Abstract
Interferon-γ (IFN-γ) is a potent inhibitor of hematopoiesis in vitro and has been implicated in the pathophysiology of human bone marrow failure syndromes. IFN-γ both inhibits cell cycling and induces expression of the Fas-receptor, resulting in subsequent apoptosis of hematopoietic progenitor cells. IFN regulatory factor-1 (IRF-1) mediates some of these suppressive effects by activation of downstream inducible genes, such as double-stranded RNA-activatable protein kinase and inducible nitric oxide synthase. However, under certain experimental conditions, IFN-γ appears to stimulate proliferation of hematopoietic cells. Based on the hypothesis that IFN-γ–receptor triggering may activate diverse signaling cascades, we designed experiments to determine which intracellular mechanisms (in addition to the IRF-1 transduction pathway) influence the biologic effects of IFN-γ. Using antisense technique, we inhibited the IRF-1–mediated pathway in KG1a cells stimulated with IFN-γ. In contrast to the suppressive effects of IFN-γ observed in control cells, untreated and IFN-γ–treated KG-1a cells that were transduced with retroviral vectors expressing IRF-1 antisense mRNA showed enhanced proliferation. The increased growth rate was associated with decreased levels of IRF-1 mRNA and protein but unchanged levels of IRF-2. We inferred that IFN-γ could also activate a stimulatory transduction pathway that, under specific conditions, may control the cellular response to this cytokine. The family of Stat proteins is involved in signal transduction of hematopoietic growth factors. We showed that, in KG-1a cells, IFN-γ also induced phosphorylation of Stat1 and Stat3, whereas p42 MAP kinase was phosphorylated regardless of the presence of IFN-γ. Using electrophoresis mobility shift assays, IFN-γ enhanced Stat1-Stat1 homodimer and Stat1-Stat3 heterodimer formation, suggesting that, in addition to inhibitory signals mediated by IRF-1, IFN-γ may activate proliferative signals by phosphorylation of Stat1 and Stat3 proteins. The observations made in experiments with KG-1a cells were confirmed in primary hematopoietic cells. After inhibition of the IRF-1 pathway by transduction of an antisense IRF-1 retrovirus into human CD34+ cells, IFN-γ produced an aberrant stimulatory effect on hematopoietic colony formation. Conversely, in control vector-transduced CD34+ cells, the typical inhibitory response to IFN-γ was seen. Our results indicate that inhibitory cytokines such as IFN-γ may exhibit diverse biologic effects depending on the intracellular balance of transcriptional regulators, in turn influenced by the activation and differentiation status of the target cells.
INTERFERON-γ (IFN-γ), a product of lymphocytes and natural killer cells, has pleiotropic effects on cells of the immune system, serving as an important regulator of lymphocyte and macrophage function (for review, see Young and Hardy1 and De Maeyer and De Maeyer-Guingard2 ). IFN-γ also exerts antiproliferative effects on many cell types2 possibly related to its antiviral and antioncogenic properties. In the hematopoietic system, IFN-γ inhibits in vitro colony formation by bone marrow (BM) and blood-derived progenitor cells of the myeloid, erythroid, and megakaryocytic lineage.3-8 Pathologic expression of IFN-γ has been implicated in immune-mediated BM failure syndromes, including acquired aplastic anemia,9-12 hemophagocytic syndrome,13 and myelodysplasia.14 Inhibitory effects of IFN-γ have been shown to be due to the inhibition of cell cycle progression and induction of apoptosis within the hematopoietic cell compartment.3,4 In tissue culture, the degree of hematopoietic inhibition by IFN-γ depends on several factors, such as the type and concentration of growth factors or presence of fetal calf serum in media.8,15 Apoptosis seems to be facilitated by upmodulation of Fas receptor expression on CD34+ cells.16 Cytotoxic effects of IFN-γ on the hematopoietic cells may be mediated by activation of several intracellular effector mechanisms, including the production and release of NO in hematopoietic cells.17
However, under some tissue culture conditions and with certain target cell populations, IFN-γ has been reported stimulate to proliferation of hematopoietic cells.18-21 For example, IFN-γ enhanced colony formation induced by interleukin-3 (IL-3) in purified CD34+ cells derived from umbilical cord blood19 and potentiated the effects of stem cell factor (SCF ) on primitive hematopoietic progenitor cells.20 IFN-γ also increased CD34+ cell expansion when added to a cocktail of hematopoietic growth factors20,22 or enhanced megakaryocyte colony formation in BM after 5-fluorouracil treatment.21 Reconciliation of these positive and negative actions of IFN-γ has been difficult. By analogy with other bifunctional cytokines, differential triggering of receptor molecules (as with tumor necrosis factor [TNF ]) or preferential use of intracellular signaling pathways are possible explanations.
The cell membrane receptor for IFN-γ appears to have a single form consisting of two separately regulated chains,23 and a correlation exists between differential expression of IFN-γRβ chain and the delivery by IFN-γ of proliferative or apoptotic signals. In addition, intracellularly, several signal transduction pathways for IFN-γ have been identified.24,25 Many inhibitory effects of IFN-γ appear to be mediated by IFN regulatory factor-1 (IRF-1), which in turn transactivates multiple effector genes.24,26 In cell lines, IRF-1 and IRF-2 appear to have reciprocal roles in the control of cell proliferation, with IRF-1 acting negatively.27 CD34+ cell population, which contains the majority of hematopoietic progenitor and stem cells, constitutively expresses IRF-1 and -2 proteins,28 and for these cells upregulation of IRF-1 expression also leads to inhibition of cell proliferation. In other recent studies, stimulation of target cells with IFN-γ resulted in phosphorylation of Janus kinase 1 (Jak1) and Jak2, which mediate activation of the signal transducer and activator of transcription 1 (Stat1).29 After transport to the nucleus, Stat1 transactivates several IFN-inducible genes.29 Importantly, Jak1, Jak2, and Stat1 are not specific for IFN-γ. Granulocyte colony-stimulating factor (G-CSF ) mediates its effects through Jak1, Jak2, Stat1, and Stat3, whereas granulocyte-macrophage colony-stimulating factor (GM-CSF ) and IL-3 signal transduction involves Jak2, Stat3, and Stat5. Recently, Stat1 was also shown to transduce negative signals of IFN-γ in fibroblasts.30 In addition, growth factors and cytokines also induce mitogen-activated protein kinases (MAPK), which, together with the Stat and Jak family of proteins, transduces mitotic signals.29-32 IFN-γ–mediated inhibition of hematopoietic cell proliferation can be counterbalanced by escalating doses of GM-CSF, G-CSF, and IL-3.15
We hypothesized that some Jak and Stat proteins were involved in the transduction of activating or proliferation-inducing effects of IFN-γ in hematopoietic cells, whereas IRF-1's role was mainly inhibitory. Therefore, our experiments were designed to investigate intracellular events after binding of IFN-γ to its receptor in hematopoietic cell lines and primary cells to clarify the biologic effects resulting from activation of distinct signal transduction pathways.
MATERIALS AND METHODS
Cell culture. KG1a, human acute myelocytic leukemia cells, were obtained from American Type Tissue Culture Collection (Rockville, MD) and were maintained in Dulbecco's modified Eagle medium (DMEM; Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), 50 U/mL of penicillin, 50 μg/mL of streptomycin, and glutamine in a humidified atmosphere containing 5% CO2 . KG1a cells were selected for these experiments due to their resemblance to BM progenitor cells and their reliably uniform suppressive response to IFN-γ.
BM was obtained from healthy volunteers by aspiration from the posterior iliac crest. Informed consent was obtained according to a protocol approved by the Institutional Review Board of the National Heart, Lung and Blood Institute. Mononuclear BM cells (BMMNC) were isolated by lymphocyte separation medium (Organon, Durham, NC) and were resuspended in Iscove's modified Dulbecco's medium (IMDM; Life Technologies) supplemented with 20% FBS.
Retroviral vector construction and production. Human IRF-1 and -2 cDNA were cloned into the pG1XSvNa retrovirus vector.33 This vector can be used to express cDNA inserts driven by the Moloney murine leukemia virus long terminal repeat (LTR) and contains a selectable marker, neomycin phosphotransferase (NeoR), driven by the simian virus 40 promoter. Termination and polyadenylation signals reside in the 3′ LTR. pG1Na containing only LTR-driven NeoR gene was used as control. A 1,339-bp fragment containing entire coding region of IRF-1 cDNA was generated from plasmid pHIRF3128 by polymerase chain reaction (PCR) using Vent DNA polymerase (New England Biolabs, Beverly, MA) with primer pairs 5′-AACTGGAAGAATTCGCGGCCGCGGCCGTGCCCGGC3′ (Not I site italicized) as a forward and 5′-GTTTGCGGTCGTCGACGGCCTGCCAGGCCCTGAGA-3′ (Sal I site italicized) as a reverse primer. The amplification product was purified and digested with Not I and Sal I and then subcloned into the Not I-Sal I site of the pG1XSvNa vector yielding pG1IRF-1S. To obtain a virus containing IRF-1cDNA with antisense direction, a new primer 5′-CAAACGCCAGGTCGACGGCCGTGCCCGGCGGCCTT-3′ (Sal I site italicized) and the previously synthesized reverse primer were used. After Sal I digestion, the 1,333-bp PCR product was subcloned into the Sal I site of pG1XSvNa (pG1IRF-1AS). The Xba I-digested 1.3-kb fragment of pHIRF4S5134 coding for entire sequence of IRF-2 was subcloned into the Xba I site of pSPORT1 vector (Life Technologies), and vectors containing IRF-2 cDNA with sense direction were selected. After excision using Not I/SnaBI, the IRF-2 insert was subcloned into the Not I-SnaBI site of pG1XSvNa (pG1IRF-2S). The fidelity of all the vectors was confirmed by dideoxy sequencing (US Biochemical, Cleveland, OH).
Retroviral gene transfer vehicles were generated from the recombinant retroviral vector plasmids as previously reported.33 Briefly, the plasmid constructs were transfected by calcium phosphate coprecipitation into the GP + E86 ecotropic packaging cell line. The supernatants from transfected GP + E86 cells were harvested after 2 days and used to infect PA317 amphotropic packaging cell line. The transduced cells were then selected in G418-containing media (geneticin sulfate; GIBCO/BRL, Gaithersburg, MD) until individual G418-resistant colonies were formed. The virus-containing supernatants from individual colonies were collected, filtered, and analyzed for the presence of retroviral RNA. Viral RNA was extracted using polyethylene glycol precipitation and after serial dilution quantitated by slot blot hybridization using the pG1Na Nco I-Nco I fragment of NeoR gene. Clones producing high titer supernatants were expanded, genomic DNA was isolated, and Southern blot hybridization was performed to confirm the presence of unrearranged proviral genome. Biotiter was measured as the number of neomycin-resistant transducing particles using NIH3T3 cells. For pG1IRF-1S virus, the biotiter was 3 × 105 antibiotic-resistance transducing particles, and for pG1IRF-1AS, pG1IRF-2S, and control virus pG1Na, the biotiter were 6 × 105, 4 × 105, and 6 × 105 antibiotic-resistance transducing particles per milliliter of supernatants, respectively.
Gene transfer into KG1a cells. For transduction of KG1a cells, all virus stocks were used at comparable titers. KG1a cells at 2 × 105 cells/mL, in the logarithmic growth phase, were incubated with retroviral supernatants in the presense of protamine sulfate (5 μg/mL final concentration). After several rounds of transduction, cells were cultured for 2 days in DMEM. After 2 days of recovery, cells were resuspended at 2 × 105 cells/mL in the presence of active G418 (0.5 mg/mL) for 4 weeks. After successful selection, cultures of uninfected KG1a cells yielded no viable cells. Neomycin-resistant cells were maintained in media containing 0.35 mg/mL G418 for more than 1 month before further experimentation.
The effects of IFN-γ on the growth of virus-transfected or wild-type KG1a cells were assayed by plating cells at a density of 1 × 105 cells/mL in 24-well plates. Increasing concentrations of IFN-γ (Boehringer, Indianapolis, IN) were added, and, after 4 days of culture, cellular viability was assayed by trypan-blue dye exclusion test. Tests were performed in quadruplicate. In some experiments, the time-course of cell growth with or without IFN-γ was assessed.
Northern blotting was performed to determine IRF-1 expression levels in transduced KG1a cells. Cells were cultured with or without 2 × 103 IU/mL of IFN-γ for 18 hours, and total RNA was extracted using the RNeasy total RNA kit (Qiagen, Chatsworth, CA). Northern blotting was performed as described previously using both IRF-1 cDNA and β-actin probes.28 The intensity of the resulting bands was quantitated by PhosphorImager (Molecular Dynamycs, Sunnyvale, CA), in which variability among the samples was compensated based on the intensity of the β-actin band.
Immunoblot and immunoprecipitation. For immunoblot, after culture for 15 minutes with or without IFN-γ, cells were washed in phosphate-buffered saline (PBS) and lysed as described previously,28 except that the cell lysing buffer contained 1 mmol/L sodium orthovanadate, 0.5% sodium deoxycholic acid, and 10 mmol/L sodium pyrophosphate (all from Aldrich Chemical Co, Milwaukee, WI). To analyze IRF-1 and -2, cells were harvested after 18 hours of culture in the presence or absence of IFN-γ. Protein concentrations in cell lysates was measured by BCA protein assay reagent (Pierce, Rockford, IL) and the same quantity of each sample was electrophoresed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The following antibodies were used: rabbit polyclonal IRF-1 and -2 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal antimouse Stat1α (p91; Upstate Biotechnology, Lake Placid, NY), rabbit polyclonal anti–phospho-specific and nonspecific MAP kinase and Stat3 (New England Biolabs). Blots were developed using either enhanced chemiluminescence reagent (Amersham, Arlington Heights, IL) or 4-Chloro-1-Naphthol (CN)/3, 3′ Diamino Benzidine Tetrahydrochloride (DAB) substrate (Pierce).
For immunoprecipitation, 500 to 1,000 μg of protein lysates was precleared by protein A agarose (Pharmacia Biotec, Piscataway, NJ) at 4°C for 2 hours, and then mouse monoclonal antiphosphotyrosine antibody (clone 4G10; Upstate Biotechnology) was added; the mixture was rocked overnight at 4°C. Immune complexes were captured by protein A agarose at 4°C for 2 hours. Agarose beads were collected by centrifugation, washed 5 times with ice-cold lysing buffer, and boiled in 2× Laemmli SDS sample buffer, and then supernatants were applied to SDS-PAGE. The membrane was immunoblotted with mouse monoclonal anti-Stat1 antibody (Transduction Laboratories, Lexington, KT).
Kinase assay. Proteins were immunoprecipitated using rabbit polyclonal antirat MAP kinase R2 (Erk1-CT; Upstate Biotechnology). Immune complexes bound to protein A agarose beads were washed 3 times in ice-cold lysing buffer and 2 times by kinase buffer (20 mmol/L HEPES, pH 7.4, 5 mmol/L MgCl2 , 1 mmol/L dithiothreitol). MAP kinase activity was assayed using a kit (Upstate Biotechnology). Briefly, washed beads were incubated for 10 minutes at 30°C in 40 μL of assay dilution buffer (20 mmol/L MOPS, pH 7.2, 25 mmol/L β-glycerol phosphate, 5 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 1 mmol/L dithiothreitol) containing inhibitor cocktail (5 μmol/L protein kinase C inhibitor peptide, 0.5 μmol/L protein kinase A inhibitor peptide, and 5 μmol/L calmodulin-dependent kinase inhibitor compound R24571), 125 μmol/L ATP, 10 μCi of [γ-32P]ATP (3,000 Ci/mmol; Amersham), and 20 μg/mL of myelin basic protein (MBP). After brief centrifugation, 25 μL of supernatant was blotted on the P81 phosphocellulose paper, rinsed 10 times with 0.75% phosphoric acid (Aldrich), and once with acetone (Mallinckrodt Chemical, Paris, KT) in the beaker, and radioactivity was then measured in a scintillation counter. Blank protein A agarose was used to correct for nonspecific binding. All samples measurements were performed in triplicate.
Electrophoresis mobility shift assay (EMSA). Cells were lysed in low-salt buffer (10 mmol/L HEPES-KOH, pH 7.9, 10 mmol/L KCl, 2.5 mmol/L MgCl2 , 0.1 mmol/L EGTA, 2% NP40, 1 mmol/L sodium orthovanadate, 0.5% sodium deoxycholic acid, 10 mmol/L sodium pyrophosphate, 0.5 mmol/L dithiothreitol, 0.5 mmol/L 4-[2-aminoethyl]-benzenesulfonyl fluoride hydrochlorine [AEBSF ], 2 mg/mL pepstatin A, and 2 mg/mL leupeptin). Nuclei were pelleted by centrifugation and then lysed in high-salt buffer (10 mmol/L HEPES-KOH, pH 7.9, 420 mmol/L NaCl, 1.5 mmol/L MgCl2 , 0.1 mmol/L EGTA, 10% glycerol, protease inhibitors, and phosphatase inhibitors, as described above). After centrifugation, the protein concentration of supernatants was measured using BCA protein assay reagent, and nuclear extracts were stored at −70°C. The oligonucleotide sequences used as probes, derived from the sequence for the high-affinity serum-inducible element of the c-fos gene (m67SIE), were 5′-GTCGACATTTCCCGTAAATC-3′ and 5′-CGACGATTTACGGGAAATGT-3′.35 Oligonucleotide pairs were annealed and labeled with [α-32P]dCTP by fill-in reaction, and aliquots equivalent to 5 × 104 cpm or approximately 10 fmol were used per reaction. Binding reactions were performed in a total volume of 20 μL, containing 10 mmol/L Tris-HCl (pH 7.9), 200 mmol/L KCL, 1 mmol/L MgCl2 , 1 mmol/L EDTA, 5% glycerol, 0.1% Triton X-100, and 2 mmol/L dithiothreitol. Nuclear extracts (10 μg of protein) were preincubated for 20 minutes on ice with 1 mg of poly (dI-dC) (Pharmacia). As indicated, 1 pmol of cold oligonucleotides, rabbit polyclonal anti-Stat1α (p91) antibody (Upstate Biotechnology), or rabbit polyclonal antimouse Stat 3 (Santa Cruz) was added during the preincubation period. The samples were further incubated for 15 minutes at room temperature with probe and then electrophoresed through 4% polyacrylamide gels (37.5:1, acrylamide to bisacrylamide) in 45 mmol/L Tris borate buffer containing 1 mmol/L EDTA, pH 8.0. Gels were dried under vacuum and subjected to autoradiography.
Isolation of bone marrow CD34+ cells. CD34+ cells were separated using an affinity column (Cellpro, Bothell, WA), as previously described.28 Briefly, nonadherent BM cells were incubated at room temperature with biotinylated murine antihuman CD34 monoclonal antibody (MoAb; clone 12.8) and washed in PBS (Life Technologies) supplemented with 1% human albumin. Cells were applied to an affinity column containing avidin-coated beads, and the CD34+ cell fraction was eluted with PBS. An aliquot of eluted cells was stained with phycoerythrin (PE)-conjugated anti-CD34 HPCA-2 MoAb (Becton Dickinson, San Jose, CA) to assess purity; usually, 70% to 90% cells were CD34+.
Gene transfer into CD34+ cells and hematopoietic cell culture. Transduction of CD34+ cells was performed as previously described.4 33 Briefly, CD34+ cells were cultured at a density of 2 × 105/mL in IMDM with 20% FCS containing 20 ng/mL recombinant human IL-3, 100 ng/mL of human SCF, 50 ng/mL of human IL-6 (all from Amgen, Thousand Oaks, CA) and an equal volume of retroviral supernatants with comparable biotiter. Protamine sulfate (5 mg/mL) was added to increase transduction eficiency. Media was exchanged 3 times daily after the subsequent challenges for a total of 3 days. Simultaneously, mock-infected samples were prepared.
Colony formation by human hematopoietic progenitors was measured in standard methylcellulose cultures: CD34+ cells were resuspended in IMDM supplemented with FCS and mixed with methylcellulose (Stem Cell Technologies, Vancouver, British Columbia, Canada) containing 50 ng/mL IL-3, 20 ng/mL GM-CSF, 50 ng/mL SCF, and 2 U/mL erythropoietin (all from Amgen) with or without 1 × 103 IU/mL IFN-γ. CD34+ cells were plated at a density of 1 × 104 cells/mL of methylcellulose. Cultures were grown in a humidified atmosphere at 37°C and 5% CO2 . Colonies were counted on day 14. Experimental procedures were performed in triplicate in endotoxin-free plasticware and, according to the manufacturers' directions, the levels of endotoxin contamination in the cytokine preparations were 3 U/mg using the limulus assay.
To confirm successful transduction by retroviruses, DNA was isolated from pools of 3 to 5 colonies. Colonies were isolated from methylcellulose culture, resuspended in 50 μL of digestion buffer (5 mmol/L Tris-HCl, pH 8.0, 0.45% NP-40, and 0.45% Tween-20), and digested with proteinase K at 55°C for 1 hour. Samples were then heated at 99°C for 10 minutes and stored at 4°C. DNA-PCR, incorporating [α-32P]dCTP for labeling, was performed using 10 μL of sample with neomycin-specific primer pairs, 5′-ACAAGATGGATTGCACGCAG-3′ and 5′-CGCCAAGCTCTTCAGCAATA-3′, which generated products of 700 bp in length. Amplification reaction conditions were 95°C for 2 minutes (1 cycle), 95°C for 1 minute, 55 °C for 1 minute, and 72°C for 2 minutes (29 cycles), followed by extension at 72°C for 8 minutes. Samples were electrophoresed on 5% PAGE followed by autoradiography.
RESULTS
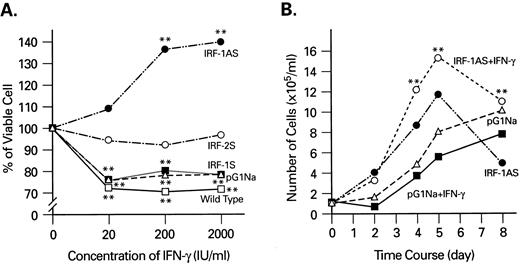
Effects of IFN-γ on KG1a cells retrovirally transfected with IRF genes. To separate the biologic effects of IFN-γ mediated by distinct intracellular signaling pathways, we first blocked the IRF-1 transduction cascade in KG1a cells by expression of IRF-1 antisense sequences. KG1a cells were transduced with IRF-1S (sense), IRF-1AS (antisense), and IRF-2S (sense) genes. The effects of IFN-γ on the growth of transduced cells were examined by trypan blue dye exclusion test (Fig 1A). Cells in the logarithmic phase of growth showed an initial viability of more than 95%. IFN-γ similarly suppressed the proliferation of both wild-type and pG1IRF-1S–transfected KG1a cells. Whereas pG1IRF-2S–transfected cells were resistant to IFN-γ at concentrations up to 2 × 103 IU/mL, pG1IRF-1AS–transduced cells showed a dose-dependent growth enhancement by IFN-γ. A time-course study of KG1a cells transfected with pG1IRF-1AS showed that 2 × 103 IU/mL of IFN-γ enhanced cell growth and prolonged cell survival (Fig 1B). These data suggested that IRF-1 mediated IFN-γ inhibition of KG1a cell growth. We concluded that, when IRF-1 expression was suppressed by an antisense retrovirus, IFN-γ can mediate stimulation of cell growth. Although IRF-2 overexpression diminished the suppressive effect of IFN-γ, this effect was not large enough to affect cell proliferation.
IFN-γ stimulates cell proliferation of IRF-1 antisense retrovirus-transfected KG1a cells. (A) Cells were stimulated with increasing concentration of IFN-γ, and the viable cell number was counted on day 4 of cultures. (B) Time course study of the effects of IFN-γ on the growth of pG1IRF-1AS–transfected and pG1NA-transfected (control) KG1a cells. Statistical analysis (t-test): **P < .01 when compared with the value without IFN-γ. Similar results were obtained in two independent experiments.
IFN-γ stimulates cell proliferation of IRF-1 antisense retrovirus-transfected KG1a cells. (A) Cells were stimulated with increasing concentration of IFN-γ, and the viable cell number was counted on day 4 of cultures. (B) Time course study of the effects of IFN-γ on the growth of pG1IRF-1AS–transfected and pG1NA-transfected (control) KG1a cells. Statistical analysis (t-test): **P < .01 when compared with the value without IFN-γ. Similar results were obtained in two independent experiments.
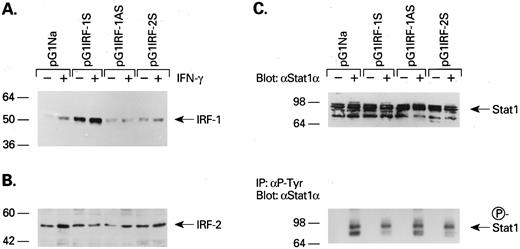
Expression of IRF-1 and -2 and Stat1 protein. Previous experiments have shown that IFN-γ increases IRF-1 levels. Blockade of IRF-1 expression resulted in reversal of the antiproliferative effect of IFN-γ. To determine the mechanism for the stimulatory IFN-γ effect, we next measured levels of IRF-1, IRF-2, and Stat1 proteins in response to IFN-γ in cells transduced with IRF-1AS sequence (Fig 2). Immunoblot and Northern blot showed that IRF-1 and -2 were constitutively expressed in KG1a cells at the protein (Fig 2A and B) and also mRNA levels (Table 1). Retroviral transduction of IRF-1 sequences increased constitutive and IFN-γ–enhanced expression levels of IRF-1, whereas both constitutive and inducible expression levels were significantly decreased in IRF-1AS virus-transfected cells (Fig 2A). Measurement of IRF-1 mRNA signal density in several autoradiograms showed similar results, with pG1IRF-1S virus increasing constitutive IRF-1 mRNA expression fourfold and inducible mRNA IRF-1 levels 1.5-fold, as compared with mock-transduced cells (Table 1). Although constitutive IRF-1 expression was not affected by pG1IRF-1AS transduction, IRF-1 expression induced by IFN-γ was decreased by about 60%. In agreement with previous experiments demonstrating abundant IRF-2 expression in hematopoietic cells and cell lines,28 IRF-2 levels were not significantly altered even in IRF-2S virus-transfected cells (Fig 2B). Stat1 was analyzed using anti-Stat1α MoAb. This antibody cannot distinguish phosphorylated form of Stat1α from nonphosphorylated form (Fig 2C; upper portion). Therefore, phosphorylation of Stat1α was analyzed using immunoprecipitation with antiphosphorylated tyrosine MoAb and Western blotting with anti-Stat1α MoAb. Our results showed that Stat1α protein, which mediates IFN-γ signaling into the nucleus, was also constitutively expressed in KG1a cells and its levels were not affected by transfected retrovirus. However, phosphorylated Stat1 was only detected after IFN-γ stimulation (Fig 2C).
Immunoblotting of IRF-1 (A), IRF-2 (B), and Stat1 (C; upper portion) protein in retrovirus transfected KG1a cells with or without IFN-γ stimulation. (C; lower portion) Cell lysates were immunoprecipitated by antiphosphotyrosine MoAb (4G10) and then immunoblotted by anti-Stat1α antibody.
Immunoblotting of IRF-1 (A), IRF-2 (B), and Stat1 (C; upper portion) protein in retrovirus transfected KG1a cells with or without IFN-γ stimulation. (C; lower portion) Cell lysates were immunoprecipitated by antiphosphotyrosine MoAb (4G10) and then immunoblotted by anti-Stat1α antibody.
Relative IRF-1 mRNA Expression Levels in Retroviraly Transfected KG1a Cells
| Type of Plasmid . | Relative Intensity (fold)* . | |||
|---|---|---|---|---|
| . | pG1Na . | pG1IRF-1S . | pG1IRF-1AS . | pG1IRF-2S . |
| Basal expression | 1 | 3.9† | 1.0 | 1.2 |
| Enhanced expression by IFN-γ | 6.6 | 9.7† | 4.1† | 8.3‡ |
| Type of Plasmid . | Relative Intensity (fold)* . | |||
|---|---|---|---|---|
| . | pG1Na . | pG1IRF-1S . | pG1IRF-1AS . | pG1IRF-2S . |
| Basal expression | 1 | 3.9† | 1.0 | 1.2 |
| Enhanced expression by IFN-γ | 6.6 | 9.7† | 4.1† | 8.3‡ |
Values represented are the average of three subsequent measurements and are shown as the fold increase when compared with expression levels in pG1Na-transfected KG1a cells. Variance was ±3%. Two independent experiments were performed. P value statistical analysis was performed using the t-test.
P < .01.
P < .05.
MAP kinase activity and Stat3 activation. To elucidate the mechanisms by which IFN-γ stimulated cell proliferation, activation of MAP kinase and Stat3 was analyzed. Stat3 and MAP kinase have been previously shown to be activated by a variety of cytokines that can mediate positive growth signals in hematopoietic cells.29,35 36 P42 MAP kinase was constitutively expressed in KG1a cells, and the levels of the phosphorylated form of the kinase were unaltered in retrovirus-transfected cells with or without IFN-γ stimulation (Fig 2A). This observation was confirmed by direct assessment of MAP kinase activity (data not shown). We used a nonphosphospecific anti-Stat3 antibody (upper portion) and a phosphospecific anti-Stat3 antibody (lower portion). Although Stat3 was also constitutively expressed in KG1a cells, phosphorylated Stat3 protein levels increased with IFN-γ exposure, regardless of IRF-1 expression (Fig 3B). Whereas Stat3 activation after IFN-γ stimulation was also observed in KG1a cells that were arrested by serum starvation for 48 hours, the phosphorylated form of MAP kinase was not detected by immunoblotting (data not shown). These results showed that IFN-γ activated Stat3 but not MAP kinase and that Stat3 activation was independent of IRF-1 expression.
Immunoblotting of nonphosphorylated (A; upper portion) and phosphorylated p42 MAP kinase (A; lower portion) and nonphosphosporylated (B; upper portion) and phosphorylated Stat3 (B; lower portion) protein in retrovirus-transfected KG1a cells with or without IFN-γ stimulation. Similar results were also obtained when MAP kinase activity in immunoprecipitates (by anti-MAP kinase MoAb) was also measured using MBP as a substrate (not shown).
Immunoblotting of nonphosphorylated (A; upper portion) and phosphorylated p42 MAP kinase (A; lower portion) and nonphosphosporylated (B; upper portion) and phosphorylated Stat3 (B; lower portion) protein in retrovirus-transfected KG1a cells with or without IFN-γ stimulation. Similar results were also obtained when MAP kinase activity in immunoprecipitates (by anti-MAP kinase MoAb) was also measured using MBP as a substrate (not shown).
IFN-γ enhanced formation of Stat1-Stat1 homodimer and also Stat1-Stat3 heterodimer. To confirm the results of immunoblotting showing that IFN-γ can mediate Stat3 activation, EMSAs were performed using an oligonucleotide probe containing a high-affinity target sequence for Stat proteins. Although three m67SIE binding activities were constitutively expressed in the nuclear extracts in the presence or absence of IFN-γ stimulation, IFN-γ induced a rapid assembly of two faster-migrating complexes regardless of the type of transfected vector (Fig 4). The bands shown the Fig 4B using anti-Stat1α and anti-Stat3 antibody demonstrate that the SIF complexes (SIF-A, SIF-B, and SIF-C) contain Stat1α and Stat3: Stat1-Stat1 homodimer (SIF-C), Stat1-Stat3 heterodimer (SIF-B), and Stat3-Stat3 homodimer (SIF-A). As reported previously, the slowest complex was identified as Stat3 homodimer, the middle complex as Stat1-Stat3 heterodimer, and the fastest one as Stat1 homodimer.37 38 All these complexes are formed by phophorylated Stat1α and Stat3. These sets of data are compatible with activation of Stat1α and Stat3 by IFN-γ. The putative Stat protein-DNA complexes were effectively competed by a 100-fold molar excess of unlabeled m67SIE probe (Fig 4A). In an effort to identify the Stat family members involved in this binding, nuclear extracts from pG1IRF-1AS transfected cells with or without IFN-γ stimulation were incubated with labeled m67SIE probe in the absence or presence of antibodies specific for Stat1α and Stat3 (Fig 4B). Anti-Stat1α antibody inhibited the major, fastest DNA binding activity induced by IFN-γ, producing the major supershifted band. Although the relatively low levels of the upper complex in the nuclei hindered the interpretation of antibody supershifting experiments, Stat3 antibody also produced the super-shifted band. Similarly, the formation of the middle m67SIE binding complex in nuclear extracts was blocked by anti-Stat1 and anti-Stat3 antibodies. We concluded that, although IFN-γ activates mainly Stat1-Stat1 homodimer formation, formation of the Stat1-Stat3 heterodimer can be enhanced by IFN-γ. EMSA results may appear in contrast to the Stat1α and Stat3 phosphorylation data shown in Fig 2C and 3B in which phosphorylated forms of Stat1α and Stat3 could be detected only when cells were stimulated by IFN-γ. However, the discrepancy might be due to the difference in the detection sensitivity of EMSA versus immunoblotting. This explanation is supported by the greater intensity of the bands corresponding to Stat proteins in comparison to those seen in immunoblotting.
EMSA using nuclear extracts from retrovirus-transduced KG1a cells cultured with or without IFN-γ (A). The nuclear extracts were incubated with or without competitor cold probe (100×). SIF, sis-inducible factor. (B) The nuclear extracts were incubated with or without normal rabbit serum (NRS) or anti-Stat1α or anti-Stat3 antibody. The arrows in the right side indicate the position of supershifted bands. This figure clearly shows three bands corresponding to SIF-A, SIF-B, and SIF-C. In IFN-γ–treated lanes, these bands were too strong to be clearly separated. However, these three lanes are clearly distinguishable in unstimulated lanes.
EMSA using nuclear extracts from retrovirus-transduced KG1a cells cultured with or without IFN-γ (A). The nuclear extracts were incubated with or without competitor cold probe (100×). SIF, sis-inducible factor. (B) The nuclear extracts were incubated with or without normal rabbit serum (NRS) or anti-Stat1α or anti-Stat3 antibody. The arrows in the right side indicate the position of supershifted bands. This figure clearly shows three bands corresponding to SIF-A, SIF-B, and SIF-C. In IFN-γ–treated lanes, these bands were too strong to be clearly separated. However, these three lanes are clearly distinguishable in unstimulated lanes.
IFN-γ enhanced hematopoietic colony formation by pG1IRF-1AS–transfected CD34+ cells. Genetically modified KG1a cells transduced with IRF-1AS sequences showed intrinsically activated MAP kinase and diminished IRF-1 expression. Experiments performed with these cells suggested that IFN-γ stimulation in the absence of IRF-1 might be a function of Stat1 and Stat3 activation. We attempted to validate these observations by experiments in primary hematopoietic stem and progenitor cells. Purified BM CD34+ cells were transfected with retroviral vectors and methylcellulose hematopoietic colony cultures were performed in a cocktail of growth factors with or without IFN-γ (Fig 5). IFN-γ consistently decreased myeloid colony growth to 50% of nontreated and control virus-transfected CD34+ cells (Fig 5A). Colony formation by pG1IRF-1S– and pG1IRF-2S–transfected CD34+ cells was significantly decreased compared with those by nontransduced cells and was not affected by the addition of IFN-γ. In contrast, IFN-γ dramatically enhanced colony formation by pG1IRF-1AS–transfected CD34+ cells. Similar but somewhat less pronounced effects were also observed for erythroid progenitor cells (data not shown). Successful transduction of retroviruses into hematopoietic cells was confirmed by PCR analysis of NeoR gene using DNA from pooled colonies (Fig 5B). Whereas IFN-γ exerts hematopoietic inhibition via IRF-1, upon blockade of IRF-1 pathway these negative effects of IFN-γ are not only reversed but the action of growth factors is further enhanced.
Effects of IFN-γ on the myeloid colony growth by retrovirus transduced BM CD34+ cells. (A) The number of myeloid colonies (colony-forming unit–granulocyte-macrophage [CFU-GM]) formed in the presence (▨) or absence (□) of IFN-γ. Statistical analysis (t-test): **P < .01 when compared with the value without IFN-γ. (B) DNA-PCR of NeoR and β-actin gene from the myeloid colonies formed. DNA was extracted from pooled colonies. Comparable results were obtained in two independent experiments.
Effects of IFN-γ on the myeloid colony growth by retrovirus transduced BM CD34+ cells. (A) The number of myeloid colonies (colony-forming unit–granulocyte-macrophage [CFU-GM]) formed in the presence (▨) or absence (□) of IFN-γ. Statistical analysis (t-test): **P < .01 when compared with the value without IFN-γ. (B) DNA-PCR of NeoR and β-actin gene from the myeloid colonies formed. DNA was extracted from pooled colonies. Comparable results were obtained in two independent experiments.
DISCUSSION
In the present study, bifunctional effects of IFN-γ on hematopoietic cell proliferation were observed in a cell line used as a model for hematopoietic progenitor cells and were confirmed in primary cultures of BM CD34+ cells. Under normal culture conditions, growth inhibition mediated by IFN-γ was dominant. When IRF-1 protein levels were suppressed by antisense IRF-1, IFN-γ acted as a growth factor. These stimulatory effects appeared to be mediated through tyrosine phosphorylation of Stat proteins, because IFN-γ did not alter the levels of constitutively activated MAP kinases but did enhance Stat1α/Stat3 activation and increase formation of Stat1-Stat3 heterodimers.
In our experiments, the level of IRF-1 expression and its decrease after IRF-1 AS-inhibition did not seem to exactly correlate as a modest decrease in the levels of IRF-1 corresponded to the marked change in the effects of IFN -γ. Other studies showed that the growth-inhibitory effects of IRF-1 can occur at very low levels of expression.39 At present, 5 members of the IRF family of transcription factors were identified (IRF-1, IRF-2, IRF-3, IRF-4, and ICSBP). Because all of these factors can bind to the same DNA sequence and it would be very difficult to examine IRF-1 transcription activity separately, we used the IFN-mediated proliferation inhibition as reporter function of IRF-1. In general, our current results were in agreement with those presented in the previous study performed with antisense-oligonucleotides, although the effects of IRF-1 antisense oligonucleotides were less pronounced.28
Our results have potential implications for understanding of the hematopoietic effects of IFN-γ. The intracellular activation pattern induced by IFN-γ may be, under certain conditions, synergistic with the action of hematopoietic growth factors such as IL-3, GM-CSF, or SCF.29,31,32 For example, IFN-γ has been shown to enhance growth factor-dependent leukemic cell proliferation.40 In primary cells, stimulatory effects of IFN-γ on the proliferation of BM progenitor cells obtained after chemotherapy or 5-fluorouracil treatment18,21 may be related to the specific activation status of regenerating cells. Similarly, increasing concentrations of growth factors can overcome IFN-γ–mediated inhibition of BM progenitor cells15 and prevent apoptosis,36 possibly due to their synergistic increase in the Stat1-Stat3 heterodimer formation outweighing the inhibitory signals of IFN-γ. These results suggest that a preexisting intracellular balance between different transduction pathways may influence the cellular response to IFN-γ. Alternatively, Stat activation may result in induction of genes that inhibit or promote proliferation. This theory is supported by the fact that IRF-1 itself is a Stat-responsive gene.41 Parallels can be drawn from IL-3–dependent cell lines, in which IRF-1 expression peaks in the growth-arrested period and declines in the initial growth phase.27 In contrast, the activated form of Stat proteins may be increased in cells induced to cycle by growth factors.29-32 Therefore, inhibition or stimulation of proliferation may depend on whether cells are initially growing or quiescent. In vitro, variable levels of hematopoietic inhibition by IFN-γ, depending on the tissue culture conditions such as concentration of serum and type of the growth factors, have been often observed8,15 and support this theory. However, it is also possible that other mechanisms influence the cellular response to IFN-γ. For example, in lymphocytes, differential expression of the IFN-γRβ chain determines whether IFN-γ delivers a proliferative or apoptotic signal.23 Such a mechanism can initiate activation of a specific cell growth-promoting (eg, Stat-mediated) or inhibitory signal (eg, IRF-1).
Whereas IRF-1 has been reported to have antioncogenic activity, IRF-2 demonstrated oncogenic effects, and both factors may affect cell cycling status.24,26,30 IRF-2 appears to antagonize the effects of IRF-1 and the transcription of IRF-1 is regulated by Stat1.41,42 Several mechanisms have been proposed to explain the inhibitory effects of IFN-γ in hematopoietic cells.28 We have previously shown that IFN-γ enhanced IRF-1 transcription in hematopoietic cells. IRF-1 can induce interleukin-1β converting enzyme (ICE) gene, a homologue of Caenorhabditis elegans cell death gene ced-3 causing apoptosis.43 IRF-1 and Stat1α are both required for the activation of double-stranded RNA (dsRNA)-activatable protein kinase (PKR), which activates the eukaryotic peptide chain initiation factor eIF-2 and impairs protein synthesis, and RNase L, which also exerts antiproliferative activity.44,45 Similarly, Stat1 can induce the p21WAF1/CIP1 gene, a cyclin-dependent kinase inhibitor, which blocks cell cycle progression.30 These reports have shown that IRF-1, as well as Stat1,46 are likely to be relevant mediators of IFN-γ–induced cell growth inhibition. Our study addresses also a number of controversial issues with regard to IFN-γ–mediated signal transduction pathways.
First of these issues is the relationship between IFNs and the activation of MAP kinases. Maximal transcription activity of Stat1 and Stat3 also requires, in addition to tyrosine phosphorylation, serine phosphorylation. Serine residues are likely targets of MAP kinases.35,47 However, in our experiments, a shift to the slow migrating (serine-phosphorylated) form of Stat3 has not been observed while a constitutive expression of MAP kinase was present. Although IFNs do not affect the Ras-pathway involved in MAP kinase activation,48 IFN-α/β–activated Stat1α coimmunoprecipitates with p42 MAP kinase, and the kinase activity of MAP is required for IFN-β–stimulated gene transcription.49 Several other cytokines and growth factors activate MAP kinase as well as the Jak-Stat pathways, and Jak2 was reported to represent a common component in the activation of p42 MAP kinase and Stat signaling.50 Therefore, whether IFN-γ can directly activate MAP kinase is important for the understanding of IFN-γ signal transduction. Our findings clearly showed that IFN-γ does not affect the levels of p42 MAP kinase activity as measured by direct and indirect assays in these cells. Because of the constitutive activation of MAP kinase in KG1a cells, we cannot rigorously exclude marginal effects on activity and subsequent phosphorylation of serine residues of Stat proteins. However, consistent with our results, MAP kinase activation has been only reported in IFN-γ–treated monocytes.51 Furthermore, constitutive activation of MAP kinase in KG1a cells may be an intrinsic property of parental KG1 cells, because constitutive activation of Shc, a protein upstream of MAP kinase, was reported in this cell line.50
Second, whether IFN-γ can activate Stat3 is also controversial. Stat1-Stat3 heterodimers have stronger transcriptional activity than either Stat1-Stat1 or Stat3-Stat3 homodimers.52 IFN-γ was previously believed to activate only Stat1, but Stat3 recently has been shown to be upregulated in human hepatoma cells and activated in mouse 3T3 fibroblasts.37,5354 IFN-α, in addition to activation of Stat1, Stat2, and ISGF-3-γ, also activates Stat3.37 We show here that IFN-γ can activate Stat3 and induce Stat3-Stat1 heterodimer formation. In this respect, our results differ from previous reports showing that IFN-γ could not activate Stat3.
The study of transduction pathways in autonomously growing cell lines has obvious limitations. Stat proteins and MAP kinase may not always be constitutively expressed in primary cells. The levels of these proteins depend on several factors, including maturation and type of the cell. However, the validity of some of the results generated using cell line KG1a was confirmed in primary hematopoietic cells. Although NeoR gene expression indicated successful transduction of IRF-1AS into progenitor cells, due to the high numbers of cells required for immunoblotting or electrophoretic mobility shift assay, these assays were not feasible in transfected CD34+ cells. Similarly, mobility shift assays are technically difficult to perform in primary CD34+ cells.
We showed that IFN-γ can, upon blockade of the IRF-1 pathway, enhance the proliferative effects of IL-3, SCF, GM-CSF, and erythropoietin used to induce colony formation by CD34+ cells. In previous reports, we have shown that both IRF-1 and -2 were expressed in CD34+ cells; antisense oligonucleotide targeted to the translation initiation site of IRF-1 gene partially abrogated inhibition of hematopoiesis by IFN-γ in these cells.28 Most likely, while IRF-1 expression was inhibited, activation of MAP kinase and Stat3 could have been activated by growth factors used in vitro. Although with antisense oligonucleotides only diminution of the inhibitory effects of IFN-γ was observed, transduction of full-length IRF-1 antisense gene resulted not only in the reversal of inhibitory activity of but also in enhancement of proliferation by IFN-γ. The difference between the activity of antisense oligonucleotide and retroviral transduced antisense mRNA for IRF-1 may be due to the specific pharmacologic features of the synthetic DNA fragment in terms of affinity, half life, and uptake of antisense oligonucleotides. However, both systems show that IRF-1 mediates the inhibitory activity of IFN-γ and decreased IRF-1 levels result in resistance to IFN-γ.
In conclusion, our results supply molecular mechanism for both negative and positive effects of IFN-γ on hematopoietic cell proliferation. If IRF-1 is expressed, proliferation is suppressed, perhaps through downstream effects on genes known to be toxic to cells, such as inducible nitric oxide synthase. The responsiveness of particular cell types to IFN-γ or growth factors under specific culture conditions may be dependent on the constitutive or inducible levels of IRF-1 in the target cell or the level of activation of alternative, common signal transduction pathways for cell proliferation. IFN-γ's effects on hematopoiesis in humans may also depend on these variables.
ACKNOWLEDGMENT
The authors are grateful to Prof T. Taniguchi for providing the IRF-1 and -2 plasmids and to Amgen for generously providing some of the cytokines.
Address reprint requests to Jaroslaw P. Maciejewski, MD, Department of Microbiology, Howard Medical Bldg 320, University of Nevada, School of Medicine, Reno, NV 89557-0046.



![Fig. 5. Effects of IFN-γ on the myeloid colony growth by retrovirus transduced BM CD34+ cells. (A) The number of myeloid colonies (colony-forming unit–granulocyte-macrophage [CFU-GM]) formed in the presence (▨) or absence (□) of IFN-γ. Statistical analysis (t-test): **P < .01 when compared with the value without IFN-γ. (B) DNA-PCR of NeoR and β-actin gene from the myeloid colonies formed. DNA was extracted from pooled colonies. Comparable results were obtained in two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/12/10.1182_blood.v90.12.4749/3/m_bl_0029f5.jpeg?Expires=1769078633&Signature=2pRVnLTa5gbkcaljosbMXEISPOFQvc6wBMKQbt0xW4BfWgtYmm5jOy5PyNMtgCgPfGo4LQaJJMY5~MxpVGqjogr2eQ-aUV613FFWbVMv3fuzGLx2yxQG4XJRD5dhkyfH16j3q20Ud0O-Dd87tdtEfOU4XEIwUxOF05mWKMany8XBgddwUD0wskHW~Z2W8TAFcqLg4AzxWLs24IgrvtWG7tU0MMlNflGB9IBJAcmp1ixOmDPYZryegpNe9tEUt3w2nQnLHO3pjmKWAQCVDSUbiM0OVdhVOmLk1iHQBLBXaygUHXHQ1Y53necC4SUf~dX0vlLur5apgFXFEOG-qU2dpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal