Abstract
Jaundiced mice, ja/ja, suffer from a severe hemolytic anemia caused by a complete deficiency of erythroid β-spectrin. We used these mice as a model to investigate the pathophysiological consequences of the deficiency, including the effects in the nonerythroid tissues where this protein is expressed. Because the ja/ja mice rarely survive beyond the fourth postnatal day, methods were assessed for extending lifespan into adulthood. Neonatal transfusion increased lifespan to a mean of 3.7 months, allowing a more complete characterization of the pathophysiology. Blood parameters and histopathology of the jaundiced mouse were compared with that from spherocytic mice, which have a hemolytic anemia caused by deficiency of erythroid α-spectrin, yet can survive the postnatal period transfusion free. The adult jaundiced and spherocytic mice present with greatly decreased hematocrit and red blood cell counts, reticulocytosis, and bilirubinemia, leading secondarily to hepatosplenomegaly and cardiomegaly. Jaundiced and spherocytic mice were analyzed histopathologically between 1.0 and 9.5 months of age. Interestingly, the complete absence of erythroid β-spectrin in jaundiced mice leads to no detectable structural defects in brain, cardiac, or skeletal muscles. However, fibrotic lesions and lymphocytic infiltration were observed in cardiac tissue from 4 of 13 jaundiced mice and 15 of 15 spherocytic mice, and thrombi were detected at either the atrioventricular valves or within the atria of 2 of 13 jaundiced mice and 15 of 15 spherocytic mice. In addition, all affected mice had a progressive renal hemosiderosis concurrent with hydronephrosis and glomerulonephritis. The severity of the renal disease and its presence in all moribund mice suggests kidney failure rather than the fibrotic heart lesions as the major cause of death in these mice.
HUMAN HEREDITARY spherocytosis (HS) is a congenital disease characterized by aberrantly shaped red blood cells and hemolytic anemia. In the majority of HS patients, the disease is caused by an aberration in one of three major erythroid cytoskeletal proteins: ankyrin, band 3, or spectrin.1,2 In the red blood cell (RBC), β-spectrin associates with α-spectrin in an antiparallel fashion to form a dimeric spectrin molecule. Dimers interact head on to form tetramers and higher order oligomers, generating a matrix underlying the lipid bilayer.3 Binding sites on β-spectrin for ankyrin, F-actin, and band 4.1 link the cytoskeletal matrix to the lipid bilayer through associations with the transmembrane proteins band 3 and glycophorin C.4-7 Defects in any of these cytoskeletal proteins lead to fragile and dysmorphic erythrocytes that are more susceptible to hemolysis during the tortuous journey through the capillaries and sinuses of the spleen.
Although the majority of human hemolytic anemias caused by primary defects in the RBC membrane are inherited as autosomal dominant,8 in mice these conditions are usually inherited as autosomal recessive.9,10 The jaundiced mouse (ja/ja ) is completely deficient in erythroid β-spectrin11 because of replacement of an arginine with a stop codon in the mRNA encoding repeat 9,12 and the spherocytic mouse (sph/sph ) is α-spectrin deficient.11 Both mutants suffer from a severe hemolytic anemia with reticulocytosis >90% and RBC lifespan ≤1 day, but the spherocytic mice survive beyond weaning whereas jaundiced mice die by neonatal day 4.9,11,13-15 Therefore, the murine diseases more closely resemble the more severe forms of human HS, which are usually presented by homozygotes or double heterozygotes.16-20 Mutations similar to those found in the jaundiced and spherocytic mice, which completely abrogate expression of one spectrin subunit, most likely lead to in utero or early neonatal death in humans. In fact, recessively inherited hereditary elliptocytosis resulting from the expression of a β-spectrin with abnormal spectrin tetramerization ability (spectrin Providence) leads to nonimmune hydrops fetalis in the third trimester.21 An almost complete absence of α-spectrin similarly results in hydrops fetalis in humans.16
In humans presenting with severe hemolytic anemia at birth, blood transfusions are administered to allow the newborns sufficient oxygen-binding capacity to survive this critical period. Subsequently, when the child is old enough and if the anemia remains severe, the patient is splenectomized to eliminate an organ critical for extramedullary hemolysis.22 Splenectomy has been used successfully to decrease hemolysis in deer mice (Peromyscus maniculatus ) with a mild form of hereditary spherocytosis.23 However, in mice with severe hemolytic anemia, removal of the spleen deprives the mouse of a critical source of hematopoiesis, and none survive after splenectomy.9 Young adult mice with hemolytic anemias have also been partially rescued by bone marrow transplantations after irradiation.24,25 However, irradiation of newborns causes radiation-induced cerebellar and retinal dysplasia.26 Alternatively, deer mice with hereditary spherocytosis have been rescued by transplanting normal marrow into unirradiated newborn mutant pups.10
Expression of erythroid α-spectrin, β-spectrin, and ankyrin is not restricted to erythroid cells but is also found in the brain, and erythroid β-spectrin and ankyrin are present in muscle.27-33 In nonerythroid tissues, β-spectrin isoforms are produced by alternative splicing, and the exact function of the isoform(s) in each tissue is not known. A deficiency in ankyrin in the murine brain causes age-related Purkinje cell degeneration and neuromuscular defects in the normoblastosis mouse.27 Similar effects were postulated to occur because of lack of β-spectrin, which is also expressed in the Purkinje cell layer and typically associates with ankyrin to attach the membrane cytoskeleton to the lipid bilayer. In this study, we use the lack of β-spectrin in the jaundiced mouse to elucidate the pathological effects of erythroid β-spectrin deficiency in erythroid tissues as well as in nonerythroid tissues such as muscle, heart, and brain. To prolong the life of jaundiced mice into adulthood, we used both bone marrow and/or peripheral blood cell (PBC) transfusions. Spherocytic mice, sph/sph, were studied in parallel to distinguish between defects that were erythroid α- and β-spectrin specific.
MATERIALS AND METHODS
Mice. Jaundiced and spherocytic mice were maintained as heterozygotes (ja/+ or sph/+) on both the WB/Re (WB) and C57BL/6J (B6) strains of mice, and homozygous (WB × B6-ja/ja or -sph/sph ) F1 hybrids for each mutation were produced by mating WB and B6 heterozygotes. Normal control mice were generally nonanemic F1 hybrid littermates (+/+ or ja/+) or age and sex-matched F1-+/+ hybrids. For jaundiced littermates, allele-specific oligonucleotide polymerase chain reaction (PCR) was developed to genotype controls as +/+ or ja/+. Mice were housed and cared for according to American Association for the Accredidation of Laboratory Animal Care (AAALAC) specifications.
Transfusion protocols. Homozygous WBB6F1-ja/ja mice were injected at postnatal days 1, 2, or 3 either intravenously (IV) or intraperitoneally (IP) with a volume of 50 to 100 μL of washed PBCs (4 to 8 × 108 RBCs), bone marrow (BM) cells (0.5 to 3.0 × 106 cells), or a 1:1 mixture of BM + PBC. IP transfusions are administered successfully to human fetuses suffering from severe hemolytic anemia in utero because of immunological incompatibilities34 as well as to mice to introduce exogenous blood cells into the circulatory system.35 Although the mice injected IP often required additional PBC transfusions, they ultimately thrived as well as those injected IV. Some mice were given PBC transfusions on a weekly basis for greater than 4 weeks to determine whether prolonged therapy increased their lifespan. Some of the treated mice were aged for lifespan determination and others were sacrificed between 1.0 and 9.5 months, at an average age of 4.2 months, at which time hematological parameters, serum chemistry, and/or histopathology were assessed. The cells used for transfusions were obtained from normal B6.Cast-Gpi1a/Gpi1a congenic mice and could be identified by glucose phosphate isomerase-1 (GPI1) isoenzyme analysis (WBB6F1 mice express the GPI1B isoenzyme). Although all of the jaundiced mice exhibited obvious signs of disease, only 10% of the mice studied were moribund at the time of analysis. The jaundiced mice did not show significant differences in hematological parameters on aging. Some normal control mice were transfused in parallel to determine that pathological conditions were not a result of the transfusion procedure. Spherocytic mice were not transfused.
Hematologic parameters. Blood for analysis was taken either from the atrium at the time of perfusion, or by retro-orbital bleeding. Blood collected by the two different methods gave very similar values. Hematocrits were measured in 100-μL microcapillary tubes. RBC counts were done on a Coulter counter Model ZBI (Coulter Immunology, Hialeah, FL). Hemoglobin concentrations were determined using the standard cyanmethemoglobin method. Plasma samples tested negative for hemoglobin, showing that the hemoglobin detected in whole blood was intracellular. The type of GPI1 isoenzyme in peripheral RBCs of transfused mice was determined by cellulose acetate chromatography of RBCs followed by a colorometric assay.36
Differential counts were performed using gentle filtration of cells onto a filter, because the jaundiced reticulocytes seemed to lyse readily under the shear stress of normal slide preparation. The cells brought down on the filter were fixed in 10% glutaraldehyde stained with Lepehne's stain, which detects heme-containing cells, and counterstained with Mayer's hematoxylin.37
Serum analysis. Blood was collected by cardiac puncture and allowed to clot at room temperature for 30 minutes before centrifugation. Serum samples were either quick frozen in liquid nitrogen and kept frozen until analysis by AniLytics Inc (Gaithersburg, MD), or analyzed immediately for serum iron with the serum total iron/unsaturated iron binding microanalysis kit (Sigma Diagnostics, St Louis, MO).
Urine analysis. Urine was collected from mice in 1-mL syringes either from spontaneous urination or directly from the bladder of anesthetized mice by needle aspiration. The urine values were determined with Ames Multistix 10 SG strips (Miles Inc, Elkhart, IN). Urine was also stained with Perl's stain, spun to collect granular material, and spread on slides to visualize cellular casts and iron deposits.
Histological sections. Spleen, liver, kidneys, brain, heart, lungs, and skeletal muscles were collected from mice perfused transcardially with phosphate buffered saline followed by Bouin's fixative and embedded in paraffin. Routine staining of all tissues was performed with hematoxylin and eosin. The presence of nonhemoglobin iron in liver, kidney, heart, brain, and spleen was assessed by staining with Gomori's stain for iron. Kidneys were stained with periodic acid-Schiff (PAS) stain for detection of glomerulonephritis. Brain sections were also stained with luxol fast blue/cresyl violet and Bodian's for visualization of Purkinje cells and nerve fibers, respectively. Sagittal or coronal serial sections of several brains were prepared to analyze all structures for correct development.
RESULTS
Transfusion of mice. Only rarely have jaundiced mice survived to adulthood unaided, and of the four such mice studied in the past 4 years, the average lifespan was 3.6 months. To hematologically rescue additional mice for these studies, hematopoietic stem cell transplantations were performed in newborn mice (1, 2, or 3 days postnatal) without prior myeloablation by marrow cell injection into the superficial temporal vein. A single injection of BM cells alone did not rescue the mice. When PBCs were combined with the BM cells or were given alone weekly (>4 weeks), these mice survived and thrived. GPI1 analyses showed that the peripheral RBCs in all of these animals were 100% host type (data not shown). It appeared that prolonged survival of the ja/ja mice was not caused by seeding with donor hematopoietic stem cells but by the PBC transfusions. Single PBC injections at postnatal days 1, 2, or 3 were as successful at extending the lifespan as multiple PBC injections for >4 weeks. The lifespan of aged ja/ja mice rescued by single or multiple PBC injections ranged from 2.6 to 6 months, with a mean of 3.7 months. A single mouse survived to 9.5 months before autopsy. In contrast, approximately 75% of the α-spectrin–deficient spherocytic mice survived the critical postnatal period without assistance and had an average lifespan of 5.75 months. Transfused normal littermates did not show any effects hematologically or histologically because of the transfusions.
Blood parameters and cell counts. The blood parameters of the jaundiced mice that were given more than 4 PBC transfusions (PBC > 4 weeks), a single PBC transfusion (PBC 1×), or a single transfusion of bone marrow and PBCs (BM + PBC) were compared at 2 to 9.5 months posttransfusion. RBC counts, mean cell volume (MCV), hemoglobin content, and mean corpuscular hemoglobin concentration (MCHC) were similar between ja/ja animals despite the type of postnatal transfusion treatment (Table 1A). However, the hematocrits of PBCs >4 weeks and PBC 1X showed a significant difference, with the continually transfused mice exhibiting a lower hematocrit. It is possible that continual transfusions lead to greater sensitization of the spleen and even greater hemolysis. Because the values were so similar otherwise, the hematologic parameters of pooled treated ja/ja mice, as well as of untreated ja/+ and sph/sph mice, were compared with those of +/+ mice. Both ja/ja and sph/sph mouse populations exhibited highly significant differences in values compared with +/+ mice in all measurements except MCV (Table 1B). Unlike human patients, jaundiced mice harbor approximately 90% reticulocytes, and so will not show the same reduction in MCV caused by microspherocytes. No significant differences were seen between +/+ and ja/+ mice except in MCV. The ja/+ erythrocytes are more osmotically fragile (Barker JE, unpublished data) and have a reduced erythrocyte lifespan compared with +/+ mice,38 indicating that they are structurally altered at the cellular level because of reduced β-spectrin content. This may lead to the moderate reduction in MCV that we observe.
Hematologic Parameters of Mice in These Studies
| A. . | |||
|---|---|---|---|
| Parameters . | Transfusion Treatment Given to Jaundiced Mice . | ||
| . | PBC >4 wks* . | PBC + BM† . | PBC 1ׇ . |
| Hematocrit (%) | 18.9 ± 1.3 (4)ρ1-155 | 19.3 ± 3.4 (3) | 22.8 ± 3.6 (15)ρ |
| RBC (×1012/L) | 4.00 ± 0.30 (4) | 3.60 ± 0.20 (3) | 4.40 ± 0.80 (15) |
| MCV ( μm3) | 47.8 ± 3.6 (4) | 53.4 ± 7.0 (3) | 52.6 ± 4.6 (15) |
| Hemoglobin (g/dL) | 3.8 ± 0.6 (4) | 3.4 ± 0.5 (3) | 4.2 ± 0.7 (15) |
| MCHC (%) | 20.1 ± 2.0 (4) | 17.8 ± 1.8 (3) | 18.3 ± 1.5 (15) |
| A. . | |||
|---|---|---|---|
| Parameters . | Transfusion Treatment Given to Jaundiced Mice . | ||
| . | PBC >4 wks* . | PBC + BM† . | PBC 1ׇ . |
| Hematocrit (%) | 18.9 ± 1.3 (4)ρ1-155 | 19.3 ± 3.4 (3) | 22.8 ± 3.6 (15)ρ |
| RBC (×1012/L) | 4.00 ± 0.30 (4) | 3.60 ± 0.20 (3) | 4.40 ± 0.80 (15) |
| MCV ( μm3) | 47.8 ± 3.6 (4) | 53.4 ± 7.0 (3) | 52.6 ± 4.6 (15) |
| Hemoglobin (g/dL) | 3.8 ± 0.6 (4) | 3.4 ± 0.5 (3) | 4.2 ± 0.7 (15) |
| MCHC (%) | 20.1 ± 2.0 (4) | 17.8 ± 1.8 (3) | 18.3 ± 1.5 (15) |
| B. . | ||||
|---|---|---|---|---|
| Parameters . | Genotype . | |||
| . | +/+ . | ja/+ . | ja/ja . | sph/sph . |
| Hematocrit (%) | 45.0 ± 4.0 (6)1-155 | 45.4 ± 4.4 (17) | 21.6 ± 3.6 (22)1-154 | 22.5 ± 5.5 (6)1-167 |
| RBC (×1012/L) | 9.15 ± 1.73 (6) | 10.60 ± 1.20 (16) | 4.20 ± 0.70 (22)1-154 | 3.74 ± 0.50 (6)1-167 |
| MCV ( μm3) | 50.0 ± 7.3 (6) | 42.7 ± 2.3 (16)1-167 | 51.8 ± 4.9 (22) | 59.5 ± 7.8 (6) |
| Hemoglobin (g/dL) | 13.5 ± 1.9 (6) | 14.1 ± 1.4 (17) | 4.0 ± 0.7 (22)1-154 | 3.7 ± 0.7 (6)1-167 |
| MCHC (%) | 29.8 ± 2.0 (6) | 31.0 ± 1.4 (17) | 18.6 ± 1.7 (22)1-154 | 16.9 ± 1.5 (6)1-167 |
| B. . | ||||
|---|---|---|---|---|
| Parameters . | Genotype . | |||
| . | +/+ . | ja/+ . | ja/ja . | sph/sph . |
| Hematocrit (%) | 45.0 ± 4.0 (6)1-155 | 45.4 ± 4.4 (17) | 21.6 ± 3.6 (22)1-154 | 22.5 ± 5.5 (6)1-167 |
| RBC (×1012/L) | 9.15 ± 1.73 (6) | 10.60 ± 1.20 (16) | 4.20 ± 0.70 (22)1-154 | 3.74 ± 0.50 (6)1-167 |
| MCV ( μm3) | 50.0 ± 7.3 (6) | 42.7 ± 2.3 (16)1-167 | 51.8 ± 4.9 (22) | 59.5 ± 7.8 (6) |
| Hemoglobin (g/dL) | 13.5 ± 1.9 (6) | 14.1 ± 1.4 (17) | 4.0 ± 0.7 (22)1-154 | 3.7 ± 0.7 (6)1-167 |
| MCHC (%) | 29.8 ± 2.0 (6) | 31.0 ± 1.4 (17) | 18.6 ± 1.7 (22)1-154 | 16.9 ± 1.5 (6)1-167 |
Age range of jaundiced and control mice studied was 2 to 9.5 months old, average 4.5 months old, one jaundiced mouse moribund at time of analysis. Age range of spherocytic mice studied was 2.75 to 4.25 months old, average 3.8 months old, none moribund at time of analysis.
Abbreviations: PBC, peripheral blood cell; BM, bone marrow; RBC, red blood cell count; MCHC, mean corpuscular hemoglobin concentration; MCV, mean cell volume.
Jaundiced mice given weekly PBC transfusions for greater than 4 weeks.
Jaundiced mice given a PBC transfusion in addition to bone marrow cells at days 1, 2, and 3 after birth; a few mice were given one additional PBC transfusion a few days later.
Jaundiced mice given a single PBC transfusion at days 1, 2, or 3 after birth.
ρ Significant difference (P < .05).
Mean ± standard deviation; number of observations in parentheses.
Extremely significant difference (P < .0005).
Very significant difference (P < .005).
For part A, significance noted is in hematocrit values between TF 1× and TF >4 weeks. For part B, significance when compared with +/+ values.
Non-nucleated heme-containing cells comprised 85.4% of total PBCs in ja/ja mice transfused a single time at days 1, 2, or 3 (PBC 1×), compared with 99.8% in normal mice. These cells in the ja/ja mice should be almost exclusively reticulocytes based on the previously published value of 91.4% reticulocytes in jaundiced mice9 (compared with 2.0% in normal mice). Nucleated RBCs comprised 8.2% and white blood cells comprised 5.6% compared with 0% and 0.26% for normal mice.
Serum and urine analyses. Serum analysis showed bilirubinemia in the transfused mice with a mean of 1.9 mg/dL bilirubin compared with 0.3 mg/dL for normal mice (Table 2). This was contributed to by both direct and indirect bilirubin. Correspondingly, measurements of urobilinogen, which is a breakdown product of bilirubin, in the urine of jaundiced and normal mice showed that the levels were increased in the jaundiced mice: mean 4 mg/dL in jaundiced and 0.3 mg/dL in normal mice. The blood urea nitrogen, which was used to estimate kidney function, was significantly higher in the jaundiced mouse indicating a decrease in renal function. In addition, serum glucose was significantly lower in the jaundiced mouse, suggesting either hypophagia, a defect in nutritional uptake or use, or a hormonal defect. Hepatosplenomegaly causes abdominal distention and may, as in β-thalassemia major,39 lead to feeding problems. The nearly complete lack of fat pads in these mice could be caused by the below normal blood sugar levels mobilizing fat stores.
Analysis of Normal and Jaundiced Serum
| Parameters . | Phenotype . | |
|---|---|---|
| . | Normal . | Jaundiced . |
| Part A | ||
| Total bilirubin (mg/dL) | 0.3 ± 0.1 (5)* | 1.9 ± 0.4 (5)† |
| Direct (mg/dL) | 0.0 ± 0.0 (5) | 0.7 ± 0.2 (5)† |
| Indirect (mg/dL) | 0.3 ± 0.1 (5) | 1.2 ± 0.2 (5)† |
| BUN (mg/dL) | 30 ± 3 (5) | 39 ± 4 (5)† |
| Glucose (mg/dL) | 206 ± 34 (5) | 151 ± 7 (5)† |
| Part B | ||
| Serum total iron ( μg/dL) | 125 ± 29 (11) | 350 ± 48 (11)‡ |
| UIBC ( μg/dL) | 184 ± 57 (6) | 96 ± 23 (6)† |
| TIBC ( μg/dL) | 312 ± 39 (6) | 448 ± 77 (6)† |
| Percent saturation (%) | 42 ± 12 (6) | 78 ± 4 (6)† |
| Parameters . | Phenotype . | |
|---|---|---|
| . | Normal . | Jaundiced . |
| Part A | ||
| Total bilirubin (mg/dL) | 0.3 ± 0.1 (5)* | 1.9 ± 0.4 (5)† |
| Direct (mg/dL) | 0.0 ± 0.0 (5) | 0.7 ± 0.2 (5)† |
| Indirect (mg/dL) | 0.3 ± 0.1 (5) | 1.2 ± 0.2 (5)† |
| BUN (mg/dL) | 30 ± 3 (5) | 39 ± 4 (5)† |
| Glucose (mg/dL) | 206 ± 34 (5) | 151 ± 7 (5)† |
| Part B | ||
| Serum total iron ( μg/dL) | 125 ± 29 (11) | 350 ± 48 (11)‡ |
| UIBC ( μg/dL) | 184 ± 57 (6) | 96 ± 23 (6)† |
| TIBC ( μg/dL) | 312 ± 39 (6) | 448 ± 77 (6)† |
| Percent saturation (%) | 42 ± 12 (6) | 78 ± 4 (6)† |
For Part A, age range of mice studied was 4 to 5.25 months old, average 4.6 months old, none moribund at time of analysis. For Part B, age range of mice studied was 2 to 5.5 months old, average 3.8 months old (serum total iron) or 3.1 months old (all other values), none moribund at time of analysis.
Abbreviations: BUN, blood urea nitrogen; UIBC, unsaturated iron binding capacity; TIBC, total iron binding capacity.
Mean ± standard deviation; number of observations in parentheses.
Very significant difference (P < .005); ‡ extremely significant difference (P < .0005) when compared to normal. Significance determined by Mann-Whitney unpaired nonparametric test.
Jaundiced mice had a very high level of serum total iron with a mean of 350 μg/dL compared with normal mice with levels of 125 μg/dL (Table 2). The unsaturated iron binding capacity in the mutant mice was decreased significantly and they had a higher total iron binding capacity (TIBC). The jaundiced mice had a higher percentage saturation of iron binding capacity of 78% compared with the normal 42%.
Morphometrics/histology. The spleens of the jaundiced mice obtained weights of 1.7 to 2 g or up to one tenth the total body weight of the mice as opposed to normal spleen (0.1 to 0.125 g, or 1/300 of total body weight). This large splenic mass extended entirely across the mouse's abdomen in both ja/ja and sph/sph mice, placing extreme pressure on other organs and often distorting them as seen in the kidney in Fig 1a. The majority of the splenic mass was increased red pulp. Large areas of necrosis and fibrosis indicative of infarctions were observed in several mice analyzed, extending entirely through the spleen and in some cases forming a visible necrotic bulge. Liver from ja/ja mice was also enlarged and showed multiple sites of hematopoiesis as well as regions of fibrosis and occasional necrosis indicative of infarcts. In spleen and liver, as well as heart, the vasculature was greatly expanded. The hearts were greatly enlarged compared with controls and contained dilated heart chambers.
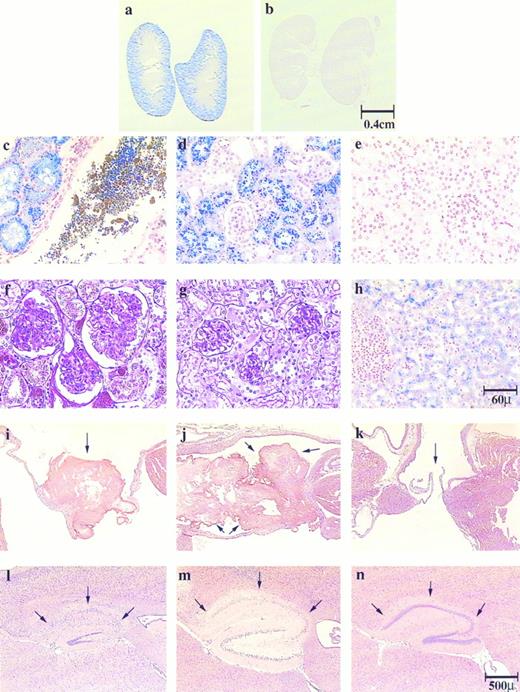
Histological sections of kidney, liver, heart and brain show hepatic and renal hemosiderosis, renal disease, thrombosis, and infarction in the pathogenesis of both jaundiced and spherocytic mice. Kidney sections (a to e) were stained with Gomori's iron stain and (f and g) with PAS: (a, c, and f ) ja/ja, (b, e, and g) control, (d) sph/sph. Liver section (h) was stained with Gomori's iron stain, ja/ja. Heart sections (i to k) were stained with H&E. Atrioventricular valves are shown as follows: (i) ja/ja and (j) sph/sph, arrows point to thrombi; (k) ja/ja, arrows point to unobstructed valve. Brain sections (l to n) were stained with H&E, hippocampus: (l) ja/ja and (m) sph/sph, arrows point to degenerated hippocampal gyri. Note that the dentate gyri are also degenerating in these mice. (n) same ja/ja as in (l), arrows point to uninfarcted hippocampal gyrus showing unilateral nature of brain infarctions. Bars designate representative size for sections preceding them. Mice: (a, c, f, and i) ja/ja#32, 9.5 months; (b, e, and g) +/+ nonlittermate control to (a, c, f, and i), 9.5 months; (d) sph/sph#163, 3 months; (h, l, and n) ja/ja#43, 3.25 months; (j) sph/sph#110, 4.25 months; (k) ja/ja#46, 3.75 months; (m) sph/sph#73, 4.25 months.
Histological sections of kidney, liver, heart and brain show hepatic and renal hemosiderosis, renal disease, thrombosis, and infarction in the pathogenesis of both jaundiced and spherocytic mice. Kidney sections (a to e) were stained with Gomori's iron stain and (f and g) with PAS: (a, c, and f ) ja/ja, (b, e, and g) control, (d) sph/sph. Liver section (h) was stained with Gomori's iron stain, ja/ja. Heart sections (i to k) were stained with H&E. Atrioventricular valves are shown as follows: (i) ja/ja and (j) sph/sph, arrows point to thrombi; (k) ja/ja, arrows point to unobstructed valve. Brain sections (l to n) were stained with H&E, hippocampus: (l) ja/ja and (m) sph/sph, arrows point to degenerated hippocampal gyri. Note that the dentate gyri are also degenerating in these mice. (n) same ja/ja as in (l), arrows point to uninfarcted hippocampal gyrus showing unilateral nature of brain infarctions. Bars designate representative size for sections preceding them. Mice: (a, c, f, and i) ja/ja#32, 9.5 months; (b, e, and g) +/+ nonlittermate control to (a, c, f, and i), 9.5 months; (d) sph/sph#163, 3 months; (h, l, and n) ja/ja#43, 3.25 months; (j) sph/sph#110, 4.25 months; (k) ja/ja#46, 3.75 months; (m) sph/sph#73, 4.25 months.
In the liver, Gomori's stain for iron showed significant iron stores in hepatic cells of both jaundiced (Fig 1h) and spherocytic mice (not shown). Negligible iron stores were seen within the hematopoietic regions of the liver, and, interestingly, Kupffer cells had very little iron storage. The kidneys of both jaundiced and spherocytic mice accumulated extraordinary amounts of iron, particularly within the proximal convoluted tubules (Fig 1a, c, and d; compare with 1b and e), but also in the distal tubules in severely siderotic ja/ja and sph/sph mice. The parietal layer of the glomerular capsule also sequestered iron and often appeared inflamed and enlarged (Fig 1f; compare with 1g). The accumulation of iron in renal tubular epithelium and subsequent sloughing of these cells leads to iron-laden cellular casts and debris in the tubules (Fig 1c), and eventually to iron-laden tubular casts in the urine. Almost all mice beyond 2 to 3 months of age had green colored urine caused by the presence of renal tubular cells laden with hemosiderin and ferritin detected by staining with Perl's iron stain (data not shown). In later stages of the disease the kidneys appeared visibly darker than normal, and showed hydronephrosis. The urine at this stage was often red caused by either hematuria or hemoglobinuria. No hemosiderosis was detected in spleen, bone marrow, myocardium, or brain.
In addition to the renal tubular disease, the mice suffered from membranoproliferative glomerulonephritis and had an increased glomerular size because of both cellular proliferation and mesangial expansion (Fig 1f; compare with 1g). The glomerulus was often surrounded by increased urinary space. Ultimately, many of the glomeruli appeared to regress and degenerate, leaving only the urinary space and a small tag of the glomerulus. The hydronephrosis and increased urinary space of the glomeruli indicated additional complications in the jaundiced mouse most likely caused by blockage of the tubules or ureters by pigment stones.
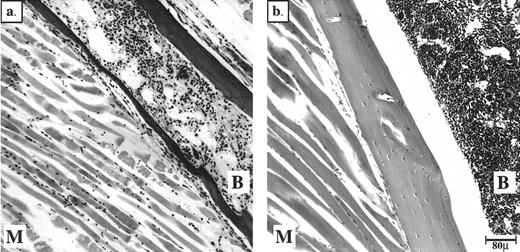
Neurologically, we observed behavior phenotypes that might indicate neuromuscular defects in only two mice. One spherocytic mouse did not use a hind limb, and one jaundiced mouse appeared paralyzed in the entire hind section. Analysis of the fixed hind limbs showed muscle degeneration in both of these mice, with the jaundiced mouse also suffering from extensive marrow degeneration (Fig 2). The degeneration reflected anoxia caused by thrombi in the vessels supplying blood to these tissues. In the case of the jaundiced mouse, the thrombus most likely occurred at the bifurcation of the aorta, therefore blocking both femoral arteries. Aside from these two cases, skeletal muscle from jaundiced mice seemed to have developed correctly and to be morphologically intact (data not shown).
Peripheral thrombi in the jaundiced and spherocytic mice can cause degeneration of BM and muscle in hind limbs. Bouin's-fixed, paraffin-embedded sections of hind limbs stained with H&E are shown. (a) ja/ja mouse #43 with probable thrombus at aortic bifurcation 3.25 months, (b) +/+ nonlittermate control 6 months old. B, bone marrow; M, muscle.
Peripheral thrombi in the jaundiced and spherocytic mice can cause degeneration of BM and muscle in hind limbs. Bouin's-fixed, paraffin-embedded sections of hind limbs stained with H&E are shown. (a) ja/ja mouse #43 with probable thrombus at aortic bifurcation 3.25 months, (b) +/+ nonlittermate control 6 months old. B, bone marrow; M, muscle.
Similarly, cardiac muscle seemed normal and morphologically intact; however, sites of infarction were observed in the myocardium in 4 of 13 (38%) jaundiced and 15 of 15 (100%) spherocytic mice. Infarcts were found in the ventricular or ventricular septal regions in both mutants, and also in the atrial walls of the spherocytic mice. In several cases, a microthrombus was present within a vessel close to the infarct. In addition, large thrombi were observed within the hearts of both jaundiced and spherocytic mice, although at different frequencies: 2 of 13 (15%) jaundiced mice and 15 of 15 (100%) spherocytic mice. The thrombi in the jaundiced mice and most of the spherocytic mice were located mainly in the mitral or left atrioventricular valve (Fig 1i; compare with 1k) often filling the atrium itself (Fig 1j). Most thrombi appeared to be old events because they were well-organized units with endothelial membrane growth surrounding the thrombus.
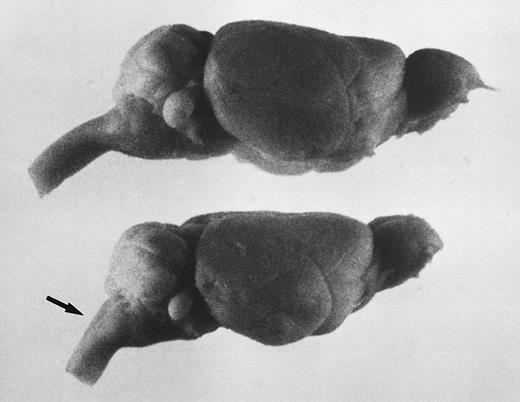
The brains of adult jaundiced mice were typically smaller and vertically flatter compared with control mice (Fig 3); however, brain sections showed no undeveloped or degenerated regions, including Purkinje cells in the cerebellum (data not shown). One striking structural difference we observed was the angle of the brain to the spinal cord, which, although in normal mice was about 135 to 140°, in jaundiced mice approximated only 120°. This altered the normally smooth curvature of the cerebellum to a sharper angle. This same anomaly was found to exist in the skull itself.
The steep angle of the brain to spinal cord in jaundiced and spherocytic mice compared with normal, also seen as a sharp angle in the skull, suggests that erythropoiesis in hind region of skull may effect brain shape. Bouin's-fixed, whole brains are shown. Top, normal littermate to ja/ja #9; bottom, ja/ja mouse #9, 5.75 months. The arrow points to spinal cord at sharpened angle. Notice also the steeper slope of the cerebellum.
The steep angle of the brain to spinal cord in jaundiced and spherocytic mice compared with normal, also seen as a sharp angle in the skull, suggests that erythropoiesis in hind region of skull may effect brain shape. Bouin's-fixed, whole brains are shown. Top, normal littermate to ja/ja #9; bottom, ja/ja mouse #9, 5.75 months. The arrow points to spinal cord at sharpened angle. Notice also the steeper slope of the cerebellum.
In addition, both jaundiced and spherocytic mice exhibited infarctions in the brain at the cerebrum, hippocampus (Fig 1l and m; compare with 1n), and cerebellum. Most infarcts were unilateral, leaving normal structure on the opposite sagittal half of the brain (compare Fig 1l and n from the same jaundiced mouse). As with cardiac infarcts, microthrombi could sometimes be detected close to the site of infarction. The presence of infarctions in the brain did not always correlate with the presence of a thrombus within the heart of the jaundiced mice (4 of 12 brain infarcts v 2 of 13 atrial/valvular thrombi). In spherocytic mice with 100% thrombosis, 14 of 14 mice showed brain infarcts. Cerebellar hemorrhage was observed in 1 of 12 jaundiced mice.
DISCUSSION
Human HS patients present with well-characterized hematologic anomalies, including nonimmune hemolytic anemia and nondiscoidal erythrocytes. Patients with HS caused by mutations in β-spectrin are generally heterozygotes, although homozygotes do exist.21 40-42 The latter patients have a disease of much greater severity, typically correlating with the corresponding reduction in spectrin levels, although the loss of spectrin is still not complete. Therefore, thorough investigations into the effects of the complete deficiency of β-spectrin in the mammalian system, including possible aberrations in nonerythroid tissues, have not been possible in humans. The jaundiced mouse offers an opportunity for such determinations subsequent to overcoming the early fatality of this mutant.
A single neonatal PBC transfusion extends the lifespan of ja/ja pups, suggesting that the high mortality rate of these mice stems from a severe neonatal crisis. In fact, once past this crisis, the mice live as long as the few jaundiced mice that survive unaided, escaping the neonatal crisis by an as yet undetermined means. The spherocytic mouse does not suffer such a severe neonatal crisis, and the majority survive without transfusions although they are essentially indistinguishable from the jaundiced mouse by all hematologic parameters at similar ages after weaning. The fact that β-spectrin, which contains the known binding sites of the spectrin molecule to the lipid bilayer, is still incorporated at reduced levels into membranes of the spherocytic mouse, may give these cells slightly more stability.
Compensatory efforts against the anemia produce hyperplasia in the BM of femurs and skull of the jaundiced mice as well as hepatosplenomegaly. The dilated cardiac chambers of both mutants also indicate attempts at cardiac compensation. Although the anemia remains uncompensated, the continued high level of reticulocytes in the blood of the jaundiced mice is strongly indicative of efficient hematopoiesis. There were no signs of aplastic crisis as is sometimes seen in HS and other chronic hemolytic anemias in humans43-46 but the mice are resistant to parvovirus. The continued anemia is therefore attributed to rapid and chronic hemolysis of RBCs and reticulocytes in the circulatory system. This high rate of hemolysis is shown by the 6-fold increase in serum bilirubin and the 10-fold increase in urobilinogen in the urine of these mice. The contribution by direct (conjugated) bilirubin to the total bilirubin in serum indicates saturation of excretion capability of the liver caused by chronic severe RBC lysis.47 As shown in Table 1A, even continued weekly transfusions in the jaundiced mice do not lessen the anemia, also suggesting a short survival time of the injected cells in the circulation. The lifespan of erythrocytes in vivo in mice with hemolytic anemia has been determined as approximately 24 hours,9 compared with the normal lifespan of 48 days. This may reflect a sensitization of the spleen that is grossly enlarged and playing dual roles of hematopoiesis and RBC destruction.
The source of grossly augmented iron stores is presumed to be both excessive intramedullary and intravascular hemolysis. Because the spherocytic mice are not transfused, yet accumulate iron in the kidneys and liver equal to that of jaundiced mice, transfusion as the source of the sequestered iron is ruled out. The increased hemolysis may lead to saturation of the hemoglobin-haptoglobin binding capacity of the liver. The accumulation of iron in the kidney may also be a result of haptoglobin and hemopexin depletion, leading very quickly to saturation of the reabsorptive capacity of renal tubules for unbound hemoglobin. At this point hemoglobinuria and hemosiderinuria appear, the latter caused by excessive sloughing of hemosiderin- and ferritin-laden renal tubules. Although we could not detect free hemoglobin in the plasma of jaundiced mice, we observed iron-laden tubular cells in the kidney and in urine, as well as red-colored urine, indicating that the haptoglobins may indeed be saturated. It has been suggested that even relatively low levels of hemoglobin present in the plasma chronically can lead to renal hemosiderosis in human HS.48
The increase in TIBC, which approximates the transferrin levels in serum, in combination with hyperferrimia (transferrin saturation above 50%), indicates that transferrin levels and absorption of iron from the intestinal mucosa may be dysregulated in these mice, remaining at increased levels even in the presence of high iron stores in the body. It has been postulated that increased erythropoietic activity, such as the hyperplasia and reticulocytosis observed in the jaundiced mice, is a dominant stimulus for increasing alimentary iron absorption, even in the presence of iron overload.47
In addition to the well-documented hematologic problems, we have shown other serious consequences of spectrin deficiency in the blood cell system of the jaundiced and spherocytic mice. Thrombi at the atrioventricular valves or within the atrium, as well as infarcts in the myocardium itself, are observed in jaundiced and spherocytic mice. Thrombi are most frequent in the mitral valve and left atrium, and microthrombi are seen in several cases in vessels close to the infarct. In two of the jaundiced mice, coronary infarcts are present in the absence of obvious atrioventricular thrombi. These data indicate that the cause of the infarcts in the heart in these mice is probably coronary artery emboli, but may also be because of primary microthrombi. Similarly, the infarcts in the spleen, liver, and brain may be caused by peripheral emboli or to intravascular thrombosis caused by hemostasis in the spleen and liver.
The limited lifespan of even continuously transfused ja/ja mice and the absence of increased severity of anemia in moribund jaundiced mice indicates that fatality cannot be attributed directly to the anemia. Most of the thrombi in the heart appear large enough to restrict blood flow through the valve to a large extent, yet because of their extensive organization, seem to have been present for a matter of weeks. Therefore, the thrombi do not appear to be the immediate cause of death, especially because most fatalities in these mice appear chronically rather than acutely. Infarctions in the liver, brain, and heart are also well established and are not found in every jaundiced mouse and, therefore, are unlikely to be the common cause of death. Previous studies on the sphha/sphha mouse showed that many of the mice suffered from pneumonitis,49 a disease not detected in our mice.
β-spectrin is normally expressed at relatively high levels in skeletal muscle, cardiac muscle, and the brain. The histological analysis of heart and skeletal muscle in these mice showed normally developed muscle without obvious signs of degeneration. These data were consistent with recent human studies on hydrops fetalis patients who had a severe defect in spectrin tetramerization ability caused by a point mutation in β-spectrin (spectrin Providence), but showed normal neonatal muscle morphology.50 In addition, the brain of the jaundiced mice showed no major morphological alterations except a steep angle between brain and spinal cord. This effect is caused by an expanded marrow cavity in the skull resulting from increased hyperplasia subsequently pressuring the developing brain into the same abnormal shape. Unlike the ankyrin-deficient mouse, normoblastosis, no age-related Purkinje cell degeneration was observed in the cerebellum, although an isoform of erythroid β-spectrin is highly expressed in these cells.
Four potential explanations for the lack of primary defects observed in the nonerythroid tissues are (1) that the age of mice and/or conditions under which our observations were made was not optimal, (2) that β-spectrin does not have a functional role in these tissues, (3) that there is compensation by a related protein, or (4) that defects are below the level of detection by light microscopy. In the case of degeneration of brain cell populations, the increased lifespan of the jaundiced mice may not have been extensive enough to observe effects. In the normoblastosis mouse maintained on the same hybrid genetic background as the ja/ja mice, Purkinje cell degeneration did not occur until 1 year of age. In the case of skeletal muscle, the tissue may not have been stressed enough in a laboratory setting to show effects. It seems unlikely that the erythroid β-spectrin isoform expressed in these tissues is nonfunctional because an alternative splicing event introduces a new carboxyl-terminus in this isoform, which is not expressed in the erythroid lineage. This alternate C-terminus contains a pleckstrin homology (PH) domain, which is a sequence shared with several molecules involved in signal transduction51 and has been proposed to associate with the bγ subunits of trimeric G proteins.52 In addition, other researchers have shown that β-spectrin is specifically localized in neurons to cell bodies, dendrites, and postsynaptic membranes, whereas a homologous but distinct gene product, β-fodrin, is localized to axons and presynaptic membranes in these cells.29,53 This differential localization implies that particular functions are performed by each of these proteins. In fact, one function that has been postulated for β-spectrin involves limiting the lateral movement of proteins within the lipid bilayer, thereby organizing topographically defined clusters of receptors at the postsynaptic membrane as has been suggested for the acetylcholine receptor54 or in cytoplasmic protein complexes.33 The potential for compensation by a related molecule such as β-fodrin for the functions usually performed by erythroid β-spectrin in these tissues is currently under investigation.
Although congestive heart failure cannot be ruled out as the cause of fatality in the jaundiced mice, the progressive increase of desquamated tubular cells in the urine, membranoproliferative glomerulonephritis, hydronephrosis, enlargement and degeneration of glomeruli, and the increase in blood urea nitrogen levels suggest that the mice die from renal failure caused by the immense accumulation of iron and subsequent organ damage. We would predict renal failure to be the cause of death in the spherocytic mice as well. A microangiopathic glomerulopathy with features similar to the glomerular disease observed in the hemolytic mice affects sickle cell anemia patients and can lead to chronic renal failure.55
Thrombosis has been observed in several blood-related diseases, including HS,48 paroxysmal nocturnal hemoglobulinuria,39,56,57 and sickle cell anemia.58 A potential contributor to thrombotic disease in sickle cell anemia is the increased exposure on the outer leaflet of blood cell lipid bilayers of the aminophospholipid phosphatidylserine (PS).59 The resultant loss of lipid asymmetry is associated with increased procoagulant activity. Although one report shows fully conserved lipid asymmetry in spherocytes of human HS patients,60 HS also involves microvesicle blebbing, and this release of PS-expressing procoagulant microvesicles may contribute to thrombotic events in the anemic mice. Theoretically, these events would occur at equal frequency in both jaundiced and spherocytic mice because of similar hemolysis and microspherocytosis in each of the mutants. However, we observe markedly increased thrombotic disease in the spherocytic mice compared with jaundiced mice. In a previous study on a second mouse mutant deficient in α-spectrin, sphha/sphha, thrombosis was detected in venules and small- to medium-sized veins with resulting ischemic damage to the spleen, myocardium, pancreas, liver, or BM in approximately 80% of the mice.49 The increased presence of thrombotic disease in both of the α-spectrin–deficient mutants compared with the β-spectrin–deficient mice suggests that α-spectrin may play an important role in a blood cell type other than erythrocytes. We find the observation remarkable that the α-spectrin–deficient mice in our study, although suffering from greatly increased levels of thrombotic disease, have a longer average lifespan and appear healthier than the jaundiced mice with similar levels of hemolysis and hemosiderosis in the two mutants. Possibly, specialized roles for β-spectrin lead to more severe defects in certain cell types, which at this level of analysis are undetectable. Ongoing work in our laboratory to determine PS levels and other differences in cellular fractions of the mutants, as well as additional functional roles of β-spectrin, may shed light on these intriguing questions.
ACKNOWLEDGMENT
The authors greatly appreciate the invaluable technical assistance of Elaine Hall, Susan Deveau, and Grace Crawford-Sharpe. We thank Connie Birkenmeier and Dr David Harrison for presubmission comments on the manuscript. We thank the Biological Imaging Service at the Jackson Laboratory for photographic assistance (partially funded by NIH Core Grant No. CA34196).
Supported by National Institutes of Health (NIH) NRSA F32 No. DK09054 (T.M.K.), NIH T32 No. DK07449 (N.J.W.), and NIH Grant No. HL29305 (J.E.B.).
Address reprint requests to Jane E. Barker, PhD, The Jackson Laboratory, 600 Main St, Bar Harbor, ME 04609.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal