9teUtUTvB59A2afm0~PKKjEjCPrn7eoGxjfIKlNmBbSI9v-A75BrkdC3tdxk4WKgl1vT18SpHj2dpa0CIDl5RacUbqx56g17NJIDq3-VuqbYs4slnAwk3w7FaFDD4KgWku7q1fg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal