Abstract
The effect of human neutrophil elastase (HNE) on human factor V (F.V) or α-thrombin–activated human factor V (F.Va) was studied in vitro by prothrombinase assays, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and NH2 -terminal sequence analysis. Incubation of F.V (600 nmol/L) with HNE (2 nmol/L) in the presence of Ca2+ resulted in a time-dependent increase in its cofactor activity. In contrast, treatment of F.Va (600 nmol/L) with HNE (60 nmol/L) in the presence of Ca2+ resulted only in a time-dependent decrease in its cofactor activity. Under the conditions of these experiments, the maximum extent of F.V activation accomplished by incubation with HNE was approximately 65% to 70% of that observed with α-thrombin in presence of Ca2+. The extent of both the HNE-dependent enhancement in F.V cofactor activity and the HNE-dependent decrease in F.Va cofactor activity was not influenced by the addition of phosphatidylcholine/phosphatidylserine (PCPS) vesicles (50 μmol/L). The HNE-derived cleavage products of F.V, which correlated with increased cofactor activity, as demonstrated by SDS-PAGE under reducing conditions, were different from those generated using α-thrombin. Treatment of F.V (600 nmol/L) with HNE (2 nmol/L) in the presence of Ca2+ resulted in the production of three closely spaced doublets of: 99/97, 89/87, and 76/74 kD whose appearance over time correlated well with the increased cofactor activity as judged by densitometry. Treatment of F.Va (600 nmol/L) with HNE (60 nmol/L) in the presence of Ca2+ resulted in the cleavage of both the 96 kD heavy chain and the 74/72 kD light chain into products of: 56, 53, 35, 28, 22, and 12 kD. Although densitometry indicated that both the heavy and light chains of F.Va were hydrolyzed by HNE, cleavage of the 96 kD heavy chain was more extensive during the time period (10 to 30 minutes) of the greatest loss of F.Va cofactor activity. NH2 -terminal sequence analysis of F.V treated with HNE indicated cleavage at Ile819 and Ile1484 under conditions during which the procofactor expressed enhanced cofactor activity in the prothrombinase complex. NH2 -terminal sequence analysis of F.Va treated with HNE indicated cleavage at Ala341, Ile508, and Thr1767 under conditions, which the cofactor became inactivated, as measured by prothrombinase activity. The activation and inactivation cleavage sites are close to those cleaved by the physiological activator and inactivator of F.V and F.Va, namely α-thrombin (Arg709 and Arg1545) and Activated Protein C (APC) (Arg306 and Arg506), respectively. These results indicate that HNE can generate proteolytic products of F.V, which initially express significantly enhanced procoagulant cofactor activity similar to that observed following activation with α-thrombin. In contrast, HNE treatment of F.Va resulted only in the loss of its cofactor activity, but again, this is similar to that observed following inactivation by APC.
THE INFUSION of a combination of Factor Xa (F.Xa) and phosphatidylcholine/phosphatidylserine (PCPS) vesicles results in thrombin generation in vivo in several mammalian species including rodents, dogs, and nonhuman primates.1 As the dose is escalated, there is significant consumption of platelets, fibrinogen, and F.V and F.VIII and substantial activation of fibrinolysis2 and protein C.3 Therefore, these effects provide an animal model that mimics in substantial detail the changes seen in human disseminated intravascular coagulation (DIC).4 Clearly, characterization of the proteolytic mechanism(s) responsible for the loss of F.V and F.VIII activity would be extremely important given the pivotal role these cofactors play in hemostasis. Disregulation of Activated Protein C (APC) inactivation of these cofactors was ruled out by the demonstration that reduced or abolished APC generation in this model promotes rather than retards the loss of cofactor activity.5 Given clear evidence of plasmin generation2 and its known capacity to proteolyse F.V and F.VIII,6,7 this protease is a potential candidate for mediating the effects seen. Nonetheless, other effects, including a substantial fall in the circulating leukocyte count and increased levels of both elastase-specific breakdown products of fibrinogen and elastase/α1 -protease inhibitor (α1 -PI) complexes, suggested that neutrophil-derived elastase should also be considered.8
Human neutrophil elastase (HNE) is a serine protease with a broad specificity for substrates that include elastin, collagen, and other extracellular matrix proteins (see Werb et al9 for review). It appears to be an important enzyme in the physiological processes of wound healing, defence against invading microorganisms, extracellular tissue remodelling, inflammation, and the breakdown and removal of bacterial debris and damaged tissue.10,11 Its catalytic activity is regulated, at least in blood plasma, primarily by α1 -protease inhibitor and secondarily by α2 -macroglobulin.12 Much experimental and clinical evidence suggests that failure to regulate its activity may represent the basis for several pathologies including pulmonary emphysema, rheumatoid arthritis, atherosclerosis, and DIC.13 14
With respect to blood coagulation, elastase inactivates several inhibitors of coagulation and fibrinolysis including antithrombin III,15 tissue factor pathway inhibitor,16 protein C,17 protein S,18 heparin cofactor II,19 and α2 -antiplasmin.20 It also degrades fibrinogen,21 factors VIII,22 IX,23 X,24 and XIII25 and initially activates factor V (F.V), but then subsequently inactivates the active cofactor.26 Thus, should its activity escape the regulation of its physiologic inhibitors during the development of DIC, it could well be involved in the pathogenesis of this condition, as has been suggested.27
Our studies have centered on the effect(s) of HNE on human F.V because it is a key regulatory cofactor of hemostasis in its α-thrombin–activated form (F.Va) within the prothrombinase complex. Because of the documented evidence of plasmin generation in this model and the known propensity of this protease to both activate and then inactivate F.V/Va,6 it was imperative to first characterize in unambiguous detail the profile of elastase induced proteolysis of F.V/Va in vitro to support what was presumed to be preliminary evidence of disregulation of elastase activity in vivo. This was achieved in vitro using purified proteins and employing synchronous prothrombinase assays and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to characterize the HNE cleavage products of F.V and F.Va. In addition, the HNE cleavage products of F.V and F.Va were subjected to NH2 -terminal sequence analysis. The results show that HNE activates human F.V, but inactivates the procoagulant activity of human F.Va.
MATERIALS AND METHODS
Materials.Purified HNE was obtained from Biodesign International (Kennebunk, ME). The specific activity of HNE was 11,500 to 12,000 U/mg protein with the peptide substrate N-Methoxy-Succinyl-Ala-Ala-Pro-Val-p-nitroanilide (MeO-Succ-AAPV-pNA; Sigma, St Louis, MO). The HNE was greater than 97% pure by SDS-PAGE and densitometry and showed negligible activity against the cathepsin G substrate, N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (Sigma). Furthermore, its activity versus both MeO-Succ-AAPV-pNA or human F.V was completely inhibited by either an elastase-specific monoclonal antibody (Pel Freeze, Rogers, AR) or Methoxy-Succinyl-Ala-Ala-Pro-Val-chloromethyl-ketone (AAPV-CMK), (Enzyme Systems Products, Livermore, CA).
Human F.V was purified from fresh frozen human plasma by the procedure of Katzmann et al.28 F.Va was generated from F.V by incubation with human α-thrombin at 2.5 NIH U/mL for 10 minutes at 37°C according to Nesheim and Mann.29 Human prothrombin was isolated according to the procedure of Bajaj et al30 and thrombin was generated from prothrombin isolated according to Lundblad et al.31 Human Factor X was isolated by the method of Bajaj et al30 and activated to F.Xa according to Fujikawa et al.32 Phosphatidylcholine/phosphatidylserine (PCPS) vesicles (75%:25% wt/wt, respectively) were made and isolated according to Barenholz et al.33 The reversible, fluorescent thrombin inhibitor dansylarginine-N-(3-ethyl-1, 5-pentanediyl) amide (DAPA) was made according to Nesheim et al.34 SDS, N-2-Hydroxylethylpiperazine-N′-2-ethanesulfonic acid (HEPES), L-α-phosphatidylserine (bovine brain), L-α-phosphatidylcholine (egg yolk), and Coomassie Blue R-250 were from Sigma. BioGelWrap was from Biodesign (Carmel, NY). Immobilon-P was from Millipore Corp (Bedford, MA). All other reagents were of analytical quality.
Human neutrophil elastase cleavage of human factor V.Purified human F.V (600 nmol/L) was incubated at 37°C with HNE (2 nmol/L) in 50 mmol/L HEPES/0.1 mol/L NaCl, pH 7.4 (HBS) in the presence of 5.0 mmol/L CaCl2 with or without the addition of 50 μmol/L PCPS vesicles. DAPA (3.0 μmol/L) was included in all HNE digests of human F.V to preclude the possibility of trace levels of contaminating thrombin altering the resultant F.V activity. Control studies showed that neither DAPA nor PCPS vesicles alone or in combination at the concentrations used had any effect on the hydrolysis of MeO-Succ-AAPV-pNA peptide by HNE (data not shown). After various times of incubation, aliquots were removed for the prothrombinase assay and characterization by SDS-PAGE (see below).
Human neutrophil elastase cleavage of human factor Va.Human F.V (660 nmol/L) was activated with human α-thrombin at 2.5 NIH U/mL in HBS, pH 7.4 in the presence of 5.0 mmol/L CaCl2 at 37°C for 10 minutes. Next, DAPA (3.0 μmol/L) was added and the sample was incubated on ice for 5 minutes. Samples of F.Va (600 nmol/L) were then incubated at 37°C with HNE (60 nmol/L) in HBS, pH 7.4, in the absence or presence of 50 μmol/L PCPS vesicles. After various times of incubation, aliquots were removed for prothrombinase assays and characterization by SDS-PAGE (see below).
Human factor V and Va prothrombinase assays.The effect of HNE on F.V and F.Va cofactor activity was determined by prothrombinase assays. Aliquots of HNE treated F.V or Va were assayed for cofactor activity in the prothrombinase complex in the presence of DAPA. After specific time intervals of incubation at 37°C, HNE activity was terminated by adding aliquots of the reaction mixture to 10 μmol/L AAPV-CMK (final concentration) in HBS (pH 7.4) containing 0.1% bovine serum albumin (BSA) and 500.0 μmol/L PCPS before assaying for prothrombinase activity. Control studies showed that 10 μmol/L AAPV-CMK completely inactivated the HNE under these conditions, but it had no effect on the prothrombinase assays at the concentrations of F.V/Va assayed. Aliquots of HNE-treated F.V or Va were added to 1.6 mL reactions containing (final concentrations): F.V or F.Va (approximately 1.0 nmol/L), PCPS vesicles (5.0 μmol/L), DAPA (3.0 μmol/L), CaCl2 (5.0 mmol/L), human prothrombin (1.4 μmol/L) in HBS, pH 7.4. Prothrombin activation was initiated with human factor Xa (5.0 nmol/L) and the time course of thrombin generation was measured by the fluorescence intensity increase caused by the binding of DAPA to thrombin. Because DAPA inhibits thrombin activity, the assay allows quantitation of F.V-dependent thrombin generation without feedback activation of any unactivated F.V. The initial rate of increase of fluorescence over time is directly proportional to the concentration of active F.V in the sample. Fluorescence measurements were made with a Perkin Elmer LS50B Luminescence Spectrometer (Perkin Elmer, Beaconsfield, UK), using excitation and emission wavelengths of 335 nm and 545 nm, respectively and a 430 nm cutoff filter in the emission beam.
Electrophoretic analysis of elastase cleavage of human factor V and Va.SDS-PAGE analysis of the HNE cleavage products of F.V and Va was performed using 4% to 10% polyacrylamide gradient gels for F.V and 5% to 15% gels for F.Va according to the procedure of Neville.35 Aliquots of the same HNE reactions with F.V or F.Va incubated at 37°C (above), were added to (final concentrations) 54.0 mmol/L Tris-HCl (pH 6.14)/1% SDS/10% glycerol/0.1 mg/mL bromophenol blue/2% mercaptoethanol (SDS Sample buffer) before heating at 90°C for 2 minutes. Samples (approximately 4 μg) were electrophoresed on 1.5 mm polyacrylamide gradient gels (4% to 10% for F.V; 5% to 15% for F.Va), and the cleavage fragments were visualized following staining in 0.25% Coomassie Blue R-250 in 50% methanol and 10% acetic acid for 30 minutes and destaining in 50% methanol and 10% acetic acid for 2 hours. The gels were then soaked for 30 minutes at room temperature in 3% glycerol/40% methanol (vol/vol) and dried between two sheets of BioGelWrap at 37°C.
Densitometry of the dried Comassie Blue stained gels was performed using a Hewlett Packard ScanJet 3C and CorelPhotoPaint (Camus, WA) software. Quantitation of the staining intensity of the individual fragments generated with HNE was performed using SigmaGel (Jandel Scientific, San Rafael, CA) after correcting for background staining intensity.
Amino acid sequencing of fragments of human factor V and factor Va obtained after incubation with human neutrophil elastase.HNE digests F.V or F.Va in the presence of CaCl2 were electrophoresed on 4% to 10% gradient SDS polyacrylamide gels according to the procedure of Neville.35 The cleavage reactions for F.V were performed at 37°C for 10 and 30 minutes in 3 mL of a solution containing (final concentrations): 600 nmol/L of human F.V; 5.0 mmol/L CaCl2 ; 3.0 μmol/L DAPA and 2 nmol/L HNE in HBS, pH 7.4. The cleavage reaction of human F.Va was performed at 37°C for 30 minutes in 1.5 mL of a solution containing (final concentrations): 600 nmol/L human F.Va; 5 mmol/L CaCl2 ; 3.0 μmol/L DAPA and 60 nmol/L HNE in HBS, pH 7.4. All reactions were terminated by the addition of 10 μmol/L AAPV-CMK (final concentration) and incubation for 10 minutes on ice. The HNE-digested F.V and F.Va samples were separately dialyzed versus 4l of 0.2 N acetic acid at 4°C using 23 mm SpectroPor dialysis tubing (6 to 8,000 molecular weight cutoff [MWCO]) for 3 hours with one change. The samples were then frozen for 1 hour at −70°C and lyophilized overnight to dryness. The samples were then dissolved in 100 μL of SDS-PAGE sample buffer (above) and heated for 5 minutes at 90°C before being electrophoresed under reducing conditions in a 4% to 10% SDS polyacrylamide gel according to the conditions of Neville.35 Electroblotting and visualization of the F.V and F.Va fragments generated by HNE was performed according to a modification of the method of Matsudaira.36 Briefly, samples were electroblotted onto an Immobilon-P membrane for 3 hours at 0.5 mA constant current in: 200 mmol/L glycine; 25 mmol/L Tris; 0.1% SDS, and 20% methanol in a Hoeffer TransPhor blotting apparatus (San Francisco, CA.). After transfer, the membrane was soaked for 5 minutes in 0.25% Coomassie Blue R-250 in 50% methanol and 10% acetic acid. The membrane was then destained in 50% methanol and 10% acetic acid (twice for 5 minutes each) and finally rinsed with distilled water (twice for 5 minutes each) and air dried overnight before storage at −20°C.
The NH2 -terminal sequencing of the F.V and F.Va protein fragments on the blot was accomplished by automated Edman Degradation using a Porton Gas-Phase Microsequencer (Model 2090; Tarzana, CA) with on-line phenylthiohydantoin (PTH) analysis by Dr Teng Song Chen (Biotechnology Service Center, Hospital for Sick Children, Toronto, Canada). The PTH-amino acids released at each cycle were determined by high-performance liquid chromatography (HPLC) analysis after comparison of retention times to those of PTH-amino acid standards except cysteine.
The results presented are representative of experiments performed on at least three separate occasions except for the NH2 -terminal sequence analysis, which was performed once.
RESULTS
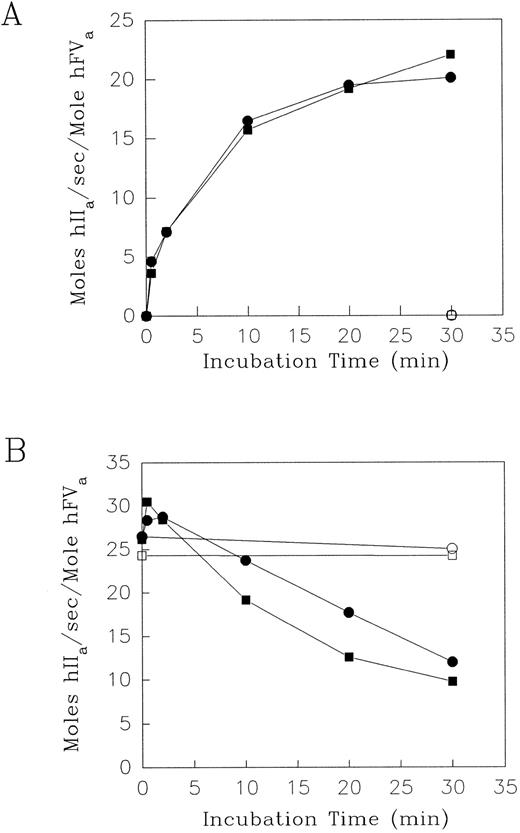
Effect of elastase on the contribution of F.V/Va to prothrombinase activity.Human F.V or F.Va were incubated with HNE in the presence of either Ca2+ alone or with the addition of PCPS vesicles. Aliquots were removed at intervals to measure the resultant cofactor activity using the prothrombinase assay. Simultaneously, timed aliquots were removed from the same reaction mixtures for analysis of the fragment profile using SDS-PAGE under reducing conditions (see below). The prothrombinase assays were performed using either F.V or F.Va at limiting concentrations (less than or equal to 1.0 nmol/L), so that the initial rates of thrombin production were directly proportional to the amount of F.Va or the HNE-activated F.V product in the reaction mixture. Initial experiments showed that with 600 nmol/L F.V in the presence of 5 mmol/L Ca2+, addition of 60 nmol/L HNE increased the resulting F.V cofactor from zero to 18.4 mol hIIa/sec/mol hF.Va by 0.5 minutes at 37°C. Subsequently, over the next 2, 10, 20, and 30 minutes, the corresponding F.V cofactor activity values (in mol hIIa/s/mol hF.Va) were: 21.7, 23.3, 24.0, and 19.5 (data not shown). Therefore, because HNE had primarily an activation effect on the cofactor activity of F.V in the presence of Ca2+, the [HNE] was decreased from 60 to 2 nmol/L, while keeping the [F.V] at 600 nmol/L to decrease the rate of the reaction to one that would be more easily measured over a longer time period. The prothrombinase assay results showing the effect of HNE on F.V are shown in Fig 1A. In the reaction mixtures containing F.V (600 nmol/L) and 5 mmol/L Ca2+, HNE (2 nmol/L) increased the cofactor activity from zero to 16.5 mol hIIa/sec/mol hFVa over the first 10 minutes (Fig 1A). Thereafter, HNE increased the cofactor activity of F.V slowly to more stable levels of approximately 20.1 mol hIIa/sec/mol hFVa by 30 minutes. The result of the addition of PCPS vesicles (50 μmol/L) to the same reaction mixture was not significantly different in terms of the resultant time course and extent of the generation of F.V cofactor activity (Fig 1A). The generation of F.V cofactor activity was, however, specific for the addition of HNE, as its omission from reaction mixtures containing F.V and Ca2+ with or without PCPS expressed negligible cofactor activity over the same time course (Fig 1A). Under the experimental conditions described, the maximal cofactor activity expressed by HNE-treated F.V in the absence or presence of PCPS vesicles was 20.1 and 22.0 mol hIIa/s/mol hF.Va, respectively. These are 64.0% and 70.0%, respectively, of the F.Va cofactor activity observed following α-thrombin activation of F.V (31.4 mol hIIa/s/mol F.Va) (data not shown). The cofactor activity of α-thrombin–activated F.V determined in this study (31.4 mol hIIa/s/mol hF.Va) was in good agreement with previously published values of between 26 to 31 mol IIa/s/mol F.Va in the bovine prothrombinase complex.37
The effect of HNE on the cofactor activity of Factor V and Va. Purified human F.V (A), and F.Va (B), (600 nmol/L) were incubated at 37°C with purified HNE (2 nmol/L in A; 60 nmol/L in B) in HBS, pH 7.4, 5 mmol/L CaCl2 and 3.0 μmol/L DAPA (•) or with 50 μmol/L PCPS (▪). Control incubation mixtures for both F.V (A) and F.Va (B) in the absence of HNE with (□) or without (○) the addition of PCPS vesicles are shown. At the times indicated, aliquots were withdrawn and HNE activity was quenched with 10.0 μmol/L AAPV-CMK in HBS, pH 7.4 containing 0.1% BSA and 500 μmol/L PCPS. F.Va cofactor activity was then determined using 1.4 μmol/L prothrombin and 5.0 nmol/L F.Xa in the prothrombinase assay (see Materials and Methods). The results shown are expressed as initial rates of mol thrombin (hIIa) generated per second per mole hF.Va.
The effect of HNE on the cofactor activity of Factor V and Va. Purified human F.V (A), and F.Va (B), (600 nmol/L) were incubated at 37°C with purified HNE (2 nmol/L in A; 60 nmol/L in B) in HBS, pH 7.4, 5 mmol/L CaCl2 and 3.0 μmol/L DAPA (•) or with 50 μmol/L PCPS (▪). Control incubation mixtures for both F.V (A) and F.Va (B) in the absence of HNE with (□) or without (○) the addition of PCPS vesicles are shown. At the times indicated, aliquots were withdrawn and HNE activity was quenched with 10.0 μmol/L AAPV-CMK in HBS, pH 7.4 containing 0.1% BSA and 500 μmol/L PCPS. F.Va cofactor activity was then determined using 1.4 μmol/L prothrombin and 5.0 nmol/L F.Xa in the prothrombinase assay (see Materials and Methods). The results shown are expressed as initial rates of mol thrombin (hIIa) generated per second per mole hF.Va.
In contrast to the predominant activation of F.V, HNE (60 nmol/L) inactivated the cofactor activity of F.Va (600 nmol/L), as measured by the prothrombinase assay (Fig 1B). Preliminary experiments indicated that addition of 2 and 60 nmol/L HNE to 600 nmol/L F.Va in the presence of 5 mmol/L CaCl2 for 30 minutes at 37°C resulted in a concentration-dependent decrease in the cofactor activity of F.Va in the prothrombinase assay from approximately 26.5 mol hIIa/s/mol F.Va to 24.3 and 12.0 mol hIIa/s/mol F.Va, respectively (data not shown). Treatment of F.Va (600 nmol/L) with HNE (60 nmol/L) in the presence of 5 mmol/L Ca2+ resulted in an initial 10% to 15% increase in the cofactor activity above the levels observed over the first 0.5 to 2 minutes at 37°C (Fig 1B). The HNE-dependent inactivation of the cofactor activity of F.Va was observed only over the next 10 to 30 minutes under these conditions and was not significantly different when PCPS vesicles (50 μmol/L) were included with the HNE, F.Va, and Ca2+ in the reaction mixture (Fig 1B). The initial values of F.Va cofactor activity were reduced to approximately 45% and 37% by HNE, in the absence and presence of PCPS vesicles, respectively, after 30 minutes. However, the inactivation of F.Va cofactor activity was dependent on HNE, with or without added PCPS vesicles, as its omission resulted in negligible alteration in F.Va cofactor activity over the entire time course of the experiment (Fig 1B).
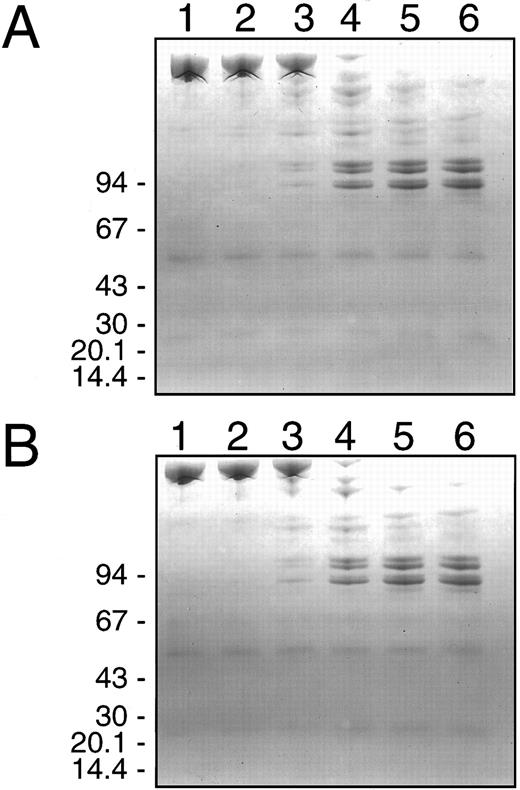
Electrophoretic analysis of HNE degradation of F.V/Va.Electrophoretic analyses under denaturing and reducing conditions and subsequent Coomassie Blue staining of the gels was performed using the same reaction mixtures and time course of sampling used for the prothrombinase assays (see Fig 1 above). This was done to investigate directly the proteolysis of these two substrates by HNE in relation to the observed changes in their cofactor activity. The results for F.V (600 nmol/L) and HNE (2 nmol/L) are shown in Fig 2, while that for F.Va (600 nmol/L) and HNE (60 nmol/L) are shown in Fig 3. The initial increase in the F.V cofactor activity following HNE treatment in the presence of Ca2+ for 0.5 to 2 minutes coincided with the loss of intact F.V and the appearance of at least six major intermediate cleavage products with apparent molecular masses of: 260, 236, 222, 175, 155, and 138 kD (Fig 2A, lanes 1 to 3). Over the next 20 to 30 minutes, the fragments generated initially were replaced by a series of three closely spaced doublets, with apparent molecular masses of 99/97 kD and 89/87 kD, and a weaker staining doublet at 76/74 kD (Fig 2A, lanes 4 to 6). The time-dependent appearance of these three doublets correlated very well with the greatest increase in F.V cofactor activity following HNE treatment as judged by densitometry of the gel (Fig 4A). Treatment of F.V (600 nmol/L) with HNE (2 nmol/L) in the presence of PCPS vesicles resulted in a proteolytic profile indistinguishable from that observed in their absence (compare Fig 2A with Fig 2B). Again, the appearance of the three doublet species of apparent molecular mass of 99/97 kD and 89/87 kD and a weaker staining doublet at 76/74 kD correlated very well with the largest increases observed in F.V cofactor activity, determined by prothrombinase assay (compare Fig 1A with Fig 2B) and densitometry of the gel (data not shown).
SDS-PAGE analysis of human neutrophil elastase cleavage of human factor V. Purified human F.V (600 nmol/L) was incubated at 37°C with purified HNE (2 nmol/L) in HBS, pH 7.4 with 5 mmol/L CaCl2 and 3 μmol/L DAPA in the absence (A) or presence (B) of 50 μmol/L PCPS vesicles and simultaneous aliquots were withdrawn for prothrombinase assays and analysis by SDS-PAGE. Samples (4 μg) were withdrawn at 0, 0.5, 2, 10, 20, and 30 minutes after HNE addition corresponding to lanes 1, 2, 3, 4, 5, and 6, respectively. HNE activity was terminated by addition of SDS-PAGE sample buffer and heating at 90°C for 2 minutes. Samples were electrophoresed on 4% to 10% gradient SDS polyacrylamide gels under reducing conditions and then stained with Coomassie Blue. The migration position of molecular weight standards in (kD) is indicated on the left of each panel.
SDS-PAGE analysis of human neutrophil elastase cleavage of human factor V. Purified human F.V (600 nmol/L) was incubated at 37°C with purified HNE (2 nmol/L) in HBS, pH 7.4 with 5 mmol/L CaCl2 and 3 μmol/L DAPA in the absence (A) or presence (B) of 50 μmol/L PCPS vesicles and simultaneous aliquots were withdrawn for prothrombinase assays and analysis by SDS-PAGE. Samples (4 μg) were withdrawn at 0, 0.5, 2, 10, 20, and 30 minutes after HNE addition corresponding to lanes 1, 2, 3, 4, 5, and 6, respectively. HNE activity was terminated by addition of SDS-PAGE sample buffer and heating at 90°C for 2 minutes. Samples were electrophoresed on 4% to 10% gradient SDS polyacrylamide gels under reducing conditions and then stained with Coomassie Blue. The migration position of molecular weight standards in (kD) is indicated on the left of each panel.
SDS-PAGE analysis of human neutrophil elastase cleavage of human factor Va. Purified human F.Va (600 nmol/L) was incubated at 37°C with purified HNE (60 nmol/L) in HBS, pH 7.4 with 5 mmol/L CaCl2 and 3 μmol/L DAPA in the absence (A) or presence (B) of 50 μmol/L PCPS vesicles and simultaneous aliquots were withdrawn for prothrombinase assays and analysis by SDS-PAGE. Samples (4 μg) were withdrawn at 0, 0.5, 2, 10, 20, and 30 minutes after HNE addition corresponding to lanes 1, 2, 3, 4, 5, and 6, respectively, and HNE activity was terminated by addition of SDS-PAGE sample buffer and heating at 90°C for 2 minutes. Samples were electrophoresed on 5% to 15% gradient SDS polyacrylamide gels under reducing conditions and then stained with Coomassie Blue. The migration position of molecular weight standards in (kD) is indicated on the left of each panel.
SDS-PAGE analysis of human neutrophil elastase cleavage of human factor Va. Purified human F.Va (600 nmol/L) was incubated at 37°C with purified HNE (60 nmol/L) in HBS, pH 7.4 with 5 mmol/L CaCl2 and 3 μmol/L DAPA in the absence (A) or presence (B) of 50 μmol/L PCPS vesicles and simultaneous aliquots were withdrawn for prothrombinase assays and analysis by SDS-PAGE. Samples (4 μg) were withdrawn at 0, 0.5, 2, 10, 20, and 30 minutes after HNE addition corresponding to lanes 1, 2, 3, 4, 5, and 6, respectively, and HNE activity was terminated by addition of SDS-PAGE sample buffer and heating at 90°C for 2 minutes. Samples were electrophoresed on 5% to 15% gradient SDS polyacrylamide gels under reducing conditions and then stained with Coomassie Blue. The migration position of molecular weight standards in (kD) is indicated on the left of each panel.
Correlation between the effect of HNE and the cofactor activities of F.V and F.Va and the appearance/dissappearance of specific protein fragments. The SDS gels for F.V (A) or F.Va (B) from Figs 2A and 3A, respectively, were scanned using a Hewlett Packard ScanJet 3C and the density of each fragment calculated as described in Materials and Methods. The data from the corresponding prothrombinase assays (F.V, Fig 1A; F.Va, Fig 1B) is plotted against the densitometry data to relate fragment appearance/disappearance after HNE treatment to changes in cofactor activity. In (A), the cofactor activity (left axis) of F.V (•) is plotted against the density (right axis) of fragments of apparent molecular mass of 99/97 kD (▪); 89/87 kD (▴); and 76/74 kD (▾). In (B), the cofactor activity (left axis) of F.Va (•) is plotted against the density (right axis) of the 96-kD heavy chain (▪) and the 74/72 kD light chain (▴).
Correlation between the effect of HNE and the cofactor activities of F.V and F.Va and the appearance/dissappearance of specific protein fragments. The SDS gels for F.V (A) or F.Va (B) from Figs 2A and 3A, respectively, were scanned using a Hewlett Packard ScanJet 3C and the density of each fragment calculated as described in Materials and Methods. The data from the corresponding prothrombinase assays (F.V, Fig 1A; F.Va, Fig 1B) is plotted against the densitometry data to relate fragment appearance/disappearance after HNE treatment to changes in cofactor activity. In (A), the cofactor activity (left axis) of F.V (•) is plotted against the density (right axis) of fragments of apparent molecular mass of 99/97 kD (▪); 89/87 kD (▴); and 76/74 kD (▾). In (B), the cofactor activity (left axis) of F.Va (•) is plotted against the density (right axis) of the 96-kD heavy chain (▪) and the 74/72 kD light chain (▴).
The initial 10% to 15% increase in the cofactor activity after incubation of F.Va with HNE for 0.5 to 2 minutes in the presence of Ca2+ was associated with the generation of two major intermediate fragments of apparent molecular mass of 35 and 29 kD (Fig 3A, lanes 2 and 3). Over the next 20 to 30 minutes, both fragments were replaced by species of lower apparent molecular mass. The greatest HNE-induced decrease in the cofactor activity of F.Va was observed between 10 and 30 minutes of F.Va exposure to HNE and resulted in the appearance of fragments of apparent molecular mass of: 56, 53, 35, 28, 22, and 12 kD (Fig 3A, lanes 4 to 6). Addition of 50 μmol/L PCPS vesicles to F.Va and HNE had no significant effect on the proteolytic profile of the fragments observed (compare Figs 3A and 3B). Densitometry of the gels indicated that although the light chain of F.Va was preferentially cleaved by HNE during the first 0.5 to 2 minutes, this resulted in a 10% to 15% increase in the resultant F.Va cofactor activity (compare Figs 1B and 3A to Fig 4B). Furthermore, the heavy chain of F.Va was only cleaved significantly to the six more stable products after 10 to 30 minutes exposure to HNE (compare Figs 3A and 4B with Fig 1B). This was the time interval during which the greatest decrease in the F.Va cofactor activity occurred (Fig 1B). This suggests that the HNE-induced cleavage of the F.Va heavy chain was the primary cause of the loss of cofactor activity observed.
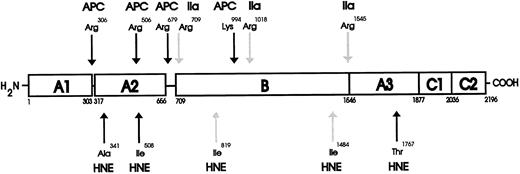
NH2 -terminal sequencing of HNE cleaved F.V and F.Va.The three peptide doublet fragments that were generated by HNE cleavage of F.V were analyzed by NH2 -terminal sequencing to identify the specific sites of hydrolysis, which correlated with the activation of the cofactor as determined by prothrombinase assay (see above, Fig 1). The results are shown in Table 1 and the position of these sites within the human F.V molecule are shown in cartoon form in Fig 5. These data indicate that HNE cleaves F.V at least at two sites, Ile819 and Ile1484 (Table 1). There are undoubtedly more HNE cleavage sites within the F.V molecule. However, in the present study, only one unique F.V NH2 -terminal sequence was identified for each of the three doublet species whose appearance correlated with increased F.V cofactor activity. The 99/97 kD doublet fragment contained a sequence matching the first six amino acids at the NH2 -terminus of the intact procofactor.38 The 89/87 kD doublet fragment contained a peptide sequence that matched the amino acid sequence of human F.V from Val1485 to Lys1489, indicating cleavage at Ile1484. The 76/74 kD doublet contained a peptide sequence that corresponded to the human F.V sequence from Arg820 to Gly825, indicating cleavage at Ile819. Because the two HNE cleavage sites of F.V at Ile819 and Ile1484 are each relatively close to the α-thrombin cleavage sites at Arg709 and Arg1545 (Fig 5), it is reasonable to propose that the generation of F.V cofactor activity by HNE is probably mediated by cleavage at these two positions.
NH2-Terminal Sequence of Human Factor V-Derived Fragments After HNE Digestion
| Cycle No. . | 99/97 kD* . | 89/87 kD . | 76/74 kD . |
|---|---|---|---|
| 1 | A(6.0) | V(7.0) | R(6.0) |
| 2 | Q(9.0) | G(8.0) | L(8.0) |
| 3 | L(10.0) | L(10.0) | L(9.0) |
| 4 | R(12.0) | S(5.0) | S(3.0) |
| 5 | Q(8.0) | K(6.0) | L(3.0) |
| 6 | F(10.0) | G(6.0) |
| Cycle No. . | 99/97 kD* . | 89/87 kD . | 76/74 kD . |
|---|---|---|---|
| 1 | A(6.0) | V(7.0) | R(6.0) |
| 2 | Q(9.0) | G(8.0) | L(8.0) |
| 3 | L(10.0) | L(10.0) | L(9.0) |
| 4 | R(12.0) | S(5.0) | S(3.0) |
| 5 | Q(8.0) | K(6.0) | L(3.0) |
| 6 | F(10.0) | G(6.0) |
Human F.V was incubated with 2 nmol/L HNE at 37°C for 2 and 30 minutes in HBS, pH 7.4 with 5 mmol/L CaCl2 as described in Materials and Methods. Approximately 900 pmol was used for each sample reaction. The apparent molecular mass of the specific fragment is shown along with the pmol of the amino acid identified (in parentheses) after each number of cycles performed.
Fragment doublets 99/97 kD; 89/87 kD; and 76/74 kD represent the HNE cleaved products of F.V.
Schematic diagram for the proteolytic cleavages of human F.V and F.Va by HNE. The domain structure of human F.V is made up of the heavy chain (residues 1-709) comprising two A domains (A1-A2) and connecting regions.38 51 The B domain spans residues 710 to 1545 and connects the heavy and light chains. The light chain (residues 1546-2196) is composed of one A and two C domains (A3-C1-C2). The results of the NH2 -terminal sequencing indicated HNE activates human F.V cofactor activity after cleavage at Ile819 and Ile1484 (HNE-labeled light arrows) and inactivates human F.Va cofactor activity after cleaving at Ala341, Ile508, and Thr1767 (HNE-labeled dark arrows). The positions of the HNE cleavage sites within the F.V/F.Va molecule are shown below the cartoon representation of intact F.V, while the thrombin (IIa-labeled light arrows) activation and the Activated Protein C (APC-labeled dark arrows) inactivation cleavage sites are shown above for comparison.
Schematic diagram for the proteolytic cleavages of human F.V and F.Va by HNE. The domain structure of human F.V is made up of the heavy chain (residues 1-709) comprising two A domains (A1-A2) and connecting regions.38 51 The B domain spans residues 710 to 1545 and connects the heavy and light chains. The light chain (residues 1546-2196) is composed of one A and two C domains (A3-C1-C2). The results of the NH2 -terminal sequencing indicated HNE activates human F.V cofactor activity after cleavage at Ile819 and Ile1484 (HNE-labeled light arrows) and inactivates human F.Va cofactor activity after cleaving at Ala341, Ile508, and Thr1767 (HNE-labeled dark arrows). The positions of the HNE cleavage sites within the F.V/F.Va molecule are shown below the cartoon representation of intact F.V, while the thrombin (IIa-labeled light arrows) activation and the Activated Protein C (APC-labeled dark arrows) inactivation cleavage sites are shown above for comparison.
The peptide fragments that were generated by HNE cleavage of F.Va were also analyzed by NH2 -terminal sequencing to determine the specific sites of hydrolysis, which correlated with the inactivation of the cofactor, as determined by prothrombinase assay (see Fig 1B above). The results are shown in Table 2 and the HNE cleavage sites within the F.Va molecule are shown in Fig 5. These data indicate that HNE cleaves F.Va at least at three sites: Ala341, Ile508, and Thr1767 (Table 2 and Fig 5). The 76-kD fragment contained two sequences matching the first five amino acids at both the NH2 -terminus of the intact procofactor and the sequence from Ser1546 to Asn1550 of the light chain.38 39 The 56- and 53-kD fragments each gave sequences matching the first seven amino acids at the NH2 -terminus of the intact procofactor. The 48-kD fragment contained two peptide sequences that matched both the first seven amino acids at the NH2 -terminus of the intact procofactor and the sequence Ser1768 to Ser1774, indicating HNE cleavage within the light chain at Thr1767. The 35-kD fragment contained a peptide sequence matching the first six amino acids from Ser1546 to Arg1551 of the light chain. The 28-kD fragment contained a peptide sequence that matched the seven amino acids from Asn342 to Arg348, indicating HNE cleavage at Ala341. The 22-kD fragment contained a peptide sequence matching six amino acids from Gln509 to Ile514, indicating HNE cleavage at Ile508. There was insufficient material present in the 12 kD fragment to permit unambiguous amino acid sequencing under these conditions (data not shown).
NH2-Terminal Sequence of Human Factor Va-Derived Fragments After HNE Digestion
| Cycle No. . | 76 kD* . | 56 kD . | 53 kD . | 48 kD . | 35 kD . | 28 kD . | 22 kD . |
|---|---|---|---|---|---|---|---|
| 1 | A(8.0)/S(31.0) | A(20.0) | A(6.0) | A(4.0)/S(13.0) | S(13.0) | N(10.0) | Q(17.0) |
| 2 | Q(4.0)/N(6.0) | Q(18.0) | Q(8.0) | Q(4.0)/S(9.0) | N(10.0) | M(5.0) | R(21.0) |
| 3 | L(4.0)/N(4.0) | L(20.0) | L(9.0) | L(2.0)/E(5.0) | N(6.0) | D(9.0) | A(16.0) |
| 4 | R(3.0)/G(4.0) | R(20.0) | R(7.0) | R(2.0)/M(3.0) | G(4.0) | K(3.0) | A(14.0) |
| 5 | Q(2.0)/N(2.0) | Q(20.0) | Q(6.0) | Q(4.0)/K(3.0) | N(4.0) | K(3.0) | D(15.0) |
| 6 | F(14.0) | F(5.0) | F(4.0)/K(3.0) | R(5.0) | Y(6.0) | I(9.0) | |
| 7 | Y(15.0) | Y(7.0) | Y(4.0)/S(4.0) | R(4.0) |
| Cycle No. . | 76 kD* . | 56 kD . | 53 kD . | 48 kD . | 35 kD . | 28 kD . | 22 kD . |
|---|---|---|---|---|---|---|---|
| 1 | A(8.0)/S(31.0) | A(20.0) | A(6.0) | A(4.0)/S(13.0) | S(13.0) | N(10.0) | Q(17.0) |
| 2 | Q(4.0)/N(6.0) | Q(18.0) | Q(8.0) | Q(4.0)/S(9.0) | N(10.0) | M(5.0) | R(21.0) |
| 3 | L(4.0)/N(4.0) | L(20.0) | L(9.0) | L(2.0)/E(5.0) | N(6.0) | D(9.0) | A(16.0) |
| 4 | R(3.0)/G(4.0) | R(20.0) | R(7.0) | R(2.0)/M(3.0) | G(4.0) | K(3.0) | A(14.0) |
| 5 | Q(2.0)/N(2.0) | Q(20.0) | Q(6.0) | Q(4.0)/K(3.0) | N(4.0) | K(3.0) | D(15.0) |
| 6 | F(14.0) | F(5.0) | F(4.0)/K(3.0) | R(5.0) | Y(6.0) | I(9.0) | |
| 7 | Y(15.0) | Y(7.0) | Y(4.0)/S(4.0) | R(4.0) |
Human F.Va (600 nmol/L) was incubated with 60 nmol/L HNE for 30 minutes in HBS, pH 7.4 with 5 mmol/L CaCl2 as described in Materials and Methods. Approximately 900 pmol was used for the F.Va reaction. The apparent molecular mass of the specific fragments is shown along with the pmol of the amino acid identified (in parentheses) after each number of cycles performed.
Fragments 76 kD; 56 kD; 53 kD; 48 kD; 35 kD; 28 kD; 22 kD represent the HNE-derived cleavage products of F.Va.
Because the HNE cleavage of F.Va at Ala341 and Ile508 are both relatively close to two of the known Activated Protein C (APC) inactivation cleavage sites at Arg306 and Arg506, it is reasonable to assume that the inactivation of F.Va cofactor activity is mediated, at least in part, by cleavage in these two positions. Additionally, because HNE is also able to cleave F.Va at Thr1767 within the A3 domain of the light chain, this cleavage may also contribute to inactivation.
DISCUSSION
This study characterizes in detail the specific cleavage events involved in the HNE-dependent activation of F.V and the HNE-dependent inactivation of F.Va in vitro. The use of purified components in a strictly controlled system permitted the correlation between the sites of HNE cleavage within Fs.V and Va with their resultant fragmentation profiles and cofactor activities within the prothrombinase complex.
The observations reported here confirm and extend the observations of Oates and Salem26 who previously showed that zymosan-activated human neutrophils released an elastase-like activity that was capable of initially activating F.V and subsequently inactivating the activated cofactor, as determined by a one-stage clotting assay. The use of clot-based assays to evaluate F.V/Va activity are confounded by the ability of thrombin to promote its own generation by feedback activation of F.V. Similarly, it is difficult to separate activation from inactivation events. The use of the DAPA-based prothrombinase assay circumvents these difficulties in interpretation and helps provide relatively unambiguous evidence of F.V cofactor activation or F.Va inactivation by HNE. Although in preliminary study, we also observed inactivation of F.V cofactor activity following its initial activation with higher concentrations of HNE (30 to 60 nmol/L) using the prothrombinase assay (data not shown), the use of lower concentrations of HNE (2 nmol/L) reported here permitted a detailed in vitro analysis of the HNE-dependent activation of F.V without interference of the competing inactivation effect. This provided the opportunity to come to quantitative conclusions with respect to the correlation of cofactor activity with the appearance and disappearance of specific protein fragments. The results therefore suggest that the functional concentration of HNE generated is critically important in determining the net effect on the cofactor activities of Fs.V and Va. Under the in vitro conditions of these experiments, significantly less HNE was required to activate F.V (2 nmol/L) than to inactivate F.Va (60 nmol/L). Interestingly, these HNE concentrations are within the approximate concentration range that has been reported in vivo. The [HNE] bound to activated human neutrophil membranes at sites of degranulation has been estimated to be in the mmol/L range,40 while the [HNE] in normal plasma has been found to be approximately 10 to 20 nmol/L.41
In comparison to the physiological activator of F.V, ie, α-thrombin, HNE was approximately 65% to 70% as efficient in enhancing the cofactor activity of F.V. The HNE-dependent generation of F.V fragments of apparent molecular mass of: 99/97 kD, 89/87 kD, and 76/74 kD was maximal during the period of greatest cofactor activity suggesting that the production of one or more of these specific fragments is diagnostic of the activation process. NH2 -terminal sequence analysis of these three protein doublets indicated HNE cleavage of F.V at Ile819 and Ile1484. Because these two HNE cleavage sites are relatively close to the known sites cleaved by α-thrombin (Arg709 and Arg1545 ) during the activation of F.V,42 43 the results strongly suggest that one or more of these three protein fragments are the active species generated from F.V by HNE. Confirmation of this will require their purification and reconstitution in functional prothrombinase assays.
In analogous experiments, treatment of F.Va with HNE indicated that while both the 96-kD heavy chain and 74/72-kD light chain of F.Va were cleaved to generate fragments of apparent molecular mass of 56, 53, 48, 35, 28, 22, and 12 kD, the time-dependent loss of the 96-kD heavy chain correlated best with the loss of F.Va cofactor activity. These results strongly suggest that the HNE-dependent production of one or more of these specific fragments from F.Va is diagnostic of the inactivation process. HNE cleavage sites within F.Va at Ala341 and Ile508 were demonstrated and are of particular interest given their proximity to the known cleavage sites for APC at Arg306 and Arg506.44 Because these APC cleavages result in inactivation of F.Va, presumably by disrupting the prothrombin and/or F.Xa binding sites, it is reasonable to assume that HNE cleavage in similar regions would also have a similar, substantial inactivating effect. In addition, NH2 -terminal sequence analysis confirmed HNE cleavage within F.Va at Thr1767, which resulted in the generation of a 48 kD protein fragment. This observation is of particular importance for two reasons. Firstly, the material migrating at 48 kD on SDS-PAGE yielded two NH2 -terminal sequences, one indicating the intact NH2 -terminus of intact F.V and another indicating cleavage at Thr1767. This correlated temporally with the loss of F.Va cofactor activity. Secondly, HNE cleavage at Thr1767 of human F.Va represents the first documented cleavage site within the A3 domain of the light chain by a protease. Thus, it would appear that the 48 kD peptide formed by HNE cleavage at Thr1767 may represent a specific HNE-derived proteolytic fragment of human F.Va and could prove to be of diagnostic use in defining elastase activity in the more complex in vivo environment.
The HNE-induced F.V activation and the HNE-induced inactivation of F.Va were not modified in vitro by a negatively charged membrane surface as provided by PCPS vesicles. These results contrast with those previously reported for plasmin and Fs.V and Va where although the activation phase with F.V was not affected by the presence of phospholipid, the inactivation processes with Fs.V and Va were both markedly enhanced in the presence of phospholipid vesicles.6 Although the α-thrombin–mediated activation of F.V does not require the presence of phospholipid,29,42,43 the inactivation of F.Va by APC is completely phospholipid-dependent.44 F.V activation by elastase and cathepsin G expressed on the human monocyte surface has been reported recently.45 This study suggested that both monocyte cathepsin G and elastase are involved in the activation of F.V, however, it is known that HNE and monocyte elastase have different proteolytic specificities.9 Therefore, it is possible that a cellular as opposed to a synthetic phospholipid membrane surface may be fundamentally different in modulating the effects of HNE on the cofactor activities of Fs.V and Va.
These results provide a theroretical basis for both a physiologic and pathophysiologic role for elastase in hemostasis and its disorders. Physiologically, the generation of fibrin around loci of infection is thought to play a major role in the prevention of systemic disease.46,47 Because neutrophils accumulate at such sites, it is reasonable to assume that elastase would become available at a concentration to promote sufficient F.Va-like cofactor activity to support the local generation of prothrombinase activity in cooperation with extrinsic pathway generated F.Xa.11 Thus, it may represent an important, albeit less efficient, pathway for supporting prothrombinase complex assembly than that generated intravascularly. In this situation, the contribution of its major inhibitor, α1 -protease inhibitor (α1 -PI), would be minimized, particularly in view of the high concentrations of elastase that are likely to exist in such situations.11
Intravascularly, where HNE's role in activating F.V would be more likely to be considered unphysiological, the potential inhibitory effect of α1 -PI requires further experimental consideration given its plasma concentration of approximately 20 μmol/L.12 Experimental evidence does exist that HNE may escape α1 -PI inhibiton in plasma. Studies of α1 -PI deficiency showed the presence of HNE-specific fibrinogen degradation products in both hetero- and homozygous forms of the condition, despite adequate plasma levels of α1 -PI in the former group of patients.48 Similarly, in the experimental model of DIC where generation of thrombin occurs in vivo following the infusion of F.Xa in combination with negatively charged phospholipid vesicles, increased levels of HNE-specific fibrinogen degradation products were also observed in combination with significant increases in the circulating levels of elastase/α1 -PI complexes.8 Therefore, it is possible that release of HNE from neutrophils could occur into a protected environment, as has been shown for elastase bound to membranes49 and elastin,50 where its natural inhibitors would be excluded or present at a concentration that could be overwhelmed by the local release of HNE.
In conclusion, the results presented in this study provide the necessary experimental basis for now considering increased HNE availability as a potentially significant factor in the pathogenesis of conditions of disregulated hemostasis such as DIC given its propensity in vitro for both initiating (through its activation of F.V) and then promoting the consumptive coagulopathy (through its inactivation of F.Va). It may now be possible to use this information to obtain definitive evidence of HNE-induced proteolysis of Fs.V/Va in vivo using Western blotting and polyclonal antibodies to F.V or HNE-specific chloromethylketone inhibitors. The generation of HNE-specific cleavage product(s) of Fs.V/Va in vivo and their detection using polyclonal antibodies to F.V may enable us to progress to diagnostic confirmation of the possible involvement of HNE in the pathologies of this important clinical disorder.
ACKNOWLEDGMENT
We acknowledge the contribution of Barbara Saunders in the preparation of the manuscript.
Supported by a grant-in-aid from the Medical Research Council of Canada (Ottawa, Ontario), Grant No. MA-7667. A.R.G. is a Distinguished Research Professor of the Heart & Stroke Foundation of Ontario.
Presented in part at the XVth Congress of the International Society on Thrombosis and Haemostasis, Jerusalem, Israel, June 11-16, 1995.
Address reprint requests to Alan R. Giles, MD, Department of Pathology, Richardson Laboratory, Queen's University, Kingston, Ontario, Canada K7L 3N6.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal