Abstract
Erythropoietin (EP) is required by late-stage erythroid progenitor cells to prevent apoptosis. Several lines of evidence suggest that it is this action of EP that regulates erythrocyte production in vivo. To study the control of apoptosis in mouse and human erythroblasts, the expression of members of the Bcl-2 family of proteins and the expression and activation of the apoptosis-linked cysteine protease Yama/CPP32/apopain were examined. These proteins have been implicated as regulators of apoptosis in several cell models. The Bcl-2 family members analyzed were Bcl-2, Bcl-X, Bax, Bad, Bak, A1, and Mcl-1. Bcl-X expression in proerythroblasts was highly EP-dependent. Bcl-X was strongly increased during the terminal differentiation stages of human and mouse erythroblasts, reaching maximum transcript and protein levels at the time of maximum hemoglobin synthesis. This increase in Bcl-X expression led to an apparent level of approximately 50 times the level in proerythroblasts. In contrast, neither mouse nor human erythroblasts expressed Bcl-2 transcript or protein. Bax and Bad proteins remained relatively constant throughout differentiation, but diminished near the time of enucleation. Bak protein was present in early erythroblasts, but diminished progressively during differentiation. EP deprivation in both mouse and human erythroblasts led to activation of the cysteine protease, apopain, as was indicated by cleavage of the proenzyme into its proteolytically active fragments. Apopain activation was detectable within 2 hours of EP deprivation in mouse erythroblasts. These findings suggest an important role for Bcl-X in late erythroid differentiation and for apopain in apoptosis of erythroblasts caused by deprivation of EP.
ERYTHROPOIETIN (EP) is the principal growth factor involved in the development of late-stage erythroid progenitors into erythrocytes. The effects of EP appear to be limited to a specific window of differentiation stages, because it has been shown that neither EP nor its receptor is necessary for erythroid lineage commitment or for proliferation of burst-forming unit-erythroid (BFU-E) progenitors.1-4 Furthermore, the EP receptor is downregulated in the late basophilic erythroblast stage,5-8 and EP has no effect on the final maturation of the cells.9 Thus, EP exerts its primary effect on mature BFU-E, colony-forming units-erythroid (CFU-E), and early erythroblasts. With EP deprivation, these erythroid progenitors undergo programmed cell death, or apoptosis.10-13 A model for regulation of erythropoiesis has been proposed in which the apoptosis or survival of EP-sensitive progenitor cells is determined by circulating EP levels.10 14 However, little is known about how EP serves to prevent apoptosis in erythroid progenitor cells.
The bcl-2 and interleukin-1β (IL-1β)–converting enzyme (ICE) gene families serve to regulate apoptosis in many eukaryotic cells.15-17 Furthermore, genes of the bcl-2 and ICE families have sequence homologies and functional similarities with ced9 and ced3, respectively (genes identified as apoptosis regulatory genes in the nematode Caenorhabdis elegans ). These relationships give weight to the notion that members of these gene families are part of a general pathway of apoptosis.18bcl-2, originally discovered as a gene activated in a particular class of human lymphomas, was shown to enhance the survival of the cells in which it was overexpressed. The Bcl-2 protein is a 26-kD protein with a transmembrane domain and unique structural domains that is located in the inner mitochondrial membranes and other specific membrane fractions of cells.19 The mechanism of Bcl-2 action is unclear, although some studies indicate that it exerts a protective effect against oxidative damage.20-22 Other genes were discovered that encode proteins with structural domains similar to bcl-2 and thus constitute a related family of genes.15,23 The protein Bcl-XL , a member of the Bcl-2 family, is a 29.2-kD protein that is also located in the inner mitochondrial membranes of cells and exerts a protective effect akin to Bcl-2 in promoting survival in cells exposed to apoptosis-inducing agents.24-26 Whereas Bcl-2, Bcl-XL , and some other family members have apoptosis-preventing properties, other members such as Bax induce apoptosis when expressed at high levels in cells. One current hypothesis is that competing dimer interactions of apoptosis-preventing and apoptosis-inducing members of this family may be involved in regulation of apoptosis in many cell systems.27
ICE encodes an inactive proenzyme form of a cysteine protease of unusual cleavage specificity that, when activated, is involved in the conversion of the peptide prointerleukin-1β to IL-1β.28 ICE was subsequently shown to have sequence homology to the ced3 death gene in C elegans29 and was shown to induce apoptosis in cell lines in which it was introduced and expressed.30 ICE was the first example discovered of a larger family of these apoptosis-inducing cysteine proteases that includes Yama/CPP32/apopain.16,17 This latter protease cleaves a number of nuclear proteins, most prominently the poly(ADP ribose) polymerase (PARP), an important DNA repair enzyme not cleaved by ICE.31 Apopain, like ICE, exists in the cell as a 32-kD inactive proenzyme, CPP32. The CPP32 proenzyme is cleaved into two subunits of 17 and 12 kD that associate to form the activated apopain complex.31 Evidence has been presented that apopain activation regulates apoptosis associated with confluence in an osteosarcoma cell line,31 in apoptosis mediated by Fas ,32 and in apoptosis induced by DNA-damaging agents.33 It appears feasible that regulation of apopain activity is key to the DNA fragmentation and nuclear changes associated with apoptosis in general.
Overexpression of certain members of the Bcl-2 family (Bcl-2, Bcl-XL , and Mcl-1) can serve to rescue cells of hematopoietic origins from stimuli that normally induce apoptosis.20,34,35 It has been recently shown that bcl-2 and bcl-x are expressed in a cell line derived from the erythroid lineage and are associated with EP exposure and continued cell survival.36,37 In HCD 57 cells, a mouse erythroleukemia cell line dependent on EP for survival, it was observed that levels of Bcl-2 and Bcl-X were downregulated upon EP deprivation.37 Furthermore, it was shown that HCD 57 cells could be rescued from apoptosis induced by EP deprivation by ectopic expression of Bcl-XL .37 However, the expression of genes of the bcl-2 family has not been analyzed in explanted erythroid progenitor cells that are fully capable of differentiation into reticulocytes.
Using both mouse and human primary erythroblast systems, we have examined the time course and the EP-dependence of expression of members of the Bcl-2 family during development through the reticulocyte stage. We have also shown that the CPP32 proenzyme is present in both cell systems, and we have examined the effect of EP deprivation on the cleavage of the inactive CPP32 proenzyme into the active form of apopain. The principal cell systems used in the study were early erythroblasts derived from spleens of mice infected with the anemia-inducing strain of Friend virus (FVA cells)38 and human erythroid colony-forming cells (Hu-ECFCs) derived by in vitro culture of partially purified peripheral blood BFU-E.39 The murine FVA cell system is a useful model for studying erythroid progenitors. These cells, which can be obtained in large numbers (2.5 to 5.0 × 108) at a purity of 95% to 98%, consist of murine CFU-Es and early erythroblasts. FVA cells require EP for survival and can differentiate through the reticulocyte stage in the presence of EP. In addition to FVA cells, some confirmatory experiments were performed with spleen cells from uninfected mice that had been made severely anemic by phlebotomy. The human ECFC system consists of normal human cells that are very similar to FVA cells in their stage of development. However, the mouse cells require only 2 days in culture to complete differentiation into reticulocytes, whereas in the human cells the same differentiation process requires 5 to 7 days.39 40
MATERIALS AND METHODS
Sources of plasmid clones and antibodies.Clones of cDNAs of murine bcl-2 and murine bax were obtained from Dr S.J. Korsmeyer (Washington University, St Louis, MO). A clone of mouse bcl-x (long) cDNA was obtained from Dr C.B. Thompson (University of Chicago, Chicago, IL), and a cDNA clone for murine A1 was obtained from Dr Amos Orlofsky (Albert Einstein College of Medicine, New York, NY). The β-globin clone was a genomic HindIII fragment containing the first two exons of the β major globin gene. For use as probes, each of the cloned gene fragments was cut from the plasmid with appropriate restriction enzymes and purified by agarose gel electrophoresis.
The primary antibodies used in Western blots were rabbit polyclonal anti–Bcl-XL (human), mouse monoclonal anti-Bad (mouse), and mouse monoclonal anti–Mcl-1 (human) from Transduction Laboratories (Lexington, KY); rabbit polyclonal anti-Bax (human) and mouse monoclonal anti–Bcl-2 (human) from UBI (Lake Placid, NY); and mouse monoclonal anti-Bak (human) from Oncogene Research Products (Cambridge, MA). Rabbit polyclonal anti-A1 (mouse) antibody was provided by Dr Amos Orlofsky. Rabbit polyclonal anti-CPP32/apopain (human) antibody was provided by Dr Donald W. Nicholson (Merck Frosst Research Center, Pointe Clair-Dorval, Quebec, Canada).
FVA cell isolation and culture.Nucleated cells were isolated from spleens of 8- to 12-week-old CD2F1 mice that had been infected 2 weeks earlier with 104 spleen focus-forming units of the anemia-inducing strain of Friend virus (FVA). Erythroblasts (FVA cells) were separated by velocity sedimentation at unit gravity on continuous 1% to 2% bovine serum albumin (BSA) gradients as previously described.38 FVA cells were cultured at 106 cells/mL in Iscove's modified Dulbecco's medium (IMDM) containing 1% deionized BSA (Intergen Co, Purchase, NY), 30% fetal bovine serum (HyClone Laboratories, Logan, UT), 0.1 mmol/L α-monothioglycerol, and (where indicated) 5 μ/mL of pure recombinant EP (Ortho Pharmaceuticals, Raritan, NJ). For some experiments, spleen cells were isolated from uninfected mice that had been subjected 48 hours earlier to phlebotomy. The hematocrits of these bled mice were 25 ± 5 (standard deviation), and the spleen cells were processed and cultured as described for FVA cells.
Blood.Blood was obtained from normal human volunteers after informed consent was given. These studies were approved by the Vanderbilt University and Department of Veterans Affairs Medical Center Institutional Review Boards. Approximately 400 mL of peripheral blood was collected in sodium heparin at a final concentration of 20 U/mL.
Generation of human day-8 ECFCs.ECFCs were prepared by a modified method previously described.39 Light-density mononuclear cells were obtained by density centrifugation using Ficoll-Hypaque (1.077 g/mL). Platelets were removed by cell centrifugation through phosphate-buffered saline containing 10% BSA. This was followed by T-lymphocyte depletion using sheep erythrocyte rosetting and by adherent cell depletion with overnight incubation in plastic tissue culture flasks at 37°C. Negative panning was then performed using CD11b/OKM*1, CD2OKT*11, CD45/MY11, and CD16/MY23 monoclonal antibodies to purify BFU-E to approximately 0.4%. The remaining cells were cultured in IMDM medium containing 0.9% methylcellulose, 30% fetal bovine serum, 0.5% deionized BSA, recombinant human EP (rEP; 2 U/mL), recombinant human IL-3 (rIL-3; 50 U/mL; Amgen, Thousand Oaks, CA), 10−4 mol/L 2-mercaptoethanol, 100 U/mL penicillin, and 40 μg/mL streptomycin at 37°C in a high humidity 5% CO2 /95% air incubator for 8 days to generate ECFCs (day-8 ECFCs). The cells were then collected from the methylcellulose and ECFCs were further enriched by centrifugation through 10% BSA and then by Ficoll-Hypaque density separation. This preparation resulted in populations of viable erythroblasts that were approximately 80% CFU-E by bioassay. Cells were resuspended and cultured with or without EP at concentrations of 106 cells/mL in culture medium as described for FVA cells.
RNA isolation and Northern analysis.Total RNA from FVA cells was extracted by a single-step guanidium thiocyanate-phenol method using RNAzol.41 Electrophoresis of RNA was performed in 6.0% formaldehyde/1.0% agarose gels. Each lane contained 20 μg of RNA. Immediately before electrophoresis, 10 μg of ethidium bromide was added to each sample. After electrophoresis, the gels were photographed and blotted onto charged nylon membrane (Hybond-N+; Amersham Life Sciences, Arlington Heights, IL). Prehybridizations and hybridizations and subsequent washes were performed as described previously.8 DNA probes were labeled by the random primer labeling method using [α-32P]dCTP (800 Ci/mmol) and purified cDNA insert from the appropriate plasmid (bcl-2, bcl-x, bax, A1, or β-globin). The probe for 28S RNA was an oligonucleotide DNA that was 5′-end–labeled with [γ-32P]dATP and T4 polynucleotide kinase. When required, blots were stripped of the radiolabeled probe by washing two times for 15 minutes in hot (>75°C) 0.01× SSC (1.5 mmol/L NaCl, 0.15 mmol/L Na3 citrate, pH 7.0), 1% sodium dodecyl sulfate (SDS) stripping buffer. Quantitation of the autoradiographic signals were performed using a LKB Bromma laser scanning densitometer (Pharmacia LKB Biotechnologies, Piscataway, NJ). The relative amounts of total RNA present in each lane were estimated by scanning an autoradiographic hybridization signal of the 28S RNA band hybridized with a probe specific for 28S RNA. Then, for each lane, the autoradiographic hybridization signals obtained for test probes were normalized to the amount of total RNA present in each lane. The signals on all the autoradiograms were within the linear range of the densitometer. Control experiments also showed that the estimates of RNA amounts based on the autoradiographs were linear with respect to the actual amounts of RNA loaded onto the gels.
Reverse transcriptase polymerase chain reaction (RT-PCR).After annealing 0.3 μg of oligo (dT) 18-mer to 7.5 μg of total cell RNA, cDNA was synthesized using the SuperScript 2.0 Reverse Transcriptase Kit (GIBCO BRL Life Technologies, Gaithersburg, MD). After synthesis of cDNA, the reaction mixture was divided into three aliquots and used directly in PCR with the three primer sets described below. PCR was performed using Taq DNA Polymerase (GIBCO BRL Life Technologies) following the protocol suggested by the manufacturer. Three bcl-x primer sets were used. The upstream primer 5′-GGAGAGCGTTCAGTGATC-3′ and the downstream primer 5′-CAATGGTGGCTGAAGAGA-3′ for mouse bcl-x,25 and the upstream primer 5′-TTGGACAATGGACTGGTTGA-3′ and the downstream primer 5′-GTAGAGTGGATGGTCAGTG-3′ used by Boise et al,24 all correspond to sequences in the mRNA for Bcl-XL . These primer sets should each amplify the mRNAs for the protein variants Bcl-XL , Bcl-XS , and Bcl-XΔTM . The upstream primer 5′-AAATGTCTCAGAGCAACCG-3′ and downstream primer 5′-GGCCTGAACAATCGGTATCT-3′ were used as described26 to amplify mRNA for Bcl-Xβ . PCR products were separated on a 2.5% NuSieve 3:1 agarose gel (FMC Bioproducts, Rockland, ME) in the presence of 0.5 μg/mL of ethidium bromide and photographed.
Protein sample preparation and Western blot analysis.Samples used in Western blots were whole cell lysates prepared in 1× Laemmli sample buffer (.0625 mol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% 2-mercaptoethanol).42 Harvested cells were counted, pelleted, and washed once in 0.1% BSA in IMDM before repelleting. Pelleted cells were lysed in 2× Laemmli buffer, and DNA was sheared by passing several times through an 18-gauge blunt needle. Samples were then diluted with an equal volume of water to make the buffer composition that of 1× Laemmli sample buffer. The final lysates contained extracts from 2 × 106 FVA cells/40 μL sample buffer or extracts from 5 × 105 human cells/40 μL sample buffer. The lysates were subjected to sonication for 2 minutes using a 60 Sonic Dismembrator sonifier (Fisher Scientific, Pittsburgh, PA) with a microtip. Samples of mouse and human cell proteins (40 μL/sample) were subjected to electrophoresis on a 1.5-mm–thick, 12.5% SDS-polyacrylamide gel electrophoresis separating gel and a 4.0% stacking gel,43 unless otherwise specified. Nitrocellulose (BA-S 83; Schleicher & Scheull, Keene, NH) or polyvinylidene difluoride (PVDF; Hybond; Amersham) membranes were used to capture the proteins electrophoretically transferred from the gels. Analysis of proteins on blots was performed by Western blotting procedures as described.43 Blots were incubated with the primary antibodies. The bound primary antibody on the blot was then detected by the appropriate peroxidase-conjugated secondary antibody (antimouse or antirabbit Ig) and chemiluminescence detection procedures of the ECL Western blotting analysis system (Amersham). The HL60 cell line was used as a positive control for Bcl-XL , Bcl-2, Bax, Bak, and Mcl-1. As positive controls, extracts of 2.5 × 105 HL60 cells in 20-μL aliquots were loaded per lane, unless otherwise specified. Quantification of band intensity was performed by use of the laser scanning densitometer.
RESULTS
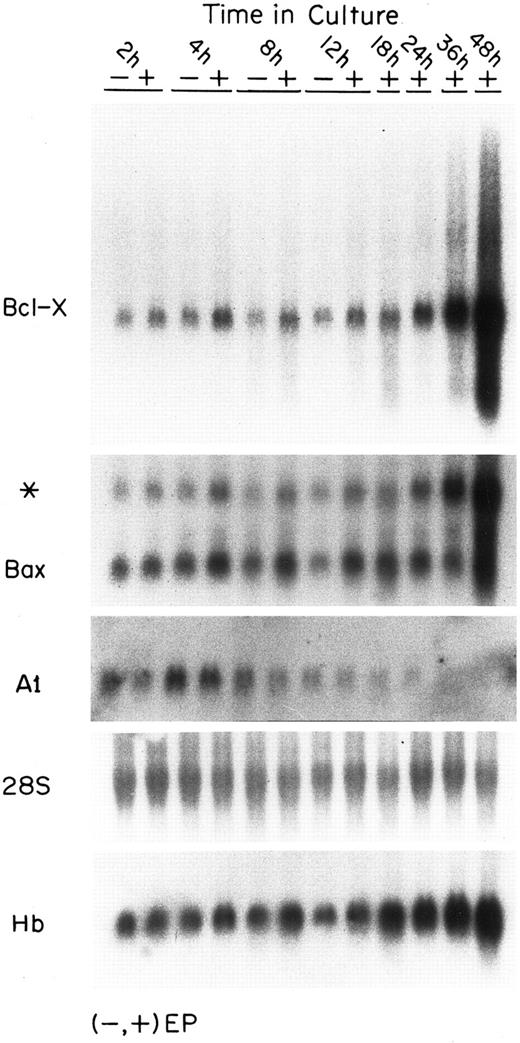
Levels of mRNAs for bcl-2 family genes in EP-treated and EP-deprived FVA cells.Figure 1 shows the mRNA levels in FVA cells for several members of the bcl-2 gene family. During the initial 12 hours of culture, mRNA levels are shown for cells with and without EP. At later times, only EP-treated cell samples are shown because extensive cell death occurs after 12 hours in EP-deprived cell populations. Bcl-X mRNA levels in the EP-treated cells increased approximately threefold during the first 4 hours and that level was sustained at each time point for about 24 hours (see quantification, Fig 4). Between 24 and 48 hours, Bcl-X mRNA levels increased to a final level 24 times higher than initial levels. In the absence of EP, the Bcl-X mRNA level remained constant during 12 hours of culture. The mRNA levels for Bax, a member of the Bcl-2 family that induces apoptosis, increased threefold in the presence of EP and remained at that level for 36 hours, after which it declined by 48 hours. Bax mRNA did not increase in the EP-deprived cells. An additional Northern blot analysis of mRNAs for Bcl-X and Bax was performed in a separate experiment using RNAs isolated from another preparation of FVA cells. The quantitative changes in the mRNAs were similar at each time point to those observed in the experiment shown. Approximately threefold elevations in Bcl-X and Bax mRNAs over baseline values were evident at 4, 8, and 12 hours in both experiments. Thus, this early elevation was observed in a total of six experimental measurements and appears to be reproducible. The later much more prominent increase in mRNA for Bcl-X is likewise reproducible. mRNA for A1, an antiapoptotic protein expressed in some hematopoietic cells, was also detected in FVA cells.44 A1 transcript levels were low compared with bcl-x and bax transcripts, and they declined significantly in the first few hours of development. Figure 1 also shows an analysis of β-globin mRNA demonstrating the time course of accumulation of that transcript during FVA cell differentiation.
Expression of mRNAs for several Bcl-2–related genes in FVA cells. Each panel shows the steady-state levels of mRNA for the indicated gene. All panels represent the same blot probed successively with various probes. Each lane contained 20 μg of total RNA taken from FVA cells cultured with or without EP and harvested at the designated time points. The first panel shows the blot probed with a bcl-x probe. The second panel shows the blot reprobed without stripping with a bax probe (* band is residual bcl-x probe activity). The third panel shows the blot after stripping and reprobing with an A1 probe. The probes for bcl-x,bax, and A1 were approximately equal in size (∼600 bp) and all labeling and hybridization conditions were similar. Film exposure times were 24 hours for bcl-x and bax and 172 hours for A1. The fourth panel shows the blot stripped and reprobed with a 28S rRNA probe. The bottom panel shows the blot stripped and reprobed with a β-globin probe (designated Hb).
Expression of mRNAs for several Bcl-2–related genes in FVA cells. Each panel shows the steady-state levels of mRNA for the indicated gene. All panels represent the same blot probed successively with various probes. Each lane contained 20 μg of total RNA taken from FVA cells cultured with or without EP and harvested at the designated time points. The first panel shows the blot probed with a bcl-x probe. The second panel shows the blot reprobed without stripping with a bax probe (* band is residual bcl-x probe activity). The third panel shows the blot after stripping and reprobing with an A1 probe. The probes for bcl-x,bax, and A1 were approximately equal in size (∼600 bp) and all labeling and hybridization conditions were similar. Film exposure times were 24 hours for bcl-x and bax and 172 hours for A1. The fourth panel shows the blot stripped and reprobed with a 28S rRNA probe. The bottom panel shows the blot stripped and reprobed with a β-globin probe (designated Hb).
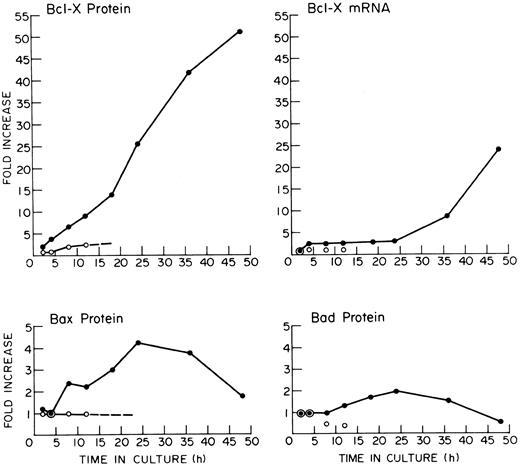
Quantification of Bcl-X, Bax, and Bad and of Bcl-X mRNA in FVA cells. The results were derived by laser densitometry of autoradiographs from experiments depicted in Figs 1 and 3. (•) Cells cultured with EP; (○) cells cultured without EP.
Quantification of Bcl-X, Bax, and Bad and of Bcl-X mRNA in FVA cells. The results were derived by laser densitometry of autoradiographs from experiments depicted in Figs 1 and 3. (•) Cells cultured with EP; (○) cells cultured without EP.
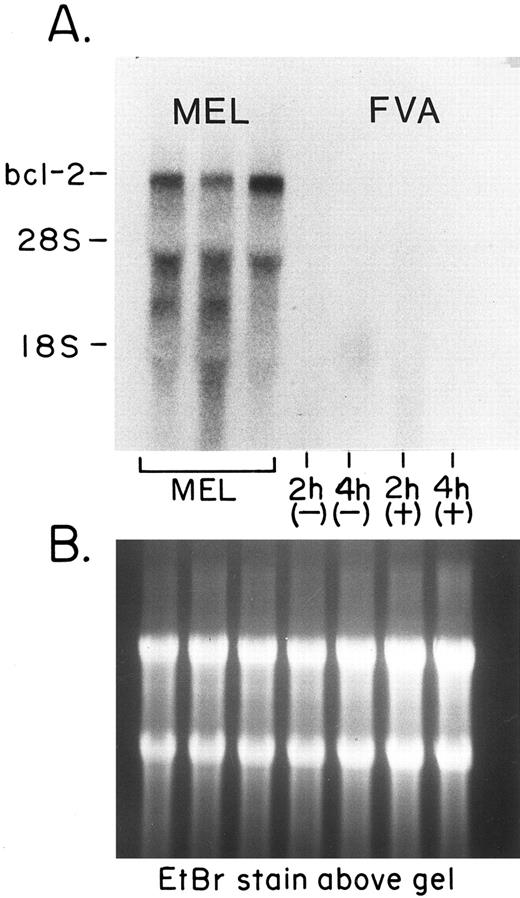
Northern analysis of bcl-2 transcript levels in EP-treated and EP-deprived FVA cells consistently indicated the absence of such transcripts. Figure 2 shows that Bcl-2 mRNA is present at high levels in a mouse erythroleukemia (MEL) cell line, but not in FVA cells. Even in Northern analyses that used up to 10 μg of poly A+ RNA from FVA cells, no mRNA for Bcl-2 could be detected (data not shown).
Lack of bcl-2 mRNA in FVA cells. (A) The steady state levels of mRNA detected by a probe specific for bcl-2. The first three lanes contained RNA taken from cells of a MEL cell line. From left to right, each lane contained 20 μg of total cellular RNA from control cells and from cells after 4 and 5 days of exposure to 2% dimethyl sulfoxide (added to induce differentiation of the cells). The last four lanes on the right each contained 20 μg of total RNA taken from EP-treated (+) or EP-deprived (−) FVA cells at 2 and 4 hours. (B) A photograph of the formaldehyde/agarose gel stained with EtBr to show the RNA in the lanes.
Lack of bcl-2 mRNA in FVA cells. (A) The steady state levels of mRNA detected by a probe specific for bcl-2. The first three lanes contained RNA taken from cells of a MEL cell line. From left to right, each lane contained 20 μg of total cellular RNA from control cells and from cells after 4 and 5 days of exposure to 2% dimethyl sulfoxide (added to induce differentiation of the cells). The last four lanes on the right each contained 20 μg of total RNA taken from EP-treated (+) or EP-deprived (−) FVA cells at 2 and 4 hours. (B) A photograph of the formaldehyde/agarose gel stained with EtBr to show the RNA in the lanes.
Analyses of Bcl-2–related proteins in FVA cells and Hu-ECFCs.Western blot analysis of FVA cells cultured with EP for increasing periods of time (Fig 3) showed a steady accumulation of Bcl-X protein. The increase occurred in an almost linear fashion in EP-treated FVA cells (Fig 4). The level was significantly increased by 4 hours, was increased 25-fold by 24 hours, and was increased 55-fold by 48 hours (Fig 4). EP-deprived cells maintained a low level of Bcl-X protein over 12 hours of culture. The chemiluminescence that is quantified in such experiments may not be directly proportional to the protein recognized by the primary antibody. Thus, the stated fold increases in Bcl-X protein are estimates subject to errors due to possible nonlinearity of signal generation and measurement. Nevertheless, the experiments do indicate a strong increase in Bcl-X during FVA cell differentiation. Bcl-X was represented in all Western blots as a doublet at 29 kD, the expected size for the full-length protein form Bcl-XL . There was no evidence for the presence of smaller variant forms such as Bcl-XS , Bcl-Xβ , or Bcl-XΔTM .25,26 Bax protein appeared to be strongly expressed in freshly isolated FVA cells. Bax protein levels increased about fourfold in the presence of EP and were maintained through 36 hours, after which they declined, but did not disappear by 48 hours. Bax protein levels in EP-deprived cells remained static throughout the 12-hour period examined. Bad, a 22.1-kD apoptosis-inducing protein, was detected as a double band in FVA cells. In the presence of EP, Bad increased twofold, peaking by 24 hours and disappearing by 48 hours. In the absence of EP, Bad levels declined to 50% of initial levels over 12 hours. Thus, EP stimulation was associated with the maintenance of Bad expression. The higher molecular weight band of the Bad doublet appeared in the EP-treated lanes but was absent in the EP-deprived lanes. This doublet band was first observed for Bad in an IL-3–dependent cell line by Yang et al.45 The slower migrating band may be a phosphorylated form of the protein, whose presence apparently depends on growth factor stimulation. Antibodies prepared against human Mcl-1 detected Mcl-1 protein at very low levels in the EP-treated FVA cells but not in the EP-deprived FVA cells (data not shown). Detectable levels of Mcl-1 protein were not present in EP-treated cells past 24 hours. Antibodies prepared against human Bak did not detect protein in blots of any of the FVA cell lysates. Also, although A1 mRNA transcripts were detected at low levels in FVA cells (Fig 1), antibody to mouse Al did not detect the protein in Western blots. Finally, Western analysis did not detect any Bcl-2 protein in FVA cells (Fig 3), although it was easily detected in the HL60 cell positive controls, confirming the negative results of the previous Northern analyses.
Western blot analysis of Bcl-2 protein family in FVA cells. The left side of each panel shows the protein levels after 2, 4, 8, and 12 hours of EP-deprived (−) and EP-exposed (+) FVA cells. The right side of each panel shows the long-term developmental expression of proteins in EP-treated cells. These lanes depict cells cultured with EP for 4, 12, 18, 24, 36, and 48 hours. The top set of panels shows blots probed simultaneously using 5 μg each of rabbit polyclonal anti–Bcl-x and anti-Bax antibodies: Bcl-X (29.2 kD) and Bax (21 kD). The second set of panels shows blots probed using 5 μg of mouse monoclonal anti-Bad antibody: Bad (22.1 kD). The bottom set of panels shows blots probed using 5 μg of mouse monoclonal anti–Bcl-2 antibody: Bcl-2 (26 kD). Far right lanes of each blot of the bottom panel contain proteins from HL60 cells that were used as a positive control for Bcl-2 (26 kD).
Western blot analysis of Bcl-2 protein family in FVA cells. The left side of each panel shows the protein levels after 2, 4, 8, and 12 hours of EP-deprived (−) and EP-exposed (+) FVA cells. The right side of each panel shows the long-term developmental expression of proteins in EP-treated cells. These lanes depict cells cultured with EP for 4, 12, 18, 24, 36, and 48 hours. The top set of panels shows blots probed simultaneously using 5 μg each of rabbit polyclonal anti–Bcl-x and anti-Bax antibodies: Bcl-X (29.2 kD) and Bax (21 kD). The second set of panels shows blots probed using 5 μg of mouse monoclonal anti-Bad antibody: Bad (22.1 kD). The bottom set of panels shows blots probed using 5 μg of mouse monoclonal anti–Bcl-2 antibody: Bcl-2 (26 kD). Far right lanes of each blot of the bottom panel contain proteins from HL60 cells that were used as a positive control for Bcl-2 (26 kD).
In addition to FVA cells, Bcl-X protein expression was also examined in cultured spleen cells of uninfected mice that had been rendered severely anemic by phlebotomy 48 hours before harvesting of their spleens (data not shown). When cultured with EP over a period of 24 hours, these cells also exhibited a strong increase in Bcl-X (∼10-fold). Such cell populations contain a large fraction of erythroblasts, but compared with FVA cells, there is a much larger portion of the initial cells that are late erythroblasts and there is significantly more contamination with lymphocytes and other cell types. Perhaps because of these factors, the initial level of Bcl-X present in spleen cells of anemic animals was higher than in FVA cells and the fold induction was slightly less than observed in FVA cells.
Western blot analysis of Bcl-2 family proteins in Hu-ECFCs.Hu-ECFCs (day-8 cells) were cultured with and without EP, and cells were harvested for analysis of proteins at several time points thereafter. Cells without EP were only analyzed after 24 hours of culture (day 9) because a large portion of the Hu-ECFC undergo apoptosis after longer periods. Figure 5 shows a steady accumulation of Bcl-X protein in human cells cultured with EP from day 8 through 13. Human ECFCs undergo differentiation over 5 to 6 days in culture rather than the 48 to 60 hours required for mouse FVA cells. It was shown that peak hemoglobin synthesis in this system is at day 10 to 11 and that by day 13 appreciable numbers of the cells form reticulocytes.40 Thus, in human ECFCs as in FVA cells, maximal Bcl-X expression occurs at or after the time of maximal hemoglobin synthesis. EP-deprived day-9 Hu-ECFCs had a low level of Bcl-X protein. In contrast to Bcl-X, the Bax protein levels in EP-treated Hu-ECFCs were high on day 9 and then declined as the cells differentiated. Bax was still present in EP-treated Hu-ECFCs at day 13. EP-deprived day-9 Hu-ECFCs expressed a high level of Bax protein, although it was somewhat higher in EP treated day-9 cells.
Western blot analysis of Bcl-2 family in Hu-ECFCs. Hu-ECFCs were purified and recultured on day 8 (see Materials and Methods) with or without EP. The lettering above the panels indicates the day at which cells were subsequently removed from culture for analysis and whether they were cultured with (+) or without (−) EP. In the top panel, both lanes marked by asterisks contained proteins from HL60 cells as positive controls. In the bottom three panels, only the lane on the far right (*) contained proteins from HL60 cells as positive controls. The top panel shows Bcl-x (29.2 kD) probed using 5 μg rabbit polyclonal anti–Bcl-x antibody. The second panel shows Bax (21 kD) probed using 5 μg rabbit polyclonal anti-Bax antibody. The third panel shows Bak (23 kD, runs at 29 kD) probed using 5 μg mouse monoclonal anti-Bak antibody. The bottom panel shows Bcl-2 (26 kD) probed using 5 μg mouse monoclonal anti–Bcl-2 antibody.
Western blot analysis of Bcl-2 family in Hu-ECFCs. Hu-ECFCs were purified and recultured on day 8 (see Materials and Methods) with or without EP. The lettering above the panels indicates the day at which cells were subsequently removed from culture for analysis and whether they were cultured with (+) or without (−) EP. In the top panel, both lanes marked by asterisks contained proteins from HL60 cells as positive controls. In the bottom three panels, only the lane on the far right (*) contained proteins from HL60 cells as positive controls. The top panel shows Bcl-x (29.2 kD) probed using 5 μg rabbit polyclonal anti–Bcl-x antibody. The second panel shows Bax (21 kD) probed using 5 μg rabbit polyclonal anti-Bax antibody. The third panel shows Bak (23 kD, runs at 29 kD) probed using 5 μg mouse monoclonal anti-Bak antibody. The bottom panel shows Bcl-2 (26 kD) probed using 5 μg mouse monoclonal anti–Bcl-2 antibody.
The expression of other members of the Bcl-2 family was examined in Hu-ECFCs. Low levels of Bak protein, a 23-kD apoptosis-inducing member of the Bcl-2 family,46 was detected in Hu-ECFCs. Bak levels were higher in day-9 EP-treated cells than in EP-deprived cells, but decreased with development and were nearly gone by day 13. A low-level, EP-dependent expression of Mcl-1 was detected in Hu-ECFCs. Mcl-1 levels were higher in day-9 EP-treated cells than in EP-deprived cells, but the expression of Mcl-1 rapidly decreased with development and was gone by day 13 (data not shown). As in the case of FVA cells, Bcl-2 protein was not present in Hu-ECFCs but was detected in the HL60 cell positive controls.
In Western blot analyses, Bak was detected in Hu-ECFCs but not in mouse FVA cells. Likewise, Bad was observed in mouse FVA cells but not in Hu-ECFCs. The primary anti-Bad antibody was prepared against mouse Bad and the primary anti-Bak was prepared against human Bak. It is likely that these antibodies did not bind to the proteins of the heterologous species under the conditions tested.
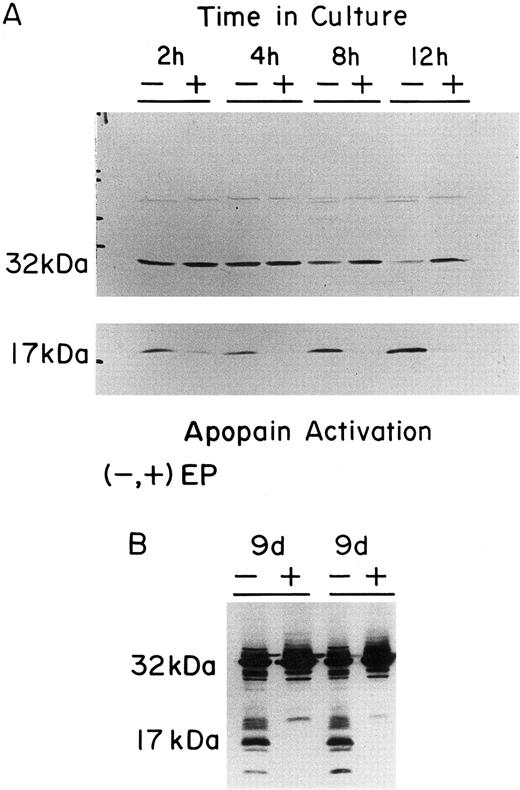
Activation of Yama/CPP32/apopain in both FVA cells and Hu-ECFCs in response to EP deprivation.Activation of the protein Yama/CPP32/apopain has been linked with the apoptotic process in several types of cells. Both FVA cells and Hu-ECFCs were examined to determine if apopain or its precursor was present and, if so, to determine if the generation of activated apopain occured in response to EP deprivation. Western blot analysis detected the CPP32 32 kD precursor in both FVA cells and the Hu-ECFCs (Fig 6). A distinct decrease in the amount of CPP32 32-kD precursor could be seen for both EP-deprived FVA cells and Hu-ECFCs, with a corresponding accumulation of its cleavage product, the 17-kD large subunit of activated apopain. In EP-deficient FVA cells, apopain activation occurred rapidly, with the 17-kD large subunit accumulating in as little as 2 hours after EP deprivation.
Western blot analysis of Yama/CPP32/Apopain in FVA cells and Hu-ECFCs. (A) The lettering at the top of the panels shows the time of culture of FVA cells (2, 4, 8, or 12 hours) without EP (−) or with EP (+). All cell samples were prepared as described and subjected to electrophoresis on a 15% acrylamide gel. The upper panel shows depletion of the 32-kD proenzyme over 12 hours without EP (30 seconds of film exposure). The lower panel shows accumulation of the 17-kD apopain large peptide in cells cultured without EP (4 minutes of film exposure). (B) The panel shows the cleavage of the 32-kD proenzyme that occurs upon EP deprivation of Hu-ECFCs. Day-8 Hu-ECFCs were cultured for 24 hours without or with EP (day-9 EP− or day-9 EP+). The results of two different preparations of cells are shown. The 32-kD and 17-kD bands are labeled. All blots were probed using 1 μL of the rabbit polyclonal antiapopain antibody.
Western blot analysis of Yama/CPP32/Apopain in FVA cells and Hu-ECFCs. (A) The lettering at the top of the panels shows the time of culture of FVA cells (2, 4, 8, or 12 hours) without EP (−) or with EP (+). All cell samples were prepared as described and subjected to electrophoresis on a 15% acrylamide gel. The upper panel shows depletion of the 32-kD proenzyme over 12 hours without EP (30 seconds of film exposure). The lower panel shows accumulation of the 17-kD apopain large peptide in cells cultured without EP (4 minutes of film exposure). (B) The panel shows the cleavage of the 32-kD proenzyme that occurs upon EP deprivation of Hu-ECFCs. Day-8 Hu-ECFCs were cultured for 24 hours without or with EP (day-9 EP− or day-9 EP+). The results of two different preparations of cells are shown. The 32-kD and 17-kD bands are labeled. All blots were probed using 1 μL of the rabbit polyclonal antiapopain antibody.
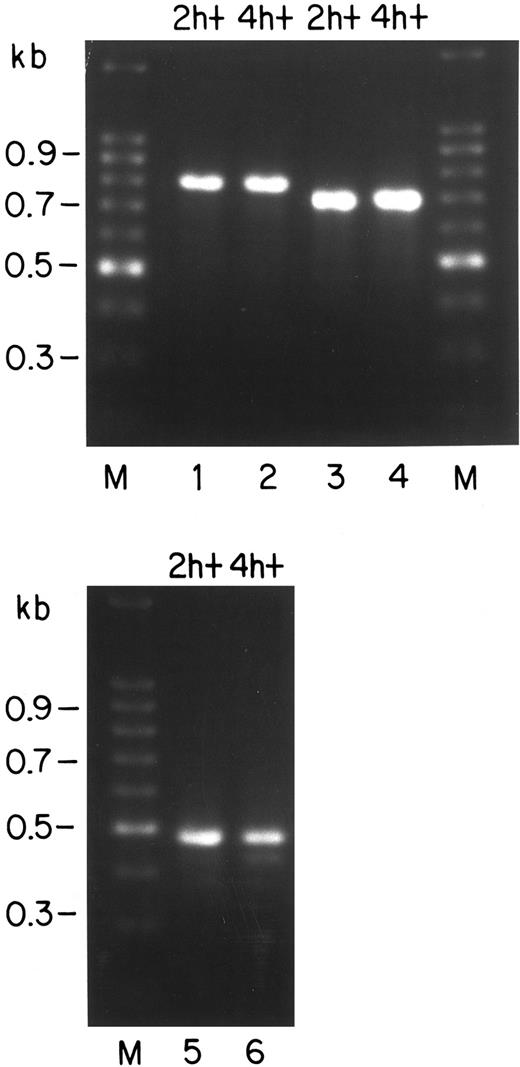
PCR analysis of the Bcl-x mRNAs in FVA cells.Several mRNAs have been described for the bcl-x gene that are generated by alternative splicing at or near the junction between the first and second protein coding exons.25,26 These alternative mRNAs code for proteins designated Bcl-XL , Bcl-XS , Bcl-XΔTM , and Bcl-Xβ . Bcl-XL and Bcl-XS possess the membrane-spanning domain necessary for placement in membranes, whereas the other variants are soluble (cytoplasmic) products. The mouse Bcl-XL product has been identified as the primary antiapoptotic form of the Bcl-X protein in mice.26 Bcl-XS , a variant described for some human cells, counteracts the protective action of Bcl-XL and causes apoptosis. We have analyzed the RNA from FVA cells by RT-PCR using three different primer sets to determine which mRNA species are present. Results are shown in Fig 7. Lanes 1 and 2 represent primers from the 5′ and 3′ untranslated regions of the mRNA for Bcl-XL .24 These primers have been shown to make PCR fragments from mRNAs of Bcl-XL and Bcl-XS with about equal efficiency,24 and they should also amplify mRNA for Bcl-XΔTM . In lanes 1 and 2, a product of the expected size for mRNA of Bcl-XL (780 bp) is seen, but no products are visible of the sizes expected for mRNAs for Bcl-XS (591 bp) or Bcl-XΔTM (710 bp). The primer set used for lanes 3 and 4 was chosen to amplify mRNA for Bcl-Xβ , the form generated by failure to splice out the intron between the coding exons for Bcl-XL (the downstream primer has sequence from the intron). A prominent PCR product of about 700 bp is present, which is the expected size for mRNA of Bcl-Xβ . A third set of primers24 was used that should yield products from mRNAs for Bcl-XL , Bcl-XΔTM , and Bcl-XS . In lanes 5 and 6, prominent bands corresponding to Bcl-XL (493 bp) are seen as well as a minor product (∼450 bp) of unknown origin; however, there are again no species corresponding to Bcl-XΔTM (423 bp) or Bcl-XS (304 bp). Based on these results, it is unlikely that the latter two mRNAs are present as more than a small fraction of bcl-x transcripts. It is unlikely that these mRNAs would fail amplification for technical reasons in a reaction that clearly has worked for amplification of Bcl-XL from common primers. Both primer sets have been used by others to show Bcl-XS and Bcl-XΔTM .24 25 We have not yet quantified the levels of mRNAs for Bcl-XL and Bcl-Xβ by a more quantitative method. Although both of these mRNAs are evident by PCR, we have not identified the protein Bcl-Xβ in Western blots (Fig 3) as a protein about 3 kD smaller than Bcl-XL .
RT-PCR identification of murine bcl-x transcripts expressed in FVA cells that were cultured for 2 or 4 hours with EP (designated 2 h+ and 4 h+, respectively). Reverse transcriptase was used to generate cDNA from total RNA of 2 h+ or 4 h+ cells. Each reverse transcriptase reaction was divided into three equal aliquots and subjected to PCR amplification using three primer sets. Marker lanes (M) show the 100-bp ladder (GIBCO-BRL), with sizes given on the left. Lanes 1 and 2 represent PCR with primers24 designed to amplify mRNAs for Bcl-XL (780 bp), Bcl-XS (591 bp), and Bcl-XΔTM (710 bp). Lanes 3 and 4 show samples generated with primers designed to detect mRNA containing an unspliced intronic sequence, ie, the mRNA for Bcl-X26β (expected size, ∼700 bp). Lanes 5 and 6 represent samples amplified by an additional set of primers designed to generate fragments from all three of the mRNAs for Bcl-XL (493 bp), Bcl-XS (304 bp), and Bcl-XΔTM (423 bp).25
RT-PCR identification of murine bcl-x transcripts expressed in FVA cells that were cultured for 2 or 4 hours with EP (designated 2 h+ and 4 h+, respectively). Reverse transcriptase was used to generate cDNA from total RNA of 2 h+ or 4 h+ cells. Each reverse transcriptase reaction was divided into three equal aliquots and subjected to PCR amplification using three primer sets. Marker lanes (M) show the 100-bp ladder (GIBCO-BRL), with sizes given on the left. Lanes 1 and 2 represent PCR with primers24 designed to amplify mRNAs for Bcl-XL (780 bp), Bcl-XS (591 bp), and Bcl-XΔTM (710 bp). Lanes 3 and 4 show samples generated with primers designed to detect mRNA containing an unspliced intronic sequence, ie, the mRNA for Bcl-X26β (expected size, ∼700 bp). Lanes 5 and 6 represent samples amplified by an additional set of primers designed to generate fragments from all three of the mRNAs for Bcl-XL (493 bp), Bcl-XS (304 bp), and Bcl-XΔTM (423 bp).25
DISCUSSION
Expression of several members of the Bcl-2 family of proteins was examined in late erythroid progenitor cells to assess their potential roles in control of apoptosis by EP. The most striking finding was the strong upregulation of Bcl-X protein and mRNA synthesis that was completely dependent on EP exposure and that led to a high level of Bcl-X accumulation. Based on the observation of a characteristic doublet band in Western blots at 29 kD, it appears that the predominant form of Bcl-X protein in erythroid cells is Bcl-XL . PCR analyses of mRNAs indicated the presence of a species that contained an unspliced intron and therefore could code for the soluble protein Bcl-Xβ .26 However, no evidence of this protein was seen in Western blots. The accumulation of Bcl-X roughly paralleled that of hemoglobin accumulation. The overall increase was about 50-fold greater than the initial levels found in early erythroblasts. In contrast to Bcl-X, neither Bcl-2 protein nor mRNA was detected in mouse or human erythroblasts. In a recent study of Bcl-X and Bcl-2 expression in an EP-dependent murine erythroleukemia cell line,37 EP was found to regulate expression of both Bcl-X and Bcl-2. We too have observed Bcl-2 expression in erythroleukemia cell lines (Fig 2), but numerous experiments did not detect Bcl-2 in explanted human or murine erythroid progenitors at the CFU-E stage or later. Perhaps the established cell lines exhibit expression of Bcl-2 as a property of an earlier differentiation stage than the cells used in the present study or as a property of their transformed state. Park et al47 showed that Bcl-X but not Bcl-2 was expressed in CD34+ primitive human hematopoietic progenitors. Studies with Bcl-X–deficient mice48 have indicated that Bcl-X is essential for hematopoiesis in the fetal liver, because massive apoptosis of hematopoietic cells is observed in the fetal livers at days 12 to 13 of gestation. Failure of hematopoiesis as well as extensive apoptotic cell death of postmitotic differentiating neurons in various regions of the brain lead to lethality for the embryos around day 13 of gestation. In contrast, Bcl-2–deficient mice have erythropoiesis that is normal.49 Bax, Bad, Bak, and Mcl-1 were present in erythroblasts, and they showed evidence of positive regulation by EP exposure, although the magnitude of these effects and the time courses were different for each one. These various patterns of expression of bcl-2 family genes in erythroid progenitors indicate that they each have individual mechanisms of regulation, although EP exposure does appear to have a positive regulatory effect on each of those that are expressed.
The expression pattern of Bcl-X during terminal differentiation of erythroblasts has several interesting aspects. In FVA cells, increases in both Bcl-X protein and mRNA are observed by 4 hours of EP exposure. The protein level increases by approximately 25-fold over the first 24 hours of FVA cell culture with EP, whereas the mRNA level remains constant during that period after the initial increase of about threefold. This result suggests that there is a major change in Bcl-X regulation at the posttranscriptional level during the first 24 hours of culture, possibly a large increase in translational efficiency of Bcl-X mRNA. However, the mechanism of this early dramatic increase in Bcl-X protein will require further investigation. It is during this period in FVA cells that hemoglobin synthesis is initiated and increases to about two thirds of its maximum rate.50 Between 24 and 36 hours of FVA cell culture with EP, at about the time that hemoglobin synthesis rate becomes nearly maximal, the Bcl-X mRNA level increases dramatically, increasing another ninefold between 24 and 48 hours in culture. The Bcl-X protein level only increases twofold during the latter period. This magnitude of increase in mRNA at this period in erythroid differentiation is unprecedented among many other genes previously analyzed such as EP receptor, actin,8 GATA1, EKLF (M.C.B., unpublished observations), and SCL/TAL1.51 After about 20 hours of FVA cell culture, total RNA synthesis decreases significantly.40 One gene whose mRNA level increases during the culture period from 24 to 48 hours is hemoglobin, but that increase is small compared with that of Bcl-X mRNA.50 At 48 hours of FVA cell culture, the majority of FVA erythroblasts have undergone enucleation to form reticulocytes,38 and the current results suggest that Bcl-X also continues to accumulate at this time. Thus, Bcl-X expression in FVA cells roughly parallels the synthesis and accumulation of heme and hemoglobin in these cells. This observation also applies to the observed Bcl-X expression in Hu-ECFCs. In the case of human erythroblasts, hemoglobin synthesis is virtually complete before enucleation. The maximum rate of hemoglobin synthesis is on day 10 to 11 of culture,40 and the β-globin mRNA levels decrease in human cells before reticulocyte formation on approximately day 13. Bcl-X protein decreases in the human cells between days 11 and 13 of culture, indicating that its level is decreasing before reticulocyte formation in these cells.
Two questions are raised by the time courses of changes in amounts of members of the Bcl-2 protein family. First, can these changes account for the regulation of apoptosis by EP in early erythroblasts such as those represented by freshly isolated FVA cells? Changes in Bcl-X protein and also changes in other family members in the cell population were seen only after 4 hours of culture (Figs 1 and 3). However, apoptosis of a significant fraction (∼30%) of cells occurs by 4 hours of EP deprivation, as indicated by DNA degradation.10 13 Furthermore, apopain activation can be seen in FVA cells cultured without EP by 2 hours (Fig 6). Thus, the timing of the increase in the amount of Bcl-X appears to be later than expected for a factor that regulates apoptosis by EP in early erythroblasts. Nevertheless, one could postulate that there are earlier changes in Bcl-X, Bax, or Bad metabolism (such as phosphorylation) that are not reflected in the total protein level. It is also possible that larger changes are occurring in a subpopulation of cells rather than the whole population and that this subpopulation represents the cells that would undergo apoptosis early unless exposed to EP. A more definitive answer about the role of Bcl-2 family members in early apoptosis in erythroblasts must await studies in which their expression can be manipulated to see if the protein can affect their EP requirements.
The high level of Bcl-XL accumulation during the later phases of erythroid differentiation raises the question as to what is the function of Bcl-XL protein in late erythroblasts and possibly in reticulocytes? There is some evidence that Bcl-2 and Bcl-XL protect cells from damage caused by reactive oxygen species. It appears that Bcl-2 in the mitochondria cannot prevent the generation of free radicals but does appear to prevent their effects of lipid peroxidation by blocking the oxidative chain reaction that such free radicals initiate.20-22 Bcl-XL is known to accumulate in mitochondria and is thought to function in a manner similar to Bcl-2.26 The developing erythroblast is extremely active in the import of iron and the formation of heme. The final steps of heme formation, which is the insertion of the iron atom into the porphyrin ring, is catalyzed by the enzyme ferrochelatase located in the mitochondria of these cells. The transition metals, especially iron, are very efficient generators of free radicals via the Fenton reaction.52 The handling of large amounts of iron in the mitochondria of erythroblasts would undoubtedly lead to the coincident free radical formation during the period of heme synthesis, necessitating cellular countermeasures to protect the maturing cell against free radical damage. The high levels of Bcl-XL protein found in the mitochondria may serve as a defense at the site of free radical generation. This is an area that warrants future investigation.
Some members of the Bcl-2 family of proteins protect cells from apoptosis, whereas other members induce apoptosis. In particular, Bax is a protein that causes apoptosis in some cells when it is in excess over the other Bcl-2 family members that inhibit apoptosis.27 A model to explain these observations has been presented that postulates that Bax/Bax homodimers are inducers of apoptosis, whereas Bcl-2/Bax or Bcl-X/Bax or A1/Bax heterodimers are inactive.27 Thus, according to the model, Bcl-2, Bcl-X, and A1 protect cells by binding Bax as heterodimers. Bad presumably favors apoptosis because it dimerizes specifically with Bcl-X and, to a lesser extent, Bcl-2, thus preventing them from forming dimers with Bax. Some evidence now suggests that modified forms of Bcl-X can protect cells from apoptosis, although they apparently cannot form heterodimers with Bax, indicating that heterodimer formation is not necessary for Bcl-X protection.53 Nevertheless, the molecular ratio of Bax and Bcl-X may be an important factor in control of apoptosis in erythroblasts. This study did not determine the molecular ratios between Bcl-X and Bax or the ratios between any of the Bcl-2 family members. The various primary and secondary antibodies used in the Western blots may not yield comparable signals on a per molecule basis for various Bcl-2 family proteins in the cells. Studies are currently underway to attempt to determine the actual ratios of these proteins. Northern analyses of mRNAs for Bcl-X and Bax do yield good estimates of the relative mRNA levels in the cells. Simultaneous hybridization of FVA cell blots with probes of similar size and specific activity for Bcl-X and Bax indicated that Bax mRNA is in significant excess at early times but that Bcl-X mRNA exceeds that for Bax after 24 hours of culture (figure not shown). Nevertheless, mRNA levels in these cases may not reflect relative protein activities due to posttranscriptional events.
The detection of the 17-kD large subunit of activated apopain by 2 hours of EP deprivation in FVA cells corresponds to the observed initiation of DNA degradation, another indicator of apoptosis in these cells. By 2 hours of EP deprivation, the DNA of 25% of FVA cells is degraded into oligonucleosomal fragments.10,13 The change in apopain activation is thus one of the earliest changes associated with apoptosis that can be measured in EP-deprived FVA cells. Importantly, there is no baseline level of activated apopain in cells that are not undergoing apoptosis, indicating that the correlation of apopain activation with apoptosis is virtually absolute. Apopain is but a single member of the ICE cysteine protease family that includes ICE, Ich-1/Nedd-2, Ich-2/TX/Rel II, Ich-2/Rel III, LAK/Mch 2α, Mch 3, Mch 4, and Mch 5.16,17 Yet, several studies using other types of cell systems have implicated apopain specifically in the mechanism of apoptosis because inhibitors of its proteolytic action can rescue cells from apoptotic cell death.31,32 It is unclear how apopain is activated in vivo during induction of apoptosis. There is evidence that the CPP32 proenzyme can undergo autocatalytic cleavage and activation or it can be activated by other ICE family members via a cascade mechanism.31 54 However, the precise signal in the apoptosis pathway that activates the ICE family members is unknown. Although we have identified Yama/CPP32/apopain in human and mouse erythroblasts, we do not yet know if there are other members of the ICE family in these cells or if they too are involved in the processes of apoptosis induced by EP deprivation.
The FVA cells that we used extensively in this study might conceivably have abnormalities in the observed properties because of their infection with Friend virus (anemia-inducing strain), which does cause expansion of early erythroid progenitor cells. However, the basic findings of EP-dependent regulation of Bcl-X and of apopain activation upon EP deprivation were confirmed in normal human erythroblasts and/or normal (uninfected) spleen cells from anemic mice. Also, in previous studies, many properties of FVA cells exhibited during late differentiation have subsequently been shown in normal erythroid progenitors. These properties include the induction of apoptosis in the absence of EP,10,11,55 the timing of expression of numerous macromolecules associated with differentiation of erythroid cells,5,8,40,50,56 and the complete differentiation into reticulocytes in culture in an EP-dependent manner. Thus, although the virus clearly initiates intracellular signals that support early development of the mouse erythroid cells, the late differentiation of FVA cells requires EP in doses comparable to those necessary for normal mouse CFU-E.12 The nature of the viral-induced signals that support early development of FVA cells is not known. Although the viral protein, gp55, of the anemia strain of Friend virus is presumed to activate the EP receptor, as has been shown for the polycythemia strain of Friend virus,57 neither phosphorylation of the EP receptor nor the activation of the JAK2/STAT5 signalling pathway is observed in FVA cells that are deprived of EP (Penta and Sawyer58 and S.T. Sawyer, personal communication, March 1997).
Supported by Veterans Health Administration Merit Review Grant (M.C.B.) and by National Institutes of Health Grant No. 5T 32 DK07186.
Address reprint requests to Maurice C. Bondurant, PhD, Division of Hematology, 2220 Pierce Ave, Room 547, Medical Research Bldg II, Vanderbilt University Medical Center, Nashville, TN 37232-6305.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal