Abstract
The human T-cell leukemia virus type I (HTLV-I) regulatory protein, Tax, has been speculated to play a major role in HTLV-I leukemogenesis. Indeed, several studies have suggested that upregulation of various cellular oncogenes and cytokines by Tax may explain the pathogenesis observed in HTLV-I–infected individuals, as well as several Tax-transgenic animal models. We report here the analysis of cytokine expression in a Tax-transgenic animal model with large granular lymphocytic (LGL) leukemia. Two different transgenic mice showed identical expression of interleukin-1α (IL-1α), IL-1β, interferon γ (IFNγ), and granulocyte-macrophage colony-stimulating factor (GM-CSF ) in peripheral tail tumors. Interestingly, LGL cell lines derived from these same tumors expressed high levels of both IFNγ and GM-CSF, which correlated with the level of Tax expression. These same LGL cell lines also expressed high levels of lymphocyte function-associated antigen-1 (LFA-1) and intracellular adhesion molecule-1 (ICAM-1). Engraftment of these LGL cell lines into severe combined immunodeficient (SCID) mice led to the development of leukemia and lymphomas. Examination of these SCID mice showed that their pathology was nearly identical to that observed in the original Tax-transgenic mouse model. Both the Tax-transgenic and engrafted SCID mouse models allow for the analysis of cellular events that are required for tumor development associated with HTLV infection and suggest that Tax expression may be responsible for the upregulation of certain cytokines and adhesion molecules that affect the infiltrating capabilities of HTLV-I–infected cells.
HUMAN T-CELL leukemia virus type I (HTLV-I) was the first pathogenic retrovirus discovered in humans and is the etiologic agent of adult T-cell leukemia/lymphoma (ATLL) and tropical spastic paraparesis (TSP).1,2 HTLV infection has also been associated with other clinical disorders such as arthropathy,3 uveitis,4 mycosis fungoides,5 and large granular lymphocytic (LGL) leukemia.6,7 The mode of leukemic transformation by HTLV infection is still unclear, but has been suggested to involve the HTLV regulatory protein, Tax, which has been shown to be a potent transcriptional activator of the HTLV long terminal repeat as well as several cellular genes.8-14 It is this promiscuous transactivating activity of Tax that has been postulated to be the mode of HTLV transformation. Indeed, expression of Tax alone has been shown to transform a number of cell types in vitro, including 3T3, Rat-1,15 Rat-2,16 primary rat embryo fibroblasts,17 and primary human CD4+ lymphocytes.18
The development of animal model systems to study the cellular events that are required for in vivo leukemogenesis and tumor transformation by HTLV is crucial.19 Several transgenic mouse models have been developed that express HTLV-I–Tax under a variety of enhancers/promoters.20-24 Although many of these transgenic mice developed neoplasias of nonlymphoid compartments, none of them resulted in the development of lymphomas and/or leukemia as a result of Tax expression. Previously, we have reported the generation of transgenic mice that target expression of Tax to the mature T-lymphocyte/natural killer (NK)-cell compartment by using the human granzyme B (GzmB) promoter.25 These mice developed LGL leukemia/lymphoma of T/NK-cell origin, showing that Tax expression in the lymphoid compartment is sufficient for the induction of leukemia. From this transgenic model, we were able to establish several T/NK-like LGL cell lines that were morphologically and phenotypically identical to those observed in vivo. We report here the differential expression of cytokines expressed in vivo and from established LGL cell lines, which may help to elucidate the cellular events that lead to the progression of tumor development in this transgenic model. In addition, we show that these LGL cell lines are capable of being engrafted into the severe combined immunodeficiency (SCID) mouse and transferring a nearly identical pathogenic phenotype as that observed in the original GzmB-Tax transgenic mice.
MATERIALS AND METHODS
Mice.Immunodeficient SCID (Balb/C.B17 scid/scid) mice were a gift from H. Skip Virgin and Samuel Stanley (Washington University, St Louis, MO). The GzmB-Tax transgenic mice were generated using the 5′ flanking region (−1170 to +36) of the human granzyme B gene as previously described.25 All mice were bred and maintained under pathogen-free conditions in accordance with Washington University School of Medicine animal care guidelines.
Cells.Tumor cell suspensions were prepared from founder F8 and F1 progeny (m388, f197) of founder M9 using sterilized frosted glass slides and cultured in Iscove's minimal essential medium with 10% (vol/vol) fetal calf serum (GIBCO, Grand Island, NY), 1% L-glutamine, 1% penicillin/streptomycin, and interleukin-2 (IL-2) at 500 to 1,000 U/mL (Cetus, Sunnyvale, CA). Cell suspensions were propagated for greater than 2 years in vitro, from which the homogeneous LGL cell lines (F8, m388, and f197) were derived. The SC cell line was derived from a peritoneal tumor of a SCID mouse engrafted with the F8 LGL cell line.
Injection of the cells into SCID mice.F8 cells (1 to 10 × 107) were washed two times in Iscove's medium. These cells were then resuspended in 1 mL of Iscove's medium and were intraperitoneally injected into SCID mice (F8 LGL cell line-injected). Mock-injected SCID mice were injected intraperitoneally with Iscove's medium.
Tissue histology and peripheral blood analysis.Tissues were fixed in 10% neutral-buffered formalin (Sigma, St Louis, MO), embedded in paraffin (Baxter, Deerfield, IL) for serial sectioning, and stained with hematoxylin/eosin (H&E; Sigma). Peripheral blood was analyzed on a Baker 9000 Coulter Counter (Serono Laboratories, Randolph, MA) and differential cell counts were reported as the mean cell count per 100 cells examined microscopically. Wright-Giemsa (Fisher, Pittsburgh, PA) staining was performed on peripheral blood smears.
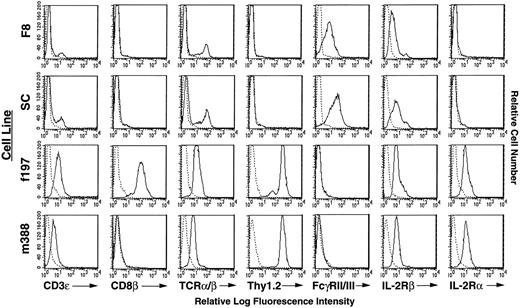
Flow cytometric analysis of cell surface markers on Tax-transgenic LGL cell lines. Reactivity of antibodies against cell surface markers is indicated with solid lines. Reactivity of a control antibody, indicated by dashed lines, is depicted in all panels.
Flow cytometric analysis of cell surface markers on Tax-transgenic LGL cell lines. Reactivity of antibodies against cell surface markers is indicated with solid lines. Reactivity of a control antibody, indicated by dashed lines, is depicted in all panels.
Reverse transcription-polymerase chain reaction (RT-PCR) for cytokine analysis.RNA from crude tumor cell suspensions and LGL cell lines was purified with guanidinium isothiocyanate as previously described.25 Eppendorfs containing 10 μg of total RNA were heated for 10 minutes at 65°C and chilled on ice for 5 minutes. Each sample was then incubated for 40 minutes at 37°C after adding 1 μL RNasin (Promega, Madison, WI), 2 μg oligo dT(12-18) (GIBCO-BRL, Gaithersburg, MD), 0.8 mmol/L deoxynucleoside triphosphates (US Biochemicals [USB], Cleveland, OH), 400 U reverse transcriptase (Moloney's murine leukemia virus [M-MLV]; USB), 10 μL of 5× reverse transcriptase buffer (USB), and diethyl procarbonate (DEPC)-treated sterile water to adjust the volume to 50 μL. An additional 400 U of reverse transcriptase was added to the reaction and the samples were incubated for an additional 40 minutes before heat-inactivating the reaction at 65°C for 10 minutes. The total volume was then adjusted to 100 μL and stored at −20°C until use.
PCR.Ten microliters of each RT-PCR sample was added to a 100 μL reaction mixture (1× PCR buffer, 0.2 mmol/L dNTPs, 2.5 U Taq DNA polymerase, 25 mmol/L MgCl2 , 0.5 μg each of 3′ and 5′ primers; USB). All reaction tubes included a 50-μL mineral oil overlay. The PCR was run in a DNA Thermal Cycler (Perkin Elmer, Norwalk, CT) as follows: 1 minute of hot start at 93°C; 30 cycles of 93°C for 1 minute, 60°C for 2 minutes, and 72°C for 1 minute; and 3 minutes of final extension at 72°C. In all experiments, negative controls were performed whereby RNA samples, which were not reverse transcribed, were amplified with primers for the murine housekeeping gene hypoxanthine phosphoribosyltransferase (HPGRT). These negative controls were performed in parallel under identical conditions as experimental samples and are indicated as HPGRT(−) samples. All cytokine primers were designed to span introns, thus ensuring that they would not detect genomic DNA sequences. Cytokine primers for IL-1α, IL-1β, IL-2, IL-4, and interferon γ (IFNγ) were a gift from Paul Allen (Washington University) and were designed at DNAX Research Institute (Palo Alto, CA). Cytokine primers for granulocyte-macrophage colony-stimulating factor (GM-CSF ) have been previously described.26 Cytokine primers for the internal control gene HPGRT were designed by Kenneth Murphy (Washington University). The following are sequences of the cytokine amplification primers (S, sense; A, antisense) with their predicted product size in parentheses: IL-1α, S 5′-CTCTAGAGCACCATGCTACAGAC-3′ (308); IL-1α A, 5′-TGGAATCCAGGGGAAACACTG-3′; IL-1β S, 5′-TGAAGGGCTGCTTCCAAACCTTTGACC-3′ (322); IL-1β A, 5′-TGTCCATTGAGGTGGAGAGCTTTCAGC-3′; IL-2 S, 5′-TGATGGACCTACAGGAGCTCCTGAG-3′ (167); IL-2 A, 5′-GAGTCAAATCCAGAACATGCCGCAG-3′; IL-4 S, 5′ACGAGGTCACAGGAGAAGGGACGCCATGCA-3′ (188); IL-4 A, 5′-TCATTCATGGAGCAGCTTATCGATCGAATCC-3′; IFNγ S, 5′-TGGAGGAACTGGCAAAAGGATGGT-3′ (336); IFNγ A, 5′-TTGGGACAATCTCTTCCCCAC-3′; GM-CSF S, 5′-TTCCTGGGCATTGTGGTCT-3′ (430); GM-CSF A, 5′-TGGATTCAGAGCTGGCCTGG-3′; HPGRT S, 5′-TGGGAGGCCATCACATTGT-3′ (471); HPGRT A, 5′-GCTTTTCCAGTTTCACTAATGACA-3′.
PCR samples were separated by electrophoresis in a 2% agarose gel (GIBCO-BRL) in Tris borate/EDTA buffer containing 0.5 μg/mL ethidium bromide. Bands were visualized by UV light exposure and photographed using a Stratagene EagleEye II (Stratagene, La Jolla, CA).
Enzyme-linked immunosorbent assay (ELISA) measurement of cytokines.Supernatant was obtained from 1 × 107 cells seeded in 10 mL of complete Iscove's medium and quantitated for cytokine production using the two-antibody sandwich assay. The monoclonal antibody H22, used as the capture antibody against murine IFNγ, was a gift from Robert Schreiber (Washington University). The secondary goat antimouse IFNγ antibody and recombinant murine IFNγ were gifts from Kenneth Murphy (Washington University). Detection in the IFNγ ELISA was performed using a peroxidase-conjugated rabbit antigoat tertiary antibody (Sigma) and the chromogenic substrate 2,2′-azino-bis(3-ethylbenzthiazoline-6)-sulfonic acid (Sigma). Substrate conversion was measured by a spectrophotometer at 405 nm. GM-CSF cytokine production was quantitated using the mouse GM-CSF Quantikine M Immunoassay (R & D Systems, Rochester, MN). All samples were performed in triplicate.
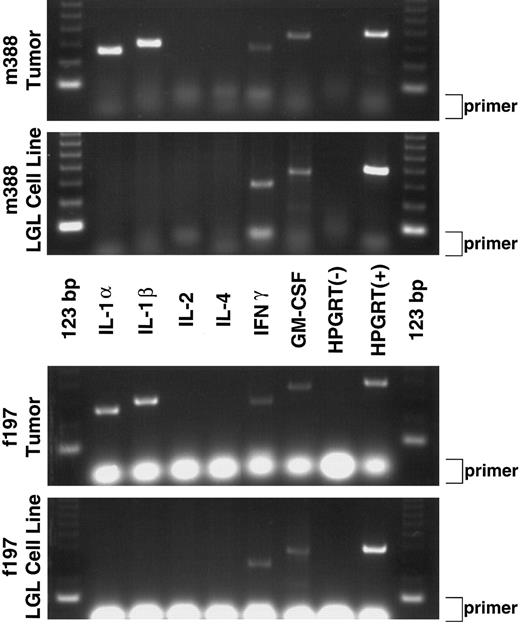
RT-PCR cytokine analysis of GzmB-Tax transgenic tumors and derived LGL cell lines. The first and third panels show mRNA expression of IL-1α, IL-1β, IFNγ, and GM-CSF in the peripheral tumors of two different transgenic mice (m388 Tumor and f197 Tumor). The second and fourth panels show that the tumor-derived LGL cell lines express mRNAs for only IFNγ and GM-CSF (m388 LGL Cell Line and f197 LGL Cell Line). Negative and positive RT-PCR controls are shown in each panel and are labeled as HPGRT(−) and HPGRT(+), respectively. Excess primer for each RT-PCR reaction is indicated at the right side of each panel (primer).
RT-PCR cytokine analysis of GzmB-Tax transgenic tumors and derived LGL cell lines. The first and third panels show mRNA expression of IL-1α, IL-1β, IFNγ, and GM-CSF in the peripheral tumors of two different transgenic mice (m388 Tumor and f197 Tumor). The second and fourth panels show that the tumor-derived LGL cell lines express mRNAs for only IFNγ and GM-CSF (m388 LGL Cell Line and f197 LGL Cell Line). Negative and positive RT-PCR controls are shown in each panel and are labeled as HPGRT(−) and HPGRT(+), respectively. Excess primer for each RT-PCR reaction is indicated at the right side of each panel (primer).
Flow cytometry and Western blot analysis.Tumor cell lines (1 × 106 cells) were incubated with 5 μg of unlabeled Fcγ receptor (FcγRII/III) antibody for 15 minutes at 4°C to block free surface FcγR (except when staining for this particular surface marker) and then counterstained for 30 minutes at 4°C with fluorescein isothiocyanate– or R-phycoerythrin–conjugated antibodies to the following surface markers: CD3ε, CD4, CD8β chain, TCR α/β, CD90.2 (Thy1.2), CD122 (IL-2Rβ chain), CD25 (IL-2Rα chain), H2Kk, PK136 (NK1.1), CD49d (VLA-4), CD11a (lymphocyte function-associated antigen-1 [LFA-1] α chain), CD54 (intracellular adhesion molecule-1 [ICAM-1]), and CD11b (Mac-1) from PharMingen (San Diego, CA); and 5E6 (Ly49C) and A1 (Ly49A) from W. Yokoyama (Washington University). These cell lines were then analyzed on a FACScan (Becton Dickinson, Mountain View, CA). For Western blot analysis, 107 cells from the F8 cell line, SC cell line, f197 cell line, m388 cell line, MT2 HTLV-I–positive cell line, and the Jurkat E6.1 HTLV-I–negative cell line were lysed in 200 μL of 2× sample buffer (1 mmol/L EDTA, 0.125 mol/L Tris [pH 6.8], 4% sodium dodecyl sulfate [SDS], 10% β-mercapatoethanol [β-ME], 30% glycerol, 0.01% bromophenol blue). These samples were heated at 85°C for 10 minutes and centrifuged at 14,000 rpm to clarify, and then 10 to 30 μL of each sample was subjected to a 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel. Gels were transferred to nitrocellulose, probed (1:5,000) with a pool of purified anti-Tax monoclonal antibodies (nos. 168A51-2, 168A51-42, and 168B17-46-34; AIDS Reagent Program, Rockville, MD),27 and visualized by using enhanced chemiluminescence (Amersham, Arlington Heights, IL).
Cell proliferation assay.The MTT proliferation assay was essentially performed according to Hansen et al.28 Briefly, 50,000 cells from each cell line were plated in triplicate into flat-bottom 96-well plates in 100 μL of complete Iscove's medium. To each of set of triplicates was added 100 μL of media or media containing cytokines to give the indicated final concentrations: IL-2 (500 U/mL; Cetus), GM-CSF (20 ng/mL), IL-1α and IL-1β (25 ng/mL), and IFNγ (250 U/mL; R & D Systems). These cultures were incubated at 37°C for 3 days, at which time 25 μL of MTT [(3,(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) 5 mg/mL in phosphate-buffered saline (Sigma)] was added to each well and allowed to incubate at 37°C for an additional 4 hours. Each sample was lysed (20% wt/vol of SDS/50% DMF in dH2O, pH 4.7) overnight at 37°C, and their optical densities at 562 nm were measured.
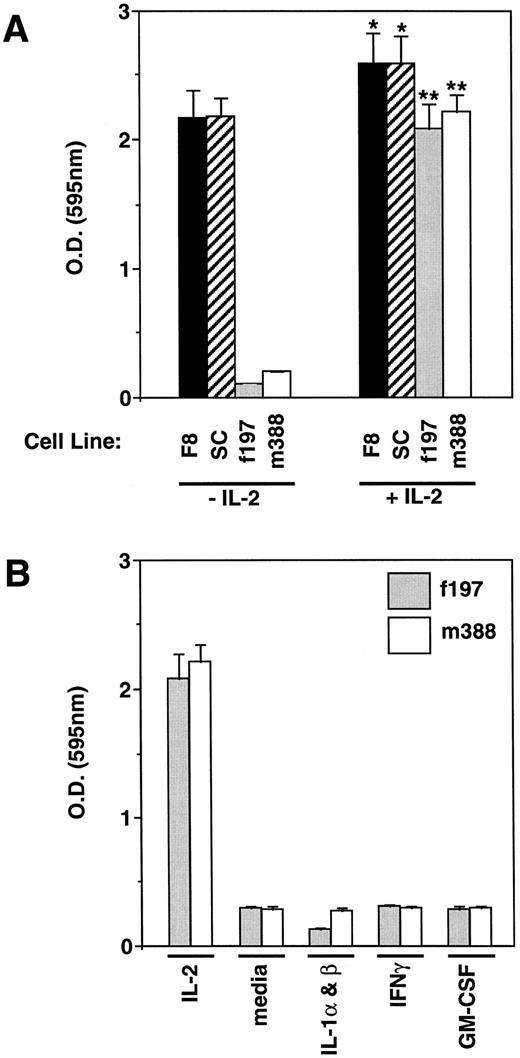
MTT cell proliferation assay on LGL cell lines. (A) LGL cell lines were incubated with or without IL-2 and measured for their proliferation. As shown, the F8 and SC LGL cell lines have become independent of IL-2 for proliferation, but have retained their capability to respond to IL-2 (*P < .05). In contrast, the f197 and m388 cell lines are highly dependent on exogenous IL-2 for their proliferation (**P < .01). (B) The IL-2–dependent LGL cell lines, f197 and m388, were incubated with the cytokines found to be expressed in the peripheral tumor from which they were derived (IL-1α and IL-1β, IFNγ, and GM-CSF ) and examined for their proliferative effects. Incubation with IL-2 and media alone served as positive and negative controls, respectively. As shown, none of these cytokines was able to support the proliferation of these IL-2–dependent LGL cell lines.
MTT cell proliferation assay on LGL cell lines. (A) LGL cell lines were incubated with or without IL-2 and measured for their proliferation. As shown, the F8 and SC LGL cell lines have become independent of IL-2 for proliferation, but have retained their capability to respond to IL-2 (*P < .05). In contrast, the f197 and m388 cell lines are highly dependent on exogenous IL-2 for their proliferation (**P < .01). (B) The IL-2–dependent LGL cell lines, f197 and m388, were incubated with the cytokines found to be expressed in the peripheral tumor from which they were derived (IL-1α and IL-1β, IFNγ, and GM-CSF ) and examined for their proliferative effects. Incubation with IL-2 and media alone served as positive and negative controls, respectively. As shown, none of these cytokines was able to support the proliferation of these IL-2–dependent LGL cell lines.
RESULTS
Phenotypic analysis of immortalized Tax-transgenic LGL cell lines.Three separate T/NK-like LGL cell lines were established from the original GzmB-Tax transgenic mice.25 These cell lines were derived from the peripheral tumors of three different mice of two different founder lines. One LGL cell line (F8) exhibited cell surface markers consistent with that of an NK-cell lineage, namely expression of FcγRII/III and the IL-2Rβ chain (Fig 1). Some of these cells did express low levels of TCR α/β and CD3ε (22% and 11%, respectively); however, these cells were a minority of the bulk population. The F8 cell line did not express any of the classical NK-cell markers, namely NK1.1, Ly49A, or Ly49C (data not shown). The other two LGL cell lines (f197 and m388) exhibited cell surface markers of the T-cell lineage, specifically CD3ε, TCR α/β, Thy1.2, and the IL-2Rβ chain. Interestingly, both of these cell lines also expressed the IL-2Rα chain (CD25), which is a characteristic surface marker of most HTLV-I–infected cells.29,30 None of the cell lines expressed CD4 (data not shown); however, the f197 cell line expressed CD8, a marker that is found on HTLV-II–infected cells and only occasionally found on HTLV-I–infected cells (Fig 1).31 This heterogeneity of distinct immortalized cell phenotypes from the tumors of different mice is consistent with the activity of the granzyme B promoter32 as well as the multiple-hit theory needed for tumor development.
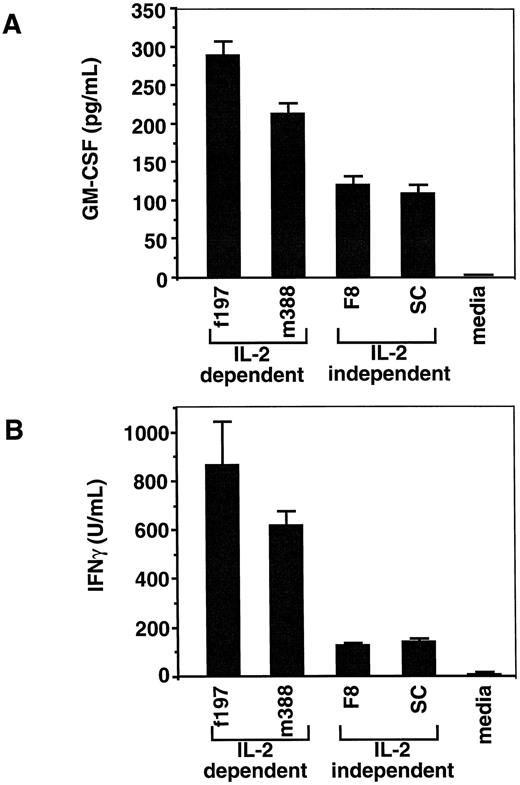
ELISAs for GM-CSF and IFNγ production of tumor-derived LGL cell lines. (A) GM-CSF ELISA showing production of soluble GM-CSF by all four cell lines (f197, 867± 75 pg/mL; m388, 620± 56 pg/mL; F8, 127 ± 9 pg/mL; and SC, 141 ± 9 pg/mL). The production of GM-CSF between IL-2–dependent cell lines (f197 and m388) is significantly different from that of the IL-2–independent cell lines (F8 and SC; **P < .01). (B) IFNγ ELISA showing production of soluble IFNγ by all four cell lines (f197, 288 ± 18 U/mL; m388, 212 ± 15 U/mL; F8, 119 ± 12 U/mL; and SC, 108 ± 11 U/mL). Similarly, the production of IFNγ between IL-2–dependent cell lines and IL-2–independent cell lines is significantly different (**P < .01).
ELISAs for GM-CSF and IFNγ production of tumor-derived LGL cell lines. (A) GM-CSF ELISA showing production of soluble GM-CSF by all four cell lines (f197, 867± 75 pg/mL; m388, 620± 56 pg/mL; F8, 127 ± 9 pg/mL; and SC, 141 ± 9 pg/mL). The production of GM-CSF between IL-2–dependent cell lines (f197 and m388) is significantly different from that of the IL-2–independent cell lines (F8 and SC; **P < .01). (B) IFNγ ELISA showing production of soluble IFNγ by all four cell lines (f197, 288 ± 18 U/mL; m388, 212 ± 15 U/mL; F8, 119 ± 12 U/mL; and SC, 108 ± 11 U/mL). Similarly, the production of IFNγ between IL-2–dependent cell lines and IL-2–independent cell lines is significantly different (**P < .01).
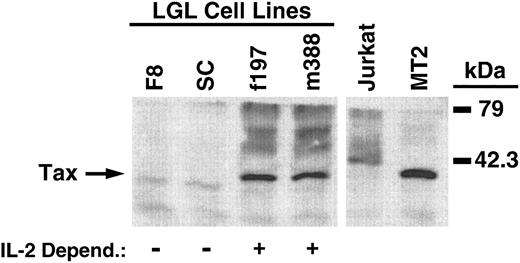
Immunoblot analysis of Tax expression in LGL cell lines. Tax expression is indicated by the arrow on the left, with molecular mass markers shown on the right. LGL cell line dependency for IL-2 is indicated below each sample lane. Jurkat and MT2 cells were used for negative and positive controls of Tax expression, respectively.
Immunoblot analysis of Tax expression in LGL cell lines. Tax expression is indicated by the arrow on the left, with molecular mass markers shown on the right. LGL cell line dependency for IL-2 is indicated below each sample lane. Jurkat and MT2 cells were used for negative and positive controls of Tax expression, respectively.
Cytokine mRNA expression in tumors and derived cell lines.Tax has been shown to upregulate the expression of numerous cytokines,13,14,33-37 some of which have been speculated to play a role in the pathogenesis observed in ATLL and TSP. Because of these observations, we decided to examine the differential mRNA expression of IL-1α, IL-1β, IL-2, IL-4, IFNγ, and GM-CSF for their possible contribution to the pathogenesis/tumor formation observed in the GzmB-Tax mice. RT-PCR was performed on RNA obtained from the peripheral tail tumors of two different GzmB-Tax transgenic mice (m388 and f197) as well as RNA from LGL cell lines derived from these tumors. Both mice exhibited identical cytokine profiles from their tumors, which were shown to express IL-1α, IL-1β, IFNγ, and GM-CSF (Fig 2). Interestingly, both LGL cell lines also displayed identical cytokine profiles. These derived cell lines were shown to express both IFNγ and GM-CSF, suggesting that the LGL cells may be responsible for the IFNγ and GM-CSF expression observed in the original tumor (Fig 2). Because T cells and NK cells have been shown to produce both IFNγ and GM-CSF,38-40 expression of these cytokines by the m388 and f197 LGL cell lines gives additional support that these cells are of T/NK-cell origin.
LGL Cell Line Tumorigenicity in SCID Mice
| Cell Line . | IL-2 Dependent (+ or −) . | Cells Injected (×10−7) . | Tumor Growth/No. of Mice . | Survival Time (mo) . | Organ Involvement . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | LN . | Lung . | Spleen* . | PB . | Liver . |
| F8 | − | 10 | 2/2 | 2.5 | ++ | ++ | + | ++ | + |
| F8 | − | 5 | 2/2 | 2 | ++ | ++ | + | ++ | + |
| F8 | − | 2 | 1/1 | 2.5 | ++ | ++ | + | ND | + |
| F8 | − | 1 | 1/1 | 2 | ++ | ++ | + | ++ | +/− |
| f197 | + | 10 | 1/4 | >6 | + | + | − | + | − |
| m388 | + | 10 | 0/4 | >6 | − | − | − | − | − |
| Cell Line . | IL-2 Dependent (+ or −) . | Cells Injected (×10−7) . | Tumor Growth/No. of Mice . | Survival Time (mo) . | Organ Involvement . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | LN . | Lung . | Spleen* . | PB . | Liver . |
| F8 | − | 10 | 2/2 | 2.5 | ++ | ++ | + | ++ | + |
| F8 | − | 5 | 2/2 | 2 | ++ | ++ | + | ++ | + |
| F8 | − | 2 | 1/1 | 2.5 | ++ | ++ | + | ND | + |
| F8 | − | 1 | 1/1 | 2 | ++ | ++ | + | ++ | +/− |
| f197 | + | 10 | 1/4 | >6 | + | + | − | + | − |
| m388 | + | 10 | 0/4 | >6 | − | − | − | − | − |
Abbreviations: ND, not determined; LN, lymph node; PB, peripheral blood (LGL lymphocytosis); ++, marked infiltration; +, slight infiltration.
Structural integrity compromised.
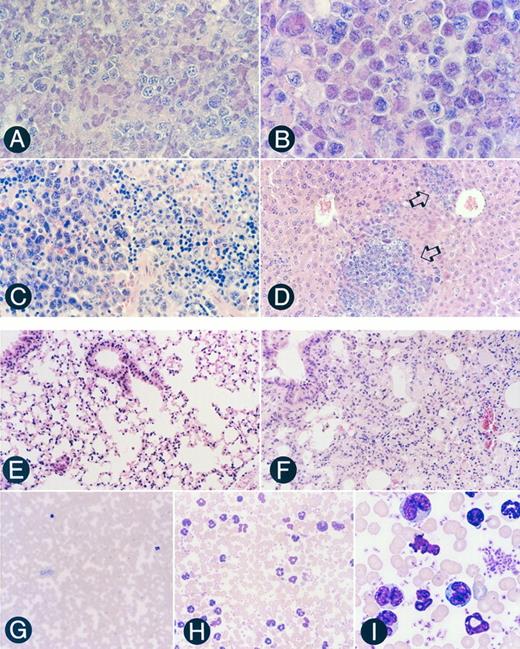
Pathohistological features of the tissues of SCID mice engrafted with the transformed Tax-transgenic F8 LGL cell line. (A) Section of a peritoneal tumor located at the site of injection, showing that it is comprised primarily of LGLs (original magnification × 720). (B) Section of a mesenteric lymph node showing massive LGL infiltration, characteristic of diffuse large-cell type lymphoma (original magnification × 1,080). (C) Section of a spleen showing infiltration of LGLs in the left portion with generalized disruption of both the white and red pulp (original magnification × 720). (D) Section of a liver showing infiltration of LGLs into the sinusoid and portal region (open arrows; original magnification × 360). (E) Section of a mock-injected SCID lung showing an intrapulmonary bronchus with associated epithelium and normal alveolar ducts and alveoli (original magnification × 360). (F ) Lung section from an LGL-engrafted SCID mouse showing massive infiltration of LGLs into the bronchus epithelium and alveoli septa (original magnification × 360). (G) Peripheral blood smear from a mock-injected SCID mouse showing a paucity of circulating lymphocytes and neutrophils (original magnification × 360). (H) Peripheral blood smear from an LGL-engrafted SCID mouse demonstrating LGL lymphocytosis and neutrophilia (original magnification × 720). (I) Higher magnification of (H) showing morphologic characteristics of the LGLs (original magnification × 2,100).
Pathohistological features of the tissues of SCID mice engrafted with the transformed Tax-transgenic F8 LGL cell line. (A) Section of a peritoneal tumor located at the site of injection, showing that it is comprised primarily of LGLs (original magnification × 720). (B) Section of a mesenteric lymph node showing massive LGL infiltration, characteristic of diffuse large-cell type lymphoma (original magnification × 1,080). (C) Section of a spleen showing infiltration of LGLs in the left portion with generalized disruption of both the white and red pulp (original magnification × 720). (D) Section of a liver showing infiltration of LGLs into the sinusoid and portal region (open arrows; original magnification × 360). (E) Section of a mock-injected SCID lung showing an intrapulmonary bronchus with associated epithelium and normal alveolar ducts and alveoli (original magnification × 360). (F ) Lung section from an LGL-engrafted SCID mouse showing massive infiltration of LGLs into the bronchus epithelium and alveoli septa (original magnification × 360). (G) Peripheral blood smear from a mock-injected SCID mouse showing a paucity of circulating lymphocytes and neutrophils (original magnification × 360). (H) Peripheral blood smear from an LGL-engrafted SCID mouse demonstrating LGL lymphocytosis and neutrophilia (original magnification × 720). (I) Higher magnification of (H) showing morphologic characteristics of the LGLs (original magnification × 2,100).
Cytokine responsiveness of LGL cell lines.All three LGL cell lines exhibited an initial growth dependence for high IL-2 concentrations. Two of these cell lines have retained this dependency for more than 2 years (m388 and f197), whereas one cell line has gained IL-2 independence (F8), but has retained the capability to respond to IL-2 (Fig 3A; P < .05). To examine whether any of the other cytokines detected in these peripheral tumors (IL-1α, IL-1β, IFNγ, and GM-CSF ) may play a role in the proliferation of the IL-2–dependent LGL cell lines (f197 and m388), a proliferation assay was performed that showed that none of these cytokines were able to support the proliferation of these cells either alone or in any combination (Fig 3B, data not shown).
Soluble IFNγ and GM-CSF levels produced by LGL cells and their relationship to Tax protein levels.To ensure that the m388 and f197 LGL cell lines produced soluble IFNγ and GM-CSF, ELISAs were performed on the supernatant from these cultured cell lines. In addition, two other IL-2–independent LGL cell lines (F8 and SC) were similarly tested. All four cell lines produced substantial levels of both cytokines. Interestingly, the IL-2–dependent cell lines (m388 and f197) produced significantly more IFNγ and GM-CSF than the IL-2–independent cell lines (F8 and SC; Fig 4A and B; P < .001). One possible explanation for the higher cytokine production in the IL-2–dependent LGL cell lines could be due to higher levels of Tax expression in these cell lines compared with the IL-2–independent cell lines. Immunoblot analysis showed significant levels of Tax protein in the IL-2–dependent cell lines, but nearly undetectable levels in the IL-2–independent cell lines (Fig 5). The lack of Tax expression in the IL-2–independent cell lines was not due to loss of transgene because Southern blot analysis of all cell lines showed identical integration patterns as originally described (data not shown). These results show a plausible correlation between high cytokine expression and high levels of Tax expression, compatible with previous observations of Tax's ability to upregulate the expression of both IFNγ and GM-CSF.14 41
Peripheral Blood Analysis of Engrafted LGL-SCID Mice
| Total and Differential WBC Counts in Tumor-Injected and Mock-Injected SCID Mice . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouse ID . | WBC . | RBC . | % of Nucleated Cells . | Hb . | R% . | ||||
| . | . | . | L . | N . | M . | E . | LGL . | . | . |
| F8 LGL cell line-injected | |||||||||
| SC-1 | 10.5 | 4.85 | 11 | 58 | 1 | 0 | 30 | 7.5 | 18.2 |
| SC-2 | 10.7 | 4.80 | 5 | 72 | 2 | 0 | 21 | 7.3 | 9.8 |
| SC-3 | 9.7 | 4.63 | 5 | 71 | 2 | 0 | 22 | 7.2 | 12.7 |
| SC-4 | 13.5 | 6.63 | 8 | 70 | 2 | 0 | 20 | 9.0 | 2.4 |
| Average | 11.1* | 5.22* | 7* | 68† | 2 | 0 | 23* | 7.8* | 10.8‡Mock-injected |
| NML-1 | 2.0 | 10.22 | 59 | 40 | 1 | 0 | 0 | 14.8 | 0.7 |
| NML-2 | 1.8 | 8.94 | 53 | 45 | 2 | 0 | 0 | 13.3 | 2.9 |
| NML-3 | 1.6 | 8.62 | 49 | 48 | 3 | 0 | 0 | 12.9 | 1.3 |
| NML-4 | 4.5 | 9.44 | 40 | 58 | 2 | 0 | 0 | 13.7 | 0.8 |
| NML-5 | 3.7 | 9.21 | 42 | 54 | 4 | 0 | 0 | 13.8 | 2.0 |
| Average | 2.7 | 9.29 | 49 | 49 | 2 | 0 | 0 | 13.7 | 1.5 |
| Total and Differential WBC Counts in Tumor-Injected and Mock-Injected SCID Mice . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouse ID . | WBC . | RBC . | % of Nucleated Cells . | Hb . | R% . | ||||
| . | . | . | L . | N . | M . | E . | LGL . | . | . |
| F8 LGL cell line-injected | |||||||||
| SC-1 | 10.5 | 4.85 | 11 | 58 | 1 | 0 | 30 | 7.5 | 18.2 |
| SC-2 | 10.7 | 4.80 | 5 | 72 | 2 | 0 | 21 | 7.3 | 9.8 |
| SC-3 | 9.7 | 4.63 | 5 | 71 | 2 | 0 | 22 | 7.2 | 12.7 |
| SC-4 | 13.5 | 6.63 | 8 | 70 | 2 | 0 | 20 | 9.0 | 2.4 |
| Average | 11.1* | 5.22* | 7* | 68† | 2 | 0 | 23* | 7.8* | 10.8‡Mock-injected |
| NML-1 | 2.0 | 10.22 | 59 | 40 | 1 | 0 | 0 | 14.8 | 0.7 |
| NML-2 | 1.8 | 8.94 | 53 | 45 | 2 | 0 | 0 | 13.3 | 2.9 |
| NML-3 | 1.6 | 8.62 | 49 | 48 | 3 | 0 | 0 | 12.9 | 1.3 |
| NML-4 | 4.5 | 9.44 | 40 | 58 | 2 | 0 | 0 | 13.7 | 0.8 |
| NML-5 | 3.7 | 9.21 | 42 | 54 | 4 | 0 | 0 | 13.8 | 2.0 |
| Average | 2.7 | 9.29 | 49 | 49 | 2 | 0 | 0 | 13.7 | 1.5 |
Abbreviations: Total WBC count is expressed as number (×10−12) per liter; Hb, hemoglobin expressed as g/dL; L, lymphocytes; N, neutrophils; M, monocytes; E, eosinophils; R, reticulocytes; LGL, large granular lymphocytes; RBC, red blood cells.
Significantly different from mock-injected SCID mice (P < .001).
Significantly different from mock-injected SCID mice (P < .01).
Significantly different from mock-injected SCID mice (P < .05).
Engraftment of immortalized LGL cells in SCID mice.The immortalized F8 LGL cell line was subsequently examined for its state of transformation by examining its ability to engraft in SCID mice. Six SCID mice were inoculated intraperitoneally with 1 to 10 × 107 F8 cells. All six F8-injected SCID mice developed LGL leukemia and lymphomas, resulting in the death of the SCID mice after 2 to 2.5 months (Table 1). Examination of the F8-injected SCID mice showed a large tumor mass in the peritoneal cavity, composed primarily of LGLs (Fig 6A). In addition, LGL infiltration was observed in numerous organs, including lymph nodes, spleen, liver, lung, and peripheral blood (Table 1 and Fig 6B through D, F, H, and I). Peripheral blood smears of F8-injected SCID mice showed marked LGL lymphocytosis and neutrophilia (Fig 6H and I). Complete and differential blood counts of F8-injected SCID mice showed a significantly higher total white blood cell (WBC) count (11.1 ± 1.6 × 1012 WBCs/L) compared with normal mock-injected SCID mice (2.7 ± 1.3 × 1012 WBCs/L; Table 2). The leukocytosis in F8-injected SCID mice was primarily due to substantial increases in the LGL and neutrophil compartments. The F8-injected SCID mice also developed severe anemia, a very common hematologic feature observed in human NK-LGL leukemia.42 These engrafted mice also exhibited reticulocytosis and decreased hemoglobin levels (Table 2). To ensure that the expanded LGL cells observed in the F8-injected SCID mice were in fact the injected LGL cell line and not an expansion of T/NK cells from the SCID mice, a cell line from a peritoneal tumor was established (SC). This cell line was found to be morphologically and phenotypically identical to the original F8 LGL cell line. This similarity was confirmed by flow cytometric analysis, which showed that the F8 and SC cell lines possessed identical surface markers (CD3ε, TCR α/β, FcγR II/III, and IL-2Rβ; Fig 1). None of the six mock-injected SCID mice developed tumors during the same period (data not shown).
In contrast to the F8 LGL cell line, only one of six SCID mice injected with the IL-2–dependent LGL cell lines (f197 and m388) developed mild leukemia/lymphoma. The reason for this difference in SCID engraftment between the different LGL cell lines is unclear; however, it is possible that the SCID mice were unable to support the highly IL-2–dependent proliferation of the f197 and m388 cell lines.
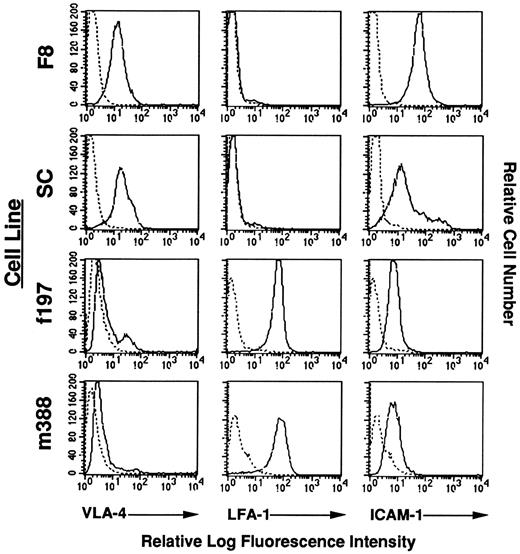
Expression of adhesion molecules on Tax-transgenic LGL cell lines.Because nearly 100% of the mice from one of the GzmB-Tax transgenic founder lines (M9) develop peripheral tumors located on the tails, ears, and limbs (data not shown), flow cytometric analysis was performed on the established LGL cell lines from this founder line (f197 and m388) for the expression of cell surface adhesion molecules. As shown in Fig 7, both the f197 and m388 LGL cell lines expressed nearly identical levels of the same adhesion molecules, namely low levels of VLA-4, intermediate levels of ICAM-1, and high levels of LFA-1. Of note is the difference in adhesion molecule expression on the F8 (and SC) LGL cell line that was not derived from peripheral tail tumors, such as the f197 and m388 LGL cell lines, but was instead derived from a cervical lymph node. In particular, the F8 LGL cell line has virtually no LFA-1 expression and instead has an increase in VLA-4 and ICAM-1 expression. This differential expression of adhesion molecules may account for the difference in the anatomic origin of these LGL cell lines, as well as an explanation for the nearly 100% penetrance of peripheral tumor development in the M9 founder line. These observations are consistent with other data that have shown that both ICAM-1 and LFA-1 expression are increased in HTLV-1 infected cells,43-45 most likely due to an increase in transactivation by Tax.46
Flow cytometric analysis of adhesion molecules on LGL cell lines. Reactivity of antibodies against adhesion molecules is indicated with solid lines. Reactivity of a control antibody, indicated by dashed lines, is depicted in all panels.
Flow cytometric analysis of adhesion molecules on LGL cell lines. Reactivity of antibodies against adhesion molecules is indicated with solid lines. Reactivity of a control antibody, indicated by dashed lines, is depicted in all panels.
DISCUSSION
In this study, we have shown that targeted expression of Tax to the T/NK-cell compartment using the human GzmB promoter results in the development of immortalized LGL cells of T/NK-cell lineages. Importantly, each individual transgenic mouse develops morphologically similar peripheral tumors; however, each individual tumor is comprised of phenotypically distinct immortalized cell types (eg, CD3−CD4−CD8−, CD3+CD4−CD8−, or CD3+CD4−CD8+). These results suggest that, although Tax expression is sufficient for the predisposition of immortalization, various additional hits/mutations are required for tumor formation and leukemia. Other cellular proteins that have been suggested to play an important role in HTLV immortalization, such as inactivation of the p53 tumor-suppressor protein47,48 and activation of the Jak/Stat signal transduction pathway,49 50 are currently under investigation.
Hypothetical model for the role of cytokines in the process of peripheral tumor development in GzmB-Tax transgenic mice. This model would suggest that there is a need for an initial injury to peripheral skin tissue (eg, ear puncture, tail clipping, and fighting). This injury would subsequently start an inflammatory response by skin epithelial cells (eg, keratinocytes [KC]) that release inflammatory cytokines such as IL-1α and IL-1β. These inflammatory cytokines could then act on ECs of nearby vessels to cause the extravasation of circulating LGLs and neutrophils (PMN) by upregulating adhesion molecules such as ICAM-1 and VCAM-1 on ECs. Circulating LGLs also constitutively express adhesion molecules on their surface (LFA-1 and VLA-4), which promote their binding to activated ECs and extravasation. Once at the site of inflammation, these LGLs would become activated and release cytokines such as GM-CSF, which could then cause a systemic increase in PMN production and PMN extravasation into the tumors. In addition, these LGLs produce large quantities of IFNγ that could potentially initiate/exacerbate this inflammatory process by upregulating the expression of adhesion molecules such as ICAM-1 and VCAM-1 at this peripheral site.
Hypothetical model for the role of cytokines in the process of peripheral tumor development in GzmB-Tax transgenic mice. This model would suggest that there is a need for an initial injury to peripheral skin tissue (eg, ear puncture, tail clipping, and fighting). This injury would subsequently start an inflammatory response by skin epithelial cells (eg, keratinocytes [KC]) that release inflammatory cytokines such as IL-1α and IL-1β. These inflammatory cytokines could then act on ECs of nearby vessels to cause the extravasation of circulating LGLs and neutrophils (PMN) by upregulating adhesion molecules such as ICAM-1 and VCAM-1 on ECs. Circulating LGLs also constitutively express adhesion molecules on their surface (LFA-1 and VLA-4), which promote their binding to activated ECs and extravasation. Once at the site of inflammation, these LGLs would become activated and release cytokines such as GM-CSF, which could then cause a systemic increase in PMN production and PMN extravasation into the tumors. In addition, these LGLs produce large quantities of IFNγ that could potentially initiate/exacerbate this inflammatory process by upregulating the expression of adhesion molecules such as ICAM-1 and VCAM-1 at this peripheral site.
We have also shown successful engraftment of a Tax-immortalized, IL-2–independent LGL cell line into SCID mice, resulting in the development of leukemia and lymphoma. The observed leukemia and lymphoma in these engrafted SCID mice closely resembled the pathological and phenotypical characteristics of the lymphoproliferative disorder that occurred in the original GzmB-Tax transgenic mice.25 The similarities between these two murine models included respiratory complications due to infiltration of LGLs, hepatomegaly due to LGL infiltration of the sinusoids, LGL infiltration of the lymph nodes and spleen, expansion of peripheral LGLs and neutrophils, reticulocytosis, and anemia. Of intrigue was the finding that the IL-2–dependent LGL cell lines were essentially incapable of being transferred to SCID mice, although the reason for this observation is unclear. Several possible explanations include (1) the inadequacy of SCID mice to support cytokine (IL-2) -dependent cell lines, (2) the lack of additional mutations in these IL-2–dependent cell lines needed for complete transformation, or (3) that these IL-2–dependent cell lines express certain surface antigens that are recognized by SCID NK cells that mediate their destruction. Recently, Stewart et al51 have reported the observation that HTLV-I–infected cell lines that lack viral gene expression are able to form tumors when inoculated into SCID mice, whereas HTLV-I–infected cells that do express viral gene products fail to form tumors. Such findings would support the difference in tumorigenicity between the IL-2–independent F8 cell line, which has lost detectable levels of Tax expression, and the IL-2–dependent f197 and m388 cell lines, which still express high levels of Tax (Fig 5). The first and third explanations are currently being investigated by coinjecting into SCID mice either recombinant human IL-2 or an NK-cell neutralizing antibody (anti–asialo-GM1) with the IL-2–dependent cell lines.
Although a definitive link between LGL leukemia and HTLV infection has yet to be established,6,52-55 the clinical features of human LGL leukemia are quite similar to those observed in the two murine models described in this report. These features include LGL leukocytosis, severe anemia, hepatosplenomegaly, bone marrow LGL infiltration, and lymphadenopathy. However, these murine models develop peripheral neutrophilia, a hematologic feature that is not common in human LGL patients. Instead, these patients tend to develop mild neutropenia.42 One plausible explanation for this difference may be due to the high level of GM-CSF expression in the GzmB-Tax transgenic mice, which is consistent with previous observations of peripheral neutrophilia and splenomegaly in mice injected with recombinant murine GM-CSF.56 Similar peripheral neutrophilia and splenomegaly were also reported in an earlier Tax transgenic mouse model that developed neurofibromas, which was shown to correspond to GM-CSF production by the transformed perineural fibroblasts.57 GM-CSF production in this model, as well as in our GzmB-Tax transgenic model, is most likely due to the ability of Tax to specifically transactivate the GM-CSF promoter.14,33 Of note, neutrophilia is a common clinical finding in ATL patients, which may be related to GM-CSF production by ATL cells.58
In addition, we have also shown that there is a similar expression profile of certain cytokines (eg, IL-1α, IL-1β, GM-CSF, and IFNγ) among different GzmB-Tax transgenic mice. This conserved cytokine profile from two different transgenic mice suggests that these cytokines may play a critical role in the observed pathogenesis. Given the initial high dependence of the derived LGL cell lines for IL-2, it was surprising that we did not observe production of IL-2 in the peripheral tumors. This observation was further explored by crossing the Tax-transgenic mice with IL-2 knockout (KO) mice.59 The GzmB-Tax/IL-2 KO mice showed no differences in tumor development, progression, or phenotype from the original GzmB-Tax model (data not shown). These results are consistent with our failure to detect IL-2 message in vivo, but they do not rule out the possibility of paracrine IL-2 production or the role of IL-15.
As suggested in Fig 8, a model for the development of these peripheral tumors would include trauma to peripheral regions such as the tail, ears, and limbs, due to activities like tail clipping, ear puncture, and fighting, which leads to an inflammatory response. These peripheral sites are the most common areas (>90%) for the formation of primary tumors (data not shown). Inflammatory cytokines from epithelial cells, such as IL-1α and IL-1β, could potentially act on nearby endothelial cells (ECs) to induce expression of adhesion molecules such as ICAM-1 and VCAM-1. Circulating LGLs seem to constitutively express adhesion molecules on their surface (LFA-1 and VLA-4), which could promote their binding to activated ECs and eventual extravasation. Once at this site of inflammation, these LGLs release GM-CSF, which is most likely the cause of the observed peripheral neutrophilia and polymorphonuclear leukocyte (PMN) recruitment into the tumors. High levels of IFNγ production by these LGL cells may potentially initiate and/or exacerbate this inflammatory process by upregulating the expression of adhesion molecules (ICAM-1 and VCAM-1) on ECs as well as epithelial cells such as keratinocytes.60 In fact, preliminary experiments suggest that injecting a neutralizing antibody against IFNγ into these transgenic mice significantly reduces the progression of these peripheral tumors. Another explanation currently being explored for the importance of IFNγ in this transgenic model is whether it is playing a protective role in preventing cellular apoptosis.61
Indeed, recent transgenic models that use either the human GzmB or GzmH promoters driving expression of the simian virus 40 large T antigen (SVlgT) also develop very similar T/NK-cell lymphomas.62 However, these transgenic mice do not develop the peripheral tumors or neutrophilia observed in the GzmB-Tax transgenic mice. These data suggest that Tax expression may be responsible for these observed phenotypes, possibly through dysregulated cytokine and/or adhesion molecule production. Data to support this hypothesis include the inability to detect IFNγ or GM-CSF production in the majority of LGL cell lines derived from GzmH-SVlgT transgenic mice (data not shown; MacIvor, Grossman, Ratner, and Ley, personal communication, January, 1997).
Overall, both the GzmB-Tax transgenic and LGL cell line-engrafted SCID models provide useful tools to examine the complex cellular mechanisms that are involved in the immortalization process by HTLV infection. In particular, these models have been shown to be useful in the dissection of the role of cytokine expression in the observed pathogenesis. Such examinations may prove to be useful in understanding the disease progression of HTLV-1–associated diseases such as ATL, TSP, and cutaneous T-cell lymphoma (mycosis fungoides), in which cytokine and adhesion molecule expression have been speculated to play an important role in organ infiltration and disease development.33,36,43,45,60 62-65
ACKNOWLEDGMENT
Transgenic mouse development was supported by a National Institute of Child Health and Human Development contract to DNX, Inc. The authors thank Dr Marie LaRegina, Niecey Hinkle, and Paula Klender at the Division of Comparative Medicine for their help in peripheral blood analysis, and Dr Timothy J. Ley for critical review of this manuscript.
Supported by Grants No. CA63417 and 5T32 HL07088.
Address reprint requests to Lee Ratner, MD, PhD, Washington University School of Medicine, Division of Molecular Oncology, Box 8069, 660 S Euclid Ave, St Louis, MO 63110.

![Fig. 8. Hypothetical model for the role of cytokines in the process of peripheral tumor development in GzmB-Tax transgenic mice. This model would suggest that there is a need for an initial injury to peripheral skin tissue (eg, ear puncture, tail clipping, and fighting). This injury would subsequently start an inflammatory response by skin epithelial cells (eg, keratinocytes [KC]) that release inflammatory cytokines such as IL-1α and IL-1β. These inflammatory cytokines could then act on ECs of nearby vessels to cause the extravasation of circulating LGLs and neutrophils (PMN) by upregulating adhesion molecules such as ICAM-1 and VCAM-1 on ECs. Circulating LGLs also constitutively express adhesion molecules on their surface (LFA-1 and VLA-4), which promote their binding to activated ECs and extravasation. Once at the site of inflammation, these LGLs would become activated and release cytokines such as GM-CSF, which could then cause a systemic increase in PMN production and PMN extravasation into the tumors. In addition, these LGLs produce large quantities of IFNγ that could potentially initiate/exacerbate this inflammatory process by upregulating the expression of adhesion molecules such as ICAM-1 and VCAM-1 at this peripheral site.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.783/4/m_bl_0022f8.jpeg?Expires=1769144397&Signature=heaf~glLE9IF4bNkDb47vfeqzXgKrNZWndv4sZv8IPsal8GI1ihaJI~4SzQMbKBf1QGKgd4p1hvo2qFOuG4AW0V1c4j2Bg6xOTaZHWhp8012iMdQ4kvItyV1yzmhyRi1tKFufexJAKP31IqatiOhAsTcoFS0vlwS~9gnPFzqn4gUYpsjYoBXpbS78wUnEFWmNuE3am33XX9OOmoS2fAgnsFapbSyzzqScV7tC6yMwjWs4wURhSqzb~~KuMXHU-~Yx1mESbUq0vJOXCxayOH-G9qQ625lyl3~mKSxHEUSmKiylogz1GbZ~m5dYkZwFRLkV-8CHr6xVMYLGZcN0g3GBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal