Abstract
The CXC-chemokines interleukin-8 (IL-8), neutrophil-activating peptide-2 (NAP-2), and melanoma growth-stimulatory activity (MGSA) are chemoattractants with high selectivity for neutrophils. Although IL-8 has been shown to act as an extremely potent mediator, reports on NAP-2 and MGSA are still contradictory. Here we show for the first time that NAP-2 and MGSA induce two distinct optima of neutrophil chemotaxis. A first optimum is elicited within a concentration range as low as it is characteristic for IL-8. However, a second optimum appears at more than 200-fold higher stimulus concentrations, at which IL-8 is inactive. Investigating the involvement of the two chemokine receptors CXCR-1 and CXCR-2 in NAP-2–mediated chemotaxis, we observe that the cells become desensitized to the first optimum of the chemokine after selective downregulation of CXCR-2, while both optima disappear upon simultaneous downregulation of both receptors. Blocking monoclonal antibodies (MoAbs) specific for CXCR-2 or CXCR-1 either suppress the first optimum of NAP-2–induced chemotaxis or drastically reduce the second one, respectively. These results provide evidence that both receptors are involved in NAP-2–induced neutrophil chemotaxis, with CXCR-2 rendering the cells responsive to low dosages of the chemokine, and with CXCR-1 extending their responsiveness to NAP-2 dosages higher by several orders of magnitude.
DURING THE ONSET of acute inflammation, polymorphonuclear neutrophil granulocytes (PMN) are the first blood cells to arrive at the inflammatory site and to provide protection against invading microorganisms. Among the various chemoattractant mediators that become released in such a situation, a number of low molecular weight cytokines belonging to the CXC-chemokine subfamily have been recognized to play an important role in the selective recruitment and functional activation of neutrophils. While the importance of the prototype CXC-chemokine, the interleukin-8 (IL-8), as a major chemoattractant for PMN has been shown in numerous in vitro and in vivo studies,1-3 there exists far less information on the physiologic roles of other members of the CXC-subfamily, such as the neutrophil-activating peptide-2 (NAP-2) and the melanoma growth stimulatory activity (MGSA, also termed GROα). NAP-2 may have a specialized function as a first line mediator, because this chemokine is rapidly generated through proteolysis of platelet-derived precursors by the neutrophils themselves.4,5 A major precursor immediately released from stimulated platelets is the connective tissue-activating peptide III (CTAP-III) that becomes converted into NAP-2 by cleavage of 15 amino acids from the N-terminus. In contrast to NAP-2, secretion of MGSA and IL-8 is later in onset because their production first must be turned on by other proinflammatory stimuli like tumor necrosis factor (TNF ) or IL-1.3 Moreover, the latter chemokines are often coinduced in various producer cells, while the only source for NAP-2 appears to be the platelet.4,6,7 Although all three chemokines contain the functionally important Glu-Leu-Arg- (ELR-) sequence motif, which has been recognized to form an absolute requirement for specific receptor binding and cell activation,8 there exist characteristic differences in the receptor binding capacities and functional activities between NAP-2 and MGSA, on the one hand, and IL-8 on the other. Binding studies performed on neutrophils have shown that NAP-2 or MGSA are able to compete for IL-8 binding, although less efficiently than IL-8 competes for itself. By contrast NAP-2 and MGSA were found to be as potent as IL-8 to compete for NAP-2 or MGSA binding.9-12 This indicated that PMN express two distinct types of binding sites, which both exhibit the same affinity for IL-8, but differ in their affinity for NAP-2 or MGSA. These binding sites were identified as the G protein-coupled seven transmembrane segment receptors CXCR-1 and CXCR-2 (previously termed IL-8RA and IL-8RB).13,14 CXCR-2 was shown to bind IL-8, NAP-2, or MGSA with high affinity, whereas CXCR-1 exhibits high affinity for IL-8 only, but about 100-fold lower affinity for NAP-2 or MGSA.15-18
In accordance with their different binding characteristics for CXC-chemokine receptors on neutrophils, it was found that IL-8 was by far more potent than NAP-2 or MGSA to activate a variety of effector functions, such as the degranulation of lysosomal enzymes,9,19-23 the respiratory burst,21,24,25 and upregulation of adhesion molecules.26 By contrast, very diverging results have been reported as to the chemotactic activity of these chemokines. Concerning NAP-2, potencies comparable to that of IL-8,20 about 12-fold lower,22 or even 100-fold lower,27 were found. Likewise MGSA was reported to be as active as IL-825,28 or about fourfold less potent.21,23 Thus, it remains an open question whether neutrophil chemotaxis in response to NAP-2 or MGSA is likewise dependent on the differential binding of these chemokines to the two receptor types or whether only one of these receptors is involved. That in principle both receptor types are able to mediate chemotaxis, at least in response to IL-8, was shown by means of Jurkat cells that had been separately transfected with CXCR-1 or CXCR-2.16 Furthermore, neutralizing antibodies to CXCR-1 or CXCR-2 were shown to at least partially inhibit IL-8–induced neutrophil migration, giving further evidence that, in principle, both receptor types activate chemotaxis in neutrophils.29 30 However, because no corresponding data are available on other CXC-chemokines, the roles of CXCR-1 and CXCR-2 in NAP-2– or MGSA-mediated neutrophil chemotaxis are still unclear.
In our present report, we have reinvestigated the chemotactic activities of MGSA and NAP-2 in direct comparison to that of IL-8 and analyzed the contribution of CXCR-1 and CXCR-2 by means of selective desensitization of these receptors, as well as by antibody inhibition studies. Our data show that NAP-2 and MGSA exhibit chemotaxis profiles quite different from that for IL-8 and that the two types of chemokine receptors are differently involved in the response.
MATERIALS AND METHODS
Chemokines. Human recombinant IL-8 (72-residue isoform) and human recombinant MGSA were purchased from PeproTech Inc (Rocky Hill, NJ). Human natural NAP-2 was purified to homogeneity from culture supernatants of platelet-containing stimulated peripheral blood mononuclear cells using sequential immunoaffinity chromatography, cation exchange chromatography, and reversed phase high-performance liquid chromatography (HPLC) according to a described protocol.4 31 Alternatively NAP-2 was also obtained by chymotrypsin digestion of homogeneous CTAP-III accordingly purified from release supernatants of thrombin-stimulated platelets. In brief, 1 mg CTAP-III in 1 mL phosphate-buffered saline (PBS) was digested with 13 μg chymotrypsin from bovine pancreas (87 U/mg; Serva, Heidelberg, Germany) for 30 minutes at 37°C, and the reaction was terminated by acidification to a pH value below 3.0 with trifluoro acetic acid. Homogeneous NAP-2, identical in sequence and biologic activity to the cell culture-derived material, was finally obtained after separation of the digest on an analytic HPLC reversed phase cyanopropyl column (4.6 × 250 mm, 5 μm, wide pore; Baker Research Products, Phillipsburg, NJ).
Antibodies against CXCR-1 and CXCR-2. Murine monoclonal antibodies (MoAbs) against CXCR-1 and CXCR-2 were generated by immunizing mice with carrier-coupled synthetic peptides, representing fragments of the extracellular N-termini of the respective receptors, according to standard protocols. In brief, an antibody against CXCR-1 (MoAb SE-2) of the IgG 2b-isotype was induced at the Department of Immunology, University of Göttingen, by immunization with a peptide (CXCR-1[1-30]) encompassing amino acid residues 1-30 of the receptor as described elsewere.32 Similarly, an antibody against CXCR-2 (MoAb RII115) of the IgG 2b-isotype was obtained at the Department of Immunology and Cell Biology, Forschungszentrum Borstel, using a synthetic peptide (CXCR-2[6-29] encompassing residues 6 to 29 of the receptor. Either antibody specifically recognized its respective peptide antigen as evidenced by total competition of its binding to the solid phase-coated peptide by an excess of free soluble peptide antigen in enzyme-linked immunosorbent assay (ELISA). The epitope for the anti–CXCR-2 antibody (MoAb RII115) was further characterized by SPOTs technique (IC Chemikalien, Ismaning, Germany). In brief, a series of overlapping octapeptides covering residues 6 to 29 of the receptor were synthesized onto a SPOTs membrane and subsequently probed for reactivity with the antibody. Binding was detected by means of β-galactosidase-linked goat α-mouse IgG and specificity of binding was examined by competition with peptide CXCR-2[6-29]. By these means, the epitope for the antibody was identified as to comprise residues 9 to 13 (sequence: FEDFW) of the receptor.
Preparation and pretreatment of neutrophils with chemokines and antibodies. Human PMN were routinely isolated from citrated blood of single healthy donors by gradient centrifugation on Ficoll-Hypaque as previously described.4 More than 98% of cells excluded trypan blue and purity was greater than 95% in all events. For desensitization experiments with chemokines 1 × 107 PMN/mL suspended in Dulbecco's PBS without Ca2+ and Mg2+ (D-PBS) and supplemented with 0.1% bovine serum albumin (BSA) (low endotoxin BSA; Serva, Heidelberg, Germany) were pretreated at 37°C with various concentrations of NAP-2 or IL-8 or left untreated for 10 minutes. After washing with a 10-fold volume of D-PBS/0.1% BSA, to remove the free chemokine, cells were assayed for surface expression of CXC-chemokine receptors or for chemotaxis as described below. For inhibition experiments with neutralizing antibodies 1 × 107 PMN/mL in D-PBS/0.1% BSA were preincubated for 60 minutes with or without 100 μg/mL of the respective antibody at 37°C under agitation. Subsequently, the cells were either used in binding experiments with 125I-labeled chemokines or in chemotaxis assays as described below. After pretreatment, the yield of cells always remained greater than 90% and viability of these cells as estimated by trypan blue dye exclusion exceeded 95% in all events.
Neutrophil chemotaxis assay. Neutrophil chemotaxis was assayed according to the method of Schröder et al19 using a modification of the endogenous component chemotaxis assay described by Creamer et al.33 Briefly, the lower compartments of blind well Boyden chambers (Costar, Bodenheim, Germany) received 100 μL of serially diluted chemoattractants in D-PBS/0.1%BSA and were covered with polycarbonate membranes (pore size 3 μm; Nucleopore GmbH, Tübingen, Germany). The upper compartments were filled with 2 × 105 PMN suspended in 100 μL D-PBS/1%BSA and supplemented with CaCl2 and MgCl2 (0.9 nmol/L and 0.5 nmol/L final concentration, respectively). After 1 hour of incubation at 37°C in a humid atmosphere, cells in the lower compartment were lysed in 0.1% Triton X-100, and endogenous β-glucuronidase enzymatic activity was measured by using p-nitrophenyl-β-glucuronide (Sigma-Aldrich Chemie, Deisenhofen, Germany) as a substrate. The number of migrated cells was calculated from a standard of lysed cells run in parallel. Control experiments showed that pretreatment of neutrophils with chemokines or antibodies did not affect subsequent measurement of endogenous enzymatic activity in the cell lysates. Furthermore, the number of migrated cells in the lower compartment of the chamber as determined by measurement of the enzyme content from lysed cells was not different from that determined in parallel by counting with a Coulter Counter connected to a Channelyzer C 256 (Coulter Electronics, Krefeld, Germany).
Flow cytometric analysis of CXCR-1 and CXCR-2 expression on neutrophils. PMN (1 × 106/mL) suspended in D-PBS/0.1%BSA were incubated with MoAb RII115 (2 μg/mL) or MoAb SE-2 (5 μg/mL) for 1 hour on ice, labeled with fluorescein-conjugated goat α-mouse IgG (H-L) Ab (Dianova, Hamburg, Germany) at a final concentration of 15 μg/mL and analyzed in a flow cytometer (model FACStar PLUS, Beckton Dickinson, Heidelberg, Germany).
Iodination of chemokines and binding assays. Chemokines were radiolabled by iodination at tyrosine residues using the chloramine T method. Before iodination, NAP-2 was chemically modified by the introduction of tyrosine residues according to a recently published method.9 Binding experiments with radiolabeled chemokines were performed as described in detail elsewhere.9 Briefly, PMN were suspended at 1 × 107 cells/mL in D-PBS/2%BSA, and duplicate samples of 1 × 106 cells were incubated on ice for 2 hours with 2 nmol/L 125I-labeled-NAP-2 or 2 nmol/L 125I-labeled IL-8 in the presence or absence of a 100-fold excess of unlabeled NAP-2 or IL-8, respectively. After removal of the unspecifically bound material by twofold washing and sedimentation through 10% surose in D-PBS, bound radioactivity was determined in a gamma counter.
RESULTS
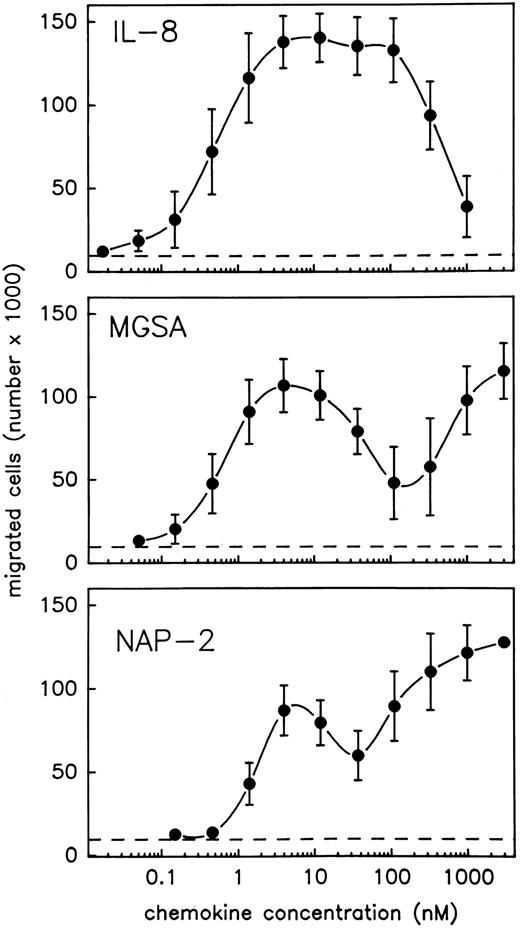
NAP-2 and MGSA induce two separate optima of neutrophil chemotaxis. In view of the contradictory results that have been reported on the chemotactic activity of MGSA and NAP-2 as compared with that of IL-8, we reinvestigated these chemokines for their relative abilities to induce migration of purified neutrophils using the Boyden chamber system. To allow for a direct comparison, the three chemokines were tested in parallel using PMN from the same preparation. Moreover, an extremely wide range of stimulus concentrations was applied (0.02 nmol/L to 3 μmol/L). To enable precise analyses of the resulting concentration kinetics, a rather narrow range of serial dilution steps (1/3) was chosen for each chemokine. As shown in Fig 1, the chemotactic response of PMN to IL-8 was characterized by a single typical optimum curve, indicating maximum efficacy of the chemokine within a rather broad concentration range of about 4 to 120 nmol/L and returning to background levels at about 1.1 μmol/L. Consistent with previous results reported by others,19,20,27 34-36 the chemotactic potency of IL-8 (stimulus concentration inducing half maximal cell migration) was characterized by an EC50 value of 0.4 nmol/L. By contrast, increasing concentrations of MGSA, as well as NAP-2, induced completely different profiles of cell migration that were each composed of two separate optimum curves (Fig 1). With either chemokine, one optimum appeared at low stimulus concentrations, and the dosages exhibiting maximum efficacies (4 nmol/L), as well as those required for half maximal cell migration (EC50 = 0.5 nmol/L for MGSA and EC50 = 1.3 nmol/L for NAP-2), were very similar to those observed with IL-8 (see above). However, another optimum appeared at rather high stimulus concentrations where the chemotactic activity of IL-8 was already declining. Within this range, the chemotactic potencies of MGSA and NAP-2 (as calculated from their maximum efficacies appearing at 3 μmol/L) amounted to EC50 = 220 nmol/L and 90 nmol/L, respectively and were thus more than 200-fold lower than that of IL-8. The entire activity range of MGSA and NAP-2 could not be determined, as dosages higher than 3 μmol/L were impracticable. Altogether these data show that MGSA and NAP-2 are, in fact, as potent as IL-8 in mediating neutrophil chemotaxis and, moreover, display chemotactic activity over a considerably wider concentration range than IL-8.
PMN chemotaxis in response to IL-8, MGSA, and NAP-2. Chemotaxis of PMN induced by increasing concentrations of IL-8, MGSA, or NAP-2 was measured in the Boyden chamber system. Results are expressed as the number of migrated cells detected in the lower compartment of the chamber after 1 hour. Random migration in the absence of stimulus is indicated (- - -). Data represent mean ± standard deviation (SD) from three independent experiments.
PMN chemotaxis in response to IL-8, MGSA, and NAP-2. Chemotaxis of PMN induced by increasing concentrations of IL-8, MGSA, or NAP-2 was measured in the Boyden chamber system. Results are expressed as the number of migrated cells detected in the lower compartment of the chamber after 1 hour. Random migration in the absence of stimulus is indicated (- - -). Data represent mean ± standard deviation (SD) from three independent experiments.
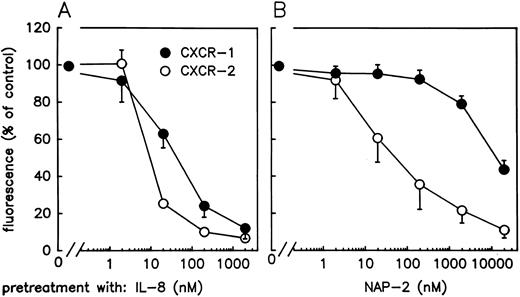
Specific detection of CXCR-1 or CXCR-2 on neutrophils by MoAbs. Our observation that MGSA and NAP-2 induce two optima of neutrophil chemotaxis, while IL-8 exhibits only one, is in striking accordance with the reported presence of two different CXC-chemokine receptor types (CXCR-1 and CXCR-2) on neutrophils, one of which binds MGSA and NAP-2 with low affinity, while the other one binds these chemokines with high affinity, but both of which bind IL-8 with similarly high affinity. To approach the question, whether either of the chemotactic optima induced by MGSA or NAP-2 was mediated through a different type of chemokine receptor or whether only the high affinity receptor (CXCR-2) was involved, we chose to use two MoAbs directed to these receptors in further studies. These antibodies were raised in mice by immunization with synthetic peptides representing fragments of the extracellular N-terminus of the respective receptor type (peptides CXCR-1[1-30] and CXCR-2[6-29], respectively). As shown in Fig 2, both the α-CXCR–1 antibody (MoAb SE-2) and the α-CXCR–2 antibody (MoAb RII115) recognized their respective antigen expressed on PMN with high sensitivity, as shown by flow cytometry analyses using fluorescein-conjugated goat α-mouse IgG for detection of the cell-bound primary antibodies. The binding of either antibody was specific, as the signals obtained were almost completely abrogated (by >93%) in the presence of a 100-fold molar excess of the respective free peptide antigen CXCR-1[1-30] or CXCR-2[6-29] (Fig 2), while no reduction in binding was observed when α-CXCR–1 antibody was used in combination with peptide CXCR-2[6-29] and vice versa (data not shown). Additional evidence for exclusive binding of the antibodies to chemokine receptors (and not to other potentially cross-reactive cell-surface proteins) was obtained by examining their reactivity with PMN that had been challenged to completely downregulate CXCR-surface expression by pretreatment with a high dose of IL-8 (2 μmol/L) at 37°C for 10 minutes. As previously reported by Samanta et al,37 PMN treated in such a way are no longer able to bind radiolabeled IL-8 due to internalization of all surface-expressed CXC-chemokine binding sites. As shown in Table 1, signals obtained with either antibody were practically abolished when PMN were pretreated with IL-8 at 37°C. This lack of reactivity was not due to competition of antibody binding through receptor occupation by the chemokine because PMN pretreated with IL-8 at 4°C (at which temperature receptor internalization does not take place),37 were still able to bind either antibody. Altogether these results show that antibodies SE-2 and RII115 selectively detect CXCR-1 and CXCR-2, respectively and are, therefore, suitable reagents for studying the surface expression of these receptors on neutrophils.
Flow cytometric analysis of CXCR-1 and CXCR-2 expression on neutrophils. Cells were incubated with α-CXCR–1 antibody SE-2 (A) or with α-CXCR–2 antibody RII115 (B). Incubation with either antibody was performed in the absence (1) or presence (2) of a 100-fold molar excess of the peptide fragments CXCR-1[1-30] (A) or CXCR-2[6-29] (B). Subsequently, cell-bound antibodies were detected by fluorescein-conjugated goat α-mouse IgG. Control cells received the secondary antibody only (3).
Flow cytometric analysis of CXCR-1 and CXCR-2 expression on neutrophils. Cells were incubated with α-CXCR–1 antibody SE-2 (A) or with α-CXCR–2 antibody RII115 (B). Incubation with either antibody was performed in the absence (1) or presence (2) of a 100-fold molar excess of the peptide fragments CXCR-1[1-30] (A) or CXCR-2[6-29] (B). Subsequently, cell-bound antibodies were detected by fluorescein-conjugated goat α-mouse IgG. Control cells received the secondary antibody only (3).
Binding of α-CXCR Antibodies to PMN Pretreated With IL-8
| IL-8 Pretreatment* . | % Specific Binding of Antibody† . | |
|---|---|---|
| . | α-CXCR–1 . | α-CXCR–2 . |
| 37°C | 12.0 ± 5.7‡ | 6.6 ± 4.7 |
| 4°C | 101.3 ± 1.5 | 98.1 ± 7.3 |
| IL-8 Pretreatment* . | % Specific Binding of Antibody† . | |
|---|---|---|
| . | α-CXCR–1 . | α-CXCR–2 . |
| 37°C | 12.0 ± 5.7‡ | 6.6 ± 4.7 |
| 4°C | 101.3 ± 1.5 | 98.1 ± 7.3 |
Neutrophils were pretreated with 2 μmol/L IL-8 at 37°C or 4°C or left untreated for 10 minutes.
Binding of α-CXCR–1 (MoAb RII115) and α-CXCR–2 (MoAb SE-2) as detected by staining with fluorescein-conjugated goat α-mouse IgG was recorded as median fluorescence intensity by flow cytometry. Unspecific fluorescence of PMN as determined in the absence of α-CXCR antibodies was subtracted (the proportion of unspecific binding was as depicted in Fig 2).
Data are given as percentage of controls that received no chemokine pretreatment and represent mean ± SD of three different experiments.
Downregulation of CXCR-1 or CXCR-2 expression differentially affects NAP-2–mediated neutrophil chemotaxis. In a first approach to analyze the individual contributions of CXCR-1 and CXCR-2 to the unusual biphasic chemotaxis dose-response curve observed with NAP-2, we examined whether it would be possible to differentially downregulate these receptors from the cell surface and then probe these cells for changes in their chemotactic profile versus NAP-2. For this purpose, PMN were preexposed to increasing concentrations of IL-8 or NAP-2 for 10 minutes at 37°C and subsequently analyzed for CXCR-1- and CXCR-2 expression using antibodies MoAb SE-2 and MoAb RII115 for receptor detection by flow cytometry. As shown in Fig 3, pretreatment with IL-8 led to a very similar concentration-dependent decrease in the surface expression of either receptor. Although CXCR-2 was slightly more susceptible to downregulation, both receptors were practically absent following incubation of cells with 2 μmol/L IL-8. By contrast, preexposure of cells to increasing dosages of NAP-2 up to 200 nmol/L resulted in the exclusive downregulation of the CXCR-2 by 65% ± 13%, while the expression of CXCR-1 remained unaffected. Only on preexposure with NAP-2, dosages higher than 2 μmol/L on CXCR-1 expression started to decline, but was still detectable on cells pretreated with 20 μmol/L of the chemokine, while CXCR-2 expression was practically abolished.
Expression of CXC-chemokine receptors on PMN after pretreatment with NAP-2 or IL-8. Neutrophils were pretreated with various concentrations of NAP-2 (A) or IL-8 (B) or left unexposed for 10 minutes. Subsequently the surface expression of CXCR-1 (•) and CXCR-2 (○) was determined by means of two specific antibodies against each receptor type (MoAb RII115 and MoAb SE-2, respectively) and recorded by flow cytometry as median fluorescence intensity after staining with fluorescein-conjugated goat α-mouse IgG. Unspecific fluorescence of PMN as determined in the absence of receptor-specific antibodies was subtracted (the proportion of unspecific binding was as depicted in Fig 2). Data were calculated as the percentage of the controls that received no chemokine pretreatment and represent mean ± SD of four independent experiments.
Expression of CXC-chemokine receptors on PMN after pretreatment with NAP-2 or IL-8. Neutrophils were pretreated with various concentrations of NAP-2 (A) or IL-8 (B) or left unexposed for 10 minutes. Subsequently the surface expression of CXCR-1 (•) and CXCR-2 (○) was determined by means of two specific antibodies against each receptor type (MoAb RII115 and MoAb SE-2, respectively) and recorded by flow cytometry as median fluorescence intensity after staining with fluorescein-conjugated goat α-mouse IgG. Unspecific fluorescence of PMN as determined in the absence of receptor-specific antibodies was subtracted (the proportion of unspecific binding was as depicted in Fig 2). Data were calculated as the percentage of the controls that received no chemokine pretreatment and represent mean ± SD of four independent experiments.
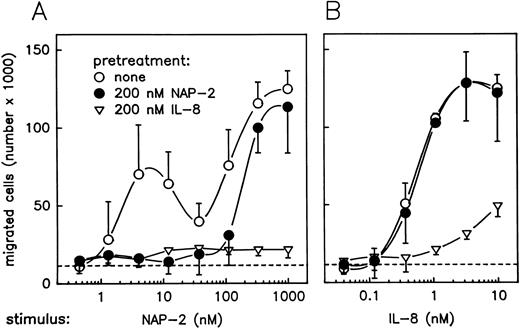
The possibility to selectively downregulate CXCR-2 surface expression by pretreatment of PMN with a moderate dosage of NAP-2 provided a helpful means for further analyses addressing the roles of CXCRs in chemokine-mediated neutrophil chemotaxis. For the experiments given in Fig 4, parallel samples of PMN were pretreated with either 200 nmol/L NAP-2 or 200 nmol/L IL-8, and washed cells were then assayed for chemotaxis versus increasing concentrations of NAP-2 and IL-8. Interestingly, pretreatment of neutrophils with NAP-2 (leading to downregulation of CXCR-2 by >60%, but leaving CXCR-1 expression unaffected; refer to Fig 3) resulted in a failure of these cells to migrate in response to low concentrations of NAP-2 (Fig 4A). In fact, the first optimum of the dose-response curve was no longer detectable, while the second optimum appearing at elevated dosages remained essentially unaffected. By contrast, the same cells were still able to mount a full chemotactic response when IL-8 was used as a stimulus (Fig 4B). Interestingly, pretreatment of PMN with IL-8 (leading to downregulation of both receptors by >75%; refer to Fig 3) rendered the cells almost totally unresponsive to NAP-2, as evident from the disappearance of the first, as well as the second, chemotactic optimum (Fig 4A), and migration of the same cells to IL-8 was drastically reduced (Fig 4B). These results provided initial evidence that CXCR-2 was probably responsible for the first peak of NAP-2–induced PMN chemotaxis, while the same receptor appeared to be less important for the IL-8–mediated response. However, the evidence for involvement of CXCR-1 in mediating the PMN chemotactic response at elevated NAP-2 concentrations remained rather indirect.
Effect of pretreatment with NAP-2 or IL-8 on chemotactic migration of PMN in response to NAP-2 or IL-8. Neutrophils were pretreated with 200 nmol/L NAP-2 (•), 200 nmol/L IL-8 (▿), or left untreated (○) for 10 minutes. Washed cells were subsequently assayed for chemotaxis in response to increasing concentrations of NAP-2 (A) or IL-8 (B). Random migration of cells that received no stimulus is indicated (- - -). Data represent mean ± SD from three independent experiments.
Effect of pretreatment with NAP-2 or IL-8 on chemotactic migration of PMN in response to NAP-2 or IL-8. Neutrophils were pretreated with 200 nmol/L NAP-2 (•), 200 nmol/L IL-8 (▿), or left untreated (○) for 10 minutes. Washed cells were subsequently assayed for chemotaxis in response to increasing concentrations of NAP-2 (A) or IL-8 (B). Random migration of cells that received no stimulus is indicated (- - -). Data represent mean ± SD from three independent experiments.
α-CXCR–1 and α-CXCR–2 antibodies selectively block chemokine binding and differentially inhibit NAP-2–mediated chemotaxis. To obtain more direct evidence for the participation of CXCR-1 and CXCR-2 in NAP-2–mediated neutrophil chemotaxis, we next examined whether antireceptor antibodies MoAb SE-2 and MoAb RII115 could also be used for selective neutralization of receptor functions through inhibition of chemokine binding. In these experiments, parallel samples of PMN were preincubated with either 100 μg/mL MoAb SE-2 or 100 μg/mL MoAb RII115 and subsequently assayed for binding of 125I-labeled NAP-2 or 125I-labeled IL-8 in comparison to control cells receiving no antibody. The final concentration of either labeled chemokine was set to 2 nmol/L, a dosage where NAP-2 reportedly binds to the CXCR-2 only and IL-8 interacts with both receptors.15-18 38 As listed in Table 2, 125I-IL–8 binding to neutrophils was partially reduced after preincubation of cells with either antibody alone, and an additive reduction in binding was observed when both antibodies were used in combination. The blocking abilities of the antibodies were specific, as no inhibition of IL-8 binding was observed in the presence of an excess of the respective peptide antigens for MoAb SE-2 and MoAb RII115 (CXCR-1[1-30] and CXCR-2[6-29], respectively). As expected, 125I-NAP–2 binding was blocked by the α-CXCR–2 antibody only. Due to the circumstance that antibody dosages higher than 100 μg/mL were impracticable, complete inhibition of chemokine binding could not be obtained. Nevertheless, these results showed that the antibodies were able to specifically block chemokine binding and thus could be expected to selectively neutralize CXCR-1– or CXCR-2–mediated neutrophil function.
Inhibition of 125I-IL-8- and 125I-NAP–2 Binding to PMN by α-CXCR–1 and α-CXCR–2 Antibodies
| Antibody* . | % Specific Binding‡ . | |
|---|---|---|
| . | 125I-IL–8 . | 125I-NAP–2 . |
| α-CXCR–1 | 65.6 ± 0.6ρ | 99.5 ± 10.6 |
| α-CXCR–2 | 75.0 ± 2.6 | 42.3 ± 5.1 |
| α-CXCR–1 + α-CXCR–2 | 45.5 ± 4.9 | ND |
| α-CXCR–1 + α-CXCR–2 + peptide-1 + peptide-2† | 105.0 ± 7.1 | ND |
| Antibody* . | % Specific Binding‡ . | |
|---|---|---|
| . | 125I-IL–8 . | 125I-NAP–2 . |
| α-CXCR–1 | 65.6 ± 0.6ρ | 99.5 ± 10.6 |
| α-CXCR–2 | 75.0 ± 2.6 | 42.3 ± 5.1 |
| α-CXCR–1 + α-CXCR–2 | 45.5 ± 4.9 | ND |
| α-CXCR–1 + α-CXCR–2 + peptide-1 + peptide-2† | 105.0 ± 7.1 | ND |
Abbreviation: ND, not determined.
PMN were left untreated or preincubated for 60 minutes with α-CXCR–1 antibody SE-2 (100 μg/mL), α-CXCR–2 antibody RII115 (100 μg/mL), or with both antibodies in combination (each at 100 μg/mL).
A 100-fold molar excess of peptide-1 (CXCR-1[1-30]) and peptide-2 (CXCR-2[6-29]) was added.
PMN were incubated with 2 nmol/L 125I-IL–8 or 2 nmol/L 125I-NAP–2. Unspecific binding was determined in the presence of a 100-fold molar excess of the respective unlabeled ligand. Specific binding was calculated by subtraction of unspecific binding from total binding.
ρ Data are expressed as the percentage of specific binding of 125I-IL–8 (4,925 ± 647 cpm) or 125I-NAP–2 (2,082 ± 601 cpm) to untreated cells and represent mean ± SD of three different experiments.
Before conducting corresponding inhibition experiments, the antibodies were examined for potential interference with the measurement of neutrophil chemotaxis. As seen with PMN in the absence of chemokines (data not shown), the antibodies did not stimulate cell migration (neither chemotaxis nor random migration) by themselves up to a final concentration of 100 μg/mL. Thus, undesirable side effects arising from antibody interaction with the cells could be excluded. The influence of the antibodies (each at 100 μg/mL) on NAP-2–dependent PMN chemotaxis is shown in Fig 5. Pretreatment with α-CXCR–2 antibody completely abrogated the first optimum of the dose-response curve, while the second optimum was not affected (Fig 5A). The opposite effect was obtained in the presence of α-CXCR–1 antibody, which drastically reduced the second optimum, but left the first optimum unaffected (Fig 5B). In the simultaneous presence of both antibodies, the first, as well as the second optimum, became profoundly reduced (Fig 5C), and finally, the antibodies were without effect upon addition of a 100-fold molar excess of synthetic peptides CXCR-1[1-30] and CXCR-2[6-29] (Fig 5D). These results clearly show that PMN chemotaxis to low dosages of NAP-2 is mediated by CXCR-2, while migration at elevated dosages involves CXCR-1. Because the two optimum curves show considerable overlap, there also exists a broad concentration range of the chemokine, where both receptors simultaneously contribute to the chemotactic response.
Effect of α-CXCR–1 and α-CXCR–2 antibodies on NAP-2–induced neutrophil chemotaxis. Neutrophils were left untreated (○) or preincubated (•) with α-CXCR–2 antibody RII115 (A), α-CXCR– antibody SE-2 (B) or a combination of both antibodies (C) for 60 minutes. The concentration of each antibody was 100 μg/mL. As a control, PMN were incubated with both antibodies in the presence of the peptides CXCR-1[1-30] and CXCR-2[6-29] (D). Subsequently, the cells were assayed for chemotaxis in response to increasing concentrations of NAP-2. Data represent mean ± SD of four different experiments (A and B) or mean ± deviation from single values of two independent experiments (C and D). Random migration of untreated cells (- - -) did not significantly differ from migration of cells incubated with the antibodies in the absence of the chemokines.
Effect of α-CXCR–1 and α-CXCR–2 antibodies on NAP-2–induced neutrophil chemotaxis. Neutrophils were left untreated (○) or preincubated (•) with α-CXCR–2 antibody RII115 (A), α-CXCR– antibody SE-2 (B) or a combination of both antibodies (C) for 60 minutes. The concentration of each antibody was 100 μg/mL. As a control, PMN were incubated with both antibodies in the presence of the peptides CXCR-1[1-30] and CXCR-2[6-29] (D). Subsequently, the cells were assayed for chemotaxis in response to increasing concentrations of NAP-2. Data represent mean ± SD of four different experiments (A and B) or mean ± deviation from single values of two independent experiments (C and D). Random migration of untreated cells (- - -) did not significantly differ from migration of cells incubated with the antibodies in the absence of the chemokines.
As seen in control experiments with two dosages (1 nmol/L and 10 nmol/L) of IL-8, both antibodies were also able to inhibit the chemotactic response to the latter chemokine. However, with either antibody, only partial inhibition was obtained (data not shown). While α-CXCR–1 caused marked inhibition (37% ± 27% and 40% ± 5% with 1 nmol/L and 10 nmol/L IL-8, respectively), α-CXCR–2 had only minor effects (16% ± 17% and 22% ± 14% of inhibition at the respective IL-8 concentrations). As expected, inhibition was increased when a combination of the antibodies was applied (49% ± 24% and 48% ± 8% of inhibition, respectively). These results, indicating a minor role for CXCR-2 in IL-8–induced chemotaxis, are in accordance with data from previous reports.29 30
DISCUSSION
Although neutrophil chemotaxis in response to CXC-chemokines has been investigated in numerous previous studies, our present report is the first one to describe a surprising difference between the chemotaxis profiles of the prototype chemokine IL-8 on the one hand, and of the related molecules, MGSA and NAP-2, on the other.
Our finding that the neutrophil chemotactic response to MGSA and NAP-2 is composed of two distinct optima, one elicited by stimulus concentrations as low as those required for IL-8–mediated chemotaxis and an additional one, appearing at more than 200-fold elevated dosages, may indicate a more pronounced role for these chemokines as chemoattractants than was previously thought. First, as opposed to several former reports,21-23 27 these observations give evidence that MGSA and NAP-2 are, in fact, equipotent with IL-8 in neutrophil chemotaxis. Secondly, we additionally found these chemokines to exhibit chemotactic activity over a considerably wider concentration range than IL-8.
There may be several reasons why the rather unusual activity profiles characteristic for MGSA and NAP-2 were not described by others before. In many studies, a rather narrow range of stimulus concentrations was tested (maximally up to about 60 nmol/L),20,25,28 excluding the detection of the second chemotactic optimum appearing at elevated dosages. Other studies investigating a wider concentration range have used only a small number of different stimulus concentrations at rather large intervals (mostly dilution steps of 1/10).21,22 27 Thus, chemokine dosages relevant to the first optimum may have been omitted.
As to the mechanisms responsible for the unusual biphasic activity profile characteristic for MGSA- and NAP-2, several possibilities have to be taken into account. In principle, the biphasic profile could be explained by assuming that the latter chemokines exist in two or more molecular forms, exhibiting different chemotactic potencies. In fact, MGSA,23 and especially NAP-2, is known to occur in N-terminally and C-terminally truncated isoforms with varying functional potencies.4,7,39 However, because homogeneous recombinant NAP-2 prepared in our laboratory and our natural NAP-2 preparation induced exactly identical chemotaxis profiles (J.E. Ehlert, Forschungszentrum Borstel, personal communication, December 1996), this assumption was rejected. Another reason could be that the neutrophil population tested subdivided into two or more subpopulations with differing susceptibilities to stimulation by chemokines. However, as seen by flow cytometry, neutrophil preparations were completely homogeneous in size and density, as well as in the surface expression of CXCR-1 and CXCR-2 (refer to Fig 2). Moreover, it has been shown previously that both receptors are coexpressed on neutrophils.32,40-42 In addition, we did not observe a biphasic chemotaxis profile with IL-8, which binds to both receptors with similarly high affinity.15-18
In our studies, we concentrated on the possibility that differential interaction of the chemokines with CXCR-1 and CXCR-2 could be responsible for their diverging potencies and ranges of activity. This assumption arose from our observation that the two chemotactic optima (as characterized by EC50 values of 1.3 nmol/L and 90 nmol/L for NAP-2, and 0.4 nmol/L and 220 nmol/L for MGSA) corresponded to the reported affinities of these chemokines for high and low affinity receptors, respectively.9-12 While it appears clear that the two chemotaxis optima detectable with NAP-2 and MGSA are a result of the well-known fact that chemotactic responses do not saturate with increasing concentrations of attractant, but rather have a bell-shaped dose-response relationship, the underlying mechanism is not completely understood. An explanation could be that directed chemotactic migration requires the perception of a chemotactic gradient by the cell. Thus, near receptor saturation, the cell will no longer be able to detect further increases in chemoattractant concentration and will stop moving. Only in a situation where another receptor with lower affinity comes into play, the cell will be able to mount a second response. In this respect, it is interesting to note that, as shown by others previously, separate transfection of CXCR-2 and CXCR-1 into Jurkat cells16 or kidney embryonic 293 cells43 rendered these cells chemotactically responsive to MGSA or NAP-2 dosages comparable to those found by us to elicit the first and second optimum in neutrophils, respectively. However, these findings could not be taken to predict our results with neutrophils, as there exist a variety of additional studies reporting quite different affinities, numbers, and functional capacities of IL-8 receptors transfected into various cell lines.14,18,38 44-47 Moreover, potential interference or cooperation of the two receptor types (that are coexpressed in neutrophils) cannot be investigated in cell lines transfected with only one receptor type.
Using neutrophils we could show by several lines of evidence that the receptors are differentially involved in the neutrophil chemotactic response to NAP-2. First, selective downregulation of CXCR-2 from the cell surface by preincubation with a low dose of NAP-2 led to the disappearance of the first chemotactic optimum without affecting the second optimum, which strongly suggested the involvement of CXCR-2 in the chemotactic response to low NAP-2 concentrations. Although it may not be excluded that ligand-mediated downregulation of the CXCR-2 could indirectly cross-desensitize the CXCR-1 at the signal transduction level, our findings that the chemotactic response to IL-8 remained unaffected under identical conditions did not support such reservations.
Secondly, as seen in antibody inhibition studies, selective blocking of ligand binding to CXCR-2 resulted in the reduction of the first optimum, whereas corresponding neutralization of CXCR-1 exclusively reduced the second optimum. Due to practical reasons, antibody dosages sufficient for complete inhibition of ligand binding were not applicable. Nevertheless, the strict specificity of the antibodies to inhibit binding of radiolabeled NAP-2, as well as IL-8 to CXCR-1 or CXCR-2, made sure that reductions observed in the chemotactic response related to neutralization of the respective receptor. These results provided evidence that apart from the CXCR-2, the CXCR-1 is also involved in NAP-2–induced neutrophil chemotaxis, with the CXCR-2 rendering the cells responsive to low dosages of NAP-2 and with the CXCR-1 extending their responsiveness up to dosages several orders of magnitude higher. Although we did not perform studies on receptor usage by MGSA, it is most likely that the chemotactic activity of this chemokine is regulated by the same mechanisms, as MGSA exhibits binding patterns to CXCR-1 and CXCR-2 almost identical to those of NAP-2. According to recent studies by others, MGSA-mediated neutrophil chemotaxis could be inhibited by antibodies to CXCR-2 only, with anti-CXCR-1 antibodies being without effect.29 30 However, these studies were performed using a single low MGSA dosage only (14 and 20 nmol/L, respectively), thus addressing exclusively the first chemotactic optimum. By contrast, corresponding antibody inhibition studies performed by the same investigators to elucidate the receptor involvement in IL-8–mediated chemotaxis showed a higher susceptibility of the cellular response to α-CXCR–1 antibodies as compared with α-CXCR–2 antibodies. This suggests that the single optimum of chemotaxis elicited by IL-8 is a composed phenomenon predominantly depending on CXCR-1 and to a minor degree on CXCR-2.
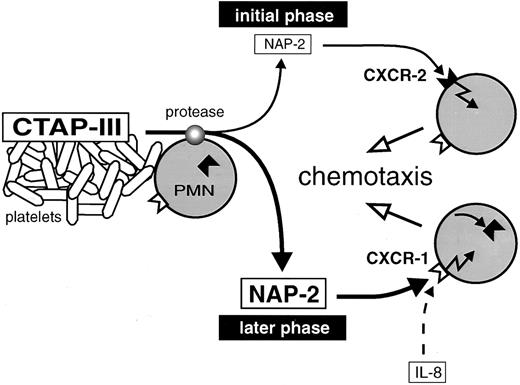
Proposed model for neutrophil recruitment by increasing concentrations of NAP-2 arising during inflammation. CTAP-III released from activated platelets is proteolytically converted into NAP-2 by neutrophils attached to a thrombus. Low dosages of NAP-2, formed in the initial phase of CTAP-III processing, attract further cells from the periphery through CXCR-2. Increasing dosages of NAP-2 accumulate in the progress of processing and cause the downregulation of CXCR-2. Further migration of the cells to the finally high concentrations of NAP-2 is mediated through CXCR-1.
Proposed model for neutrophil recruitment by increasing concentrations of NAP-2 arising during inflammation. CTAP-III released from activated platelets is proteolytically converted into NAP-2 by neutrophils attached to a thrombus. Low dosages of NAP-2, formed in the initial phase of CTAP-III processing, attract further cells from the periphery through CXCR-2. Increasing dosages of NAP-2 accumulate in the progress of processing and cause the downregulation of CXCR-2. Further migration of the cells to the finally high concentrations of NAP-2 is mediated through CXCR-1.
With respect to antibody inhibition studies, it is interesting to note that all α-CXCR–2 antibodies (including our MoAb RII115) capable of blocking chemokine binding,30,48 have been found to recognize the N-terminal amino acid sequence FEDFW (positions 11-1517 or 6-10,14 the numbering depending on which of the two potential translation initiation sites is considered). Therefore, this part of the receptor was suggested to play an important role in ligand-binding. Considering that our neutralizing antibody RII115 (although blocking subsequent chemokine binding) was nevertheless able to detect CXCR-2 after its occupation by NAP-2 or IL-8, the ligand binding site on the receptor does not appear to directly involve the epitope for the antibody. Instead, antibody interaction with its epitope may prevent ligand-binding by causing a conformational change of the receptor or by sterical hindrance.
Apart from chemotaxis, other effector functions of neutrophils in response to CXC-chemokines have been reported to involve both receptors, eg, IL-8–induced degranulation, as well as integrin activation.30,49 However, this may not be a general phenomenon because it was also shown that the IL-8–induced respiratory burst depends on the CXCR-1 only.49 Correspondingly, a number of intracellular signals were found to become transduced by both receptors, such as the transient elevation in intracellular Ca2+-ion concentration47 and phosphorylation of mitogen-activated protein kinase,50 while stimulation of phospholipase D activity appears to be mediated by CXCR-1 only.49 It remains to be elucidated which of these signals are linked to the elicitation of a chemotactic response.
Ever since the discovery of the chemokines, there have been questions as to why there are so many members of the CXC-subfamily displaying largely overlapping functional patterns, as for example seen with IL-8, MGSA, NAP-2, and still others, all of which are able to stimulate neutrophils to undergo chemotaxis, degranulation of lysosomal enzymes, and adhesion. One explanation may be the requirement for individual mediators that are functionally adapted to the special physical and physiologic conditions existing at different sites of the tissue. Concerning IL-8, this chemokine is a rather widespread one, becoming secreted by a wide spectrum of tissue and immune cells after induction by proinflammatory stimuli. Thus, only relatively short distances will have to be bridged by diffusion to establish a chemotactic gradient for the continuous recruitment of neutrophils from nearby postcapillary venules. It is conceivable, as found with IL-8, that in such a situation a rather narrow chemotactic gradient at low stimulus concentrations will be sufficient for the attraction of cells to the inflammatory site. Moreover, the recruitment will be highly efficient due to almost simultaneous usage of both CXC-receptors by the chemokine. A quite different situation is likely to exist within blood vessels that have become occluded by the formation of a thrombus. Due to the standstill of blood flow, only limited numbers of neutrophils are available that are moreover distributed over relatively large distances within the vessel. This may require a chemokine such as NAP-2, which has been found to arise from platelet-derived precursors (predominantly CTAP-III) and to accumulate very rapidly (within minutes) through proteolytic processing by the neutrophils themselves.4 5 It may be envisaged that neutrophils making contact with the thrombus and becoming exposed to the high concentration of released CTAP-III (normal serum concentration about 3 μmol/L, as determined in our laboratory) will start to generate a wide gradient of NAP-2 extending into the micromolar range and bridging long distances within the vessel. Concerning the roles of the two chemokine receptors in this setting, the CXCR-2 will be more relevant during the initial phase of PMN attraction, when NAP-2 concentrations are still low (compare with schematic representation in Fig 6). Neutrophils accumulating at this stage would contribute to the processing of CTAP-III and further extend the chemokine gradient, resulting in the recruitment of cells from the periphery. However, while the CXCR-2 in cells migrating along the gradient will become downregulated due to increasing NAP-2 concentrations, the CXCR-1 now can take over and enable the cells to arrive at the thrombus. Taken together, through differential interaction with high- and low-affinity receptors, NAP-2 may efficiently attract the neutrophils available in an occluded vessel and thus, function as an important first line mediator in a situation of acute injury.
ACKNOWLEDGMENT
We thank G. Kornrumpf for her expert work in cell culture and antibody generation and gratefully acknowledge the perfect technical assistance of C. Pongratz and I. von Cube.
Supported in part by Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 367, Projekt C4 and Sonderforschungsbereich 236, Projekt B6.
Address reprint requests to Andreas Ludwig, Department of Immunology and Cell Biology, Forschungszentrum Borstel, Parkallee 22, D-23845 Borstel, Germany.

![Fig. 2. Flow cytometric analysis of CXCR-1 and CXCR-2 expression on neutrophils. Cells were incubated with α-CXCR–1 antibody SE-2 (A) or with α-CXCR–2 antibody RII115 (B). Incubation with either antibody was performed in the absence (1) or presence (2) of a 100-fold molar excess of the peptide fragments CXCR-1[1-30] (A) or CXCR-2[6-29] (B). Subsequently, cell-bound antibodies were detected by fluorescein-conjugated goat α-mouse IgG. Control cells received the secondary antibody only (3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4588/3/m_bl_0034f2.jpeg?Expires=1769573009&Signature=CCfuWAKiUJvNomrWUgW~nrJatX3BR1zHxQO11S01QkmaRsGlsu6TpGoEn-RrLyPe5xMk3ik1mzuBV9wFWEyiO84U6eU0x-R8~a6qmXRkI8nIYWFQp53h59jGsCCceU9w8I-fcwsq--cd3MiiSVSgPN45AbSTdfzNW0W6i3MqelSs03cNi4uccQ5KvnFi2833LmbtZxLRdAPBvwJP5cb0h0gmsjHz6~LzMQCmmjr51I6bFQbwMVw-KtuQd~ztnsxxJi2y2o3YGkodwlZmRoTqAZPF-n~GKHQHvxmLOsUleIj0l9pf788YydDz0ZGpKGRV-8fOz8nOlxMNdLzrXLs6vA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effect of α-CXCR–1 and α-CXCR–2 antibodies on NAP-2–induced neutrophil chemotaxis. Neutrophils were left untreated (○) or preincubated (•) with α-CXCR–2 antibody RII115 (A), α-CXCR– antibody SE-2 (B) or a combination of both antibodies (C) for 60 minutes. The concentration of each antibody was 100 μg/mL. As a control, PMN were incubated with both antibodies in the presence of the peptides CXCR-1[1-30] and CXCR-2[6-29] (D). Subsequently, the cells were assayed for chemotaxis in response to increasing concentrations of NAP-2. Data represent mean ± SD of four different experiments (A and B) or mean ± deviation from single values of two independent experiments (C and D). Random migration of untreated cells (- - -) did not significantly differ from migration of cells incubated with the antibodies in the absence of the chemokines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4588/3/m_bl_0034f5.jpeg?Expires=1769573009&Signature=lo9JnLqv~ZPPPUknLDgvG7BBf2oo5YDtjdE9RJvyBOcseAp6JZxKeq0ExLM1-aqvr321GZrMEUtclrsqx5vX9CUXYjYy6lN7DuJDwcSDWe58seUSRrifVhnV86fzT1wbiShA-FsVd6sFsgkl~sbjl4wY0k-u3TkaKasRiQKs4-wtIQ6n0RLIuIZCaWbBp2iViVqBFKDt4NwRRhJlPuPQSi8xRXTY~ZjtVvmy5VcW4hPj3la6Tv7Y5G7JLPrtwIYyYvMHdogOJXb8sCO-J3btqFd5GqggGKtS4hhrrkeb~kH3oufec3TTBdJHeJiwStqgXHF~GRaVAREzfTJwnnUy-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal