Abstract
The aim of this study was to ascertain prevalence and natural history of hepatitis C virus (HCV) infection in a large cohort of patients cured of childhood leukemia who had been followed prospectively for liver disease for at least 10 years since chemotherapy withdrawal: 114 consecutive patients entered the study. Liver function tests and ultrasonography were used to assess presence of liver disease. Patients were tested for antibody to HCV and for serum HCV-RNA at the end of chemotherapy and at the end of follow-up. At chemotherapy withdrawal, 56 patients (49%) were HCV-RNA positive, often without detectable anti-HCV, and in these cases, transaminase levels were more elevated during (P = .08) and after (P = .04) chemotherapy compared with HCV-RNA negative cases. Patients were then followed-up 13 to 27 years (mean, 17) after chemotherapy withdrawal. During this period, 38 initially anti-HCV negative patients seroconverted to anti-HCV and 17 initially anti-HCV positive cases lost reactivity. Forty patients were persistently HCV-RNA positive in serum, while 16 initially viremic patients became HCV-RNA negative during follow-up. At the end of the observation period, a persistent transaminase elevation was detected only in four HCV-RNA positive and anti-HCV positive cases, while no patient developed signs or symptoms of decompensated liver disease. Thus, hepatitis C was a frequent finding in long-term survivors after chemotherapy. It was associated with an atypical serologic profile and did not cause severe liver impairment over a period of 13 to 27 years.
BEFORE THE DISCOVERY of the hepatitis C virus (HCV) and the implementation of anti-HCV tests for the screening of blood donors, patients with hematologic malignancies were at a very high risk of HCV infection due to the large transfusional support they often needed and to the immunodeficiency status caused by the underlying disease and by chemotherapy. At that time, about 70% to 80% of children with acute leukemia were found to have a persistent elevation of transaminase levels with liver histologic lesions suggestive of chronic viral hepatitis that, in our own early studies, could be related to hepatitis B virus infection in about half of the children, the remaining being cases of non-A, non-B hepatitis.1-3 More recently, several studies based on the detection of HCV markers in serum, mainly anti-HCV by enzyme-linked immunosorbent assay (ELISA), have reported variable prevalences of positive HCV serology in this clinical setting, in association with a wide spectrum of liver involvement, ranging from minimal enzyme elevation to severe, life-threatening hepatic failure.4-6 Most patients, however, were found to have a persistent elevation of transaminase levels with no significant impairment of liver function. The high rate of chronic hepatitis in this clinical setting has become a major concern for the long-term outcome of the patients, as the prognosis of childhood leukemia has dramatically improved over the last 20 years,7 while chronic HCV infection has been recognized as a major cause of liver cirrhosis and hepatocellular carcinoma.8
This report is an analysis of HCV infection and of related chronic hepatic sequelae in a large series of consecutive children cured of leukemia and monitored for 13 to 27 years after cessation of chemotherapy.
MATERIALS AND METHODS
Among 386 consecutive children with leukemia diagnosed and treated at the Department of Pediatric Hematology of the S. Gerardo Hospital in Monza-Milan, Italy, between 1968 and 1982, 261 (68%) died within 1 to 96 months after diagnosis; 210 of leukemia, 25 of hemorrhagic complications, and 26 of infectious complications. Stored sera were available from 256 of these patients: anti-HCV was detected in 14 (5.4%) patients.
The remaining 125 patients appeared to be cured and 114 of them were followed prospectively during the off-therapy period with yearly biochemical and clinical assessment. This series was made up of 59 men and 55 women, with a mean age (± standard deviation [SD]) of 4 years ± 2.6 at the time of diagnosis of the hematologic disease (acute lymphoblastic leukemia, 109 cases; acute myeloblastic leukemia, 5 cases). The follow-up period lasted 13 to 27 years (mean ± SD, 17 ± 3.2). All 114 patients were alive and were still prospectively followed-up when this evaluation was performed in June 1996. None of them received antiviral or interferon therapy during the observation period. The remaining 11 cases (9%) did not undergo periodic controls, but could be evaluated in June 1996, and all of them were still alive with no clinical evidence of decompensated liver disease.
Assessment of liver disease. The clinical records of the 114 patients who had been followed prospectively were reviewed in June 1996, and the presence of liver disease was assessed on the basis of clinical data and biochemical findings, which included yearly determination of serum alanine aminotransferase (ALT, measured using a standard autoanalyzer, according to the International Federation of Clinical Chemistry (IFCC) recommendations; upper normal limit, 42 IU/L), alkaline phosphatase, total and fractionated bilirubin, albumin, gammaglobulins, as well as white blood cell and platelet counts. At the end of follow-up, all patients were also evaluated by abdominal ultrasonography. Liver biopsies were available only in a minority of our patients. Thirty-six patients had undergone liver biopsy at the time of chemotherapy withdrawal between 1978 and 1981. These biopsy samples were taken to assess whether liver histology could be predictive of severe outcome after chemotherapy withdrawal. In two patients with persistent high ALT levels during follow-up, a second liver biopsy was taken after 5 and 11 years of follow-up, respectively.
HCV markers. Antibodies to HCV (anti-HCV) were detected by II generation Ortho-ELISA tests (Ortho Diagnostic Systems, Raritan, NJ) and radioimmuno blotting assay (RIBA; Chiron Corp, Emeryville, CA) at regular intervals throughout the observation period using serum samples that had been stored at −20°C. The RIBA test was performed in all samples independently of the anti-HCV ELISA results. Serum specimens with only one band of reactivity were defined indeterminate, while those reacting with at least two viral antigens were defined positive, according to the manufacturer's instructions. HCV-RNA was investigated by nested polymerase chain reaction (PCR) in serum samples obtained at the end of chemotherapy and at the end of follow-up. HCV-RNA sequences were detected using primers of the 5′ UTR part of the virus genome.9 When HCV-RNA was amplified, the HCV genotype was then defined by spot hybridization with genotype specific oligonucleotide probes, as previously described.9
Hepatitis B surface antigen (HBsAg) was tested in serum by commercially available radioimmunoassay (Abbott Laboratories, North Chicago, IL).
Blood components administered to the patients were obtained from HBsAg-negative volunteer donors. ALT values were used as screening test for non-A, non-B hepatitis. First generation anti-HCV test was routinely introduced as screening test from January 1990 and was replaced by second generation ELISA tests in June 1991.
Statistical methods. Data for unpaired groups were analyzed by t-test and frequencies of positive findings were compared by χ2 test. The development of anti-HCV positivity in groups was computed and plotted by the Kaplan-Meier method and compared into groups by one-way analysis of variance (ANOVA).
RESULTS
HCV serum markers and liver disease at the end of antileukemic treatment. At the end of antileukemic treatment, 56 patients (49%) were HCV-RNA positive, 55 (48%) were negative, while only 3 cases could not be tested due to lack of well-preserved serum specimens. Fourteen of 114 children (12%) (10 HCV-RNA positive and 4 HCV-RNA negative) were anti-HCV positive (Table 1). Mean transaminase peak values during chemotherapy and mean ALT values at the end of treatment were significantly higher in cases with viremia compared with HCV-RNA negative patients (486.46 IU/L v 380.65 IU/L, P = .08 and 101.26 IU/L v 58.95 IU/L, P = .04, respectively). The number of patients with abnormal ALT levels at chemotherapy withdrawal was significantly higher among HCV-RNA positive cases (34 of 56 v 20 of 55, P = .01) (Table 1). Thirty-six children (25 HCV-RNA positive and 11 HCV-RNA negative) underwent a diagnostic liver biopsy: as shown in Table 1, a wide spectrum of histologic lesions ranging from nonspecific minimal changes to chronic lobular or persistent hepatitis and to chronic active hepatitis was observed without significant differences between viremic and nonviremic patients.
HCV Markers and Liver Disease at the End of Chemotherapy in 114 Children With Acute Leukemia
| . | Serum HCV-RNA at the End of Chemotherapy . | Not Tested . | ||
|---|---|---|---|---|
| . | Positive . | . | Negative . | . |
| N patients (114 cases) | 56 (49%) | 55 (48%) | 3 (3%) | |
| Anti-HCV positive | 10/56 (18%) | 4/55 (7%) | 0/3 | |
| Mean ALT* peak ±SD during chemotherapy | 486.46 (±342.37) | †P = .08 | 380.65 (±303.42) | 224.3 (±253.6) |
| Patients with abnormal ALT levels at chemotherapy withdrawal | 34/56 (64%) | ‡P = .01 | 20/55 (36%) | 1/3 |
| ALT* (±SD) at chemotherapy withdrawal | 101.26 (±122.66) | †P = .04 | 58.95 (±95.18) | 41 (±22.51) |
| Patients with liver biopsy | 25 | 11 | 0/3 | |
| Histologic diagnosisρ | ||||
| NSRH | 3/25 | 2/11 | ||
| CLH | 5/25 | 4/11 | ||
| CPH | 12/25 | 3/11 | ||
| CAH | 5/25 | 2/11 | ||
| . | Serum HCV-RNA at the End of Chemotherapy . | Not Tested . | ||
|---|---|---|---|---|
| . | Positive . | . | Negative . | . |
| N patients (114 cases) | 56 (49%) | 55 (48%) | 3 (3%) | |
| Anti-HCV positive | 10/56 (18%) | 4/55 (7%) | 0/3 | |
| Mean ALT* peak ±SD during chemotherapy | 486.46 (±342.37) | †P = .08 | 380.65 (±303.42) | 224.3 (±253.6) |
| Patients with abnormal ALT levels at chemotherapy withdrawal | 34/56 (64%) | ‡P = .01 | 20/55 (36%) | 1/3 |
| ALT* (±SD) at chemotherapy withdrawal | 101.26 (±122.66) | †P = .04 | 58.95 (±95.18) | 41 (±22.51) |
| Patients with liver biopsy | 25 | 11 | 0/3 | |
| Histologic diagnosisρ | ||||
| NSRH | 3/25 | 2/11 | ||
| CLH | 5/25 | 4/11 | ||
| CPH | 12/25 | 3/11 | ||
| CAH | 5/25 | 2/11 | ||
Alanine aminotransferase; normal values ≤42 IU/L.
HCV-RNA positive versus HCV-RNA negative group. P calculated by t-test.
HCV-RNA positive versus HCV-RNA negative group. P calculated by χ 2 test.
ρ NSRH, nonspecific reactive hepatitis; CLH, chronic lobular hepatitis; CPH, chronic persistent hepatitis; CAH, chronic active hepatitis.
Liver disease during follow-up. None of the 114 patients followed prospectively after chemotherapy developed signs or symptoms of decompensated liver disease such as jaundice, ascites, bleeding from portal hypertension, hepatic encephalopathy, and liver failure. The ALT values, assessed at yearly intervals, were constantly normal in 81 cases (71%), showed fluctuations ranging from normal to abnormal values in 29 (25.4%), and were constantly abnormal in only 4 cases (3.5%).
Anti-HCV and ALT profiles during follow-up in relation to HCV-RNA behavior. Forty-seven of 114 patients were negative for HCV-RNA at chemotherapy withdrawal and at the end of follow-up (Table 2). Among these 47 patients, 40 (85%) were also anti-HCV negative for the entire period of observation, 2 (4%) were constantly anti-HCV positive during follow-up, 1 (2%) seroconverted to anti-HCV positivity after chemotherapy withdrawal, while in 4 (8%) cases, a seroreversion from anti-HCV positive to anti-HCV negative was observed. Transaminase values normalized during follow-up in 85% of patients. Forty of 114 patients (34%) showed detectable viremia both at cessation of treatment and at the end of follow-up. Anti-HCV reactivity was never detected in 6 of 40 (15%); a seroreversion was documented in nine (22.5%) patients. Two of 40 patients (5%) were constantly anti-HCV positive and the remaining 21 (52.5%) seroconverted to anti-HCV positivity after chemotherapy withdrawal. ALT levels were persistently high or fluctuating during the off-therapy observation in 22 of 40 patients (55%), while a stable normalization was observed in the remaining 18 (45%). Two patients with chronic viremia and persistently high transaminase levels underwent a second liver biopsy 5 and 11 years after the first, taken at chemotherapy withdrawal: in both cases, there was an improvement from chronic active hepatitis to chronic persistent and chronic lobular hepatitis, respectively. Of the 56 patients found HCV-RNA positive at chemotherapy withdrawal, 16 (28.6%) became HCV-RNA negative during follow-up: all of them showed anti-HCV reactivity (two were already positive at chemotherapy withdrawal and the other 14 seroconverted later during follow-up). In this group of patients, ALT normalized in 14 (87.5%), while two (12.5%) showed ALT fluctuations from normal to abnormal values during the off-therapy observation. Eight of 114 patients (7%) were HCV-RNA negative at chemotherapy withdrawal, but were viremic at the end of follow-up: in four of them, anti-HCV was never detected, one was anti-HCV positive at the end of chemotherapy, two seroconverted while off-therapy, and the last one lost anti-HCV reactivity during follow-up. The majority of these patients (6 of 8) showed ALT normalization during follow-up (Table 2). The last three patients could not be tested for HCV-RNA at chemotherapy withdrawal and were negative at the end of follow-up. All of them seroconverted to anti-HCV positivity and showed ALT normalization during follow-up.
Anti-HCV and ALT Profiles During Off-Therapy Follow-up in Relation to HCV-RNA Behavior in 114 Patients Cured of Acute Leukemia
| Serum HCV-RNA . | No. of Patients . | Anti-HCV During Follow-up . | ALT . | |||||
|---|---|---|---|---|---|---|---|---|
| End of Treatment . | End of Follow-up . | . | Always Negative . | Always Positive . | Seroconversion . | Seroreversion . | Persistently High or Fluctuating (%) . | Normalized (%) . |
| Negative | Negative | 47 | 40 | 2 | 1 | 4 | 7 (15) | 40 (85) |
| Positive | Positive | 40 | 6 | 4 | 21 | 9 | 22 (55) | 18 (45) |
| Positive | Negative | 16 | 0 | 2 | 14 | 0 | 2 (12.5) | 14 (87.5) |
| Negative | Positive | 8 | 4 | 1 | 2 | 1 | 2 (25) | 6 (75) |
| Not tested | Negative | 3 | 0 | 0 | 3 | 0 | 0 | 3 |
| Serum HCV-RNA . | No. of Patients . | Anti-HCV During Follow-up . | ALT . | |||||
|---|---|---|---|---|---|---|---|---|
| End of Treatment . | End of Follow-up . | . | Always Negative . | Always Positive . | Seroconversion . | Seroreversion . | Persistently High or Fluctuating (%) . | Normalized (%) . |
| Negative | Negative | 47 | 40 | 2 | 1 | 4 | 7 (15) | 40 (85) |
| Positive | Positive | 40 | 6 | 4 | 21 | 9 | 22 (55) | 18 (45) |
| Positive | Negative | 16 | 0 | 2 | 14 | 0 | 2 (12.5) | 14 (87.5) |
| Negative | Positive | 8 | 4 | 1 | 2 | 1 | 2 (25) | 6 (75) |
| Not tested | Negative | 3 | 0 | 0 | 3 | 0 | 0 | 3 |
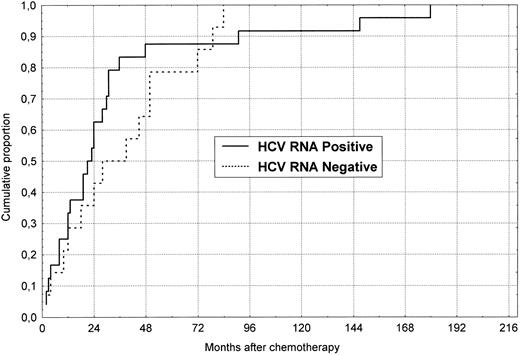
Cumulative probability of anti-HCV seroconversion during follow-up after chemotherapy withdrawal in 38 initially anti-HCV negative patients divided according to the HCV-RNA status at the end of follow-up.
Cumulative probability of anti-HCV seroconversion during follow-up after chemotherapy withdrawal in 38 initially anti-HCV negative patients divided according to the HCV-RNA status at the end of follow-up.
Overall, 14 of the 114 patients (12%) were anti-HCV positive by ELISA in serum samples obtained at the end of chemotherapy and specificity was confirmed by RIBA in all of them. Five of these 14 patients lost anti-HCV during follow-up (3 to 110 months after cessation of chemotherapy). On the other hand, 38 patients seroconverted to anti-HCV during follow-up. Figure 1 describes the cumulative rate of anti-HCV seroconversion (Kaplan-Meier curves) observed in these patients when divided according to serum HCV-RNA reactivity at the end of follow-up. More than 50% of the cases seroconverted within 2 years of off-therapy and more than 80% did so within 5 years.
HCV markers at the end of follow-up. At the end of follow-up, 48 patients were HCV-RNA positive in serum by PCR, while the remaining 66 were HCV-RNA negative (Table 3). The 114 patients could therefore be classified into four serologic groups according to the anti-HCV and HCV-RNA status at the end of follow-up: 47 patients (41.2%) were anti-HCV and HCV-RNA negative (group A), 20 (17.5%) were anti-HCV negative, but HCV-RNA positive (group B), 28 (24.6%) were anti-HCV and HCV-RNA positive (group C), and 19 (16.6%) were anti-HCV positive, but HCV-RNA negative (group D).
ALT Behavior During Follow-up in Patients With Different Serologic Profiles at the End of the Observation Period
| Serologic Profile at End of Follow-up . | ALT Profile During Follow-up: No. of Patients (%) . | |||||
|---|---|---|---|---|---|---|
| Group . | Anti-HCV . | HCV-RNA . | No. of Patients . | Constantly Normal . | Constantly Elevated . | Fluctuating . |
| A | − | − | 47 | 42 (89) | 0 | 5 (11) |
| B | − | + | 20 | 17 (85) | 0 | 3 (15) |
| C | + | + | 28 | 6 (22) | 4 (14) | 18 (64) |
| D | + | − | 19 | 16 (84) | 0 | 3 (16) |
| Serologic Profile at End of Follow-up . | ALT Profile During Follow-up: No. of Patients (%) . | |||||
|---|---|---|---|---|---|---|
| Group . | Anti-HCV . | HCV-RNA . | No. of Patients . | Constantly Normal . | Constantly Elevated . | Fluctuating . |
| A | − | − | 47 | 42 (89) | 0 | 5 (11) |
| B | − | + | 20 | 17 (85) | 0 | 3 (15) |
| C | + | + | 28 | 6 (22) | 4 (14) | 18 (64) |
| D | + | − | 19 | 16 (84) | 0 | 3 (16) |
Clinical and biochemical features in relation to HCV serology. The behavior of ALT values during follow-up in the four groups of patients is described in Table 3. Patients positive for both anti-HCV and HCV-RNA (group C) had a statistically significant higher prevalence of ALT abnormalities during follow-up compared with the other three groups (P = .008). Interestingly, the pattern of ALT profiles during follow-up in patients who were positive for HCV-RNA in the absence of anti-HCV (group B) was the same as that observed in patients without viremia (groups A and D).
The amount of transfusional support was similar in all patients: mean number of blood units was 3.4, 2.6, 2.7, and 2.4 in groups A, B, C, and D, respectively, with no significant difference among the four groups.
Hepatitis B surface antigen was detected during follow-up in 6 of 114 patients (5.2%). Two of them became chronic carriers (1 belonged to group A and the other to group D) and 4 (3 in group A and 1 in group C) became HBsAg negative during further observation.
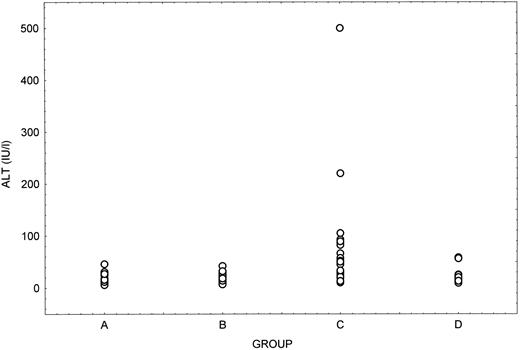
Table 4 summarizes the main findings obtained at the end of follow-up in the 114 patients, grouped in relation to their HCV serologic status. Anti-HCV positive patients were significantly older (P = .0009) and had been followed for a longer period of time (P = .009) than anti-HCV negative cases. Mean ALT values were significantly higher in patients positive for both anti-HCV and HCV-RNA compared with the other serologic groups (Fig 2). On the other hand, no significant differences were noted between the four groups regarding albumin, bilirubin, gammaglobulin levels, or platelet counts at the end of follow-up. Ultrasound examination of the liver showed normal findings in all patients.
Main Findings at the End of Follow-up in the Four Serologic Groups
| . | Group . | |||
|---|---|---|---|---|
| . | A . | B . | C . | D . |
| Anti-HCV | − | − | + | + |
| HCV-RNA | − | + | + | − |
| Male/female | 22/25 | 9/11 | 17/11 | 9/9 |
| Mean age (mo ± SD) | 266 ± 50 | 253 ± 34 | 300 ± 54 | 297 ± 40 |
| Mean follow-up (mo ± SD) | 166 ± 35 | 168 ± 23 | 187 ± 32 | 197 ± 37 |
| Mean ALT ± SD (IU/L) | 17 ± 7 | 18 ± 9 | 68 ± 94 | 21 ± 14 |
| Mean albumin ± SD (g/L) | 4.9 ± 0.31 | 5.1 ± 0.4 | 5.06 ± 0.3 | 5.0 ± 0.4 |
| Mean γ globulin ± SD (g/L) | 1.03 ± 0.19 | 1.02 ± 0.19 | 1.15 ± 0.22 | 0.9 ± 0.29 |
| Mean bilirubin ± SD (mg/dL) | 0.17 ± 0.10 | 0.26 ± 0.17 | 0.20 ± 0.09 | 0.17 ± 0.08 |
| Mean platelets ± SD (× 1,000/μL) | 231 ± 57 | 222 ± 53 | 225 ± 43 | 237 ± 49 |
| . | Group . | |||
|---|---|---|---|---|
| . | A . | B . | C . | D . |
| Anti-HCV | − | − | + | + |
| HCV-RNA | − | + | + | − |
| Male/female | 22/25 | 9/11 | 17/11 | 9/9 |
| Mean age (mo ± SD) | 266 ± 50 | 253 ± 34 | 300 ± 54 | 297 ± 40 |
| Mean follow-up (mo ± SD) | 166 ± 35 | 168 ± 23 | 187 ± 32 | 197 ± 37 |
| Mean ALT ± SD (IU/L) | 17 ± 7 | 18 ± 9 | 68 ± 94 | 21 ± 14 |
| Mean albumin ± SD (g/L) | 4.9 ± 0.31 | 5.1 ± 0.4 | 5.06 ± 0.3 | 5.0 ± 0.4 |
| Mean γ globulin ± SD (g/L) | 1.03 ± 0.19 | 1.02 ± 0.19 | 1.15 ± 0.22 | 0.9 ± 0.29 |
| Mean bilirubin ± SD (mg/dL) | 0.17 ± 0.10 | 0.26 ± 0.17 | 0.20 ± 0.09 | 0.17 ± 0.08 |
| Mean platelets ± SD (× 1,000/μL) | 231 ± 57 | 222 ± 53 | 225 ± 43 | 237 ± 49 |
ALT levels at the end of follow-up in the four serologic subgroups of patients. Group A, anti-HCV and HCV-RNA negative; Group B, anti-HCV negative and HCV-RNA positive; Group C, anti-HCV and HCV-RNA positive; Group D, anti-HCV positive and HCV-RNA negative.
ALT levels at the end of follow-up in the four serologic subgroups of patients. Group A, anti-HCV and HCV-RNA negative; Group B, anti-HCV negative and HCV-RNA positive; Group C, anti-HCV and HCV-RNA positive; Group D, anti-HCV positive and HCV-RNA negative.
Anti-HCV patterns in patients with or without viremia. All patients were tested for anti-HCV by RIBA, independently of the anti-HCV ELISA results. As shown in Table 5, no differences in the prevalence of antibodies to C100, C33, C22, and NS5 determinants were identified by RIBA in patients with or without HCV-RNA in serum. Interestingly, the RIBA assay detected an isolated reactivity against C100, C33, or C22 in a significant proportion of HCV-RNA positive patients who were negative for anti-HCV by ELISA.
Riba Profiles in ELISA Anti-HCV Positive and Negative Patients and Relation to Serum HCV-RNA
| RIBA Anti-HCV Profile . | % HCV-RNA Positive Patients . | % HCV-RNA Negative Patients . |
|---|---|---|
| In ELISA anti-HCV positive | ||
| Anti-C 100 positive | 75 | 66 |
| Anti-C 33 positive | 89 | 93 |
| Anti-C 22 positive | 96 | 73 |
| Anti-NS5 positive | 60 | 66 |
| RIBA positive | 100 | 100 |
| RIBA indeterminate | 0 | 0 |
| In ELISA anti-HCV negative | ||
| Anti-C 100 positive | 14 | 0 |
| Anti-C 33 positive | 28 | 0 |
| Anti-C 22 positive | 21 | 0 |
| Anti-NS5 positive | 0 | 0 |
| RIBA positive | 0 | 0 |
| RIBA indeterminate | 28 | 0 |
| RIBA Anti-HCV Profile . | % HCV-RNA Positive Patients . | % HCV-RNA Negative Patients . |
|---|---|---|
| In ELISA anti-HCV positive | ||
| Anti-C 100 positive | 75 | 66 |
| Anti-C 33 positive | 89 | 93 |
| Anti-C 22 positive | 96 | 73 |
| Anti-NS5 positive | 60 | 66 |
| RIBA positive | 100 | 100 |
| RIBA indeterminate | 0 | 0 |
| In ELISA anti-HCV negative | ||
| Anti-C 100 positive | 14 | 0 |
| Anti-C 33 positive | 28 | 0 |
| Anti-C 22 positive | 21 | 0 |
| Anti-NS5 positive | 0 | 0 |
| RIBA positive | 0 | 0 |
| RIBA indeterminate | 28 | 0 |
HCV genotypes in HCV-RNA positive patients. The HCV genotype was investigated in all HCV-RNA positive patients and could be classified as HCV-1 in 34 cases (71%), HCV-2 in 2 cases (4%), and HCV-3 in 1 patient (2%). Two patients (4%) were infected by a mixed genotype population (HCV-1 and HCV-2, one case; HCV-1 and HCV-3, one case), while in nine cases (19%), the HCV type could not be defined with our method. No differences in the prevalence of HCV genotypes were observed between anti-HCV positive and anti-HCV negative patients.
DISCUSSION
The natural history of chronic HCV infection is still poorly understood. While there is evidence that a subgroup of patients progress rather slowly to severe liver damage with cirrhosis, hepatic decompensation, and hepatocellular carcinoma,10 other cases, perhaps the majority, have a much less aggressive disease that remains stable and mild for decades.11,12 The determinants of disease progression are obscure. It has been suggested that the virus load or genotype may play a role in determining the course of hepatitis C and HCV-1 has been suggested as causing a more rapidly progressive disease compared with other genotypes,13-15 but not all studies have confirmed this.16 17 Host and environmental factors certainly play some role. In our study, which contains a large series of individuals cured of leukemia and followed for more than a decade after therapy withdrawal, we analyzed the long-term outcome of chronic hepatitis C in patients who had acquired the infection during malignant lymphoproliferative disorders, requiring aggressive immunosuppressive chemotherapy. Although about 40% of these patients showed serologic evidence of chronic HCV infection, most likely acquired during the hematologic disease, none of them had developed evidence of severe chronic liver disease when studied 13 to 27 years after the estimated time of infection. The serologic profile observed in our patients was rather peculiar, as 20 of the 48 HCV-RNA positive cases were negative in serum for anti-HCV when tested by ELISA, although weak monospecific antibody reactivity, mainly directed against structural proteins of HCV, were detectable by RIBA in 19% to 28% of the patients. This would suggest that acquisition of the infection during intrinsic and extrinsic immunosuppression could have prevented the development of a valid antiviral immune response and might also explain the mild form of liver disease assuming that host rather than virus factors play a major role in liver pathology. Indeed, no sign of liver damage was observed in most of the patients with this atypical serologic profile in contrast to HCV-RNA positive and anti-HCV (ELISA) positive patients, most of whom had elevated ALT, although without any biochemical or echographic evidence of severe liver involvement. Although many cases were infected by HCV-1, thought to be the most aggressive virus genotype, the observation that HCV infection had not developed into a severe liver disease in any of the patients over a period of one or two decades, indicates that chronic hepatitis C is mild and has an extremely low rate of progression in this clinical setting. Furthermore, a proportion of these individuals became HCV-RNA negative during long-lasting follow-up, although none of them was treated with interferon or other antivirals during follow-up. These data would suggest that hepatitis C might resolve or enter into a protracted remission phase in a significant number of cases. This finding and the observation that liver disease was not clinically progressive in our patients would indicate that the rationale for early therapeutic intervention with interferon or antivirals is rather weak in these patients.
Continued follow-up of our cohort of patients will clarify whether these conclusions remain valid beyond the third decade of infection.
ACKNOWLEDGMENT
The authors thank J. Upton for her secretarial and linguistic assistance.
Supported by Progetto Epatiti Virali, Istituto Superiore Di Sanità and Comitato ML Verga per lo Studio e la Cura delle Leucemie del Bambino.
Address reprint requests to Anna Locasciulli, MD, Ematologia Pediatrica, Nuovo Ospedale S. Gerardo, Via Donizetti 106, 20052 Monza (MI), Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal