Abstract
Platelet-type von Willebrand disease (vWD) is a congenital bleeding disorder characterized by heightened ristocetin-induced platelet aggregation caused by abnormally high affinity between the platelet membrane glycoprotein (GP) Ib/IX complex and von Willebrand factor (vWF ). Two distinct point mutations, Gly233 to Val and Met239 to Val, have been reported in GPIbα. We have constructed a recombinant GPIbα fragment containing the latter mutation, Met239 to Val (M239V) and characterized the mutant molecule using two methods, ie, interaction between soluble vWF and immobilized M239V and inhibition of platelet aggregation by purified soluble M239V. Spontaneous binding (ie, binding without any inducers) was observed between 125I-vWF and immobilized M239V but not between 125I-vWF and immobilized wild-type (WT) GPIbα. The addition of low concentrations of ristocetin (0.2 mg/mL) induced specific 125I-vWF binding to immobilized M239V, but not to WT GPIbα. At high concentrations of ristocetin (1.2 mg/mL), both WT GPIbα and M239V specifically bound to 125I-vWF. Thus, M239V reproduced the unique functional abnormality of the GPIb/IX complex in platelet-type vWD. Moreover, the purified soluble M239V inhibited platelet aggregation induced by low concentration of ristocetin (0.3 mg/mL) in platelet-rich plasma from a patient having Met239 to Val mutation, whereas purified WT did not. These results provide direct evidences that the reported point mutation is the responsible molecular basis of this disorder.
PLATELET-TYPE (or pseudo-) von Willebrand disease (vWD) is a congenital bleeding disorder with unique characteristics. In addition to the decreased levels of plasma von Willebrand factor (vWF ) common to several subtypes of vWD, heightened ristocetin-induced platelet aggregation is noteworthy. Aberrant interaction between the glycoprotein (GP) Ib/IX complex on the platelet surface and its ligand, vWF, in plasma is thought to initiate the activation of platelets that leads to intravascular platelet clumping, followed by consumption of platelets and vWF. An intrinsic defect in the GPIb/IX complex has been postulated that causes a hypersensitive interaction between the receptor and the ligand. A similar subtype of vWD designated as type 2B has been described, but the hypersensitivity was attributed to the abnormal function of the vWF molecule.
GPIb/IX complex is a hetero-oligomeric platelet membrane receptor that consists of three subunits, GPIbα (140 kD), GPIbβ (24 kD), and GPIX (17 kD).1-5 vWF binding domain on GPIb/IX complex has been analyzed in detail and determined to be located on the N-terminal 45-kD domain of GPIbα.6-8 Studies using synthetic peptides have shown that the amino acid sequences from 251 to 279 and 235 to 262 in 45-kD domain are thought to be an important site in vWF binding.9 10
Four distinct families of platelet-type vWD have been reported in three different races.11-15 Recent molecular analyses have shown two distinct point mutations in GPIbα among these families. The first one is a Gly233 to Val substitution in a Caucasian family16 and the second one is a Met239 to Val substitution in three families, one of Puerto Rican ancestry17 and two of Japanese ancestry.18 However, GPIbα is only one component of the GPIb/IX complex on the platelet surface and whether other components such as GPIbβ, GPIX, or another part of GPIbα participate in the abnormal function of this complex is still unclear.
To show that the Met239 to Val mutation is the direct cause of platelet-type vWD, we introduced the molecular abnormality into a mammalian expression system, expressed a recombinant GPIbα fragment containing the mutation, and described the unique binding characteristics of the mutant molecule for soluble vWF.
MATERIALS AND METHODS
Construction of expression vectors and site-directed mutagenesis.A recombinant expression plasmid was synthesized to contain cDNA encoding a partial GPIbα sequence (His1-Ala302 ) corresponding to the N-terminal 45-kD extracytoplasmic domain that includes the vWF binding site.6-10 The plasmid has a neomycin cassette for selection. A recombinant plasmid encoding mutant GPIbα, Met239 to Val, was synthesized by site-directed mutagenesis, as described.8,19 20 The DNA sequence was verified by sequence analysis and it was confirmed that the introduced mutation was restricted to residue 239.
Transfection, cell culture, and collection of conditioned medium.Transfection and cell culture were performed essentially as described.8,19 Briefly, the two recombinant plasmids, encoding normal and mutant GPIbα, were independently transfected into CHO-K1 cells by the calcium phosphate procedure.21 Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 0.5 mmol/L nonessential amino acid solution, 10% heat-inactivated fetal calf serum (GIBCO, Grand Island, NY) and 800 μg/mL of geneticin (Sigma, St Louis, MO) to select stable transfectants. After the establishment of cell lines, culture supernatants were collected and assayed for GPIbα antigen. Transfected cells were grown to 80% confluence in serum-containing medium, washed with serum-free medium, and incubated in serum-free medium for 24 hours. The supernatant containing the recombinant protein was collected. Serum-free culture medium of nontransfected (NT) CHO-K1 cells was simultaneously collected as a control.
Quantitation and immunologic evaluation of the recombinant GPIbα fragments by anti-GPIbα antibodies.Recombinant GPIbα fragments with normal (wild-type [WT]) or mutant (M239V) sequence secreted in culture medium from CHO cells were quantitated based on immunologic reactivity with mouse anti-GPIbα monoclonal antibody (MoAb) LJ-Ibα1 (generous gift of Dr Z.M. Ruggeri, La Jolla, CA), which recognizes a epitope within the first 237 residues of GPIbα and reacts better under reducing condition.6 7 Serum-free culture media containing the recombinant fragments were serially diluted, reduced by treatment with 30 mmol/L dithiothreitol at 37°C for 1 hour, and immobilized onto a nitrocellulose membrane (0.45-μm pore size; Bio-Rad, Hercules, CA) using an enzyme-linked immunofiltration assay (ELIFA) apparatus (Pierce Chemical Co, Rockford, IL) with a peristaltic pump. The membrane was soaked in Blotto (50 mg/mL fat-free dry milk, 0.25 mmol/L phenylmethylsulfonyl fluoride, 0.15 mol/L NaCl in phosphate buffer, pH 7.3) for 2 hours at 22°C to 25°C with constant shaking and incubated with LJ-Ibα1 (5 to 10 mg/mL) for 2 hours at 22°C to 25°C. After washing three times with Blotto, the membrane was transferred into a solution of 125I-labeled rabbit antimouse IgG for 2 hours at 22°C to 25°C, washed again three times with Blotto, and exposed to Kodak AR film (Eastman Kodak, Rochester, NY). Spots on the membrane corresponding to each medium sample were cut out and evaluated for radioactivity by γ-scintillation spectrometry. A standard semiquantitative curve was drawn and media containing WT or M239V were appropriately diluted with nontransfected CHO cell culture medium to ensure application of equivalent antigen amounts on the nitrocellulose membrane.
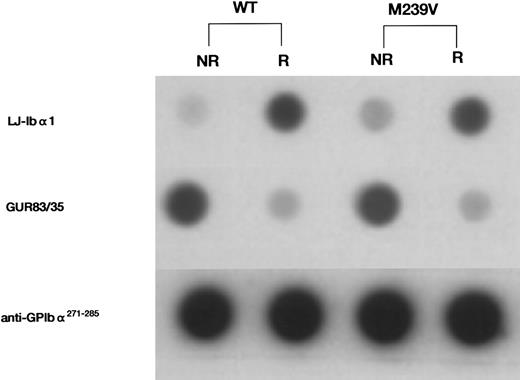
Dot-blot analysis of the recombinant GPIbα fragments, WT and M239V. Immunologic reactivity of the two recombinant proteins was evaluated using a panel of anti-GPIbα antibodies. To quantitate the amount of GPIbα antigen for dot-blot analysis, serum-free CHO-cell culture media containing either WT or M239V were first serially diluted and the amount of GPIbα-related antigen was assessed using anti-GPIbα MoAb LJ-Ibα1. LJ-Ibα1 recognizes an epitope within the first 237 residues in the N-terminal domain on GPIbα and reacts better under reducing condition. Culture media containing either WT or M239V were then appropriately diluted with nontransfected CHO-cell culture medium and the equivalent amount of antigen was applied onto the nitrocellulose membrane. After incubation with one of the first antibodies (LJ-Ibα1, GUR83/35, or anti-GPIbα271-285 ), nitrocellulose membranes were incubated with 125I-antimouse IgG and autoradiographed. GUR83/35 recognizes conformation-dependent epitope in the N-terminal division of GPIbα, whereas anti-GPIbα271-285 recognizes linear epitopes created by a polypeptide sequence of GPIbα271-285. Note that WT and M239V presented similar immunochemical reactivities to each anti-GPIbα antibody tested, regardless of the presence (R) or absence (NR) of 50 mmol/L dithiothreitol in the medium before spotting on nitrocellulose membranes. NR, not reducing; R, reducing.
Dot-blot analysis of the recombinant GPIbα fragments, WT and M239V. Immunologic reactivity of the two recombinant proteins was evaluated using a panel of anti-GPIbα antibodies. To quantitate the amount of GPIbα antigen for dot-blot analysis, serum-free CHO-cell culture media containing either WT or M239V were first serially diluted and the amount of GPIbα-related antigen was assessed using anti-GPIbα MoAb LJ-Ibα1. LJ-Ibα1 recognizes an epitope within the first 237 residues in the N-terminal domain on GPIbα and reacts better under reducing condition. Culture media containing either WT or M239V were then appropriately diluted with nontransfected CHO-cell culture medium and the equivalent amount of antigen was applied onto the nitrocellulose membrane. After incubation with one of the first antibodies (LJ-Ibα1, GUR83/35, or anti-GPIbα271-285 ), nitrocellulose membranes were incubated with 125I-antimouse IgG and autoradiographed. GUR83/35 recognizes conformation-dependent epitope in the N-terminal division of GPIbα, whereas anti-GPIbα271-285 recognizes linear epitopes created by a polypeptide sequence of GPIbα271-285. Note that WT and M239V presented similar immunochemical reactivities to each anti-GPIbα antibody tested, regardless of the presence (R) or absence (NR) of 50 mmol/L dithiothreitol in the medium before spotting on nitrocellulose membranes. NR, not reducing; R, reducing.
The recombinant fragments (WT and M239V) were evaluated by dot-blot analysis for immunologic reactivity with mouse anti-GPIbα MoAb GUR83/35, and a rabbit anti-GPIbα271-285 antiserum. GUR83/35 recognizes a conformation-specific epitope between residues 1 and 302 of GPIbα and blocks the binding of vWF to platelets in the presence of ristocetin (Tokuhira et al, unpublished observation). Anti-GPIbα271-285 (kindly provided by Dr Z.M. Ruggeri) was raised by immunization with a peptide containing the GPIbα sequence, Gly271-Glu285.22
Isolation of the recombinant GPIbα fragments.Recombinant GPIbα fragments were purified by thrombin-affinity chromatography, as described.23 Purified human thrombin was reacted with Hitrap NHS-Activated Sepharose 4B (Pharmacia, Uppsala, Sweden) according to the standard procedure. The column was preincubated in 50 mmol/L Tris-HCl (pH 7.4) containing 100 mmol/L NaCl, incubated with protein contained medium, and washed with the same buffer. The bound proteins were then eluted with 50 mmol/L Tris-HCl (pH 7.4) containing 500 mmol/L NaCl.
125I-vWF binding to the recombinant GPIbα fragments.vWF was radiolabeled with 125I by the IODO-GEN (Pierce Chemical Co) procedure.24 WT and M239V GPIbα fragments, either in culture media or in purified form, were evaluated for their ability to bind soluble vWF. For conditioned media, the amount of GPIbα fragments was first quantitated by immunologic reactivity with LJ-Ibα1 as described above. Media were then diluted with nontransfected culture medium to contain equal concentrations of GPIbα antigen. For purified WT and M239V, the concentration was adjusted to 2 μg/mL. Recombinant GPIbα fragments were immobilized onto nitrocellulose membranes by vacuum-drawing of 200 μL of culture medium using an ELIFA apparatus with a peristaltic pump. Protein binding sites were saturated by filtering 600 μL of HEPES/bovine serum albumin (BSA) buffer (20 mmol/L HEPES, 150 mmol/L NaCl, 1% BSA, pH 7.4) through the nitrocellulose membranes. Ristocetin or botrocetin was mixed with 125I-vWF and was incubated for 30 minutes. Fifty microliters of the mixture was then filtered through the nitrocellulose membrane with GPIbα fragments immobilized on it for a period of 5 minutes. Membranes were washed with 200 μL of HEPES/BSA buffer, and the bound reactivity was determined. In some experiments, the inhibitory effect on the binding was evaluated by MoAbs to assess the specificity of the binding. NMC-4, an anti-vWF MoAb 25 (generous gift of Dr A. Yoshioka, Nara Medical University, Nara, Japan) and GUR83/35, an anti-GPIbα MoAb, were used at final concentrations of 12 μg/mL and 15 μg/mL, respectively.
Preparation of platelet-rich plasma (PRP) and platelet aggregation studies.Blood was drawn from a platelet-type vWD patient (propositus of family B, see Takahashi et al18 ) and mixed with 1:10 vol of 3.14% sodium citrate solution. PRP was prepared by centrifugation at 100g for 15 minutes. To estimate the inhibitory effects of the WT and M239V fragments against platelet aggregation, purified recombinant fragments were first incubated with PRP for 5 minutes. PRP was then added with ristocetin and was applied to platelet aggregometer (Nema Tracer T-638; Niko Bioscience, Inc, Tokyo, Japan).
Statistical analyses.Analysis of variance (ANOVA) was used for the comparison of repeated measurements in multiple independent experiments. Either one-way or two-way layout ANOVA was used, as appropriate. The Bonferroni Post-Hoc test was applied to identify differences among the various groups in one experiment. For comparison of corresponding data between WT and M239V under various concentrations of ristocetin or botrocetin, two-way factorial ANOVA was performed with the use of the StatView (Abacus Concepts, Berkeley, CA) statistical software program on a Macintosh computer (Apple Computer Inc, Cupertino, CA). Scatchard-type analysis of the experimental data was performed using a computer-assisted program StatView. The dissociation constant (kd) and maximum binding (Bmax) were expressed as ranges of 95% confidence interval (95% CI). Analysis of covariance (ANCOVA) was used to test the difference in the two regression curves. A probability value (P value) of less than .05 was considered statistically significant.
RESULTS
Immunologic analysis of the recombinant GPIbα fragments (WT and M239V).Immunologic reactivity of equivalent amounts of recombinant GPIbα fragments was assessed under reducing and nonreducing conditions using anti-GPIbα antibodies (GUR83/35 and anti-GPIbα271-285) in dot-blot analysis. Both WT and M239V showed similar immunologic reactivities with each antibody (Fig 1), suggesting that the mutant fragment is secreted similarly to the native molecule to the culture medium and is immunologically indistinguishable from native GPIbα molecule.
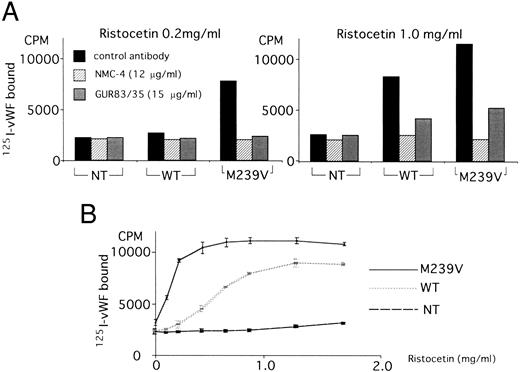
Binding of 125I-vWF to the recombinant GPIbα fragments (WT and M239V) in the absence of inducers.Consistent with previous results, no significant binding between WT and 125I-vWF was detected in the absence of inducers such as ristocetin or botrocetin (Fig 2). On the other hand, M239V specifically bound to 125I-vWF in the absence of inducers, as shown by the complete inhibition of the binding by an anti-vWF MoAb, NMC-4, although the extent of binding was much less than that attainable with ristocetin stimulation. Statistical analyses of 10 independent experiments using two-way layout ANOVA showed that 125I-vWF binding to M239V was significantly higher than that to NT (P < .01), but 125I-vWF binding to WT was statistically not different from that to NT. The statistical results shown in Fig 2 were the consequence of Bonferroni Post-Hoc test on 1 experiment that was representative of the 10 experiments.
Binding of 125I-vWF to WT or M239V in the absence of inducers. Nontransfected (NT) medium was used as a control (□). Two hundred microliters of culture media from WT-transfected cells or M239V-transfected cells, containing equivalent amounts of GPIbα antigen, was immobilized onto a nitrocellulose membrane. After blocking, 125I-vWF (0.15 μg/mL) was passed through the membranes and a bound radioactivity of each was assessed. Although no statistically significant 125I-vWF binding could be shown to WT (), specific spontaneous binding of 125I-vWF was observed to the mutant recombinant fragment M239V (▪; P < .01) and 125I-vWF, and this binding was inhibited by an anti-vWF MoAb, NMC-4 (▨). This conclusion was based on the statistical analyses of 10 independent experiments of the same kind by the use of two-way layout ANOVA. However, the statistical results shown in this figure were the consequence of Bonferroni Post-Hoc test on 1 experiment that is representative of the 10 independent experiments. This analysis showed that only the column indicated (*) was significantly (P < .01) higher than the remaining five columns. Also, comparisons of 125I-vWF binding to M239V in two different conditions [ie, NMC-4 (−) or (+)] showed that there was a statistically significant difference. However, no differences were demonstrated in the binding to NT [NMC-4 (−) or (+)] or to WT [NMC-4 (−) or (+)]. The results shown are the mean (±SD) of triplicate determinations.
Binding of 125I-vWF to WT or M239V in the absence of inducers. Nontransfected (NT) medium was used as a control (□). Two hundred microliters of culture media from WT-transfected cells or M239V-transfected cells, containing equivalent amounts of GPIbα antigen, was immobilized onto a nitrocellulose membrane. After blocking, 125I-vWF (0.15 μg/mL) was passed through the membranes and a bound radioactivity of each was assessed. Although no statistically significant 125I-vWF binding could be shown to WT (), specific spontaneous binding of 125I-vWF was observed to the mutant recombinant fragment M239V (▪; P < .01) and 125I-vWF, and this binding was inhibited by an anti-vWF MoAb, NMC-4 (▨). This conclusion was based on the statistical analyses of 10 independent experiments of the same kind by the use of two-way layout ANOVA. However, the statistical results shown in this figure were the consequence of Bonferroni Post-Hoc test on 1 experiment that is representative of the 10 independent experiments. This analysis showed that only the column indicated (*) was significantly (P < .01) higher than the remaining five columns. Also, comparisons of 125I-vWF binding to M239V in two different conditions [ie, NMC-4 (−) or (+)] showed that there was a statistically significant difference. However, no differences were demonstrated in the binding to NT [NMC-4 (−) or (+)] or to WT [NMC-4 (−) or (+)]. The results shown are the mean (±SD) of triplicate determinations.
Binding of 125I-vWF to the recombinant GPIbα fragments (WT and M239V) in the presence of inducers.Because the enhanced ristocetin-induced platelet aggregation induced by low concentrations of ristocetin is the phenotypic abnormality of platelet-type vWD, we compared the interaction between the recombinant GPIbα fragments and constant concentration of 125I-vWF (0.15 μg/mL) under conditions of low (0.2 mg/mL) versus high (1.0 mg/mL) concentrations of ristocetin, using the ELIFA apparatus with immobilized recombinant fragments on nitrocellulose membrane as described in the Materials and Methods. At 0.2 mg/mL ristocetin, no specific 125I-vWF binding to WT was observed (P > .05), whereas specific binding to M239V was shown (P < .05) that could be inhibited by NMC-4 or GUR83/35 (Fig 3A, left panel). At 1.0 mg/mL ristocetin, specific 125I-vWF binding was observed in both WT (P < .05) and M239V (P < .05), but much more prominent in the latter (Fig 3A, right panel). In each case, NMC-4 and GUR83/35 inhibited 125I-vWF binding to WT or M239V. All statistical analyses were performed by one-way layout ANOVA that compared values with control antibody, NMC-4, and GUR83/35.
Binding of 125I-vWF to WT or M239V in the presence of ristocetin. Using the same method as described in the legend to Fig 2, WT and M239V were assayed for binding to 125I-vWF (0.15 μg/mL) in the presence of ristocetin. (A) The inhibitory effect on the binding was evaluated by MoAbs to assess the specificity of the binding. NMC-4 (▨), an anti-vWF MoAb (generous gift of Dr A. Yoshioka); GUR83/35 (), an anti-GPIbα MoAb; and an indifferent antibody (▪) were used. (Left panel) At a low concentration of ristocetin (0.2 mg/mL), WT did not specifically bind to 125I-vWF (P < .05), whereas M239V did (P < .05). (Right panel) At a high concentration of ristocetin (1.0 mg/mL), WT bound to 125I-vWF and M239V binding to vWF was enhanced. Anti-vWF MoAb NMC-4 and anti-GPIbα MoAb GUR83/35 inhibited 125I-vWF binding in all of the conditions shown. The statistical analyses were perfomed using one-way layout ANOVA, which showed that, at 0.2 mg/mL ristocetin, there were no statistically significant difference between the three conditions (control antibody, NMC-4, and GUR83/35) in 125I-vWF binding to NT or WT (P < .05), but there was a difference between the three conditions in the binding to M239V (P < .05). On the other hand, at 1.0 mg/mL ristocetin, there were significant differences between the three conditions in WT and M239V (P < .05), but not in NT (P < .05). (B) 125I-vWF binding was measured in the presence of various concentrations of ristocetin. Nontransfected (NT) medium was used as a control. At low ristocetin concentrations (0.2 to 0.6 mg/mL), the difference between WT and M239V in 125I-vWF binding was marked, whereas the difference was minimized at ristocetin concentrations greater than 1.0 mg/mL. Two-way factorial ANOVA showed significant interaction between the effect of ristocetin concentration and the two recombinant GPIbα fragments (WT or M239V), ie, the two dose-response curves (WT and M239V) were statistically different (P < .05). The results shown are the mean (±SD) of triplicate determinations from one experiment that is representative of four.
Binding of 125I-vWF to WT or M239V in the presence of ristocetin. Using the same method as described in the legend to Fig 2, WT and M239V were assayed for binding to 125I-vWF (0.15 μg/mL) in the presence of ristocetin. (A) The inhibitory effect on the binding was evaluated by MoAbs to assess the specificity of the binding. NMC-4 (▨), an anti-vWF MoAb (generous gift of Dr A. Yoshioka); GUR83/35 (), an anti-GPIbα MoAb; and an indifferent antibody (▪) were used. (Left panel) At a low concentration of ristocetin (0.2 mg/mL), WT did not specifically bind to 125I-vWF (P < .05), whereas M239V did (P < .05). (Right panel) At a high concentration of ristocetin (1.0 mg/mL), WT bound to 125I-vWF and M239V binding to vWF was enhanced. Anti-vWF MoAb NMC-4 and anti-GPIbα MoAb GUR83/35 inhibited 125I-vWF binding in all of the conditions shown. The statistical analyses were perfomed using one-way layout ANOVA, which showed that, at 0.2 mg/mL ristocetin, there were no statistically significant difference between the three conditions (control antibody, NMC-4, and GUR83/35) in 125I-vWF binding to NT or WT (P < .05), but there was a difference between the three conditions in the binding to M239V (P < .05). On the other hand, at 1.0 mg/mL ristocetin, there were significant differences between the three conditions in WT and M239V (P < .05), but not in NT (P < .05). (B) 125I-vWF binding was measured in the presence of various concentrations of ristocetin. Nontransfected (NT) medium was used as a control. At low ristocetin concentrations (0.2 to 0.6 mg/mL), the difference between WT and M239V in 125I-vWF binding was marked, whereas the difference was minimized at ristocetin concentrations greater than 1.0 mg/mL. Two-way factorial ANOVA showed significant interaction between the effect of ristocetin concentration and the two recombinant GPIbα fragments (WT or M239V), ie, the two dose-response curves (WT and M239V) were statistically different (P < .05). The results shown are the mean (±SD) of triplicate determinations from one experiment that is representative of four.
125I-vWF (0.15 μg/mL) binding to the recombinant fragments increased with increasing ristocetin concentrations, but a binding plateau was reached at much lower concentrations in M239V (0.4 mg/mL) than in WT (1.2 mg/mL). The difference between WT and M239V in vWF binding was much more prominent at the ristocetin level between 0.2 mg/mL and 0.6 mg/mL (Fig 3B). Two-way factorial ANOVA showed significant interaction between the effect of ristocetin concentration and the two recombinant GPIbα fragments (WT or M239V), ie, the two dose-dependent curves were statistically different (P < .05).
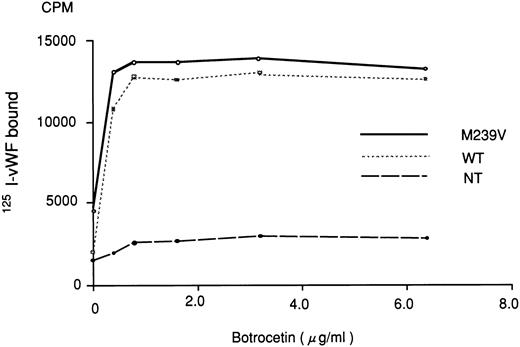
Botrocetin reportedly gives rise to an active complex form with vWF.25 In the presence of various concentrations of botrocetin, no significant difference was observed between WT and M239V in their ability to bind 125I-vWF (0.15 μg/mL; Fig 4). Two-way factorial ANOVA showed no significant interaction between the effect of botrocetin concentration and the two recombinant GPIbα fragments (WT or M239V; P > .05).
Binding of 125I-vWF to WT or M239V in the presence of various concentrations of botrocetin. The experiment was essentially the same as that described in Fig 3, except that botrocetin was used to induce vWF-GPIbα binding. Nontransfected (NT) medium was used as a control. There was no significant difference between WT and M239V in terms of botrocetin dose-dependent binding of 125I-vWF. Two-way factorial ANOVA showed no significant interaction between the effect of botrocetin concentration and the recombinant GPIbα fragments (WT or M239V).
Binding of 125I-vWF to WT or M239V in the presence of various concentrations of botrocetin. The experiment was essentially the same as that described in Fig 3, except that botrocetin was used to induce vWF-GPIbα binding. Nontransfected (NT) medium was used as a control. There was no significant difference between WT and M239V in terms of botrocetin dose-dependent binding of 125I-vWF. Two-way factorial ANOVA showed no significant interaction between the effect of botrocetin concentration and the recombinant GPIbα fragments (WT or M239V).
Scatchard-type analysis of vWF binding to the purified recombinant GPIbα fragments.Recombinant GPIbα fragments were isolated by thrombin-affinity chromatography, and the purity of the protein was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coumassie blue staining. Saturation binding of 125I-vWF to WT or M239V was evaluated using the purified recombinant GPIbα fragments. In the presence of 0.2 mg/mL ristocetin, scatchard-type analysis showed no saturable binding between 125I-vWF and WT, whereas specific binding was shown between 125I-vWF and M239V. On the other hand, at 1.2 mg/mL of ristocetin, both fragments showed saturable binding of vWF. Table 1 shows the calculated dissociation constant (kd) with 95% CI between purified GPIbα fragments and 125I-vWF. At a ristocetin concentration of 0.2 mg/mL, no measurable affinity was shown between purified WT and 125I-vWF, whereas purified M239V and 125I-vWF gave a kd of 0.92 (0.29 ∼ 1.55) × 10−8 mol/L. At a ristocetin concentration of 1.2 mg/mL, the calculated kds of WT and M239V were 1.21 × 10−8 mol/L (0.66 ∼ 1.76 × 10−8 mol/L) and 0.60 × 10−8 mol/L (0.22 ∼ 0.98 × 10−8 mol/L), respectively. These calculated kds were statistically equivalent as analyzed by ANCOVA (P < .05).
Binding Parameters of Ristocetin-Induced vWF Interaction With Purified Recombinant GPIbα Calculated From Scatchard-Type Analysis
| . | Ristocetin (0.2 mg/mL) . | Ristocetin (1.2 mg/mL) . | ||
|---|---|---|---|---|
| . | kD (×10−8 mol/L) . | Bmax (nmol/L) . | kD (×10−8 mol/L) . | Bmax (nmol/L) . |
| WT | NM | NM | 1.21 (0.66∼1.76) | 14.8 (9.7∼19.9) |
| M239V | 0.92 (0.29∼1.55) | 10.7 (5.4∼16.0) | 0.60 (0.22∼0.98) | 11.1 (6.2∼16.0) |
| . | Ristocetin (0.2 mg/mL) . | Ristocetin (1.2 mg/mL) . | ||
|---|---|---|---|---|
| . | kD (×10−8 mol/L) . | Bmax (nmol/L) . | kD (×10−8 mol/L) . | Bmax (nmol/L) . |
| WT | NM | NM | 1.21 (0.66∼1.76) | 14.8 (9.7∼19.9) |
| M239V | 0.92 (0.29∼1.55) | 10.7 (5.4∼16.0) | 0.60 (0.22∼0.98) | 11.1 (6.2∼16.0) |
Purification of the recombinant fragments was performed as described in the Materials and Methods. Binding of 125I-vWF (specific activity, 7.8 × 108 cpm/mg) to purified WT and M239V was evaluated by scatchard-type analysis at low or high concentrations of ristocetin. At low concentrations of ristocetin (0.2 mg/mL), no significant 125I-vWF binding was observed in WT, whereas saturable binding was shown in M239V. On the other hand, at high concentrations of ristocetin (1.2 mg/mL), saturable binding to 125I-vWF was shown both in WT and M239V. Dissociation constant (kd) and maximum binding (Bmax) were calculated from each binding isotherms by simple regression using a computer-assisted program StatView. ANCOVA showed that kds and Bmaxs calculated from each saturation curve (M239V at 0.2 mg/mL ristocetin, WT at 1.2 mg/mL ristocetin, and M239V at 1.2 mg/mL ristocetin) were statistically equivalent. Values in parenthesis indicate the 95% CI.
Abbreviation: NM, not measurable, ie, no defined value could be obtained by scatchard-type analysis.
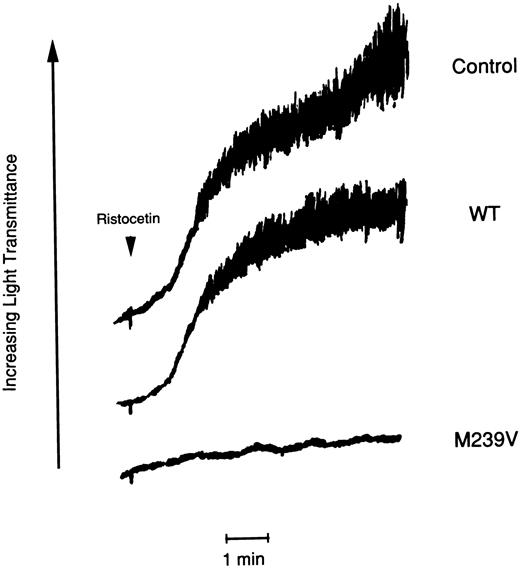
Effect of purified soluble recombinant GPIbα fragments (WT and M239V) on platelet aggregation.Low concentration of ristocetin (0.3 mg/mL) induced platelet aggregation in PRP of the platelet-type vWD patient but not in PRP of normal control subject (data not shown). Preincubation of purified WT (200 μg/mL) in PRP did not interfere the aggregation of the patients' platelets. On the other hand, the same concentration of purified M239V completely inhibited the platelet aggregation (Fig 5).
Effect of purified recombinant GPIbα fragments on platelet aggregation of the platelet-type vWD patient induced by low concentration of ristocetin. PRP (1.4 × 105 platelets/mL) of the patient with Met239 → Val substitution was mixed with purified recombinant GPIbα (WT and M239V) at a final concentration of 200 μg/mL. After 3 minutes of incubation, ristocetin (0.3 mg/mL) was added to PRP. Aggregation of the patient's platelets was measured as the increase in light transmittance as a function of time. Platelet aggregation was completely inhibited by purified M239V, whereas it was not by purified WT in the same condition.
Effect of purified recombinant GPIbα fragments on platelet aggregation of the platelet-type vWD patient induced by low concentration of ristocetin. PRP (1.4 × 105 platelets/mL) of the patient with Met239 → Val substitution was mixed with purified recombinant GPIbα (WT and M239V) at a final concentration of 200 μg/mL. After 3 minutes of incubation, ristocetin (0.3 mg/mL) was added to PRP. Aggregation of the patient's platelets was measured as the increase in light transmittance as a function of time. Platelet aggregation was completely inhibited by purified M239V, whereas it was not by purified WT in the same condition.
DISCUSSION
The initial phase of hemostasis at the site of vascular injury begins with adhesion of platelets to the exposed subendothelium, leading to platelet activation and the formation of platelet thrombi. Among several receptors and ligands involved in the platelet-subendothelium interaction, the GPIb/IX complex on the platelet membrane and vWF in the subendothelium play a pivotal role in both normal hemostasis and pathological thrombosis. vWF is also present in plasma, but it does not bind to GPIb/IX complex under normal circumstances, because the interaction is believed to be strictly regulated to maintain normal blood flow conditions. It is assumed that conformational changes in either or both of the two molecules are necessary to initiate the interaction. In in vitro experiments, the interaction can be induced by several ways, including chemical agonists such as ristocetin, shear stress,26 and using asialo vWF in place of normal vWF.27
Platelet-type vWD and type 2B vWD are congenital bleeding disorders characterized by the hypersensitivity of the vWF-GPIb interaction. The consumption of vWF and/or platelets is believed to account for the bleeding diathesis in these disorders. The pathophysiology of the disease has been characteristically represented in in vitro experiments in two distinct ways: one using the aggregation inducer ristocetin and the other using a physical shear stress stimulus. Ristocetin-induced platelet aggregation is heightened in both of the bleeding disorders and, more importantly, low concentrations of ristocetin, which never cause aggregation in normal platelets, induce the aggregation of patients' platelets. vWF-dependent shear-induced platelet aggregation was observed under low shear stress (15 dyne/cm2 ) in both of the disorders, which never gives rise to platelet aggregation in normal individuals,28 for which more than 80 dyne/cm2 is necessary.29 In type 2B vWD, the qualitive abnormality of vWF is thought to explain the hypersensitivity of the interaction, and recent molecular analyses have shown several point mutations in the GPIb binding domain of vWF.30-32
In platelet-type vWD, two single point mutations in GPIbα have been described. The first one is Gly233 to Val substitution,16 and the second, Met239 to Val, was reported in three distinct families, one from Puerto Rican ancestry17 and two from Japanese ancestry.18 An in vitro mammalian expression system has successfully represented the phenotypical abnormality of the Gly233 to Val substitution.33 In the present study, we have constructed a recombinant GPIbα fragment that contains the second substitution, Met239 to Val (designated M239V), and intended to show the direct evidence that the mutation could cause the unique characteristics of the hypersensitive interaction of GPIbα with a normal vWF molecule.
The mutant fragment M239V bound to 125I-vWF in the absence of any inducers, whereas the wild-type fragment (WT) did not. Moreover, M239V bound to 125I-vWF at a lower concentration of ristocetin as compared with WT. These results are consistent with one of the clinical laboratory features of platelet-type vWD, ie, platelets of these patients aggregate in vitro in the presence of low concentrations of ristocetin that never induce aggregation of normal platelets. With respect to the spontaneous interaction of vWF and GPIb/IX, purified type 2B vWF in solution has been reported to bind to normal GPIb in the absence of any modulating substances.34 Moreover, normal vWF in solution directly interacts with platelets in platelet-type vWD and induces aggregation.35-37 In a mammalian expression system, Ware et al30 have shown that a recombinant vWF molecule containing a type 2B mutation (Trp550 → Cys) binds to GPIb without ristocetin modulation. However, analysis of interaction between normal vWF and recombinant GPIbα fragment containing Gly233 to Val mutation showed no spontaneous binding, although the low concentration of ristocetin (0.34 mg/mL) supported specific binding between the two molecules.33 In the present study, we used the same assay system as in the previous analysis,33 but clearly detected spontaneous binding between normal vWF and GPIbα (Met239 → Val). The discrepancy in the two results is currently unexplained, but may reflect subtle conformational differences between the two molecules (Gly233 → Val and Met239 → Val) that determine the difference in the sensitivity to soluble vWF in our assay. However, it is still unclear whether the spontaneous binding is of pathophysiological relevance in patients with platelet-type vWD, because binding of vWF to affected GPIbα would rather be attributable to or affected by other factors such as shear stress.
Unlike the results using ristocetin, no significant difference between WT and M239V was observed with respect to the botrocetin concentration necessary to induce the binding. The differences observed in the two inducers reflect the distinct mechanisms by which they induce aggregation. Ristocetin presumably acts as a dimer and mediates the binding interaction of vWF and GPIbα by bridging both molecules,38 whereas botrocetin affects only vWF, forming an active complex with vWF that can bind to the GPIb/IX complex regardless of whether the receptor is upregulated.39
Scatchard-type analysis for the affinity between the recombinant fragments and vWF showed several interesting characteristics of the binding interaction (Table 1). At low ristocetin concentrations (0.2 mg/mL), no binding affinity between vWF and WT could be detected, whereas dissociation constant was calculated for M239V binding. At high ristocetin concentrations (1.2 mg/mL), both WT and M239V showed statistically equivalent dissociation constants for 125I-vWF, the values of which are very comparable to that of WT reported in a previous study.8 It is surprising that 125I-vWF bound to recombinant GPIbα with statistically equivalent affinity under different conditions, ie, to M239V at low or high ristocetin concentrations and to WT at high ristocetin concentrations. These findings suggest that the enhanced binding of M239V is not due to a change in the vWF binding site itself, but rather to a change in the modulatory site on GPIbα that upregulates the receptor function. Recent analyses of the vWF binding site identified amino acid residues 251 to 279 and 235 to 262 in the 45-kD domain of the GPIbα sequence as important sites for the interaction.9,10 Moreover, an epitope analysis of an MoAb raised against platelet-type vWD platelets with the other mutation, Gly233 → Val, showed the regulatory effects of this region on vWF-dependent platelet aggregation.40 Therefore, the substitutions of Val for Met at residue 239 and Val for Gly at residue 233 described in platelet-type vWD might determine a change in the modulatory sites, leading to a persistently active vWF binding site. In other words, the amino acid region around residues 233 to 239 might work as an on and off switch effector that regulates the status of the vWF binding site.
The inhibitory effect of the purified M239V but not of WT on platelet aggregation of platelet-type vWD induced by low concentrations of ristocetin further confirms that the characteristic phenotype of the disorder is caused by Met239 → Val substitution of GPIbα.
In conclusion, our results using a mammalian expression system clearly established a molecular basis of platelet-type vWD. The phenotype of the Met239 to Val substitution reported in two Japanese families and a family of Puerto Rican ancestry was represented in our in vitro functional assays; ie, binding of soluble vWF to the mutant GPIbα fragment was induced by low ristocetin concentrations or even in its absence. Moreover, the mutant recombinant GPIbα fragment may be a model for the active form of GPIbα, a molecule that plays a key role in regulating the initial steps of platelet activation. Such a model might provide important information in approaches to preventing thrombosis and will show a new aspect of antiplatelet therapy.
ACKNOWLEDGMENT
The authors thank Dr Z.M. Ruggeri and Dr A. Yoshioka for providing antibodies to GPIbα and vWF. The expert editorial assistance of Marina Hoffman is gratefully appreciated.
Supported in part by a Grant-in-Aid for Scientific Research No. 05670930 (to M.M.) from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Mitsuru Murata, MD, Division of Hematology, Department of Medicine, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo 160, Japan.

![Fig. 2. Binding of 125I-vWF to WT or M239V in the absence of inducers. Nontransfected (NT) medium was used as a control (□). Two hundred microliters of culture media from WT-transfected cells or M239V-transfected cells, containing equivalent amounts of GPIbα antigen, was immobilized onto a nitrocellulose membrane. After blocking, 125I-vWF (0.15 μg/mL) was passed through the membranes and a bound radioactivity of each was assessed. Although no statistically significant 125I-vWF binding could be shown to WT (), specific spontaneous binding of 125I-vWF was observed to the mutant recombinant fragment M239V (▪; P < .01) and 125I-vWF, and this binding was inhibited by an anti-vWF MoAb, NMC-4 (▨). This conclusion was based on the statistical analyses of 10 independent experiments of the same kind by the use of two-way layout ANOVA. However, the statistical results shown in this figure were the consequence of Bonferroni Post-Hoc test on 1 experiment that is representative of the 10 independent experiments. This analysis showed that only the column indicated (*) was significantly (P < .01) higher than the remaining five columns. Also, comparisons of 125I-vWF binding to M239V in two different conditions [ie, NMC-4 (−) or (+)] showed that there was a statistically significant difference. However, no differences were demonstrated in the binding to NT [NMC-4 (−) or (+)] or to WT [NMC-4 (−) or (+)]. The results shown are the mean (±SD) of triplicate determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.698/4/m_bl_0011f2.jpeg?Expires=1769907202&Signature=5OCmBimWrIx0qP49KY-QwO0f7c2hu2vEtjqZP038POErifXGtFFIL466Ce3uIYgIRw9R-M9Abw4cLAUBH-azsdGJpSlHUixoWP9fbjeepdMLc6G3z-kl5t-tzLb7vSes1fSJHfzo4AxrqaWygTPyOtCE2mmt7aTnkz~hmJF-v1bf9Ae5Abft27n9YyK5cGOxMeCmoshoGOGczd00j5J8Rr5IJluYPx5-M1hZ6n5qkMdOgd1wVnRJy4EwVtlzTJL92fiqRWEULMjQNKMKik7D9-tG9hM8dOmCPKZTGUmkakJrGBZAnPDax5UXmeC-Iydqx7Fl5ekcVm1hv3OrWyY6qw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal