Abstract
We previously reported that the abl promoter (Pa) undergoes de novo DNA methylation in the course of chronic myelocytic leukemia (CML). The clinical implications of this finding are the subject of the present study in which samples of CML patients, including a group treated with interferon α (IFNα) were surveyed. The methylation status of the abl promoter was monitored by polymerase chain reaction (PCR) amplification of the Pa region after digestion with several site-methylation sensitive restriction enzymes. Some 74% of the DNA samples from blood and marrow drawn in the chronic phase were nonmethylated, similar to control samples from non-CML patients. The remaining 26% were partially methylated in the abl Pa region. The latter samples were derived from patients who were indistinguishable from the others on the basis of clinical presentation. Methylated samples were mostly derived from patients known to have a disease of longer duration (26 months v 7.5 months, P = .01). Samples of 30 IFNα-treated patients were sequentially analyzed in the course of treatment. Fifteen patients with no evidence of Pa methylation before treatment remained methylation-free. The remainder, who displayed Pa methylation before treatment, reverted to the methylation-free status. The outcome is attributed to IFNα therapy, as the Pa methylation status was not reversed in any of the patients treated with hydroxyurea. Methylation of the abl promoter indicates a disease of long-standing, most likely associated with a higher probability of imminent blastic transformation. It appears to predict the outcome of IFNα therapy far better than the cytogenetic response.
CHRONIC MYELOCYTIC leukemia (CML) is a fatal, clonal stem-cell disorder characterized by a triphasic course that reflects the malignant evolution. In the initial, chronic phase, which often develops insidiously, there is an increased pool of committed myeloid progenitor cells whose differentiation remains uncompromised.1 The chronic phase lasts for variable lengths of time, typically about 3 years. The disease then progresses, sometimes through an accelerated phase, to an acute leukemic or blast crisis phase with a rapidly fatal outcome.2 Transformation appears to occur randomly: the actual time that it will occur for any individual patient cannot be predicted.3 Survival in CML is essentially determined by time to transformation. The annual transformation/death rate within the first 2 years is low, increases to 25% by the third year, and remains fairly constant in subsequent years, with survival determined mainly by the intrinsic biology of the disease.3
Several groups have constructed models that include a series of parameters to divide patients with CML into low-risk, intermediate-risk, and high-risk groups.3-5 The International CML Prognosis Study Group identified age, spleen size, platelet count, and percentage of blasts at diagnosis as features with prognostic significance. This model was tested and proved successful in categorizing patients according to three groups. The proportion of patients surviving more than 2 years from diagnosis in the high-, intermediate-, and low-risk groups was 70%, 80%, and 93%, respectively.4 Other studies suggested to use the response to initial treatment as a prognostic tool.6 In practice, individual assessment of prognosis solely according to clinical features is problematic.5
Whereas the initiating event in CML is well established: generation of the bcr/abl hybrid gene and consequent expression of the p210BCR/ABL oncoprotein,7-9 genetic factors associated with disease progression to the blast crisis, are far less defined.10-15 Furthermore, none of the latter genetic aberrations reported in CML precede blastic transformation and, therefore, cannot predict this outcome.12 In contrast, we have observed a common epigenetic event in CML, CpG-rich islands (CpG) methylation at several sites in the proximal abl promoter (Pa). This process was evident in blast crisis samples and cell lines, but could also be found in certain chronic phase samples.16 Analysis of a small series of patients followed through the chronic stage to the blast crisis showed that whereas DNA samples from the chronic phase were mostly methylation-free, corresponding blast crisis samples were methylated.
On the basis of these initial observations, we argued that, due to its progressive nature, DNA methylation may serve as a marker for tumor progression in CML.16 Validation of this hypothesis is of major importance in timing bone marrow transplantation (BMT), which although potentially curative in the chronic phase, is generally ineffective once the disease has entered the blast crisis.17 Many CML patients can also benefit from interferon (IFN)α therapy: about 70% of patients treated with IFNα show a good hematologic response and 22% to 50% achieve a significant cytogenetic response. Furthermore, most randomized trials showed prolonged survival in IFNα-treated patients.18-20 Nevertheless, IFNα treatment is recommended only for patients who are not candidates for BMT, mainly because a significant proportion of CML patients do not respond to the drug and are, therefore, at risk of progressing to blast crisis.20 If there was a tool for monitoring advance of the disease in this phase, IFNα therapy could be initiated as the treatment of choice, while BMT, with its attendant risks, would be reserved for patients showing disease progression despite IFNα treatment.
To evaluate the feasibility of abl promoter methylation as a marker of tumor progression in the chronic phase, we investigated the Pa methylation status in a large series of samples from patients at this stage of the disease. Furthermore, we initiated a prospective study of IFNα-treated patients to examine the effect of treatment on Pa methylation parallel to the clinical response.
MATERIALS AND METHODS
Patients. We obtained 201 bone marrow or peripheral blood samples from 99 Ph positive, bcr/abl positive, CML patients, two thirds of which were sent for routine molecular analysis (bcr/abl ). The remainder were referred from five institutions from different countries for analysis of tumor progression. The patients' ages ranged from 8 to 74 years (mean, 35.5 years); 54% were men and 46% were women. A total of 148 samples were drawn from 69 patients in the chronic phase, 23 samples from 17 patients in the accelerated phase, and 30 samples from 25 patients in blast crisis. Disease stage was determined according to the criteria of the Bone Marrow Transplant Registry for classification of CML phases.21 The chronic phase was defined according to the following criteria: no significant symptoms (after treatment), none of the features of accelerated or blastic phase, particularly no chromosomal changes other than the Ph′ chromosome. Accelerated phase: rapid doubling of white blood cell (WBC) count, >10% blasts in blood or marrow, >20% blasts and promyelocytes in blood and marrow, >20% basophils and eosinophils in blood, anemia or thrombocytopenia, persistent thrombocytosis, additional chromosomal changes, increased splenomegaly, development of chloromas or myelofibrosis. Blastic phase: >30% blasts and promyelocytes in the blood or bone marrow. Using the Sokal index,3 4 the relative risk group was determined for 39 chronic phase patients with methylation pattern A for which samples were obtained on diagnosis. Sequential samples were obtained from 63 patients: 39 patients contributed two sequential samples, 14 patients, three samples; 7 patients, four samples; 1 patient had five samples; and 2 patients, six samples. The time intervals between samples were 3 to 24 months.
Twelve bone marrow control samples were obtained from 8 normal bone marrow donors, 2 non-Hodgkin's lymphoma patients, 1 multiple myeloma patient, and 1 with Hodgkin's disease.
IFNα therapy. Among the patients treated with IFNα, serial samples were obtained from 30 patients. Eleven patients contributed a first sample before treatment; the rest were sampled for the first time within 3 to 20 months (average, 8.5 months) of initiation of therapy. The second sample was obtained on an average of 10 months later. Twenty-five patients were first sampled in the chronic phase, five in the accelerated phase. The cytogenetic response is defined by more than 30% Ph′ negative cells.
Methylation assay. Most of the methylation assays were performed on bone marrow cells. Only if the WBC count was more than 100 × 109/L was peripheral blood used. We found no differences in methylation patterns when simultaneous blood and marrow samples from the same patients were analyzed.
In probing for CpG island methylation, we took advantage of several site-methylation-sensitive enzymes. After enzymatic digestion of the genomic DNA, the samples were amplified by polymerase chain reaction (PCR). The enzymes are unable to cleave DNA at sites of methylation and a PCR product is generated. On the other hand, nonmethylated DNA is cut and, therefore, there is no PCR product.
Peripheral blood or bone marrow cells were isolated on a Ficoll-Hypaque density gradient. A 1-μg quantity of genomic DNA was digested with 30 U of one of the following enzymes: BamHI, Hpa II, Msp I, Sac II, or Bgl II for 6 hours in 40 μL aliquots. To ensure complete cleavage, an additional 20 U of enzyme was added and digestion was allowed to proceed in a total volume of 70 μL for an additional 16 hours. The reaction products were concentrated and purified using Microcon-100 microconcentrators (Amicon, Inc, Beverly, MA). A total of 5 μL (≈400 ng DNA) of the concentrated product was added to 50 μL of a PCR mixture containing: all four dNTPs (550 μmol/L each), 10 mmol/L Tris HCL (pH 9), 50 mmol/L KCl, 1.5 mmol/L MgCl, Triton x-100 0.1%, bovine serum albumin (BSA) or gelatin 0.2 mg/mL, and 10% (vol/vol) dimethyl sulfoxide. A total of 10 pmol of the antisense primer Pa3 (5′ccagataacagctggaggac3′) and the sense primer Pa5 (5′ctccgggccctttgttaaca3′) was added. The reaction mixture was preheated for 10 minutes at 98°C and then 1 U of Taq DNA polymerase was added at 86°C, followed by 40 cycles of 1 minute at 94°C, 1 minute at 56°C, and 1 minute at 72°C, terminated by 10 minutes at 72°C, in a thermocycler. The amplified product was separated by electrophoresis in 2% D-1 agarose gel, and the bands were visualized with ethidum bromide. The person who performed the assay was blind to the stage of the disease.
Statistical analysis. The difference in mean months since diagnosis was assessed using the t-test. The proportion of cases displaying reversed methylation was compared in IFNα versus hydroxyurea-treated patients using the χ2 test. A P value of <.05 was considered significant.
RESULTS
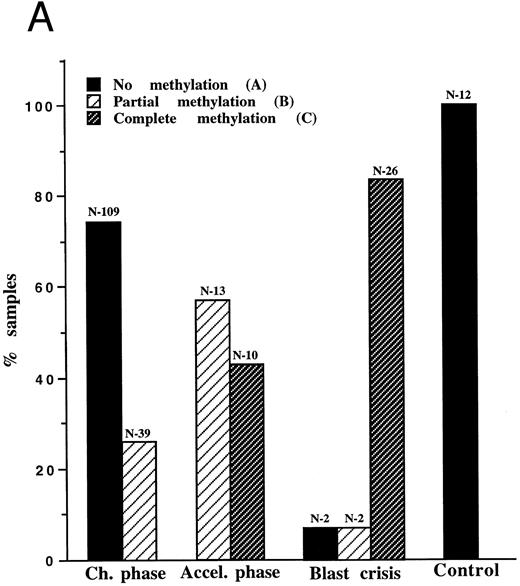
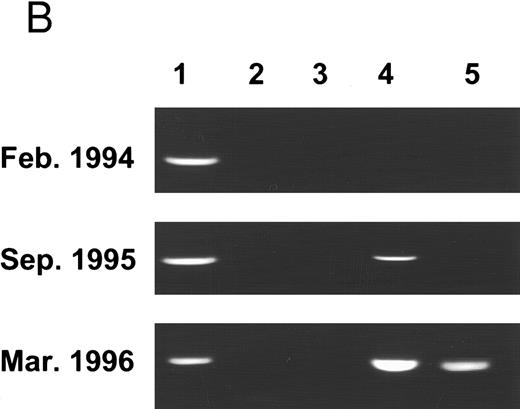
Molecular follow-up of CML according to methylation of the abl promoter (Pa). A total of 201 samples from 99 CML patients underwent molecular analysis according to the previously defined methylation status16: Pattern A, no evidence of Pa methylation; pattern B, partial methylation, reflected by Sac II resistance; pattern C, total methylation, reflected by both Sac II and Hpa II resistance (Fig 1A). As in the previous study, the vast majority (93%) of samples from the blast crisis showed evidence of Pa methylation, mostly pattern C (complete methylation). Two blast crisis patients displayed pattern B, and the other two were classified as pattern A. The latter was distinguished by a lymphatic common acute lymphoblastic leukemia antigen (CALLA)-positive, P210 bcr/abl, blast crisis. Samples from accelerated phase patients were invariably methylated, exhibiting pattern B or C. In an attempt to determine whether pattern C is associated with a more advanced stage of the accelerated phase, we compared the laboratory parameters of the samples with the methylation status. Yet, none of the parameters were significantly more severe in the C samples (data not shown), indicating that the methylation status is not a simple reflection of the frequencies of blood cell populations. Of the 148 samples drawn in the chronic phase, 74% were nonmethylated, like the 12 control samples from normal and non-CML patients. In 13 patients, samples were obtained from both the chronic phase and blast crisis (see representative examples in Fig 1B). In 11 of these patients, the methylation status evolved from pattern A to B or C, or from pattern B to C. Only two of the 13 patients maintained the same methylation pattern over the 9-month study period.
(A) Pa methylation analysis in different phases of CML: 201 samples of 99 CML patients and 12 samples of non-CML patients underwent molecular analysis. The bars represent the ratio of samples characterized by patterns A, B, or C in each phase of the disease. The numbers (N) above the bars indicate the number of samples of a given pattern. (B) PCR analysis of Pa methylation of an individual patient in different phases of the disease: genomic DNA was cut with BamHI (1), Msp I (2), Bgl II (3), Sac II (4), and Hpa II (5). After digestion, samples were amplified by PCR and analyzed on ethidium-bromide agarose gel. On diagnosis, the patient displayed pattern A. Nineteen months later, the patient was still in the chronic phase, but the Sac II site was already methylated (pattern B). After an additional 5 months, the patient was in blastic crisis with evidence of methylation at both the Sac II and the Hpa II sites (pattern C).
(A) Pa methylation analysis in different phases of CML: 201 samples of 99 CML patients and 12 samples of non-CML patients underwent molecular analysis. The bars represent the ratio of samples characterized by patterns A, B, or C in each phase of the disease. The numbers (N) above the bars indicate the number of samples of a given pattern. (B) PCR analysis of Pa methylation of an individual patient in different phases of the disease: genomic DNA was cut with BamHI (1), Msp I (2), Bgl II (3), Sac II (4), and Hpa II (5). After digestion, samples were amplified by PCR and analyzed on ethidium-bromide agarose gel. On diagnosis, the patient displayed pattern A. Nineteen months later, the patient was still in the chronic phase, but the Sac II site was already methylated (pattern B). After an additional 5 months, the patient was in blastic crisis with evidence of methylation at both the Sac II and the Hpa II sites (pattern C).
Whereas pattern A was virtually confined to the chronic phase (97% specificity; likelihood ratio, 24), pattern C was specific to blast crisis, and/or the accelerated phase (100% specificity; likelihood ratio of infinity). Hence, the two extreme patterns (A and C) are reliable markers for the chronic phase and transformed phases, respectively.
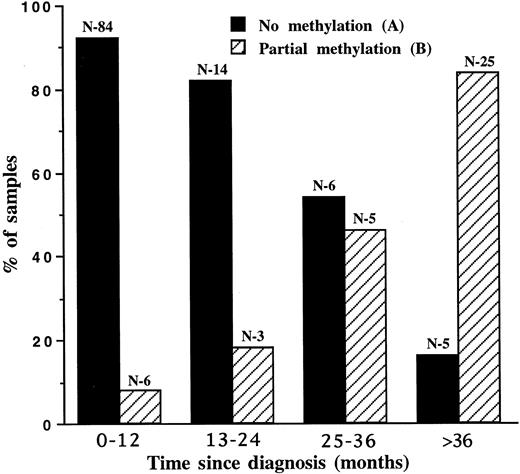
Partial methylation in the chronic phase indicates a disease of longer standing. Clinical parameters and the time since diagnosis were compared in pattern B chronic patients versus pattern A patients (Table 1). Pattern B patients were indistinguishable from pattern A patients on the basis of clinical presentation, particularly parameters indicative of a poor prognosis. There was no correlation between the methylation pattern and the breakpoint site within the bcr gene. The frequencies of methylation pattern B were 29% and 25% in the b2/a2 and b3/a2 translocation types, respectively. The only difference between the two groups was the time interval between diagnosis and sampling, with a mean of 13.9 months for pattern A and 29.3 months for pattern B patients (P = .01). In an attempt to find a correlation between the methylation pattern and prognostic risk group based on clinical presentation, we analyzed 39 patients with methylation pattern A according to the Sokal index.3,4 The distribution of the three prognostic groups among our patients was not different from the distribution of nonselected chronic phase patients in the prospective study by the Italian Cooperative Study Group on CML4 (Table 2). Hence, pattern A represents a parameter independent of the “good risk” Sokal index.3 4 The distribution of methylation patterns A and B was plotted against the time elapsed since diagnosis (Fig 2). On analysis of the patients' first samples, pattern B was significantly associated with a disease duration of more than 24 months (P < .0001). Although most samples displayed pattern A at diagnosis, within 2 to 3 years postdiagnosis, the predominant pattern was B. Hence, pattern B is associated with a longer duration of disease.
Partial Methylation is Correlated With Elapse of Time Since Diagnosis
| . | Pattern A . | Pattern B . | P . |
|---|---|---|---|
| . | N = 109 . | N = 39 . | . |
| Age (years) at Dx | 33.5 ± 12.2 | 35.7 ± 17.6 | .219 |
| WBC (109/L) | 32.5 ± 39.4 | 48.3 ± 72.5 | .231 |
| Platelets (109/L) | 468 ± 352 | 489 ± 348 | .223 |
| Blasts in BM (%) | 2.3 ± 2.6 | 2.9 ± 3.2 | .339 |
| BM Basophils and Eosinophils (%) | 6.1 ± 5.4 | 6.6 ± 3.4 | .261 |
| Spleen size (cm below costal margin) | 2.5 ± 4.3 | 4.2 ± 5.2 | .321 |
| Time since Dx (months) | 13.9 ± 18.5 | 29.3 ± 33.8 | .010 |
| Median-7.5 | Median-26 |
| . | Pattern A . | Pattern B . | P . |
|---|---|---|---|
| . | N = 109 . | N = 39 . | . |
| Age (years) at Dx | 33.5 ± 12.2 | 35.7 ± 17.6 | .219 |
| WBC (109/L) | 32.5 ± 39.4 | 48.3 ± 72.5 | .231 |
| Platelets (109/L) | 468 ± 352 | 489 ± 348 | .223 |
| Blasts in BM (%) | 2.3 ± 2.6 | 2.9 ± 3.2 | .339 |
| BM Basophils and Eosinophils (%) | 6.1 ± 5.4 | 6.6 ± 3.4 | .261 |
| Spleen size (cm below costal margin) | 2.5 ± 4.3 | 4.2 ± 5.2 | .321 |
| Time since Dx (months) | 13.9 ± 18.5 | 29.3 ± 33.8 | .010 |
| Median-7.5 | Median-26 |
Clinical parameters and time since diagnosis were compared in pattern B chronic phase samples versus pattern A samples. One hundred nine samples were derived from methylation-pattern A patients; 39 samples from pattern B patients. We incorporated all samples including multiple samples from any particular patient taken in the chronic phase. The clinical parameters (aside from age) relate to the number of samples.
Abbreviations: Dx, diagnosis; WBC, white blood cells.
Classification of Chronic Phase Patients According to Sokal Index
| Rsk . | Pattern A Patients (%) . | Italian Study Group (%) . |
|---|---|---|
| . | N = 39 . | N = 508 . |
| I <0.8 | 36 | 31.5 |
| II-0.8-1.2 | 43 | 41.5 |
| III >1.2 | 21 | 27 |
| Rsk . | Pattern A Patients (%) . | Italian Study Group (%) . |
|---|---|---|
| . | N = 39 . | N = 508 . |
| I <0.8 | 36 | 31.5 |
| II-0.8-1.2 | 43 | 41.5 |
| III >1.2 | 21 | 27 |
Distribution of Pa methylation patterns during the chronic phase. A total of 148 samples were drawn in the chronic phase. The bars represent the percentage of each pattern at a given time (months) following diagnosis. The numbers (N) above the bars indicate the number of samples of a given pattern.
Distribution of Pa methylation patterns during the chronic phase. A total of 148 samples were drawn in the chronic phase. The bars represent the percentage of each pattern at a given time (months) following diagnosis. The numbers (N) above the bars indicate the number of samples of a given pattern.
Reversed Pa methylation during IFNα, but not hydroxyurea treatment. A total of 60 samples from 30 patients treated with IFNα were analyzed sequentially during treatment, at a mean interval of 9.9 ± 6.6 months (Table 3). Fifteen patients who displayed pattern A on initial sampling remained methylation-free. More significantly, 12 of the remaining 14 patients, who displayed pattern B on first sampling, reverted to pattern A during treatment (Table 3). A single patient who was in the accelerated phase and displayed pattern C, reverted to pattern B within the surveillance period (Table 3) and later, to pattern A (Fig 3). Interestingly, among the 15 patients who reverted to a less advanced methylation pattern, only three showed a cytogenetic response. Methylation reversal can most probably be attributed to IFNα treatment, as this phenomenon was not recorded among hydroxyurea-treated patients during a similar surveillance period. In fact, there was evidence of progressive methylation in 37% of such patients during this interval (Table 3). On initial sampling, the hydroxyurea-treated group was not different from the IFNα-treated group on the basis of clinical parameters. The reason they received hydroxyurea was either because of refusal to be treated with IFNα (6 patients) or due to insurance problems (10 patients).
Reversed Pa Methylation After IFNα, but not Hydroxyurea Treatment
| . | abl Methylation . | Time Interval Between . | ||
|---|---|---|---|---|
| . | First Sample . | Second Sample Pattern/ . | Cytogenetic Response . | Samples (months) . |
| . | Pattern/ . | No. of Patients . | . | . |
| . | No. of Patients . | . | . | . |
| IFNα | ||||
| A | 15 | A 15 | 5/15 | 9.1 ± 4.3 |
| B 0 | ||||
| A 12 | 3/12 | 10.8 ± 6.5 | ||
| B | 14 | B 1 | ||
| C 1 | ||||
| C | 1 | B 1 | ||
| HU | ||||
| A 8 | 0/12 | 11.0 ± 4.5 | ||
| A | 12 | B 2 | ||
| C 2 | ||||
| A 0 | 0/4 | 13.6 ± 4.6 | ||
| B | 4 | B 2 | ||
| C 2 | ||||
| . | abl Methylation . | Time Interval Between . | ||
|---|---|---|---|---|
| . | First Sample . | Second Sample Pattern/ . | Cytogenetic Response . | Samples (months) . |
| . | Pattern/ . | No. of Patients . | . | . |
| . | No. of Patients . | . | . | . |
| IFNα | ||||
| A | 15 | A 15 | 5/15 | 9.1 ± 4.3 |
| B 0 | ||||
| A 12 | 3/12 | 10.8 ± 6.5 | ||
| B | 14 | B 1 | ||
| C 1 | ||||
| C | 1 | B 1 | ||
| HU | ||||
| A 8 | 0/12 | 11.0 ± 4.5 | ||
| A | 12 | B 2 | ||
| C 2 | ||||
| A 0 | 0/4 | 13.6 ± 4.6 | ||
| B | 4 | B 2 | ||
| C 2 | ||||
Two consecutive samples were drawn from patients treated with IFNα with or without hydroxyurea or hydroxyurea alone and assayed for Pa methylation. The cytogenetic response (>30% Ph′ negative cells) was recorded at the time of second sampling, two patients did not undergo second cytogenetic analysis. The mean interval between consecutive sampling is indicated.
Abbreviation: HU, hydroxyurea.
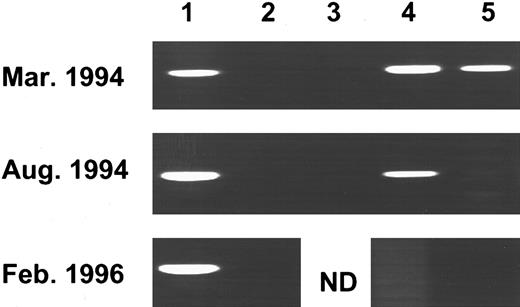
PCR analysis of Pa methylation of an individual patient before and during IFNα treatment. Genomic DNA was cut with BamHI (1), Msp I (2), Bgl II (3), Sac II (4), and Hpa II (5) and processed for the Pa methylation assay (see Fig 1B). The patient diagnosed in the accelerated phase was classified as pattern C. Six months later, under IFNα therapy, pattern B was evident, and 23 months after diagnosis, the methylation pattern was A.
PCR analysis of Pa methylation of an individual patient before and during IFNα treatment. Genomic DNA was cut with BamHI (1), Msp I (2), Bgl II (3), Sac II (4), and Hpa II (5) and processed for the Pa methylation assay (see Fig 1B). The patient diagnosed in the accelerated phase was classified as pattern C. Six months later, under IFNα therapy, pattern B was evident, and 23 months after diagnosis, the methylation pattern was A.
Therefore, IFNα, in contrast to hydroxyurea, has a profound effect on Pa methylation: it either delays further methylation or reverses the process, regardless of the extent of cytogenetic response. This is particularly significant, as it has never been encountered among non–IFNα-treated CML patients. It should be noted that a considerable number of samples analyzed for Pa methylation at the chronic phase (Fig 2), were obtained from patients who had been treated with IFNα. Thus, an association between disease duration and Pa methylation was evident, despite the IFNα effect.
DISCUSSION
Epigenetic alterations are often encountered in cancer, some of them characteristic of an advanced stage. De novo DNA methylation is one of the most common epigenetic phenomena and has recently been recognized as an important factor in malignancy.22-29 While analyzing the function of the abl promoters in CML, we found that the CpG island associated with the proximal c-abl promoter Pa, is abnormally methylated in CML cell lines and in samples from CML patients.19
De novo methylation of the abl Pa promoter may represent a significant step in the evolution of the leukemia. Whereas the CpG islands of normal cells are invariably methylation-free,23,25 those associated with several tumor suppressor genes (Rb, p16, and Von Hippel-Lindau) are often subject to de novo methylation and, consequently, the tumor suppressor genes are silenced.26-29 Cumulative evidence indicates that c-abl has a growth inhibitory effect.30 31 The observed DNA methylation may act to repress it during the chronic phase, thereby facilitating blastic transformation of Ph′ positive cells.
Irrespective of its biologic significance, the extent of Pa methylation appears to be a useful indicator for tumor progression in CML. This feature is particularly important in the chronic phase of the disease, as there is no molecular parameter that reflects tumor progression at that stage. Several other studies reported methylation changes in CML.32-34 However, none of these, in contrast to Pa methylation, was found to evolve at the chronic stage.
Although we have detected Pa methylation in some patients at the time of diagnosis, it was encountered significantly more often in those known to have CML for over 2 years and may, therefore, indicate a disease of longer standing. It is conceivable that chronic phase CML of long duration is prone to secondary genetic aberrations that culminate in blastic transformation. Indeed, the mortality rate of CML patients increases proportionately with the length of the disease.3,4,19 35 If the duration of the disease could be extrapolated from the methylation status, it would help to calculate the risk of an imminent blastic transformation. From the limited number of samples sequentially analyzed by a large panel of restriction enzymes (data not shown), it seems that the rate of methylation is a faithful chronometer of the disease.
In contrast to chemotherapy, which has so far failed to modify the natural course of CML, there is evidence that IFNα can favorably affect the outcome of the disease and prolong survival,18-20 irrespective of cytogenetic response.2 We, therefore, posed the question whether the IFNα effect on the pace of CML progression would be reflected in Pa methylation status. Indeed, at a median period of 9 months, IFNα therapy not only delayed the progression of Pa methylation, but also reversed its extent, so that a pattern typical of more recent CML was obtained.
Although the full applicability of the Pa methylation assay awaits a large prospective study, several interim conclusions regarding the management of CML patients may be drawn. Individual risk assessment is critical in CML, mainly when considering BMT. Currently, assessment is made only on the basis of clinical and laboratory presentation at diagnosis. Statistically, this provides valuable information, but it is not sufficiently informative in predicting the actual time of transformation, the prime concern for timing BMT.17 Therefore, many studies advocate BMT within a year of diagnosis.17 Pa methylation appears to represent a disease criterion that is independent of the clinical score (Tables 1 and 2).
Furthermore, it clearly represents a superior monitoring tool at any time point in the course of the disease (Figs 1 and 2) and precedes any other sign of disease progression. Therefore, in the absence of any other molecular tool for monitoring disease progression throughout the chronic phase, it could be considered, among other parameters, an aid for deciding on the appropriate mode of treatment.
An important example for the use of Pa methylation is the monitoring of IFNα therapy. Whereas most CML patients benefit from IFNα therapy, a major cytogenetic response is observed in a minority of treated patients.19 In our follow-up of IFNα therapy, we observed 85% methylation reversals in contrast to 25% cytogenetic responses for the same group of patients (Table 3). Hence, it appears that Pa methylation provides a better tool for monitoring the efficacy of IFNα treatment. Methylation analysis could pick up early signs of IFNα therapy failure and prompt an alternative mode of treatment, such as BMT.
The advantages of studying de novo CpG methylation may not be limited to the abl promoter and CML, but could prove applicable to many other tumor suppressor genes implicated in various types of cancer. Critical information on tumor progression may be obtained by monitoring the methylation status of such genes and could be applied to clinical management of the disease.
Supported by grants from the Israel Ministry of Health, Jerusalem, Israel; Strang Cancer Prevention Center, New York, NY; and Friends of the Lautenberg Center for Immunology, New York, NY.
Address reprint requests to Dina Ben-Yehuda, MD, Department of Hematology Hadassah University Hospital, Ein-Kerem, PO Box 12000, Jerusalem 91120, Israel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal