Abstract
We have investigated the cytopathic effects induced by the T-lymphotropic human herpesvirus 7 (HHV-7) on the CD4+ T-lymphoblastoid SupT1 cell line and primary CD4+ T lymphocytes. Acute in vitro HHV-7 infection induced (1) the formation of giant multinucleated syncytia, which eventually underwent necrotic lysis, and (2) single-cell apoptosis. Both cytopathic effects increased with the progression of infection and were blocked by phosphonoformic acid, a specific inhibitor of herpetic DNA polymerase. Using electron microscopy analysis of various samples, we found that all syncytia contained large amounts of virions and that most of them exhibited clear evidence of necrosis, whereas apoptosis was predominantly observed in single cells. Although empty viral capsids could be identified in the cytoplasm of approximately 25% of single cells exhibiting an apoptotic morphology, mature virions were hardly observed in these cells. In both coculture and cell-free HHV-7 infection experiments, a significant correlation was observed between the degree of single-cell apoptosis, evaluated by quantitative flow cytometry after propidium iodide staining, and the decrease in the total number of viable cells. Moreover, in cell-free infection experiments, apoptosis showed a positive correlation also with the viral load, monitored by quantitative HHV-7 DNA polymerase chain reaction. Thus, it appears that apoptosis occurred predominantly in uninfected bystander cells but not in productively HHV-7–infected cells.
HUMAN HERPESVIRUS-7 (HHV-7) is a CD4+ T-lymphotropic herpesvirus, isolated for the first time in 1990.1 Its genetic content and organization are closely related to HHV-6 and cytomegalovirus.2-5 In fact, the overall structure of the HHV-7 genome is identical to that of HHV-6 with a long unique component of approximately 133 kb flanked by a single direct repeat unit (DR; approximately 6 kb) at each end.3-5
HHV-7 was first isolated from the peripheral blood lymphocytes of healthy individuals and patients affected by chronic fatigue syndrome,1,6,7 and it turned out to be a prevalent virus toward which the great majority (>90%) of the population is seropositive by adulthood.8-10 Nevertheless, the portal of entry of HHV-7 into the human host and the sites of primary infection and of latency have yet to be determined. At present, the human salivary system is the only known source for persistent production of infectious HHV-7.11 12
Conclusive evidence linking infection of HHV-7 to a particular disease or syndrome is yet to be documented. At present, association of primary HHV-7 infection with exanthem subitum has been established by various investigators.13-17 More recently, a causative role for HHV-7 in pityriasis rosea has been proposed.18 Because HHV-7 is widespread in the adult population,8-10 it has also been proposed that it may represent a potential opportunistic agent in immunocompromised hosts.19 Once reactivated, acute HHV-7 infection might worsen the state of immunodeficiency due to its selective tropism for CD4+ T-lymphocytes.1,2 20 However, the consequences of virus replication events for host cells are only poorly understood.
Previous studies have already shown that HHV-7 displays in vitro cytopathic effects,1,2,7 which present some characteristic features similar to those induced by HHV-6.21 In fact, few days after infection with either HHV-6 or HHV-7, a proportion of cells starts to exhibit peculiar cytomorphologic alterations, becoming homogeneously rounded, enlarged in size, and refractile. Giant multinucleated syncytia, which result from fusion of single cells, are typically observed at later time points. Although cells infected with HHV-7 have a limited life expectancy in culture, the mechanisms of HHV-7–induced cell death remain unclear.
Because many viruses induce both necrotic lysis and apoptotic cell death as a part of their interactions with the target cell populations,22 we have investigated the presence and degree of necrosis and apoptosis during acute HHV-7 infection in SupT1 lymphoblastoid CD4+ T-cell line and primary CD4+ T lymphocytes. Both cell-to-cell (cocultivation) and cell-free models of HHV-7 infection were used. At various time points postinfection (pi), apoptosis was evaluated by transmission electron microscopy, direct immunofluorescence after annexin-V staining, and flow cytometry after propidium iodide (PI) staining. In parallel, the presence of necrosis lytic was evaluated by transmission electron microscopy, and the kinetics of infection was monitored by quantitative polymerase chain reaction (PCR).
MATERIALS AND METHODS
Cells and HHV-7 infection. The SupT1 lymphoblastoid CD4+ T-cell line was obtained from the American Type Tissue Collection (Rockville, MD). SupT1 cells were routinely cultured in RPMI 1640 (GIBCO BRL Life Technologies Inc, Gaithersburg, MD) containing 10% fetal calf serum (GIBCO BRL).
Enriched populations of CD4+ T cells derived from the peripheral blood of healthy blood donors were purified by negative immunomagnetic selection, using a mixture of monoclonal antibodies to CD8, CD14, CD19, CD20, and CD56 (all from Becton Dickinson, San José, CA) and magnetic beads coated with goat antimouse IgG antiserum (Dynal, Great Neck, NY), as previously described.21 Primary cells were cultured in RPMI containing 10% fetal calf serum and activated with 5 μg/mL of purified phytohemagglutinin (PHA; Sigma Chemicals, St Louis, MO) plus 20U/mL of human recombinant interleukin-2 (IL-2; Boeringher Mannheim, Postfack, Germany). After 3 days of culture, cells were washed twice and seeded again in complete medium plus 5 U/mL of IL-2 alone, which was readded every 5 days.
The HHV-7 isolate AL, which was used in this study, and the preparation procedures of viral stock have been previously described.7 SupT1 or preactivated primary CD4+ T cells were either mock-infected or adsorbed with HHV-7 for 10 hours at 37°C at a multiplicity of infection (MOI) of approximately 0.1. After adsorption, cells were washed to remove viral inoculum and diluted to the concentration of 5 × 105 cells/mL with fresh medium in the absence or presence of 100 μg/mL of phosphonoformic acid (PFA; Sigma). In other experiments, HHV-7 infection was performed by coculture of productively infected SupT1 cells with uninfected SupT1 cells at a ratio of 1:5, respectively.
The infection was allowed to proceed at 37°C. Viable cells, including syncytia, were scored at light microscopy by Trypan blue exclusion staining every other day, and the cultures were refed with fresh medium. Because the optimal cell density for both SupT1 cell line and primary CD4+ T cells was between 5 × 105 cells and 1 × 106 cells/mL, cell density was readjusted to 5 × 105 cells/mL whenever the cell number exceeded 1 × 106.
Evaluation of necrosis and apoptosis. Necrosis was evaluated exclusively by transmission electron microscopy, whereas apoptosis was examined by multiple techniques: transmission electron microscopy, flow cytometry after PI staining, and annexin V staining (Clontech, Palo Alto, CA) shown by fluorescence microscopy.
For the morphologic studies, SupT1 cells were fixed with 1% glutaraldehyde in 0.1 mol/L phosphate-buffered saline (PBS), postfixed with 1% osmium tetroxide, and embedded in Epon according to routine techniques. Thin sections were mounted on nickel grids and examined by transmission electron microscopy after staining with uranyl acetate and lead citrate. The ultrastructural characterization of necrosis included the loss of plasma membrane integrity, microvescicle formation, and swelling of individual nuclei with lack of chromatin condensation. The distinction between apoptotic and necrotic cells was mainly based on the presence of chromatin condensation in the presence of plasma membrane integrity or discontinuation of the plasma membrane with a lack of chromatin condensation, respectively.
For flow cytometry studies, cells were harvested by centrifugation at 200g for 10 minutes at 4°C, fixed with cold 70% ethanol for at least 1 hour at 4°C, and treated as previously described.23 Briefly, samples were pelletted, treated with 0.5 μg RNAse (type I-A; Sigma), and resuspended in PBS containing 50 μg/mL PI. Analysis was performed on a FACScan flow cytometer (Becton Dickinson) with the FL2 detector in logarithmic mode, using Lysis II software (Becton Dickinson). PI fluorescence was collected on a log scale. The threshold was triggered on the same Fl2 (PI fluorescence) signal, in which a clear-cut distinction between cell debris and apoptotic cells was virtually always present. Quantitative evaluation of apoptotic cells was performed by the Lysis II analysis software (Becton Dickinson), and data were expressed as the percentage of apoptotic versus nonapoptotic cells, regardless the specific cell cycle phase.23
Annexin-V staining was performed according to the manufacturers' recommendations (Clontech).
Quantitation of viral DNA in HHV-7–infected cells. To quantitate HHV-7 replication, an identical number of cells was removed from each infected culture at different time points. After washing with PBS, the cell pellets were stored at −20°C until PCR analysis. DNA was extracted by crude digestion with proteinase-K buffer (0.1 mg/mL of proteinase K in 50 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2 , 0.5% Tween 20, 0.5% Nonidet P-40), as described previously.24 Dilutions of the cellular extracts were used as the template for HHV-7 quantitative PCR.25
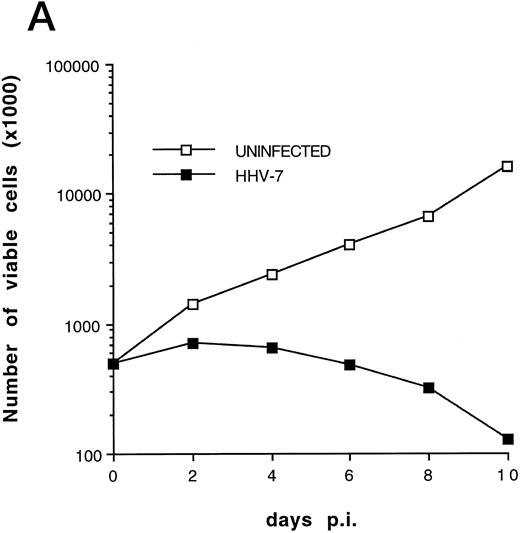
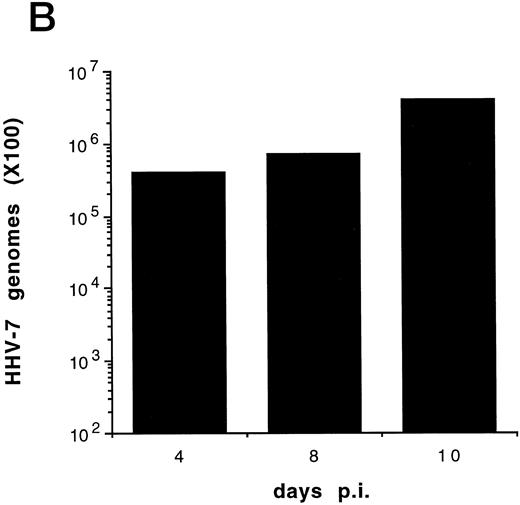
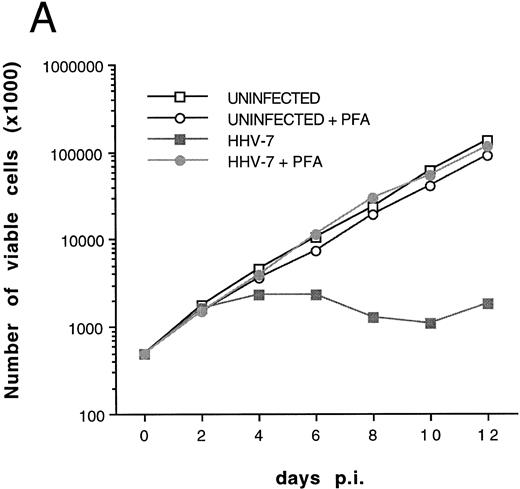
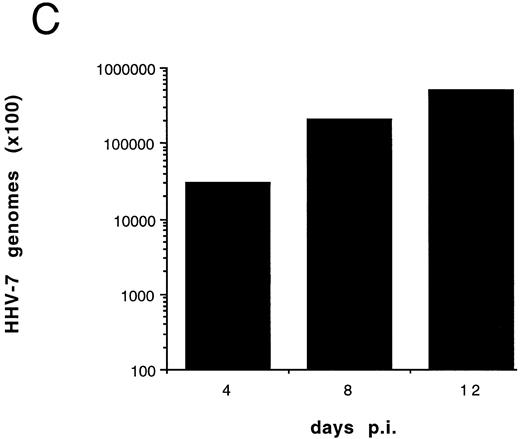
(A) Evaluation of the total number of viable cells by Trypan blue dye exclusion at different days pi. (B) Cell-associated HHV-7 DNA evaluated at different days pi by quantitative PCR (values are referred to 250 cells). Results of one experiment representative of four separate experiments performed in duplicate are shown.
(A) Evaluation of the total number of viable cells by Trypan blue dye exclusion at different days pi. (B) Cell-associated HHV-7 DNA evaluated at different days pi by quantitative PCR (values are referred to 250 cells). Results of one experiment representative of four separate experiments performed in duplicate are shown.
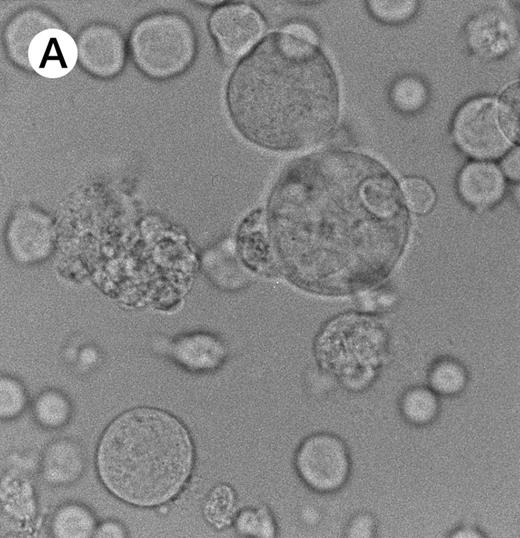
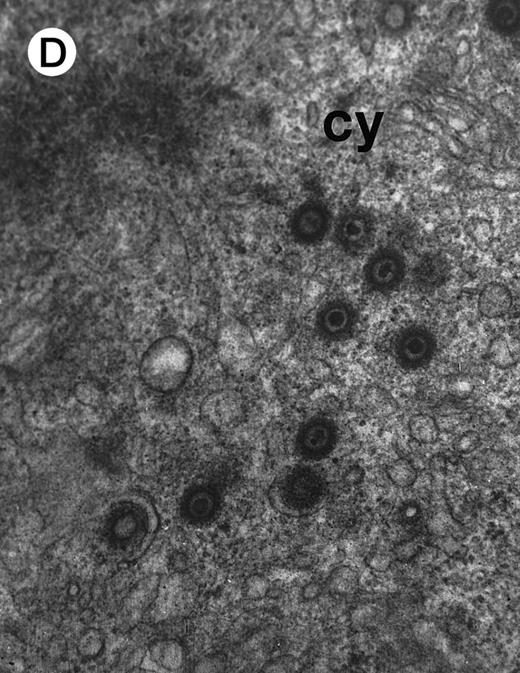
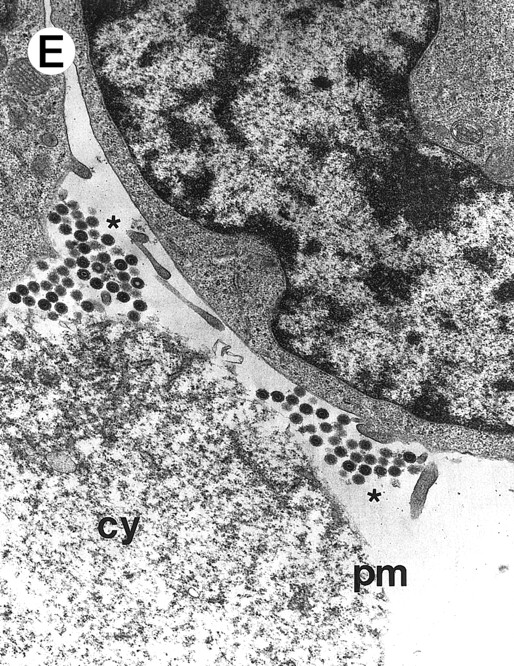
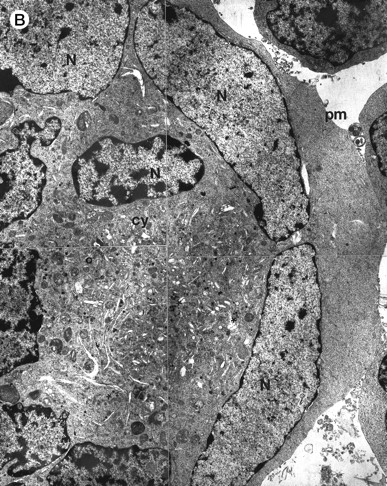
Morphologic examination of syncytia in HHV-7–infected SupT1 cultures (8 day pi) by light (A) and electron (B through E) microscopy. (A) Field with three syncytia and various single cells. (B) Multinucleated syncytium with an intact morphology. (C) Particular of a necrotic syncytium with vacuolized cytoplasm and initial discontinuation of the plasma membrane. Also note the lack of nuclear chromatin condensation. In (B) and (C), virions appear as dark points (asterisks). (D) Ringed nucleocapsids with an electron-dense core in the cytoplasm of a necrotic syncytium. (E) Particular of a syncytium in advanced stage of necrosis (bottom left), releasing mature virions (asterisks) in proximity of an intact cell (top right). N, nucleus; cy, cytoplasm; v, vacuole; pm, plasma membrane. Original magnifications: (A) ×400; (B and C) ×6,000; (D) ×36,000; and (E) ×7,000.
Morphologic examination of syncytia in HHV-7–infected SupT1 cultures (8 day pi) by light (A) and electron (B through E) microscopy. (A) Field with three syncytia and various single cells. (B) Multinucleated syncytium with an intact morphology. (C) Particular of a necrotic syncytium with vacuolized cytoplasm and initial discontinuation of the plasma membrane. Also note the lack of nuclear chromatin condensation. In (B) and (C), virions appear as dark points (asterisks). (D) Ringed nucleocapsids with an electron-dense core in the cytoplasm of a necrotic syncytium. (E) Particular of a syncytium in advanced stage of necrosis (bottom left), releasing mature virions (asterisks) in proximity of an intact cell (top right). N, nucleus; cy, cytoplasm; v, vacuole; pm, plasma membrane. Original magnifications: (A) ×400; (B and C) ×6,000; (D) ×36,000; and (E) ×7,000.
Statistics. Statistical analysis was performed using the two-tailed Student's t-test and Pearson's test for correlation.
RESULTS
Formation of syncytia and necrotic cell lysis in productively HHV-7–infected SupT1 cell cultures. Previous studies have reported that direct cell-to-cell transmission of HHV-7 is the most efficient system for viral propagation7,20 and that SupT1 is the only one among a variety of CD4+ lymphoblastoid T-cell lines that shows high susceptibility to HHV-7 infection.2,7 26 Therefore, to characterize the HHV-7–induced cytopathic effects (CPE), in the first group of experiments we performed cocultures of uninfected and infected SupT-1 cells at a ratio (5:1) optimal for the detection of HHV-7–mediated CPE. Results of one representative experiment of four separate experiments are shown in Figs 1-4.
Although some variability in the degree and kinetics of cytopathic effects was observed in the presence of different viral inocula, we constantly found that the total cell number in HHV-7–infected SupT1 culture hardly reached twice of the initial input (5 × 105 cells/mL), and a drastic decrease in viable cells was rather observed between 8 and 12 days pi, as shown in Fig 1A. Using quantitative DNA PCR, we found that the viral load progressively increased with the time of infection (Fig 1B).
Evaluation of the Ultrastructural Features of HHV-7–Induced Syncytia in SupT1 Cell Cultures
| Total no. of syncytia examined | 100 |
| Kind of morphology | |
| Normal | 36/100 |
| Necrotic | 60/100 |
| Apoptotic | 4/100 |
| Total no. of syncytia examined | 100 |
| Kind of morphology | |
| Normal | 36/100 |
| Necrotic | 60/100 |
| Apoptotic | 4/100 |
The samples examined by transmission electron microscopy were obtained from two separate experiments and different (6 to 8) days pi.
Evaluation of apoptosis by annexin V staining shown by fluorescence microscopy at day 8 pi. (Left panel) phase contrast light microscopy; (right panel) immunofluorescence microscopy of the same field. Note the presence of one syncytium (left panel) that is negative after annexin V staining (right panel). On the other hand, a single cell in the bottom right part of the photograph shows intense green membrane staining. Original magnification × 400.
Evaluation of apoptosis by annexin V staining shown by fluorescence microscopy at day 8 pi. (Left panel) phase contrast light microscopy; (right panel) immunofluorescence microscopy of the same field. Note the presence of one syncytium (left panel) that is negative after annexin V staining (right panel). On the other hand, a single cell in the bottom right part of the photograph shows intense green membrane staining. Original magnification × 400.
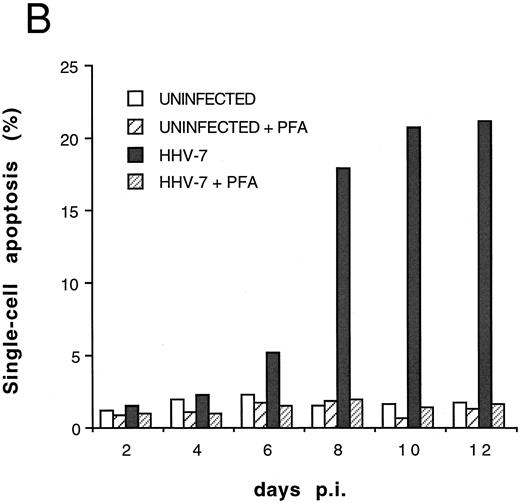
Cocultivation quantitative evaluation of apoptosis after PI staining and flow cytometry at different days pi. Results of one representative experiment of four experiments performed in duplicate are shown.
Cocultivation quantitative evaluation of apoptosis after PI staining and flow cytometry at different days pi. Results of one representative experiment of four experiments performed in duplicate are shown.
As expected,1,2 7 the morphologic examination performed at light microscopy showed the presence of giant multinucleated syncytia (Fig 2A). Because in this group of experiments the viral inoculum was represented by HHV-7–infected SupT1 cells, syncytia were already present at day 0 of infection, but their number progressively increased with the time of infection (data not shown).
At ultrastructural examination, a significant fraction of syncytia still maintained an intact appearance with little loss of cytoplasmic and nuclear organization despite the presence of numerous virions in the cytoplasmic compartment (Fig 2B). Although cytoplasmic virions were also observed in single cells, all syncytia invariably contained large amounts of typical herpesvirus nucleocapsids, which appear as single, ringed particles with an electron-dense center representing the core.27 28
However, most syncytia exhibited a vacuolized cytoplasm and discontinuation of the plasma membrane with lack of chromatin condensation, which are considered typical ultrastructural features of necrosis (Fig 2C). The morphologic features of necrosis were always associated with the presence of nucleocapsids in the cytoplasm (Fig 2D) and often with the release of mature virions in the extracellular space (Fig 2E). To ascertain that the ultrastructural features of syncytia shown above were truly representative of HHV-7 infection, various syncytia obtained from two separate experiments were analyzed. The presence of large amounts of cytoplasmic virions was confirmed in all syncytia examined, strongly suggesting that these giant multinucleated cells represented the major source of viral particles in the culture. Moreover, the majority of syncytia showed morphologic evidences of necrosis, whereas less than 5% showed apoptotic features (Table 1).
Progressive increase of apoptosis in HHV-7–infected SupT1 cell cultures. In parallel, we investigated whether the HHV-7–mediated CPE on lymphoid CD4+ T cells might comprise additional mechanisms, besides the induction of necrotic lysis. Because many viruses induce apoptotic cell death,22 the presence of apoptosis was investigated by using different techniques, ie, (1) annexin-V staining (Fig 3) and (2) PI uptake followed by flow cytometry examination (Fig 4), that specifically recognize early and late events of the apoptotic cell death program.
In HHV-7–infected cultures, membrane fluorescence identifying apoptotic cells was present predominantly in single cells rather than in syncytia, confirming the electron microscopy findings. A representative experiment of annexin-V staining is shown in Fig 3. Similar observations were made using different infected cultures.
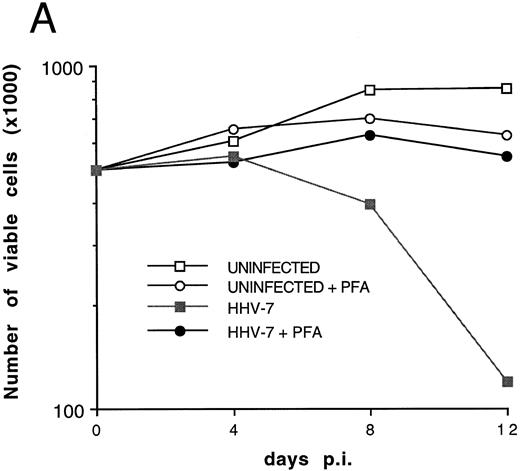
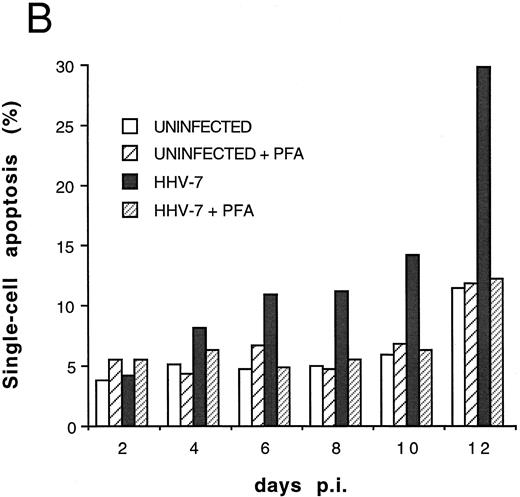
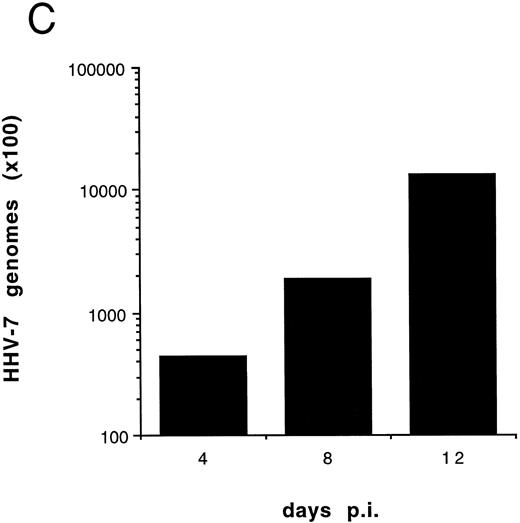
Effect of cell-free HHV-7 inoculation on SupT1 cells evaluated at different days pi. (A) Total number of viable cells scored by Trypan blue dye exclusion. (B) Percentage of apoptosis evaluated by flow cytometry after PI staining. (C) Cell-associated HHV-7 DNA evaluated by quantitative PCR (values are referred to 250 cells). Results of one representative of three separate experiments performed in duplicate are shown.
Effect of cell-free HHV-7 inoculation on SupT1 cells evaluated at different days pi. (A) Total number of viable cells scored by Trypan blue dye exclusion. (B) Percentage of apoptosis evaluated by flow cytometry after PI staining. (C) Cell-associated HHV-7 DNA evaluated by quantitative PCR (values are referred to 250 cells). Results of one representative of three separate experiments performed in duplicate are shown.
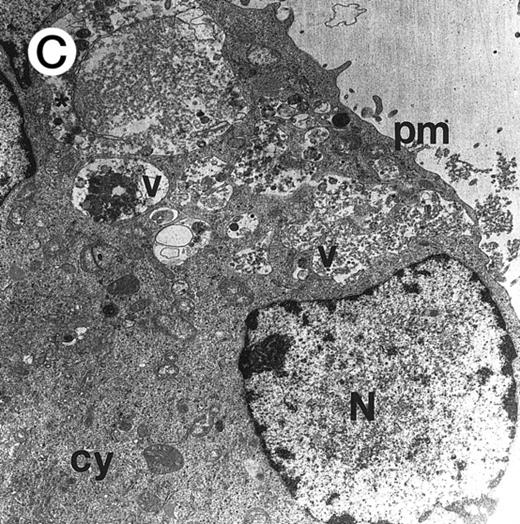
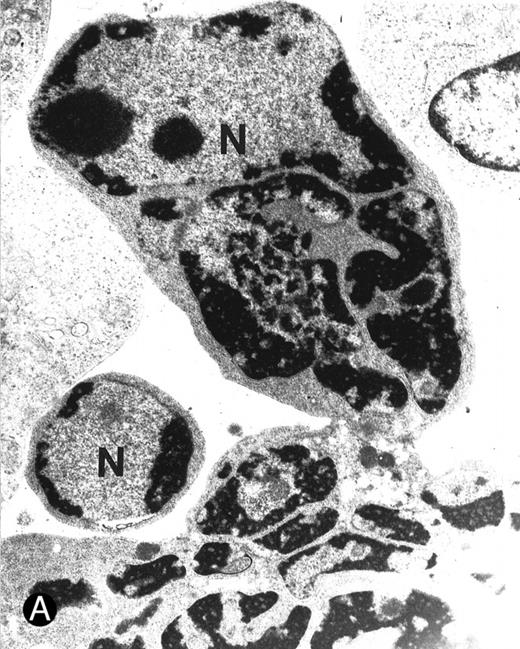
Ultrastructural examination of apoptotic SupT1 cells by transmission electron microscopy (8 day pi). In (A), two apoptotic nuclei in cells that do not show viral particles.
In (B), note the presence of empty capsids (asterisk) in the cytoplasm of an apoptotic cell (see also the insert at the top left). All cells show chromatin condensation, a characteristic feature of apoptosis. N, nucleus; cy, cytoplasm. Original magnifications: (A) ×4,400 and (B) ×8,800; insert in (B) ×40,000.
Ultrastructural examination of apoptotic SupT1 cells by transmission electron microscopy (8 day pi). In (A), two apoptotic nuclei in cells that do not show viral particles.
In (B), note the presence of empty capsids (asterisk) in the cytoplasm of an apoptotic cell (see also the insert at the top left). All cells show chromatin condensation, a characteristic feature of apoptosis. N, nucleus; cy, cytoplasm. Original magnifications: (A) ×4,400 and (B) ×8,800; insert in (B) ×40,000.
Presence of HHV-7 Nucleocapsids in SupT1 Apoptotic Single Cells
| Total no. of apoptotic cells examined | 100 |
| Kind of cytoplasmic virions | |
| Mature nucleocapsids | 0/100 |
| Empty nucleocapsids (6-10/cell) | 25/100 |
| None | 75/100 |
| Total no. of apoptotic cells examined | 100 |
| Kind of cytoplasmic virions | |
| Mature nucleocapsids | 0/100 |
| Empty nucleocapsids (6-10/cell) | 25/100 |
| None | 75/100 |
The samples examined by transmission electron microscopy were obtained from two separate experiments and different (6 to 8) days pi.
The relative extent of programmed cell death in HHV-7–infected cultures was also determined by flow cytometry using quantitative fluorescent staining of nuclear DNA to enumerate single cells undergoing apoptosis.23 Infection of SupT1 cells resulted in a progressive increase in numbers of apoptotic nuclei, which remained minimal in uninfected cells for all the experimental culture times (Fig 4). The increase in single-cell apoptosis showed a significant positive correlation (r = .97, P < .01), with the decrease in the total number of viable cells found in HHV-7–infected cultures (Fig 1A). On the other hand, no significant correlation (r = .69, P = .5) could be established between the degree of apoptosis and the viral load in HHV-7–infected cultures (Fig 1B).
To further investigate the role of HHV-7 replication in the induction of SupT1 apoptosis, the next experiments were performed using cell-free HHV-7 inocula (MOI 0.1) in the absence or presence of PFA, a specific inhibitor of herpetic DNA polymerase.25 29 The decrease in viable cell counts (Fig 5A), the induction of apoptosis (Fig 5B), and the appearance of syncytia (data not shown) in cell-free HHV-7 infections were generally delayed compared with those initiated by cell-to-cell transmission (Figs 1A and 4). Moreover, the viral load calculated at different days pi (Fig 5C) was generally 1 log lower than in coculture experiments (Fig 1B).
It is of note that the addition of PFA to HHV-7–infected SupT1 cell cultures completely blocked the detrimental effect of HHV-7 on the total number of viable cells (Fig 5A). This was accomplished by inhibition of syncytium formation (data not shown) and of single-cell apoptosis (Fig 5B). PFA, by itself, did not show any toxic effect on uninfected SupT1 cells. As shown previously in coculture experiments, a statistically significant inverse correlation was observed between the total number of viable cells and the percentage of apoptosis (r = .83, P < .05), strongly suggesting that apoptosis contributes to the progressive loss of viable SupT1 cells in HHV-7–infected cultures. Moreover, in this group of experiments, a positive correlation was also observed between the viral DNA copy numbers and the percentage of apoptosis (r = .79, P < .05).
Absence of mature virions in apoptotic SupT1 cells. Although the findings given above suggested that an increased viral load was causally related to the induction of apoptotic cell death, we had no information on whether productive HHV-7 infection directly induced apoptotic cell death or whether this effect was indirectly mediated by HHV-7 gene products or by soluble factors released by infected cells. We approached this problem in next group of experiments by analyzing the presence of HHV-7 virions in cells with an apoptotic morphology by transmission electron microscopy (Fig 6A and B and Table 2).
It is notable that most cells showing the characteristic morphologic features of apoptosis at the ultrastructural analysis did not show the presence of virions in either the nuclear or the cytoplasmic compartments (Fig 6A). On the other hand, in about 25% of the cells examined it was possible to document the anomalous presence of empty viral capsids in the cytoplasmic compartment (Fig 6B). Because the assembly of nucleocapsids occurs within the nucleus,27 28 these empty cytoplasmic capsids suggested the presence of abortive virus assembly in some apoptotic cells. Table 2 reports the data from the analysis of 100 apoptotic cells, which were randomly obtained from two different infection experiments.
HHV-7–mediated induction of apoptosis in primary CD4+ T lymphocytes. To evaluate whether the induction of apoptosis observed in SupT1 T-cell line was of physiologic relevance, our next experiments were performed on primary CD4+ T lymphocytes that were stimulated with 5 μg/mL of PHA and 20 U/mL of IL-2 for the first 3 days of culture. Prestimulated CD4+ T cells were next infected with cell-free viral inoculum (MOI 0.1) and kept in culture in the presence of 5 U/mL of IL-2. This low IL-2 concentration is required for an optimal viral growth,28 but does not support a high level of primary T-cell proliferation.
Inoculation with cell-free HHV-7 induced a progressive decline in the total number of viable primary lymphoid CD4+ T cells (Fig 7A) and was accompanied by the appearance and progressive increase of syncytia (data not shown) and single-cell apoptosis (Fig 7B). PFA blocked syncytium formation, induction of apoptosis, and viral replication also in these cells (Fig 7C). As previously shown for the SupT1 cell line, the increase of apoptosis in primary cells was significantly correlated with the loss of viable cell numbers (r = .98, P < .05) as well as with the amount of viral DNA, as evaluated by DNA PCR (r = .97, P < .001).
Effect of cell-free HHV-7 inoculation on primary CD4+ T cells evaluated at different days pi. (A) Total number of viable cells scored by Trypan blue dye exclusion. (B) The percentage of apoptosis evaluated by flow cytometry after PI staining. (C) Cell-associated HHV-7 DNA, evaluated by quantitative PCR (values are referred to 250 cells). Results of one experiment of two separate experiments performed in duplicate are shown.
Effect of cell-free HHV-7 inoculation on primary CD4+ T cells evaluated at different days pi. (A) Total number of viable cells scored by Trypan blue dye exclusion. (B) The percentage of apoptosis evaluated by flow cytometry after PI staining. (C) Cell-associated HHV-7 DNA, evaluated by quantitative PCR (values are referred to 250 cells). Results of one experiment of two separate experiments performed in duplicate are shown.
DISCUSSION
Cell death can occur by two major distinct mechanisms, necrosis and apoptosis, which differ both morphologically and biochemically.30 Necrosis is considered to be a pathologic reaction in response to toxic agents or lytic viral infections, whereas apoptosis is a physiologic process that is part of the homeostatic regulation during normal tissue turnover30 and can be triggered by several viruses.22 31-37
The most readily observable cytopathic effect of HHV-7 infection of lymphoid CD4+ T cells is the process of syncytium induction.1,2 7 In this study, by combining information derived from electron microscopy and annexin-V staining studies, the following points could be clearly established: (1) HHV-7–induced giant multinucleated syncytia always contain a large number of cytoplasmic virions; (2) they mainly undergo lytic necrosis with release of mature virions and therefore are likely to represent the main source of infectious particles; and (3) they rarely show apoptotic features.
Although the formation of large multinucleate syncytia likely accounts for a significant proportion of the T-cell death observed in HHV-7–infected cultures, a major achievement of the present study was the demonstration that HHV-7 infection also triggers apoptosis in both SupT1 lymphoblastoid cell line and primary CD4+ T lymphocytes. By using annexin-V staining and PI uptake, followed by flow cytometry and electron microscopy analyses, we could show that (1) apoptosis occurred predominantly in single cells; (2) in both coculture and cell-free HHV-7 infection experiments, a significant correlation was present between the loss of viable cells and the degree of apoptosis; (3) in cell-free HHV-7 infection experiments, a significant positive correlation was also present between the percentage of apoptosis and the viral yield; (4) inhibition of HHV-7 replication by PFA, a specific inhibitor of herpetic DNA polymerase, resulted in a block in the induction of apoptosis; and (5) cells with an apoptotic morphology very rarely contained mature viral particles, but approximately 25% of these cells showed the presence of empty nucleocapsids.
Both cell-to-cell and cell-free transmission routes of HHV-7 infection were able to induce formation of syncytia and apoptosis. The faster kinetics and the higher degree of cytopathic effects observed in the cell-to-cell transmission model with respect to the cell-free model likely reflect the accelerated spread of infection in the first model.
The viral mechanisms by which a productive HHV-7 infection is able to induce formation of necrotic syncytia and apoptosis in its target cells and the relationships between these two phenomena remain to be elucidated and await the availability of selective molecular tools and the recognition of key regulatory and structural HHV-7 proteins. At this point, we were unable to investigate these mechanisms in depth. However, the induction of apoptosis, after viral infection of a cell, is generally viewed as an attempt by the host cell at limiting virus replication. In fact, many viruses have their own antiapoptosis genes or upregulate antiapoptotic cellular genes, which can block premature death of infected cells and thereby facilitate a persistent infection or prolong the survival of lytically infected cells to maximize the production of a viral progeny.22,31 A major conclusion that can be drawn from the data presented in this study is that a productive HHV-7 replication is not a main mechanism inducing apoptotic cell death, which occurred predominantly in uninfected bystander cells. Our findings are consistent with a recent report on HHV-6 that was published while our study was under consideration for publication.36 In their study, Inoue et al36 showed that HHV-6 is also able to induce apoptosis, in JJHAN lymphoid T cells, and that apoptosis occurs predominantly in HHV-6 antigen-negative cells.
In other viral models, three main indirect mechanisms have been proposed to explain the induction of apoptosis in the target cells22,31: (1) surface interactions between extracellular virions and cell membrane receptors; (2) defective viral maturation; and (3) upregulation of cellular genes encoding for inhibitory cytokines.
The first of the three mechanisms listed above is typically explored by testing the ability of inactivated virus to induce apoptosis.35 In preliminary experiments performed with UV-inactivated cell-free HHV-7 inoculum, we found a slight but reproducible increase of apoptosis with respect to mock-treated cells in the first days posttreatment. If confirmed in more detailed studies, these data may favor the hypothesis that early binding events contribute to the HHV-7–mediated apoptosis of uninfected single cells.
The second mechanism of virus-mediated apoptosis postulates that accumulation of viral proteins in cytoplasmic compartment, resulting in abortive virus assembly, may induce an intracellular stress and thereby activate the apoptotic pathway. Thus, intracellular accumulation of viral proteins, rather than virus assembly and release, can trigger apoptosis, as shown, for example, in the model of Dengue type 1 infection of neuronal cells.37 Interestingly, in this study, we have demonstrated the anomalous presence of empty nucleocapsids in the cytoplasm of 25% of the cells with morphologic features of apoptosis. However, it should also be considered the possibility that the triggering of apoptosis might interfere with viral DNA replication and lead to the formation of empty viral particles.
The third of the three general mechanisms proposed to explain the induction of apoptosis in uninfected cells postulates the upregulation of inhibitory cytokines by viral gene products.38 39 At present, the possibility that also HHV-7 might dysregulate the production of cytokines cannot be excluded and clearly requires further investigation.
The role of apoptosis has long been debated as the potential cause of immunosuppression and T-cell depletion during many acute or persistent infections caused by other herpesviruses, such as Epstein-Barr virus (EBV),40 or human immunodeficiency virus–type 1 (HIV-1).41-44 For instance, it has been shown that T lymphocytes from individuals with acute EBV or HIV-1 infection are conditioned in vivo to die by apoptosis in vitro.40,44 An interesting parallelism exists between two distantly related viruses such as HHV-7 and HIV-1, because they both show the same selective tropism for CD4+ T cells, use the same cellular receptor for infection (the CD4 molecule),20,45,46 and are both capable of inducing apoptotic cell death of their target cells. Although the contribution of only HIV-1 to CD4+ T-cell apoptotic cells is still controversial, reactivation of latent infection with herpesviruses in the immunocompromised host has been recently proposed as one determinant of the clinical course in HIV-1–infected subjects by triggering apoptosis.47
In conclusion, we have shown that HHV-7 demonstrates the ability to induce both necrotic lysis and apoptosis in human CD4+ T cells. Efforts to define the role of this recently identified lymphotropic virus in human diseases are still ongoing. Because HHV-7 is able to induce functional alterations in CD4+ T cells,19 further studies are needed to evaluate a possible role of HHV-7–triggered apoptosis to the T-cell loss that characterizes various immunocompromised subjects, including patients with HIV-1 disease.
Supported by “AIDS and Blood projects” of the Italian Ministry of Health. P.S. is the recipient of an ANLAIDS Fellowship.
Address reprint requests to Giorgio Zauli, MD, PhD, Institute of Human Anatomy, Via Fossato di Mortara 66, 44100 Ferrara, Italy.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal