Abstract
AC133 is one of a new panel of murine hybridoma lines producing monoclonal IgG antibodies (mAbs) to a novel stem cell glycoprotein antigen with a molecular weight of 120 kD. AC133 antigen is selectively expressed on CD34bright hematopoietic stem and progenitor cells (progenitors) derived from human fetal liver and bone marrow, and blood. It is not detectable on other blood cells, cultured human umbilical vein endothelial cells (HUVECs), fibroblast cell lines, or the myeloid leukemia cell line KG1a by standard flow cytometric procedures. All of the noncommitted CD34+ cell population, as well as the majority of CD34+ cells committed to the granulocytic/monocytic pathway, are stained with AC133 antibody. In vitro clonogenicity assays have demonstrated that the CD34+AC133+ double-positive population from adult bone marrow contains the majority of the CFU-GM, a proportion of the CFU-Mix, and a minor population of BFU-E. The CD34dim and AC133− population has been shown to contain the remaining progenitor cells. AC133-selected cells engraft successfully in a fetal sheep transplantation model, and human cells harvested from chimeric fetal sheep bone marrow have been shown to successfully engraft secondary recipients, providing evidence for the long-term repopulating potential of AC133+ cells. A cDNA coding for AC133 antigen has been isolated, which codes for a polypeptide consisting of 865 amino acids (aa) with a predicted size of 97 kD. This antigen is modeled as a 5-transmembrane molecule, a structure that is novel among known cell surface structures. AC133 antibody provides an alternative to CD34 for the selection and characterization of cells necessary for both short- and long-term engraftment, in transplant situations, for studies of ex vivo expansion strategies, and for gene therapy.
EFFICIENT SELECTION of hematopoietic stem and progenitor cells is important for both experimental investigation and the clinical strategies of stem cell transplantation and gene therapy.1 Functionally, isolated progenitors may permit study of the lineage commitment process and the capacity for ex vivo expansion.2 Transplantation of enriched bone marrow or peripheral blood progenitors devoid of T lymphocytes may minimize serious complications of bone marrow allografts such as graft-versus-host disease, and tumor cell purging may be crucial to reducing tumor recurrence in autologous transplantation. The use of growth factors in ex vivo expansion of progenitor subsets could provide the next generation of cellular therapeutics for a variety of malignant and inherited diseases.2-4 Monoclonal antibodies (MoAbs) to cell surface antigens are now routinely used for identification and subsequent isolation of subsets of progenitors, and have been useful for elucidation of receptor/ligand interactions involving progenitors and humoral or cellular regulatory elements.5-7
Bone marrow– and blood-derived progenitors share expression of a great many surface markers with other hematopoietic cells, and they typically constitute less than 1% of normal bone marrow and 0.5% of peripheral blood.8 Unfortunately, direct conventional strategies to develop novel antibodies specific for progenitors have been difficult due to the relative paucity of stem cells for immunization and screening purposes, and the immunodominance of class II and other stem cell antigens, which dominate the immune response when using standard immunization procedures.
The marker of choice in progenitor selection strategies is CD34,9 and over the past decade, the use of CD34 as a progenitor surface marker has resulted in a rapid appearance of more sophisticated purification techniques and clinical trials in the field of hematopoiesis.1,10,11 CD34 is a stage-specific antigen associated with hematopoietic stem and progenitor cells that is also expressed on small-vessel endothelial cells9,12 and embryonic fibroblasts.13 CD34 antigen density is highest on early progenitors, and the density decreases progressively as cells mature.14 Fully differentiated hematopoietic cells are CD34−. Cells described as early multipotential CFU-Mix, CFU-blast, and long-term culture-initiating cells appear as lymphocyte-like cells and express high amounts of CD34 antigen.9,15,16 The CD34bright population includes cells with features of long-term B-cell lymphopoiesis and myelopoiesis in vitro, as well as mediating T, B, myelomonocytic, and megakaryocytic repopulation in vivo, while the CD34dim population contains lineage-committed progenitors, and these cells have no ability to provide long-term hematopoietic activity in vitro and in vivo.17,18 The CD34bright population containing primitive stem cell activity is therefore the target population for study of progenitor reconstitutive and self-renewal abilities. However, only an arbitrary distinction between CD34bright and CD34dim subsets can be made on the basis of CD34 expression alone. CD38, CD71, CD50, HLA-DR, CD90, TIE, CD117, and other markers18-24 have been proposed as additional tools to further enrich the CD34+ population for long-term engrafting cells. The consensus phenotype of primitive hematopoietic stem cells is CD34bright, CD38dim/-, HLA-DRdim/-, CD90+, CD117+, lineage−, and rhodamine 123lo.24-27
In this report, we describe an immunization and screening procedure that has allowed the production of a novel MoAb, AC133. AC133 identifies a structurally novel 5-transmembrane antigen with expression restricted to CD34bright progenitor populations derived from blood and bone marrow. In contrast to CD34, AC133 does not react to HUVECs, the KG1a cell line, or fibroblasts. AC133 defines a population of immature/primitive and GM-committed cells that can subsequently be isolated by AC133 antibody using fluorescence or magnetic-activated cell sorting. Our observations show that AC133 defines a desirable population for experimental study of hematopoiesis and ex vivo expansion of progenitors. In providing a source of primitive stem cells, together with progenitor cells committed to the granulomonocytic maturation pathway, AC133 may have an important role in stem cell selection for transplantation.
MATERIALS AND METHODS
Fetal tissues and cord blood were obtained from Advanced Bioscience Resources Inc, (Alameda, CA), a nonprofit corporation that provides tissue in compliance with state and federal laws. Peripheral blood and cytokine-mobilized leukapheresis products were from Stanford Medical School Blood Center (Palo Alto, CA). Bone marrow cells were aspirated from the posterior iliac crest of consenting healthy adult donors following guidelines approved by the Institutional Review Board for Human Research, Medical University of South Carolina, the Ralph H. Johnson Department of Veterans Affairs Medical Center, University of Nevada, Reno, and Stem Cell Laboratories, Division of Hematology and Oncology, University of California, San Diego, CA.
Mononuclear Cell Preparation
Peripheral blood or cord blood was diluted with phosphate-buffered saline (PBS), pH 7.4 (Sigma, St Louis, MO) and centrifuged over Histopaque-1077 (Sigma) for 30 minutes at 400g.28 Mononuclear cells recovered from the interface were washed and stored in liquid nitrogen if not used immediately.
Fetal Liver Preparation
Second-trimester fetal livers were mechanically dissociated in PBS, pH 7.4, containing 5 mmol/L EDTA, 0.5% bovine serum albumin (BSA), and 0.05% sodium azide (Sigma). The cell suspension was filtered sequentially through a Cellector Tissue Sieve (VWR, San Diego, CA) and 70-μm nylon mesh. Red blood cells, nucleated red blood cells, and mononuclear cells were separated by Histopaque-1077 density sedimentation. The CD34-rich fragment was collected from the interface. Cells were then washed and stored in liquid nitrogen for further use. Normally, 108 to 109 cells were harvested from one specimen, and the proportion of intact CD34+ cells obtained was between 5% and 15%.
Fetal and Adult Bone Marrow Preparation
For phenotypic analysis, fetal bone marrow was obtained from aborted fetuses of 17 to 22 weeks' gestation. Marrow suspension was prepared by flushing intramedullary cavities of upper- and lower-extremity bones with PBS, pH 7.4, containing 5 mmol/L EDTA, 0.5% BSA, and 0.05% sodium azide. The cells were pelleted and resuspended in the same buffer. Adult bone marrow and cell suspensions from fetal bone marrow were diluted 10-fold in buffer containing 0.17 mmol/L NH4Cl, 10 mmol/L KHCO3 , and 0.1 mmol/L tetrasodium EDTA in double-distilled H2O (pH 7.2, 20°C) to lyse the mature red blood cells. After 5 minutes of lysing, cells were centrifuged, washed, and passed through a 70-μm nylon filter (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) for subsequent staining.
Adult bone marrow for in vitro colony assays or in vivo studies was diluted with an equal volume of PBS without calcium or magnesium (PBS− ) and centrifuged over Accupaque (1.086 g/mL; Accurate Chemical and Scientific Corp, Westbury, NY) for 30 minutes at room temperature at 450g. The cells at the interface were washed three times with PBS− and resuspended in α-medium (α-modification of Eagle's medium; ICN, Aurora, OH) containing 10% fetal bovine serum (Intergen, Purchase, NY) for overnight storage at 4°C. Before analysis, cells were resuspended in PBS− containing 2% human AB serum, 0.1 mg/mL DNase (Sigma), and 0.1% sodium azide.
CD34+ Cell Purification
For immunization and FACS analysis, CD34+ cells were enriched from fetal liver preparations, fetal and adult bone marrow, cord blood, peripheral blood, or mobilized leukapheresis product. Cells from these sources were first prepared with Histopaque-1077 density gradient centrifugation. Mononuclear cells were then incubated with CD34 (QBEnd10)-conjugated magnetic microbeads (AmCell Corp, Sunnyvale, CA) and processed through a MACS magnetic separation column (Miltenyi Biotec, Bergisch Gladbach, Germany) to obtain purified CD34+ cells.29 For higher purity of CD34+ cells, a second column run was used. Purity of isolated CD34+ cells was generally greater than 90%, and cell viability as evaluated by propidium iodide exclusion was always higher than 95%.
Antibody Production
Immunization. For the AC133 hybridoma, 6-week-old female New Zealand Black (NZB) mice (Simonsen Laboratories, Gilroy, CA) were inoculated with human CD34+ cells under anesthesia on days 0, 3, 6, 9, 13, 17, and 20 in the right hand footpad, and peripheral blood mononuclear cells in the left hand footpad on days −3, 0, 3, 6, 9, 13, 17, and 20. CD34+ cells were immunomagnetically selected (QBEnd10 microbeads; AmCell Corp, Sunnyvale, CA) from a leukapheresis pack prepared from a cytokine-mobilized donor. Both cell types were incubated with 1:100 phytohemagglutinin (PHA) (GIBCO/BRL, Gaithersburg, MD) for 10 minutes before injection. Approximately 3 to 5 × 105 cells in 0.03 mL PBS, pH 7.4, were inoculated per footpad. For AC133-like hybridomas, 6-week-old female Balb/c mice (Simonsen) were immunized with WERI-Rb-1 cells. A total of seven injections of 5 × 105 cells were given via each footpad twice weekly.
Fusion. On day 21, right hand popliteal lymph nodes from the CD34 cell injection site of NZB mice or both popliteal nodes from WERI-Rb-1–injected Balb/c mice were removed. A lymphocyte suspension was prepared and fused to SP2/0 Ag14 myeloma cells using a modification of the method described by Köhler and Milstein.30 Fused cells were plated on 10 96-well plates in DMEM supplemented with 20% fetal bovine serum (FBS), 2 mmol/L L-glutamine, 15 mmol/L HEPES, 10−4 mmol/L hypoxanthine, and 2 μg/mL azaserine31 (FCS from HyClone Laboratories Inc, Logan, UT; other reagents from Sigma), and placed in a 37°C incubator with 9% CO2 .
Primary screening and subcloning. Hybridoma supernatants were screened on fetal liver cell preparations using two-color flow cytometric analysis. Binding of secreted mouse Ig from supernatants to the fetal liver cells was traced with phycoerythrin (PE)-conjugated rat antimouse IgG1 and IgG2a+2b (BDIS), and counterstaining with fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD34 (HPCA-2, BDIS; QBEnd10, AmCell Corp). Dead cells in the fetal liver preparation were excluded from analysis by propidium iodide staining.32 CD38PE and HLA-DRFITC (PharMingen, San Diego, CA) were used for FACScan compensation. All selected antibodies used later for this study were derived from cells subcloned by limiting dilution. The isotype of new antibodies was determined by the ISOTYPE Ab-STAT Kit (SangStat Medical Corp, Palo Alto, CA) or by FACS analysis using rat anti-mouse isotype fluorescent conjugates (BDIS).
Antibody Purification and Conjugation
Pure AC133 or AC133-like antibodies were prepared from hollow-fiber (Cellmax QUAD Artificial Capillary System; Cellco Inc, Germantown, MD) cultures or from ascites fluid by protein A chromatography.33 FITC and PE conjugates were prepared using standard procedures.34 35 AC133 magnetic bead conjugates were obtained from Miltenyi Biotec.
Cell Lines and Activation
Cell lines used for this study were obtained from the ATCC (Rockville, MD) and maintained in DMEM with 10% FBS, 2 mmol/L-glutamine, and 15 mmol/L HEPES in a 9% CO2 atmosphere. HUVECs were from Clonetics (San Diego, CA), and NT-2 and hNT neurons were kindly provided by Dr M. McGrogan (Layton BioSciences, Gilroy, CA). Anchorage-dependent cells were removed from the flasks by Trypsin-EDTA digestion (GIBCO/BRL). For activation, 1:100 PHA was added to aseptically prepared peripheral blood mononuclear cells in culture medium and placed in a 37°C humidified incubator with 9% CO2 . Cell lines KG1a, K562, HEL92.1.7, Jurkat, Daudi, HPB-ALL, SK-N-MC, SK-N-SH, H4, 134H, 8402, Y79.1, and WERI-Rb-1 were activated with 1 ng/mL phorbol myristate acetate ([PMA]-GIBCO/BRL). Activated cells were harvested at 24-, 48-, 72-, 96-, and 120-hour intervals and washed with PBS before FACS analysis.
Cell Staining
To minimize nonspecific antibody binding, cells were preincubated with PBS containing 0.5% human γ-globulin (Sigma). For indirect immunofluorescence staining, nucleated cells from bone marrow, cord blood, or fetal liver were incubated with an unlabeled MoAb, washed twice in PBS-FCS, and stained with fluorochrome-conjugated secondary antibodies. Before additional labeling with fluorochrome-conjugated MoAbs, the cells were washed twice and incubated for 20 minutes with 30% normal mouse serum to block remaining binding sites for antimouse IgG. All incubation steps were performed on ice for 25 minutes. Immunostained cells were either kept on ice and analyzed immediately by flow cytometry or fixed in 0.5% paraformaldehyde and analyzed within 48 hours.
Flow Cytometric Analysis
One-, two-, three-, or four-color FACS analysis was used for AC133 antibody screening, determination of AC133 expression on cell lines, AC133+ cell phenotyping, and stem/progenitor cell evaluation after magnetic selection. CD90PE (Thy-1) was obtained from Pharmingen. Anti-Flt3 MoAb SF 1.340 and CD117 MoAb 104D2 were obtained from the Sixth International Workshop on Leukocyte Differentiation Antigens. MoAbs used to discriminate between noncommitted and various lineage-committed progenitor cells included CD34 (HPCA-2), CD38 (Leu-17), CD19 (SJ25C1), CD71 (transferrin receptor) (all from BDIS), CD50 (140-11; kindly provided by Dr Ramon Vilella, Servei d'Immunologia, Hospital Clinic, Barcelona, Spain), and CD64 (M22; MedaRex Inc, Annandale, NJ). The mAbs were conjugated to FITC, PE, peridinin chlorophyll protein (PerCP), or allophycocyanin (APC). Fluorochrome conjugates that are not listed in the BDIS catalog were made through the BDIS custom conjugation program. Isotype control mouse MoAbs included anti–keyhole limpet hemocyanin (IgG1; BDIS) and NC2 (IgG1; Fifth International Workshop on Leukocyte Differentiation Antigens). Polyclonal PE-conjugated F(ab′ )2 goat anti-mouse IgG heavy + light chain was purchased from Jackson ImmunoResearch (West Grove, PA). Normal mouse serum was from Caltag Laboratories (South San Francisco, CA).
For phenotypic analysis, immunostained cells were analyzed using an experimental high-sensitivity flow cytometer designed by Dr Robert Hoffman at BDIS. This flow cytometer is equipped with three lasers: a 488-nm argon ion laser (15 mW) for excitation of PerCP and FITC, a 633-nm helium-neon laser (10 mW) for excitation of APC, and a 532-nm YAG laser (20 mW) for excitation of PE. The 532-nm excitation of PE yields six times the signal to noise ratio obtained with 488-nm excitation (not shown). For each sample, 10,000 to 50,000 events were acquired in list mode with Cell Quest software (BDIS). Forward light scatter, orthogonal light scatter, and four fluorescence signals were determined for each cell, and the listmode data files were analyzed on a Macintosh computer with Paint-A-GatePRO (BDIS).
Colony Assay
Adult bone marrow cells were stained with PE-conjugated AC133 antibody at a concentration of 200 ng/106 cells in a volume of 100 μL and with FITC-conjugated CD34 antibody7 at a concentration of 2 μg/106 cells in 100 μL for 30 minutes at 4°C. Propidium iodide was added to the final wash as an indicator of nonviable cells. A BDIS FACS Vantage was used to analyze and sort bone marrow mononuclear cells into fractions of CD34+, CD34+/AC133+, and CD34+/AC133−.
Clonal cell cultures were performed in 35-mm culture dishes using serum-free conditions.7 36 One milliliter of the culture mixture contained 500 sorted cells, 2% deionized crystallized BSA, 10 μg/mL soybean lecithin, 6 μg/mL cholesterol, 1 × 10−7 mol/L sodium selenite, 300 μg/mL human transferrin, 10 μg/mL insulin (reagents from Sigma), and 1.2% 1,500-centipoise methycellulose (Shinetsu Chemical, Tokyo, Japan).
The source of recombinant human cytokines was as previously described.7 Concentrations of the cytokines were interleukin-3 (IL-3) 100 U/mL, stem cell factor (Steel factor, kit ligand) 100 ng/mL, thrombopoietin (TPO) 100 ng/mL, IL-6 100 ng/mL, and erythropoietin (EPO) 2 U/mL. Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 , 5% O2 , and 90% N2 . Colonies were identified on day 14 of incubation, on an inverted microscope using the criteria described previously.37
Preliminary In Vivo Analysis
After two washes with PBS, pH 7.4, containing 0.5% BSA and 5 mmol/L EDTA, bone marrow mononuclear cells were incubated with AC133-conjugated magnetic beads (Miltenyi Biotec) for 30 minutes at 4°C and passed through a VS column in the magnetic field of a VarioMACS Apparatus (Miltenyi Biotec). To obtain a higher purity of CD34bright cells, eluted AC133 cells were recaptured on a second VS column. Recovered bone marrow cells were evaluated by FACS analysis with CD34PE (HPCA-2 ; BDIS) against CD45FITC, CD15FITC, and Glycophorin-A FITC (Pharmingen). Ninety-five percent of AC133 cells obtained from bone marrow magnetic separation were CD34bright. AC133-selected cells were transplanted into primary preimmune fetal sheep (earlier than 65 gestation days; term, 145 days) recipients using the amniotic bubble technique.38 Five fetuses received a single intraperitoneal injection of 1.5 × 105 AC133-purified cells. All recipients survived the procedure. One fetus was killed at 60 days posttransplantation (ie, 120 days gestation, 25 days before birth) and evaluated for human donor cell activity by flow cytometry. The remaining animals were carried to term. These lambs were evaluated soon after birth and at intervals thereafter. For transplantation of secondary recipients, human CD45+ cells were isolated from the marrow of a primary chimeric recipient by immune adherence “panning” and transplanted into preimmune fetal sheep.39 Each of four fetuses received 6 × 104 CD45+ cells isolated from the marrow of a primary recipient made chimeric with AC133-purified cells. Donor cell engraftment and expression were determined by flow cytometry and karyotyping of colonies derived from hematopoietic progenitors (CFU-Mix, CFU-GM, and BFU-E) as previously described.40
RESULTS
Antibody Production and Screening
The contralateral immunization of NZB mouse popliteal lymph node cells with purified progenitors has allowed the production of an IgG1-secreting hybrid culture, 5F3, which stained only the CD34bright subset of a fetal liver preparation (Fig 1). This hybridoma was subcloned by limiting dilution and renamed AC133. AC133 was of immediate interest in that it was not reactive to unfractionated peripheral blood mononuclear cells (PBMCs), granulocytes, platelets, fibroblasts, cultured HUVECs, or various CD34-expressing cell lines such as KG-1, KG-1a, or 84029 (Table 1). By FACS analysis, AC133 specifically stained CD34bright cells in human fetal livers (n = 27; mean, 29.88% of CD34+ cells) and bone marrow (n = 3; mean, 31.6%), adult bone marrow (n = 2; mean, 70%), cord blood (n = 2; mean, 35.13%), and leukapheresis products from cytokine-mobilized donors (n = 3; mean, 54.9%) (Fig 1). Staining of PHA-activated PBMCs was negative (data not shown). AC133 antigen expression by FACS analysis of a large panel of hematopoietic and nonhematopoietic cell lines was uniformly negative, with the exception of two human retinoblastoma cell lines, Y79.1 and WERI-Rb-1, and one teratocarcinoma-derived cell line, NT2 (Table 1 and Fig 2.) These NT2 precursor cells differentiate into postmitotic cells with properties of central nervous system neurons in vitro by retinoic acid induction.55 hNT neurons derived from the NT2 cell line were found to have downregulated AC133 antigen expression to undetectable levels (not shown). While AC133 antigen expression levels were low on Y79.1 cells, they were approximately 10-fold higher on WERI-Rb-1 cells. The 24-hour PMA activation of Y79.1 resulted in upregulation of AC133 antigen expression, but no upregulation was seen with the WERI-Rb-1 cell line. PMA activation of a panel of cell lines for 24 to 96 hours did not induce AC133 antigen expression (Table 1). Retinoblastoma cell line expression led us to examine AC133 expression on other cell lines of neural origin; however, AC133 was found to be nonreactive with SK-N-SH, SK-N-MC, or IMR32, three neuroblastoma cell lines, with H4, a human neuroglioma cell line, or with 134H, a glioblastoma cell line (Table 1). Baboon bone marrow cells (kindly provided by Dr Jonathon Allen, Southwest Foundation for BioMedical Research, San Antonio, TX) did not express the AC133 antigen (data not shown).
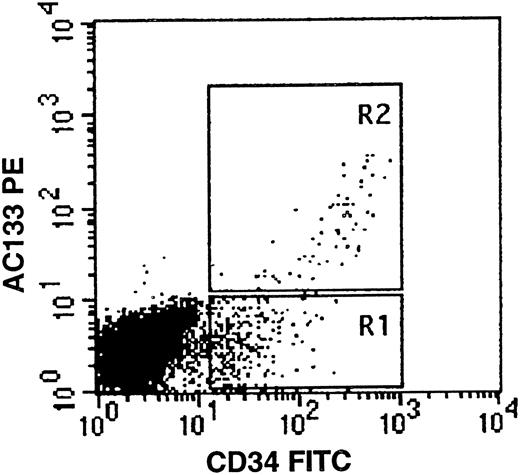
AC133 expression on fetal liver cells. Fetal liver mononuclear cells were stained with AC133PE (vertical axis) and CD34FITC (horizontal axis) as described. CD34bright/AC133+ cells are seen in region R2. The gates R1 and R2 are representative of those used in selection of bone marrow CD34/AC133 subpopulations for in vitro colony assays.
AC133 expression on fetal liver cells. Fetal liver mononuclear cells were stained with AC133PE (vertical axis) and CD34FITC (horizontal axis) as described. CD34bright/AC133+ cells are seen in region R2. The gates R1 and R2 are representative of those used in selection of bone marrow CD34/AC133 subpopulations for in vitro colony assays.
AC133 Expression Profile
| Primary Cells and Cell Lines . | Protein Detected by FACS Staining . | Primary Cells and Cell Lines . | Protein Detected by FACS Staining . | ||
|---|---|---|---|---|---|
| . | . | . | . | . | . |
| Primary cells | |||||
| Nucleated red blood cells | − | ||||
| Lymphocytes | − | ||||
| Monocytes | − | ||||
| Granulocytes | − | ||||
| Platelets | − | ||||
| Fetal thymocytes | − | ||||
| Fetal splenocytes | − | ||||
| CD34+ cells from all tissues | + | ||||
| PHA-activated PBMCs | − | ||||
| Baboon CD34+† | − | ||||
| NZB + LPR mouse splenocytes | − | ||||
| NZB + LPR mouse thymocytes | − | ||||
| NZB + LPR mouse hepatocytes | − | ||||
| Hematopoietic cell lines | |||||
| 8402*† | |||||
| T-cell line | − | ||||
| JM | |||||
| T-cell line | − | ||||
| Jurkat* | |||||
| T-cell line | − | ||||
| HPB-ALL* | |||||
| T-ALL | − | ||||
| Molt-4 | |||||
| T-ALL | − | ||||
| HUT-78 | |||||
| Cutaneous T-cell lymphoma | − | ||||
| HEL.92.1.7* | |||||
| Erythroleukemia | − | ||||
| K562* | |||||
| Erythroleukemia | − | ||||
| 8866 | |||||
| EBV-transformed B-cell line | − | ||||
| IM-9 | |||||
| EBV-transformed B-cell line | − | ||||
| Daudi* | |||||
| EBV-transformed B-cell line | − | ||||
| Raji | |||||
| EBV-transformed B-cell line | − | ||||
| HS-Sultan | |||||
| EBV-transformed B-cell line | − | ||||
| T.5.1 | |||||
| EBV-transformed B-cell line | − | ||||
| BJAB | |||||
| North American Burkitt's lymphoma | − | ||||
| KG1† | |||||
| AML | − | ||||
| KG1a*† | |||||
| AML | − | ||||
| HL-60 | |||||
| Promyelocytic leukemia | − | ||||
| RPMI-8226 | |||||
| Myeloma | − | ||||
| U937 | |||||
| Histiocytic lymphoma | − | ||||
| Nonhematopoietic cell lines | |||||
| 134H* | |||||
| Glioblastoma | − | ||||
| AZ676 | |||||
| Carcinosarcoma, breast | − | ||||
| BT474 | |||||
| Ductal carcinoma, breast | − | ||||
| BT549 | |||||
| Ductal carcinoma, breast | − | ||||
| T47D | |||||
| Ductal carcinoma, breast | − | ||||
| BT20 | |||||
| Carcinoma, breast | − | ||||
| Du4475† | |||||
| Metastatic cutaneous nodule, breast carcinoma | − | ||||
| MCF-7 | |||||
| Adenocarcinoma, breast | − | ||||
| H4* | |||||
| Neuroglioma | − | ||||
| SK-N-MG* | |||||
| Neuroepithelioma, metastasis to supraorbital area | − | ||||
| SK-N-SH* | |||||
| Neuroblastoma, metastasis to bone marrow | − | ||||
| IMR-32 | |||||
| Neuroblastoma | − | ||||
| WERI-Rb-1 | |||||
| Retinoblastoma | + | ||||
| Y79 | |||||
| Retinoblastoma | + | ||||
| CACL74-36 | |||||
| Melanoma | − | ||||
| HT1080 | |||||
| Fibrosarcoma | − | ||||
| CC90 | |||||
| Diploid fibroblast | − | ||||
| CC93 | |||||
| Diploid fibroblast | − | ||||
| CC95 | |||||
| Diploid fibroblast | − | ||||
| HT29 | |||||
| Adenosarcoma, colon | − | ||||
| COLO 205 | |||||
| Adenosarcoma, colon | − | ||||
| SK HEP-1 | |||||
| Adenocarcinoma, liver | − | ||||
| Chang Liver | |||||
| Hela markers | − | ||||
| HUVEC† | |||||
| Umbilical vein endothelial cells | − | ||||
| NT2 | |||||
| Teratocarcinoma line | + | ||||
| hNT | |||||
| Neuron cell derived from NT2 | − | ||||
| Primary Cells and Cell Lines . | Protein Detected by FACS Staining . | Primary Cells and Cell Lines . | Protein Detected by FACS Staining . | ||
|---|---|---|---|---|---|
| . | . | . | . | . | . |
| Primary cells | |||||
| Nucleated red blood cells | − | ||||
| Lymphocytes | − | ||||
| Monocytes | − | ||||
| Granulocytes | − | ||||
| Platelets | − | ||||
| Fetal thymocytes | − | ||||
| Fetal splenocytes | − | ||||
| CD34+ cells from all tissues | + | ||||
| PHA-activated PBMCs | − | ||||
| Baboon CD34+† | − | ||||
| NZB + LPR mouse splenocytes | − | ||||
| NZB + LPR mouse thymocytes | − | ||||
| NZB + LPR mouse hepatocytes | − | ||||
| Hematopoietic cell lines | |||||
| 8402*† | |||||
| T-cell line | − | ||||
| JM | |||||
| T-cell line | − | ||||
| Jurkat* | |||||
| T-cell line | − | ||||
| HPB-ALL* | |||||
| T-ALL | − | ||||
| Molt-4 | |||||
| T-ALL | − | ||||
| HUT-78 | |||||
| Cutaneous T-cell lymphoma | − | ||||
| HEL.92.1.7* | |||||
| Erythroleukemia | − | ||||
| K562* | |||||
| Erythroleukemia | − | ||||
| 8866 | |||||
| EBV-transformed B-cell line | − | ||||
| IM-9 | |||||
| EBV-transformed B-cell line | − | ||||
| Daudi* | |||||
| EBV-transformed B-cell line | − | ||||
| Raji | |||||
| EBV-transformed B-cell line | − | ||||
| HS-Sultan | |||||
| EBV-transformed B-cell line | − | ||||
| T.5.1 | |||||
| EBV-transformed B-cell line | − | ||||
| BJAB | |||||
| North American Burkitt's lymphoma | − | ||||
| KG1† | |||||
| AML | − | ||||
| KG1a*† | |||||
| AML | − | ||||
| HL-60 | |||||
| Promyelocytic leukemia | − | ||||
| RPMI-8226 | |||||
| Myeloma | − | ||||
| U937 | |||||
| Histiocytic lymphoma | − | ||||
| Nonhematopoietic cell lines | |||||
| 134H* | |||||
| Glioblastoma | − | ||||
| AZ676 | |||||
| Carcinosarcoma, breast | − | ||||
| BT474 | |||||
| Ductal carcinoma, breast | − | ||||
| BT549 | |||||
| Ductal carcinoma, breast | − | ||||
| T47D | |||||
| Ductal carcinoma, breast | − | ||||
| BT20 | |||||
| Carcinoma, breast | − | ||||
| Du4475† | |||||
| Metastatic cutaneous nodule, breast carcinoma | − | ||||
| MCF-7 | |||||
| Adenocarcinoma, breast | − | ||||
| H4* | |||||
| Neuroglioma | − | ||||
| SK-N-MG* | |||||
| Neuroepithelioma, metastasis to supraorbital area | − | ||||
| SK-N-SH* | |||||
| Neuroblastoma, metastasis to bone marrow | − | ||||
| IMR-32 | |||||
| Neuroblastoma | − | ||||
| WERI-Rb-1 | |||||
| Retinoblastoma | + | ||||
| Y79 | |||||
| Retinoblastoma | + | ||||
| CACL74-36 | |||||
| Melanoma | − | ||||
| HT1080 | |||||
| Fibrosarcoma | − | ||||
| CC90 | |||||
| Diploid fibroblast | − | ||||
| CC93 | |||||
| Diploid fibroblast | − | ||||
| CC95 | |||||
| Diploid fibroblast | − | ||||
| HT29 | |||||
| Adenosarcoma, colon | − | ||||
| COLO 205 | |||||
| Adenosarcoma, colon | − | ||||
| SK HEP-1 | |||||
| Adenocarcinoma, liver | − | ||||
| Chang Liver | |||||
| Hela markers | − | ||||
| HUVEC† | |||||
| Umbilical vein endothelial cells | − | ||||
| NT2 | |||||
| Teratocarcinoma line | + | ||||
| hNT | |||||
| Neuron cell derived from NT2 | − | ||||
Cell lines were maintained as described, and single-cell suspensions were prepared in PBS containing 0.5% BSA and 0.1% sodium azide. Following staining with AC133PE or control antibodies, cells were washed and fixed in 1% paraformaldehyde in PBS before flow cytometric analysis.
Tested negative for AC133 expression following PMA activation.
CD34+ but AC133−.
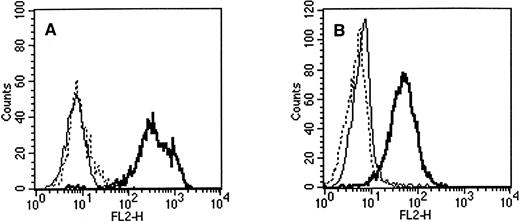
Flow cytometric staining of (A) retinoblastoma cell line WERI-Rb-1 and (B) teratocarcinoma cell line NT2 with PE-conjugated antibodies. Solid line, IgG1 control; dashed line, QBEnd10 (CD34); bold line, AC133. All three antibodies were isotype IgG1.
Flow cytometric staining of (A) retinoblastoma cell line WERI-Rb-1 and (B) teratocarcinoma cell line NT2 with PE-conjugated antibodies. Solid line, IgG1 control; dashed line, QBEnd10 (CD34); bold line, AC133. All three antibodies were isotype IgG1.
The WERI-Rb-1 cell line that expressed high levels of AC133 antigen was used as immunogen in two further immunizations to produce a panel of antibodies to the AC133 antigen. Balb-c mice were immunized via the footpad with WERI-Rb-1 cells, and two separate fusions of popliteal lymph node cells from these animals resulted in a number of new hybridomas that secreted AC133-like antibodies (Table 2). Specificity of these new antibodies was confirmed by two- or three-color FACS analysis, cross-blocking studies, immunoprecipitation, and/or by reactivity with AC133-transfected COS cells.41 Cross-blocking studies using an excess (5 μg) of unlabeled antibody to compete with the binding of AC141PE conjugate to the AC133+ cell line WERI-Rb-1 indicates that at least two and maybe three spatially unrelated epitopes are recognized by the panel of antibodies in Table 2.
Antibodies With Specificity for AC133 Antigen
| Antibody . | Immunogen . | Isotype . | AC141PE Cross-Blocking . |
|---|---|---|---|
| AC133 | HSC | IgG1-κ | −− (E) |
| AC139 | WERI-Rb-1 | IgG1-κ | +/− |
| AC140 | WERI-Rb-1 | IgG1-κ | −− |
| AC141 | WERI-Rb-1 | IgG1-κ | +++ |
| AC142 | WERI-Rb-1 | IgG1-κ | +/− |
| Antibody . | Immunogen . | Isotype . | AC141PE Cross-Blocking . |
|---|---|---|---|
| AC133 | HSC | IgG1-κ | −− (E) |
| AC139 | WERI-Rb-1 | IgG1-κ | +/− |
| AC140 | WERI-Rb-1 | IgG1-κ | −− |
| AC141 | WERI-Rb-1 | IgG1-κ | +++ |
| AC142 | WERI-Rb-1 | IgG1-κ | +/− |
AC133 was raised by immunization with hematopoietic stem cells (HSC), and AC139 to AC142 were raised by immunization with the WERI-Rb-1 retinoblastoma cell line.
Abbreviations: +++, complete cross-blocking of AC141PE conjugate by unlabeled AC141; +/−, partial cross-blocking; −−, zero cross-blocking ability; (E), 20% enhancement of AC141PE conjugate binding in the presence of unlabeled AC133 antibody.
Phenotyping Studies
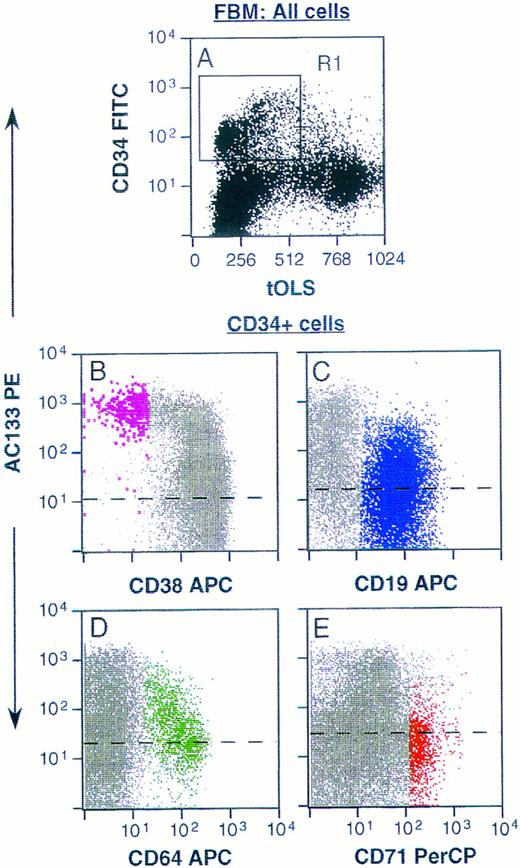
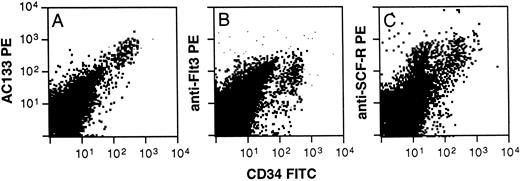
Phenotyping of AC133+ cells was accomplished using multicolor FACS analysis with a panel of conjugated antibodies directed to leukocyte surface structures known to be expressed on human hematopoietic stem, progenitor, or lineage-committed progenitor cells. Typically, AC133 stained 20% to 60% of all CD34+ cells derived from fetal liver and bone marrow, adult bone marrow, cord blood, and peripheral blood. Only CD34+ cells from all of these tissue sources were AC133+. Fetal bone marrow (FBM) cells were stained with AC133 and also CD38, CD50, CD64, CD71, and CD19 to allow identification of noncommitted and various lineage-committed progenitor cells (Fig 3). AC133 did not stain above isotype control levels on CD34negative cells (not shown). Figure 3B through E represent CD34+ cells gated from region R1 in Fig 3A, and 26% to 37% of CD34+ cells were AC133+ (n = 3). Noncommitted progenitor cells were identified as CD34brightCD38−CD50+ cells,17,19,42 and were among the CD34+ cells showing the brightest staining with AC133 (Fig 3B, violet dots). In contrast, only a subset of lymphoid-committed progenitors, identified as CD34+CD19+ cells,43 stained with levels above the isotype control (Fig 3C, blue dots). Granulomonocytic-committed progenitor cells (CD34+CD64+, green dots in Fig 3D) showed decreasing staining with AC133 with increasing levels of CD64 expression, indicating downregulation of the AC133-binding molecule with granulomonocytic differentiation.26,44 However, the mean fluorescence intensity (MFI) for this subset was higher than the MFI for the lymphoid-committed (Fig 3C) and erythroid-committed (red dots, Fig 3E) progenitors. The erythroid-committed progenitor cells (CD34brightCD71brightCD64− )26 45 stained with AC133 only slightly above isotype control levels. The staining pattern of adult bone marrow (ABM) CD34+ and CD34− cells with AC133 closely resembled that shown for FBM cells (Fig 3). However, a larger fraction of CD34+ ABM cells stained positively with AC133 (64% to 76%, n = 2) as compared with FBM cells. Figure 4 furthermore shows that when labeling with CD34 and AC133, CD117 (anti-SCF-R), or anti-Flt3, respectively, AC133 fluorescence shows the closest positive correlation with CD34 fluorescence. Both Flt3 (Fig 4B) and CD117 (Fig 4C) were expressed also on subsets of CD34− cells.
Staining of FBM cells with AC133. Ammonium chloride–lysed FBM cells were labeled with AC133 and goat anti-mouse PE, followed by staining with CD34FITC (A through E) and CD38APC and CD50PerCP (B), or CD19APC (C), or CD64APC and CD71PerCP (D and E). (A) Unfractionated FBM cells. (B through E) Staining of CD34+ cells only, gated from region R1 in A. Noncommitted progenitor cells (CD34hiCD38loCD50+ ) are represented by enlarged violet dots in B, and lymphoid committed progenitors (CD34+CD19+ ) are depicted in blue in C. Granulomonocytic committed progenitors (CD34+CD64+ ) are represented by green dots in D, and erythroid committed progenitors (CD34hiCD71hiCD64− ) are depicted in red in E. Dashed lines represent isotype control levels for each of the colored populations. tOLS, transformed orthogonal light scatter. The results are representative of 3 experiments.
Staining of FBM cells with AC133. Ammonium chloride–lysed FBM cells were labeled with AC133 and goat anti-mouse PE, followed by staining with CD34FITC (A through E) and CD38APC and CD50PerCP (B), or CD19APC (C), or CD64APC and CD71PerCP (D and E). (A) Unfractionated FBM cells. (B through E) Staining of CD34+ cells only, gated from region R1 in A. Noncommitted progenitor cells (CD34hiCD38loCD50+ ) are represented by enlarged violet dots in B, and lymphoid committed progenitors (CD34+CD19+ ) are depicted in blue in C. Granulomonocytic committed progenitors (CD34+CD64+ ) are represented by green dots in D, and erythroid committed progenitors (CD34hiCD71hiCD64− ) are depicted in red in E. Dashed lines represent isotype control levels for each of the colored populations. tOLS, transformed orthogonal light scatter. The results are representative of 3 experiments.
Staining of ABM cells with AC133, CD117 (SCF-R), or anti-Flt3. Ammonium chloride–lysed ABM cells were labeled with AC133 (A) or anti-Flt3 (SF 1.340, B), or anti-SCF-R (104D2, C) and goat anti-mouse PE, followed by staining with CD34FITC. The results are representative of 2 experiments.
Staining of ABM cells with AC133, CD117 (SCF-R), or anti-Flt3. Ammonium chloride–lysed ABM cells were labeled with AC133 (A) or anti-Flt3 (SF 1.340, B), or anti-SCF-R (104D2, C) and goat anti-mouse PE, followed by staining with CD34FITC. The results are representative of 2 experiments.
AC133 cDNA Cloning and Protein Structure
The isolation and sequencing of a novel cDNA encoding the AC133 antigen is described in detail elsewhere.41 Briefly, the cDNA encodes a single open reading frame of 2,595 nucleotides, and predicts an 865–amino acid protein with a MW of 97 kD. The AC133 antigen is modeled as a 5-transmembrane protein with an extracellular N-terminus and a cytoplasmic C-terminus. The structure of the AC133 antigen appears to be novel among known hematopoietic cell surface antigens.46 47 Immunoprecipitation detected AC133 antigen with a molecular weight of 120 kD in PMA-activated Y79.1 and WERI-Rb-1 cells.
In Vitro Colony Assays
Quadruplicate culture dishes were seeded with 500 adult bone marrow cells sorted from regions similar to those depicted in region R1 + R2 (CD34+ ), R1 (CD34+, AC133− or R2 (CD34+, AC133+ ) (Fig 1). There were no AC133+ cells that were negative for CD34. On day 14 of incubation, culture dishes were scored for colony growth as previously described.37 The results shown in Table 3, which is an example of one of three similar experiments, clearly indicate that the majority of CFU-GM are contained in the CD34+/AC133+ fraction, while the majority of BFU-E and CFU-Mix are in the CD34+/AC133− fraction. This finding is surprising, as CFU-Mix have been previously considered to be more primitive and therefore to express a high density of CD34.9
Colony Formation From CD34+ Cells Separated on the Basis of AC133 Expression
| Fraction . | % of Population . | GM . | E . | CFU-Mix . | Total . |
|---|---|---|---|---|---|
| CD34+ | 1.6 | 15 ± 1 | 20 ± 4 | 8 ± 3 | 42 ± 4 |
| CD34+AC133− | 1.3 | 13 ± 1 | 30 ± 5 | 16 ± 3 | 59 ± 7 |
| CD34+AC133+ | 0.3 | 82 ± 8 | 24 ± 2 | 18 ± 2 | 124 ± 9 |
| Fraction . | % of Population . | GM . | E . | CFU-Mix . | Total . |
|---|---|---|---|---|---|
| CD34+ | 1.6 | 15 ± 1 | 20 ± 4 | 8 ± 3 | 42 ± 4 |
| CD34+AC133− | 1.3 | 13 ± 1 | 30 ± 5 | 16 ± 3 | 59 ± 7 |
| CD34+AC133+ | 0.3 | 82 ± 8 | 24 ± 2 | 18 ± 2 | 124 ± 9 |
Five hundred cells were incubated in serum-free medium containing cytokines for 14 days. Colonies were identified in situ.3
Abbreviations: GM, granulocyte and/or macrophage colonies; E, erythroid colonies; CFU-Mix, mixed colonies containing erythroid and myeloid cells and/or megakaryocytes.
In Vivo Engraftment Studies
Xenogeneic transplantation studies were performed using the fetal sheep model as previously described.38 In this set of experiments, fetal sheep were inoculated with 1.5 × 105 CD34/AC133 double-positive cells. The bone marrow of one member of this group of primary recipients was analyzed for the presence of human cells at 60 days posttransplant, which is approximately 25 days before birth, and these human cells were used to inoculate secondary recipients. Other animals were carried to term and examined at various times after birth using CD45 expression or in vitro colony formation for the presence of human donor cells in bone marrow. The percentage of colonies exhibiting a human karyotype and the percentage of marrow cells expressing the CD45 antigen in these primary recipients are listed in Table 4. The percentage of CD45+ cells in the marrow of primary recipients ranged from 0.7% to 1.7% of total marrow mononuclear cells, and in vitro derived colonies demonstrate the human and multilineage nature of these cells. Only primitive repopulating cells have the ability to engraft in secondary recipients,48 and therefore, human progenitors isolated from secondary recipients must necessarily be derived from primitive stem cells obtained from the primary recipients. The data in Table 5 clearly demonstrate that multilineage cells were identified and isolated from the marrow of secondary recipients harvested at 60 days posttransplant. These cells, 0.1% to 2.14% of total marrow mononuclear cells, were identified as human both by karyotyping and by CD45 antigen expression. Given that human progenitor cells are only minimally stimulated by sheep growth factors49 and considering the relatively small number of cells inoculated (6 × 104 per fetus), the level of engraftment of human progenitor cells in these chimeric sheep marrows can be considered very significant.
Donor (human) Cell/Progenitor Activity in Bone Marrow of Primary Sheep Recipients
| Animal . | Colonies With Human Karyotype . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | CFU-Mix . | CFU-GM . | BFU-E . | % CD45+ Cells4-150 . | . | . | . | |||
| . | No. . | % . | No. . | % . | No. . | % . | . | . | . | . |
| 770 | 9/290 | 3.1 | 60/1,000 | 6.0 | 14/609 | 2.3 | 1.41 ± 0.11 | |||
| 771 | 8/186 | 4.3 | 29/763 | 3.8 | 1/281 | 0 | 1.3 ± 0.23 | |||
| 772 | 0/162 | 0 | 17/370 | 4.6 | 11/500 | 2.2 | 0.7 ± 0.22 | |||
| 775 | 8/381 | 2.1 | 79/887 | 8.9 | 19/306 | 6.2 | 1.7 ± 0.32 | |||
| Animal . | Colonies With Human Karyotype . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | CFU-Mix . | CFU-GM . | BFU-E . | % CD45+ Cells4-150 . | . | . | . | |||
| . | No. . | % . | No. . | % . | No. . | % . | . | . | . | . |
| 770 | 9/290 | 3.1 | 60/1,000 | 6.0 | 14/609 | 2.3 | 1.41 ± 0.11 | |||
| 771 | 8/186 | 4.3 | 29/763 | 3.8 | 1/281 | 0 | 1.3 ± 0.23 | |||
| 772 | 0/162 | 0 | 17/370 | 4.6 | 11/500 | 2.2 | 0.7 ± 0.22 | |||
| 775 | 8/381 | 2.1 | 79/887 | 8.9 | 19/306 | 6.2 | 1.7 ± 0.32 | |||
Data from primary fetal recipients transplanted with 1.5 × 105 CD34+ cells/fetus are presented. As expected, donor cell activity was also detected in bone marrow of some animals transplanted with 1 × 106 primarily CD34+, AC133− cells/fetus. Bone marrow mononuclear cells obtained 1 week after birth were cultured in multiple methylcellulose plates as previously described.40 Total colony numbers were counted on days 9 and 19 of incubation; all 3 progenitor types (CFU-Mix, CFU-GM, and BFU-E) were detected on both days. On day 19, colonies were removed from the plate and individually processed for karyotyping.40 Percent values indicate the percentage of total colonies enumerated on day 9 (denominator) of culture that were determined to be of human origin (numerator) and were calculated as % human colonies = (no. of human colonies on day 19 × 100)/total no. of colonies on day 9.
Each value represents the mean ± 1 SEM of results from 4 separate determinations at 1 week, 1 month, 2 months, and 3 months after birth. Expression of human cells was multilineage as determined by flow cytometric analysis of bone marrow with the following antibodies: CD34, 0.26%; CD4, 0.2%; CD5, 0.35%; CD13, 1.12%; CD14, 0.19%; gly A, 0.05%; CD33, 0.37%; CD7, 4.17%; CD13/CD45, 0.31%; CD5/CD10, 0.32%.
Relative Distribution of Donor (human) Hematopoietic Progenitors/Cells in Bone Marrow of Secondary Sheep Recipients at 60 Days Posttransplant
| Animal . | Colonies With Human Karyotype5-150 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | CFU-Mix . | CFU-GM . | BFU-E . | % CD4+ Cells . | . | . | . | |||
| . | No. . | % . | No. . | % . | No. . | % . | . | . | . | . |
| A14 | 0/164 | 0 | 1/498 | 0 | 1/268 | 0 | 0.1 | |||
| A15 | 1/310 | 0 | 98/1,607 | 6.1 | 17/850 | 2.0 | 1.06 | |||
| A16 | 16/421 | 3.8 | 73/1,177 | 6.2 | 16/485 | 3.3 | 2.14 | |||
| A17 | 11/262 | 4.2 | 14/467 | 3.0 | 8/500 | 1.6 | 1.30 | |||
| Animal . | Colonies With Human Karyotype5-150 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | CFU-Mix . | CFU-GM . | BFU-E . | % CD4+ Cells . | . | . | . | |||
| . | No. . | % . | No. . | % . | No. . | % . | . | . | . | . |
| A14 | 0/164 | 0 | 1/498 | 0 | 1/268 | 0 | 0.1 | |||
| A15 | 1/310 | 0 | 98/1,607 | 6.1 | 17/850 | 2.0 | 1.06 | |||
| A16 | 16/421 | 3.8 | 73/1,177 | 6.2 | 16/485 | 3.3 | 2.14 | |||
| A17 | 11/262 | 4.2 | 14/467 | 3.0 | 8/500 | 1.6 | 1.30 | |||
Primary recipients served as donors for secondary transfer studies.
The numerator indicates the actual number of colonies exhibiting human karyotype, and the denominator indicates the total number of colonies enumerated on day 9 of culture.
Phenotypic analysis of AC133+ cells strongly suggested that primitive repopulating stem cells were included within the AC133+/CD34+ population, which was confirmed by the data presented here from bone marrow analysis of sheep made chimeric with human AC133+ cells.
DISCUSSION
Human hematopoietic stem and progenitor cells share a large number of immunogenic cell surface structures with mature hematopoietic cells.46,47 Molecules such as HLA class I and II, β1 and β2 integrins, CD26, CD31, CD43, CD44, CD45, CD50, CD53, and CD71 are examples of structures expressed on CD34+ cells, and these can dominate the immune response to a level where less immunogenic/novel molecules are not recognized by the host animal. Other investigators have approached this problem with techniques such as subtractive immunization,50,51 where animals are exposed to the tolerizing agent cyclophosphamide to suppress the immune response to immunodominant antigens. Our approach to this problem has been a contralateral footpad immunization procedure in mice, where the left footpad is injected with mature hematopoietic cells that express many of the antigens expressed on progenitors and the right footpad is injected with CD34+ progenitors. This system relies on the ability of antigen-specific lymphocytes to traffic to the site of initial antigenic stimulation. Therefore, in this example, the left footpads of the mice were injected on days −3, 0, 3, 6, 9, 13, 17, and 20 with PBMCs, while the right footpads were injected with purified progenitors on days 0, 3, 6, 9, 13, 17, and 20. Antigen-specific lymphocytes specific for shared antigens were decoyed to the left popliteal lymph node, while lymphocytes draining to the right popliteal node were more likely to respond to less immunogenic and hopefully novel structures. This powerful immunization procedure combined with the utilization of human fetal liver cell populations that provide large numbers of CD34+ progenitors for screening has permitted the recognition of a novel MoAb, AC133, that recognizes only the CD34bright subset of human progenitors. This antibody reacts with only two human retinoblastoma cell lines and a teratocarcinoma cell line from a panel of 46 human cell lines tested. It is of interest that we have not encountered a cultured cell line that is double-positive for both AC133 and CD34. CD34 and AC133 antigens are coexpressed on primitive progenitors and on some leukemias,41 and therefore, both can be considered early antigens. However, the lack of reactivity of AC133 antibody to endothelial cells or fibroblasts, together with the cell line staining data and immunohistology,41 demonstrates that the AC133 antigen is restricted to stem cells and possibly a few other tissues such as retina, pancreatic islet cells, and placenta. While protein expression has not been demonstrated on these last three tissues, ESTs (expressed sequence tags) derived from human tissues such as retina, pancreatic islets, and placenta matching the AC133 gene sequence are present in Genebank.41 Therefore, although progenitor cells coexpress these two early antigens, they appear to share no other known relationship, as the function of either molecule is not known and the expression on other cells and tissues and the degree of antigen glycosylation and protein structure41 are markedly different.
Expression of the AC133 antigen has been shown to be restricted to human progenitors and to a teratocarcinoma cell line, NT2, as well as two retinoblastomas, using FACS analysis of multiple primary and continuous cell lines. The two retinoblastoma lines are composed of neuron-committed cells and are karyotypically abnormal.52 NT2 cells from a human malignant testicular germ cell tumor may contain elements that resemble extraembryonic tissues of the trophoblast, yolk sac, or various embryonic tissues. NT2 cells have been reported to represent a committed neuronal precursor stage of differentiation,53,54 which differentiate to mature neurons on induction with retinoic acid. The differentiated cells, called hNT neurons, lose neuroepithelial markers such as VLA-4 (CD49d) and cytokeratins detected by the antibody CAM-5.2 (A.Y. and D.B., unpublished data, September 1996). AC133 antigen expression is undetectable on hNT neurons, and therefore appears to be a very early antigen, which is rapidly lost during cell differentiation. No sequence similarity with known tyrosine kinase receptor families or with other multiple transmembrane antigens has been found.41
We have shown that AC133 is expressed on a population of stem and progenitor cells that contain all of the CD34bright and CD38− progenitors, as well as the CD34bright cells committed to the granulocytic/monocytic pathway. These cells engraft and home to the bone marrow of fetal sheep in a xenogeneic transplantation model. Moreover, cells harvested from primary sheep recipients successfully engraft secondary recipients, demonstrating the long-term repopulating potential of AC133-derived progenitors. One surprising finding in this series of experiments was that the majority of the CFU-Mix population, which is generally considered to be a primitive population of progenitors, were found in the CD34dim/AC133− fraction. In the murine system, single CD34dim cells have been shown to reconstitute lethally irradiated mice,55 suggesting the possibility that the true stem cell may in fact be CD34− or CD34dim. Whether a similar cell type exists in man will require further study.
While the AC133 antigen has no known ligand or function at this time, we have shown it to have a novel expression pattern and 5-transmembrane structure.41 Downregulation to undetectable levels in mature hematopoietic cells and on terminally differentiated teratocarcinoma cells, as described for growth factor receptors such as fibroblast growth factor receptor,56 may suggest a functionally restricted role for this molecule. Alternatively, the presence of a leucine zipper motif in the second extracellular loop of the AC133 molecule41 may suggest a role in protein-protein interactions, perhaps with bone marrow stromal antigens. The AC133 antigen system that provides all CD34+/CD38− primitive cells, including presumably the SCID repopulating cell57 as well as CFU-GM needed for short-term engraftment,58 may provide an alternative to the widely used CD34 antigen in hematopoietic stem cell manipulations.
ACKNOWLEDGMENT
We thank Kerry MaGee and Gary Frenzel for excellent technical assistance, and Michael McGrogan for the generous gift of NT-2 cells and hNT neurons.
Supported in part by National Institutes of Health Grants No. A135940, HL52955, HL49042, and DK51427.
Reprint requests to David W. Buck, AmCell Corp, 1190 Bordeaux Dr, Sunnyvale, CA 94089.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal