Abstract
Using homologous recombination, both EKLF alleles in murine embryonic stem (ES) cells were inactivated. These EKLF−/− ES cells were capable of undergoing in vitro differentiation to form definitive erythroid colonies that were similar in size and number to those formed by wild-type ES cells. However, the EKLF−/− colonies were poorly hemoglobinized and enucleated erythrocytes in these colonies contained numerous Heinz bodies. Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses revealed that adult and embryonic globin genes were appropriately regulated, with the exception of βh1-globin, which continued to be expressed at a very low level. The ratio of adult β-globin/α-globin mRNA in the mutant ES cells was 1/15 of that in wild-type ES cells. When the EKLF−/− cells were injected into blastocysts, they did not contribute at a detectable level to the mature erythrocyte compartment of the chimeric animals, based on analysis of glucose phosphate isomerase-1 (GPI-1) isozymes and hemoglobins that distinguish ES cell-derived erythrocytes from host blastocyst-derived erythrocytes. In contrast, semiquantitative RT-PCR analysis of RNA from reticulocytes of the same chimeric animals suggested that the ES cell-derived reticulocytes were present at a level of 6% to 8%. This indicated that the EKLF−/− erythrocytes in adult animals must be short-lived, apparently due to the imbalance of β-versus α-globin chains, leading to the precipitation of excess α-globin chains to form Heinz bodies. Consistent with this hypothesis, the short life span was ameliorated by introduction into the EKLF−/− ES cells of a human LCR/γ-globin gene, as evidenced by the presence of ES cell-derived reticulocytes as well as mature erythrocytes in the blood of the chimeric animals.
ONE OF THE distinctive features of erythroid cell ontogeny is the switch from embryonic to fetal to adult hemoglobin in a developmental stage-specific manner. In the mouse, the embryonic β-like globin genes (εy and βh1) are expressed at very high levels in the primitive erythroblasts of the yolk sac. At a later stage of development, there is a switch from embryonic erythropoiesis in the yolk sac to the definitive erythropoiesis in the fetal liver, and during this transition, the embryonic β-like globin genes are downregulated and the adult β-globin genes are upregulated.1-3 High-level expression of the β-like globin genes is dependent on an upstream stretch of DNA known as the locus control region (LCR).4-7 Some studies have indicated that while cis-acting elements within and flanking each globin gene can confer stage specificity,8-13 competition between different globin genes for interaction with the LCR may also be important in determining the stage-specific expression of the globin genes.14-16 Based on studies of mice transgenic for various permutations of human LCR and γ- and β-globin gene constructs, as well as on hemoglobin switching in the chick, it has been postulated that hemoglobin switching from the fetal to the adult stage involves the expression of adult stage-specific transcription factors that favored the interaction of the promoter of the adult β-globin promoter with the LCR over that of the fetal β-globin promoter.4,14 17-19
One transcriptional activator implicated in these regulatory events is Erythroid Kruppel-like Factor (EKLF ).20,21 EKLF binds via its three zinc fingers to a 9-base pair (bp) target sequence that encompasses the adult β-globin CACCC motif, one of a trio of red cell promoter and enhancer DNA binding sites known to be crucial for transcription of globin and other erythroid cell-specific genes.22 Specific amino acids within these fingers form close contacts to each of the guanine residues on the G-rich strand of this extended binding element.23 Point mutations of some of these important residues, including those that are known to lead to β-thalassemia, abolish the transactivation ability of EKLF by disrupting its ability to bind these modified target sites.23 Molecular dissection of the protein has indicated that EKLF contains a strong transcriptional activation domain that is specifically required for erythroid cell-restricted and β-globin promoter-specific activation.24 Biological analyses reveal that murine EKLF is expressed in primitive erythroid cells by embryonic day 7.5 (E7.5), and in definitive erythroid cells within the hepatic primordium by E9.25 However, EKLF does not recognize equally well all CACCC motifs within the developmentally regulated β-like globin cluster and its ability to preferentially activate a β-globin promoter over a linked γ-globin promoter directly implicates EKLF as an important component for efficient γ- to β-globin gene switching.20 Consistent with this idea, genetic ablation of EKLF resulted in a profound β-thalassemia leading to fetal death at the time of the switch to β-globin synthesis in the fetal liver.26 27 EKLF-deficient mice exhibited drastically low β-globin chain expression at the transcriptional level, which accounted for the severe β-thalassemia phenotype.
To investigate the role of EKLF in definitive erythropoiesis in the adult (as opposed to fetal definitive erythropoiesis) and to characterize further the effects of the lack of EKLF on erythropoiesis using in vitro differentiation, we inactivated both EKLF alleles in ES cells. In this report, we show that these EKLF−/− ES cells were capable of differentiating in vitro to form definitive red cells. However, the level of adult β-globin mRNAs was decreased by 15-fold relative to the level in EKLF+/+ definitive red cells. This resulted in an imbalance of α- versus β-globin chains, as evidenced by the presence of numerous Heinz bodies that resulted from precipitation of excess α-globin chains. When the EKLF−/− cells were injected into mouse blastocysts, they contributed to the reticulocytes but not to the mature red cells of the resulting chimeric mice, suggesting that these ES cells are capable of undergoing erythropoiesis in the mouse, but produced erythrocytes with a short half-life. The short half-life was ameliorated when a human γ-globin gene linked in cis to the LCR was introduced into the EKLF−/− ES cells before generating chimeric mice.
MATERIALS AND METHODS
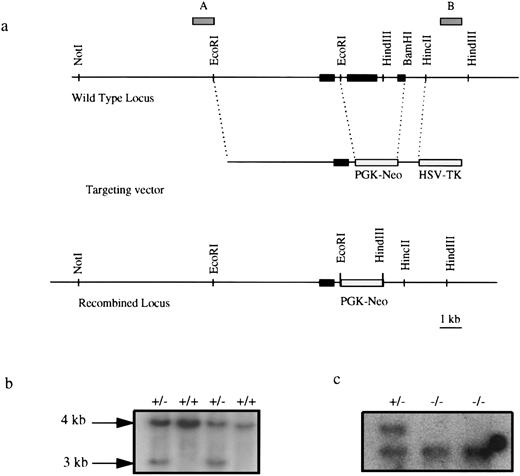
Generation of EKLF−/− and EKLF−/−, LCRγ ES cells.EKLF genomic sequences were isolated from a 129/SvEv mouse DNA library using an EKLF cDNA probe.21 The targeting vector consisted of a 5.5-kb EcoRI fragment containing upstream sequences and exon 1 of the EKLF gene, a 1-kb BamHI-HincII fragment downstream of the EKLF gene, a neoR gene driven by the phosphoglycerate kinase promoter (PGK) and the herpes simplex virus thymidine kinase (HSV-tk) gene (see Fig 1). Not I linearized DNA was electroporated into R1 ES cells,28 which were selected with G418 and ganciclovir. EKLF targeted ES cells were identified by Southern blot hybridization.29 Homozygous mutant (EKLF−/−) ES cells were generated by culturing the heterozygous ES cells in an elevated level (4.5 mg/mL) of G41830 and also identified by Southern blot hybridization. To introduce the LCRγ-globin gene into EKLF−/− ES cells, a 26-kb Sal I fragment that includes DNase I hypersensitive sites I-V linked to the human Aγ-globin gene14 (see Fig 6b) was mixed with a puromycin resistance gene in a 10:1 molar ratio and electroporated into EKLF−/− ES cells, which were selected with puromycin (1 μg/mL). EKLF−/−, LCRγ+ ES cell clones were identified by Southern blot hybridization with a probe derived from the HindIII/Xho I fragment of the human LCRγ-globin hybrid gene, which includes 5′ portion of the human γ-globin gene.
Targeted disruption of the EKLF gene. (a) Targeting strategy. Solid boxes denote exons 1, 2, and 3. Gray boxes represent probes used in Southern blot analysis. Exons 2 and most of exon 3 were deleted in a targeted allele, effectively removing 239 amino acids of the 358-amino acid protein.21 (b) Southern blot analysis of EKLF+/+ and EKLF+/− ES cell clones using HindIII digestion and probe B. The 4-kb fragment represents the WT allele and the 3 kb represents the targeted allele. (c) Southern blot analysis using HindIII digested DNA from EKLF+/− and EKLF−/− ES cells derived by selection in elevated G418, using probe B. Hybridizations with probe A and a Neo probe confirmed that a homologous recombination event had occurred (data not shown).
Targeted disruption of the EKLF gene. (a) Targeting strategy. Solid boxes denote exons 1, 2, and 3. Gray boxes represent probes used in Southern blot analysis. Exons 2 and most of exon 3 were deleted in a targeted allele, effectively removing 239 amino acids of the 358-amino acid protein.21 (b) Southern blot analysis of EKLF+/+ and EKLF+/− ES cell clones using HindIII digestion and probe B. The 4-kb fragment represents the WT allele and the 3 kb represents the targeted allele. (c) Southern blot analysis using HindIII digested DNA from EKLF+/− and EKLF−/− ES cells derived by selection in elevated G418, using probe B. Hybridizations with probe A and a Neo probe confirmed that a homologous recombination event had occurred (data not shown).
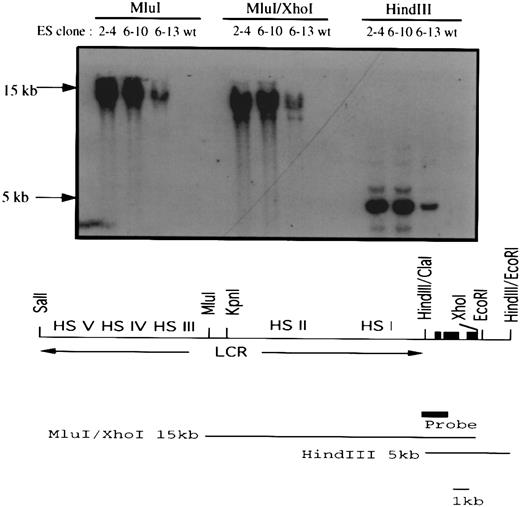
Transfection of a human LCRγ-globin gene into the EKLF−/− ES clones. A 26-kb Sal I fragment containing a human LCR/γ-globin construct and a PGK-puromycin resistance gene were coelectroporated into EKLF−/− ES cells. Three clones (2-4, 6-10, and 6-13) out of 27 puromycin-resistant clones carried the human LCR/γ-globin hybrid gene. The top panel shows a Southern blot analysis of Mlu I, Mlu I/Xho I, and HindIII restricted genomic DNAs using the indicated probe. The presence of a 15-kb Mlu I/Xho I and an overlapping 5-kb HindIII fragment in all three clones suggested at least 16 kb of the hybrid gene had integrated into the genome of the ES cells. The human LCR/γ-globin gene is shown at the bottom. The three solid black boxes represent the three exons of the human γ-globin gene and the solid gray box represents the probe used in the Southern blot analysis.
Transfection of a human LCRγ-globin gene into the EKLF−/− ES clones. A 26-kb Sal I fragment containing a human LCR/γ-globin construct and a PGK-puromycin resistance gene were coelectroporated into EKLF−/− ES cells. Three clones (2-4, 6-10, and 6-13) out of 27 puromycin-resistant clones carried the human LCR/γ-globin hybrid gene. The top panel shows a Southern blot analysis of Mlu I, Mlu I/Xho I, and HindIII restricted genomic DNAs using the indicated probe. The presence of a 15-kb Mlu I/Xho I and an overlapping 5-kb HindIII fragment in all three clones suggested at least 16 kb of the hybrid gene had integrated into the genome of the ES cells. The human LCR/γ-globin gene is shown at the bottom. The three solid black boxes represent the three exons of the human γ-globin gene and the solid gray box represents the probe used in the Southern blot analysis.
In vitro differentiation of the ES cells.ES cells were differentiated in vitro using the two-step method of Keller et al.31 Briefly, in the first step of the differentiation protocol, 1 × 105 cells were cultured in methylcellulose for 8 days to form embryoid bodies. The embryoid bodies were subsequently disaggregated and replated in methylcellulose. The sources and concentrations of hematopoietic growth factors used were 20 ng/mL recombinant rat stem cell factor (SCF; Amgen, Thousand Oaks, CA), 1.5% pokeweed mitogen-stimulated SCM (Stem Cell Technologies, Inc, Vancouver, British Columbia, Canada) and 2 U/mL recombinant human Epo (R&D Systems, Minneapolis, MN). The colonies were scored on day 8 of culture. Single colonies were picked, dispersed on slides, and stained with crystal violet. RNA was also extracted from individual colonies as described.32 An initial reverse transcriptase-polymerase chain reaction (RT-PCR) assay using the α- and β-globin specific primers was performed to confirm that the colonies were erythroid before cDNAs of three colonies were pooled for further semiquantitative RT-PCR analysis.
RNA, glucose phosphate isomerase-1 (GPI-1) isozymes, and hemoglobin analysis.ζ-, α-, and β-globin cDNAs were described by Weiss et al.33 To determine the ratio of βsingle-globin mRNA versus βmaj/βmin-globin mRNAs, RT-PCR was performed as described.33 To distinguish between the 578-bp RT-PCR products of βsingle-globin mRNA versus βmaj/βmin-globin mRNAs, the RT-PCR products were extracted with phenol-chloroform, ethanol precipitated and digested with Msc I. RT-PCR products from βmaj- and βmin-globin mRNAs but not those from βsingle-globin mRNA are digested by Msc I to form 487-bp and 91-bp fragments. The relative ratio of the βsingle-globin RT-PCR product and the 487-bp Msc I-restricted, βmaj-, βmin-globin RT-PCR was calculated by first quantitating the signals by phosphorimaging. The signal from the smaller 487-bp fragment was then normalized by a factor of 1.14 to compensate for the loss of the 91-bp fragment after Msc I digestion. The factor of 1.14 was calculated based on the 266 GC nucleotides in the 578-bp fragment versus 233 GC nucleotides in the 487-bp fragment. Separations of GPI-1 isozymes and globin chains were performed as previously described.34 35
RESULTS
Inactivation of both EKLF loci in embryonic stem cells by homologous recombination.The EKLF gene was inactivated by homologous recombination using a replacement-type targeting vector as shown in Fig 1a. Briefly, the targeting vector consisted of a 5.5-kb 5′ EcoR1 fragment and a 1-kb 3′ BamHI-HincII fragment of the EKLF gene, a neoR gene driven by the PGK and the HSV-tk. The construct was electroporated into R1 ES cells followed by positive/negative selection using G418 and ganciclovir.36 A total of 250 doubly resistant ES clones were isolated and analyzed by Southern blot hybridization and in two of these clones, one of the EKLF alleles had been disrupted by the targeting vector (Fig 1b). Further Southern blot analyses using different enzymes and probes confirmed that the appropriate recombination event had occurred (data not shown). To select for cells in which the second EKLF allele had been spontaneously inactivated, 1 × 104 cells derived from one of the targeted clones were cultured in an elevated level of G418.30 Eleven colonies survived this selection and two clones #2 and #6 were found to be homozygous for the targeted allele of EKLF (Fig 1b).
In vitro differentiation of ES cells to erythroid cells.Since earlier studies have shown that EKLF is a β-globin specific erythroid transcription factor,20,21 and that primitive erythropoiesis is normal in the EKLF−/− mouse embryos,26,27 the definitive erythropoietic potential of the EKLF−/− ES cells versus wild-type R1 ES cells was examined using a two-step in vitro differentiating protocol.31,33 To favor the formation of definitive erythroid colonies, 8-day-old embryoid bodies were harvested and replated. Erythroid colonies were scored and harvested after an additional 8 days of culture. Erythroid colonies derived from wild-type or EKLF+/− ES cells were well-hemoglobinized and appeared reddish while erythroid colonies derived from EKLF−/− ES cells were morphologically similar but had only a slight tinge of red, indicating a low level of hemoglobinization (Fig 2a). The number of erythroid colonies per 5 × 105 cells from the embryoid bodies derived from the WT and EKLF−/− cells were similar (116 ± 7 and 105 ± 12, respectively). Therefore, the lack of EKLF did not affect the survival of the erythroid progenitor cells. Furthermore, the size of erythroid colonies derived from wild-type, EKLF+/− and EKLF−/− cells appeared to be similar (Fig 2a), suggesting that the proliferative capacity of the erythroid progenitor cells were not affected by the lack of EKLF. Consistent with the results of Perkins et al,27 no effect on the differentiation of myeloid cells or megakaryocytes was observed (data not shown).
(a) In vitro differentiated erythroid colonies of EKLF+/+, EKLF+/−, and EKLF−/− ES cell-derived erythroid colonies at day 8 of replating. Both EKLF+/+ and EKLF+/− colonies were hemoglobinized as indicated by the reddish coloration, whereas the EKLF−/− colony was poorly hemoglobinized with a slight reddish tinge. Colonies are shown at the same magnification. (b) Staining of EKLF+/+ and EKLF−/− colonies with crystal violet. Single colonies were picked, spread on slides, and stained with crystal violet. Small dark Heinz bodies indicative of excess α-globin chains were present in the enucleated EKLF−/− erythrocytes (N, nucleus; E, erythrocytes).
(a) In vitro differentiated erythroid colonies of EKLF+/+, EKLF+/−, and EKLF−/− ES cell-derived erythroid colonies at day 8 of replating. Both EKLF+/+ and EKLF+/− colonies were hemoglobinized as indicated by the reddish coloration, whereas the EKLF−/− colony was poorly hemoglobinized with a slight reddish tinge. Colonies are shown at the same magnification. (b) Staining of EKLF+/+ and EKLF−/− colonies with crystal violet. Single colonies were picked, spread on slides, and stained with crystal violet. Small dark Heinz bodies indicative of excess α-globin chains were present in the enucleated EKLF−/− erythrocytes (N, nucleus; E, erythrocytes).
To analyze the erythroid colonies at the molecular level, single colonies were picked, RNA was purified and analyzed by RT-PCR for the presence of α- and β-globin mRNAs. Colonies that were identified as erythroid based on morphology were always positive for α- and β-globin mRNAs. The cDNAs from three colonies of each genotype were then pooled so that there would be sufficient cDNA to determine the level of embryonic βh1-, εy-, and ζ-globin mRNAs by semiquantitative RT-PCR. εy- and ζ-globin mRNAs were not detected in EKLF−/−, EKLF+/−, or EKLF+/+ erythroid colonies (Fig 3a and 3b). On long exposure, βh1-globin mRNA was, however, detected at a low level in EKLF−/− and EKLF+/− but not EKLF+/+ erythroid colonies (Fig 3d). The low-level persistence of βh1-globin expression in EKLF−/− definitive erythroid colonies appeared to be a specific consequence of the lack of EKLF, rather than a contamination of the colonies with primitive erythroid cells, because of the absence of the embryonic εy-and ζ-globin mRNAs.
RT-PCR using RNA from EKLF+/+, EKLF+/−, and EKLF−/− ES cell-derived erythroid colonies. RNA from each erythroid colony was purified and 1/10 of the RNA was reversed transcribed and amplified by PCR using α- and β-globin specific primers to confirm that the colonies were erythroid. The rest of the RNAs were then reverse transcribed and cDNAs from three erythroid colonies of each genotype were then pooled. The resulting cDNAs were diluted 1× and 10×, and amplified by PCR in the presence of 32P-dCTP. The PCR products were separated on a polyacrylamide gel, quantitated by phosphorimaging, and exposed to autoradiography. (a) α- and ζ-globin specific primers. Controls are embryonic yolk sac (YS) and fetal liver (FL). (b) α- and εy-globin specific primers. (c) α- and β-globin specific primers. (d) α- and βh1-globin specific primers. βh1-globin mRNA was not detected in the WT colonies, but was present in EKLF+/− colonies at about 1/10 the level in EKLF−/− colonies after normalizing against α-globin mRNA. εy-globin and ζ-globin mRNAs were not detectable in colonies of any of the three genotypes.
RT-PCR using RNA from EKLF+/+, EKLF+/−, and EKLF−/− ES cell-derived erythroid colonies. RNA from each erythroid colony was purified and 1/10 of the RNA was reversed transcribed and amplified by PCR using α- and β-globin specific primers to confirm that the colonies were erythroid. The rest of the RNAs were then reverse transcribed and cDNAs from three erythroid colonies of each genotype were then pooled. The resulting cDNAs were diluted 1× and 10×, and amplified by PCR in the presence of 32P-dCTP. The PCR products were separated on a polyacrylamide gel, quantitated by phosphorimaging, and exposed to autoradiography. (a) α- and ζ-globin specific primers. Controls are embryonic yolk sac (YS) and fetal liver (FL). (b) α- and εy-globin specific primers. (c) α- and β-globin specific primers. (d) α- and βh1-globin specific primers. βh1-globin mRNA was not detected in the WT colonies, but was present in EKLF+/− colonies at about 1/10 the level in EKLF−/− colonies after normalizing against α-globin mRNA. εy-globin and ζ-globin mRNAs were not detectable in colonies of any of the three genotypes.
The levels of β-globin mRNA in EKLF−/− erythroid colonies were determined using a semiquantitative RT-PCR assay, and normalized against the level of α-globin mRNA. We observed that the ratio of β-globin/α-globin mRNA in EKLF−/− colonies was approximately 1/15 of that in EKLF+/+ cells (Fig 4c ), in agreement with the observations in the EKLF−/− mouse embryos.26 27 The low level of β-globin mRNA in EKLF −/− erythroid cells would presumably lead to an imbalance of α- versus β-globin chains resulting in an excess of free α-globin chains that would precipitate to form inclusion bodies known as Heinz bodies. Therefore, single erythroid colonies were dispersed and stained with crystal violet for the presence of Heinz bodies. Small darkly stained bodies indicative of Heinz bodies were detected in the enucleated EKLF−/− cells while such darkly stained bodies were not detected in enucleated EKLF+/+ cells (Fig 2b).
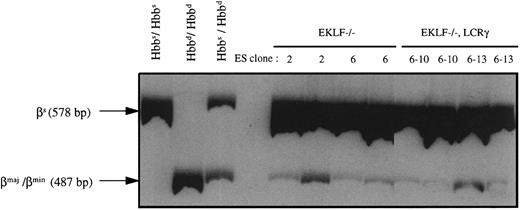
RT-PCR analysis of peripheral blood of chimeric mice produced with EKLF−/− or EKLF−/−, LCRγ ES cells. Chimeras were generated by injecting EKLF−/− clones 2 and 6, and EKLF−/−, LCRγ clones 6-10 and 6-13 into C57BL/6J blastocysts. RNA was prepared from peripheral blood of eight chimeric mice at approximately 3 to 4 months of age and RT-PCR was performed using β-globin primers in the presence of 32P-dCTP. The RT-PCR products were then digested with Msc I and separated on a 5% polyacrylamide gel, quantitated by phosphorimaging, and exposed to autoradiography. The 578-bp fragment represents the Msc I-resistant, βsingle-globin RT-PCR product and the 487-bp fragment represents the Msc I-restricted, βmaj-, βmin-globin RT-PCR products (the 91-bp fragment was run off the gel to better resolve the 578 and 487 fragments). mRNAs from blood of C57BL/6J mice (Hbbs/Hbbs), 129Sv/Ev mice (Hbbd/Hbbd), and (C57BL/6J × CBA/J) F1 mice (Hbbs/Hbbd) were used as controls.
RT-PCR analysis of peripheral blood of chimeric mice produced with EKLF−/− or EKLF−/−, LCRγ ES cells. Chimeras were generated by injecting EKLF−/− clones 2 and 6, and EKLF−/−, LCRγ clones 6-10 and 6-13 into C57BL/6J blastocysts. RNA was prepared from peripheral blood of eight chimeric mice at approximately 3 to 4 months of age and RT-PCR was performed using β-globin primers in the presence of 32P-dCTP. The RT-PCR products were then digested with Msc I and separated on a 5% polyacrylamide gel, quantitated by phosphorimaging, and exposed to autoradiography. The 578-bp fragment represents the Msc I-resistant, βsingle-globin RT-PCR product and the 487-bp fragment represents the Msc I-restricted, βmaj-, βmin-globin RT-PCR products (the 91-bp fragment was run off the gel to better resolve the 578 and 487 fragments). mRNAs from blood of C57BL/6J mice (Hbbs/Hbbs), 129Sv/Ev mice (Hbbd/Hbbd), and (C57BL/6J × CBA/J) F1 mice (Hbbs/Hbbd) were used as controls.
ES cells with both inactivated EKLF genes can contribute to the formation of reticulocytes in the blood of mice.To assess the ability of EKLF−/− cells to form erythrocytes in vivo, the ES cells were injected into the 3.5 days post coitum blastocysts of C57BL/6J mice. Several adult chimeric mice of approximately 20% to 30% chimerism by coat color were generated and analyzed. No chimera with greater than 30% chimerism by coat color was ever generated, while chimeras with greater than 50% coat color chimerism were routinely generated with EKLF+/− and wild-type R1 ES cells. Since our in vitro differentiation assays showed that EKLF−/− cells can form enucleated erythrocytes, the EKLF−/− chimeric mice were analyzed for their ability to produce either reticulocytes or mature erythrocytes from EKLF−/− ES cells. Reticulocytes, which constitute about 1% of total red cells in the adult mice, contain high levels of globin RNA whereas the mature red cells have negligible amounts of RNA but high levels of hemoglobin. Therefore, RNA prepared from the total red cells is derived mainly from the reticulocytes whereas protein prepared from total red cells is derived mainly from the mature erythrocytes.
To determine if EKLF−/− ES cells contribute to the reticulocyte compartment of the chimeric mice, RNA was extracted from total red blood cells and analyzed by RT-PCR. The host blastocysts from C57BL/6J strain of mice are homozygous for the Hbbs haplotype and therefore synthesize one species of β-globin chain, the βsingle chain. The R1 ES cells, which are derived from the 129 strain of mice, are homozygous for the Hbbd haplotype and synthesize two β-globin chains, βmaj and βmin. Using primers previously described,33 β-globin cDNAs from both Hbbs and Hbbd haplotypes can be amplified by PCR to give a 578-bp fragment. To distinguish between RT-PCR products of Hbbs and Hbbd haplotypes, the RT-PCR products were digested with Msc I, which cleaves RT-PCR products from βmaj and βmin mRNAs but not those from βsingle-globin mRNA, to form 487-bp and 91-bp fragments. As shown in Fig 4, some of the β-globin RT-PCR products derived from the RNA of EKLF−/− chimeric animals were susceptible to Msc I digestion as evidenced by the presence of the 487-bp fragment. Therefore, βmaj- and βmin-globin mRNAs were present in the blood of the chimeric mice indicating that the double knockout ES cells can contribute to the reticulocytes. Quantitation by phosphorimaging indicated that the 487-bp fragment was about 0.4% to 0.5% of the 578-bp fragment in 4 chimeric mice (two produced with EKLF−/− clone 2 and two produced with EKLF−/− clone 6) analyzed. Our in vitro differentiation experiments have shown that EKLF−/− erythrocytes expressed approximately 1/15 as much adult β-globin mRNA as EKLF+/+ erythrocytes. Therefore, by multiplying by a factor of 15 to correct for the decrease in the adult β-globin, we can estimate that the EKLF−/− reticulocytes make up approximately 6% to 7.5% of the total reticulocytes in the chimeras.
Red blood cells from chimeras were then analyzed at the protein level to determine if the EKLF−/− reticulocytes could form mature erythrocytes. The proteins analyzed were GPI-1 isozymes and adult β-globin chains. R1 ES cell-derived erythrocytes express the GPI-1A isozyme and, the βmaj and βmin globins, which are easily distinguishable from the GPI-1B isozyme and βsingle globin of the C57BL/6J-derived host erythrocytes. Based on the RNA analysis of the reticulocytes, the expected proportions of GPI-1A isozyme and βmaj- and βmin-globin chains were ∼6% to 7.5% and ∼0.5%, respectively. However, neither marker was detectable in the red cell fraction of all four chimeras (Fig 5). While the expected contribution of 0.5% by β-globin of the Hbbd haplotype may be beyond the detection limit of the assay, the GPI-1 assays can readily detect a 3% contribution of GPI-1A (data not shown). We conclude that although EKLF−/− ES cells can contribute to the formation of reticulocytes, these cells are underrepresented in the mature erythrocyte population. This is consistent with our in vitro differentiation assays which showed that EKLF−/− erythrocytes suffer from a deficiency of adult β-globin chains, which results in an excess of α-globin chains that precipitate to form Heinz bodies. It is known that erythrocytes with Heinz bodies are rapidly culled from the circulation by the spleen, which could account for the shortened life span of EKLF−/− erythrocytes.
Analysis of ES cell contributions to the red cells of chimeras. (a) Acid urea-Triton X-100 (Sigma, St Louis, MO) polyacrylamide gel electrophoretic analysis of globin chains from the blood of EKLF−/− and EKLF−/−, LCRγ chimeras. Blood from a mouse transgenic for the human LCRγ hybrid gene (TGγ), a C57BL/6J mouse of Hbbs haplotype and a (C57BL/6J × CBA/J) F1 mouse of Hbbs/Hbbd haplotype were used as controls. The gel was deliberately overstained with Protostain silver stain to enable detection of the human γ-globin chain and, the βmaj and βmin -globin chains in the EKLF−/−, LCRγ chimeric mice. (b) GPI-1 analysis of red blood cells. Red cell extracts from chimeras produced with EKLF+/− clone #12, EKLF−/− clone 2 and 6 and, EKLF−/−, LCRγ clone 6-10 and 6-13 were separated by cellulose acetate electrophoresis and stained for GPI-1 enzymatic activity. Red cell extracts from 129/SvEv mice and C57BL/6J mice were used as controls for GPI-1A and GPI-1B isozymes, respectively. R1 ES cells produce GPI-1A and cells derived from the host blastocyst produce GPI-1B. To detect GPI-1A activity in the blood of EKLF−/−, LCRγ 6-10 and 6-13 chimeras, the stain was overdeveloped such that detection of GPI-1B from the host blastocysts was past saturation limits of the assay.
Analysis of ES cell contributions to the red cells of chimeras. (a) Acid urea-Triton X-100 (Sigma, St Louis, MO) polyacrylamide gel electrophoretic analysis of globin chains from the blood of EKLF−/− and EKLF−/−, LCRγ chimeras. Blood from a mouse transgenic for the human LCRγ hybrid gene (TGγ), a C57BL/6J mouse of Hbbs haplotype and a (C57BL/6J × CBA/J) F1 mouse of Hbbs/Hbbd haplotype were used as controls. The gel was deliberately overstained with Protostain silver stain to enable detection of the human γ-globin chain and, the βmaj and βmin -globin chains in the EKLF−/−, LCRγ chimeric mice. (b) GPI-1 analysis of red blood cells. Red cell extracts from chimeras produced with EKLF+/− clone #12, EKLF−/− clone 2 and 6 and, EKLF−/−, LCRγ clone 6-10 and 6-13 were separated by cellulose acetate electrophoresis and stained for GPI-1 enzymatic activity. Red cell extracts from 129/SvEv mice and C57BL/6J mice were used as controls for GPI-1A and GPI-1B isozymes, respectively. R1 ES cells produce GPI-1A and cells derived from the host blastocyst produce GPI-1B. To detect GPI-1A activity in the blood of EKLF−/−, LCRγ 6-10 and 6-13 chimeras, the stain was overdeveloped such that detection of GPI-1B from the host blastocysts was past saturation limits of the assay.
Amelioration of excess α-globin chains in EKLF−/− erythrocytes by human γ-globin chains.To determine if the shortened life span of EKLF−/− erythrocytes is due mainly to the imbalance of β- versus α-globin chains, a human γ-globin gene linked in cis to the LCR13 was introduced into the EKLF−/− ES cells. The promoter of human γ-globin gene does not have the optimal binding sequences for EKLF and is not transactivated by EKLF in cotransfection assays, unlike the promoter of the β-globin gene.20 Thus, the LCR linked in cis with the human γ-globin gene would continue to drive expression of the gene in the adult erythroid cells, albeit in a reduced amount.4,14 16 It was anticipated that expression of the γ-globin gene would ameliorate the imbalance of β- versus α-globin chains by dimerization with the excess free α-globin chains, thereby extending the life span of the EKLF−/− erythrocytes in the chimeric animals.
A 26-kb fragment from a cosmid containing a human γ-globin gene linked in cis to the LCR14 was introduced into the EKLF−/− ES cells by coelectroporation with a puromycin resistance gene. Three out of 11 puromycin-resistant ES cell clones isolated also contained the human γ-globin gene, including at least a 16-kb segment of the LCRγ construct (Fig 6). Chimeras (15% to 20% by coat color) were generated with two of the clones, 6-10 and 6-13, and their blood was analyzed for the presence of β-globin mRNA from the Hbbd haplotype, and GPI-1A isozyme, as described above. Attempts to differentiate clones 6-10 and 6-13 in vitro to form erythroid colonies failed to produce sufficient viable embryoid bodies for replating in the 2-step differentiation protocol. Although the reasons for this defect are unknown, the extensive culture and drug selection required to generate the double knockout/LCR γ-globin cells may have compromised the ability of the ES cells to develop into embryoid bodies in vitro, while in a chimeric environment this deficiency may be compensated for by the host blastocyst.
RT-PCR analysis indicated that βmaj- and βmin-globin mRNAs constituted approximately 0.5% to 0.7% of the total β-globin mRNAs in all four chimeras analyzed, two with EKLF−/−, LCRγ clone 6-10 and two with clone 6-13 (Fig 4). Based on the level of βmaj- and βmin-globin mRNAs (0.5% to 0.7%) and on the expected 15-fold reduction in the β/α mRNA ratio, we estimated that 7% to 10% of the reticulocytes in the chimeric mice are ES cell derived (as explained above). Erythrocytes from these chimeras were also analyzed for the presence of GPI-1A isozyme and βmaj- and βmin-globin chains. Unlike the blood from EKLF−/− chimeras, blood from EKLF−/−, LCRγ chimeras contained detectable amounts of GPI-1A isozyme and βmaj-globin chains as well as human γ-globin chains (Fig 5). Thus, the expression of the human γ-globin gene appears to extend the life span of the EKLF−/− erythrocytes, possibly by forming hemoglobin tetramers with some of the excess α-globin chains.
DISCUSSION
In this report, we have used targeted mutagenesis in mouse ES cells to investigate the role of EKLF in adult erythropoiesis. EKLF has been previously shown to bind the CACCC motif in the promoters of human and mouse adult β-globin genes,21 and to transactivate a reporter gene when linked in cis to an adult β-globin promoter.20 However, it does not bind well to the CAC-like motif of the human γ-globin or the mouse βh1-globin promoters, and it will not activate a reporter gene when linked in cis to a fetal γ-globin promoter.20 In agreement with this apparent specificity for adult β-like globin genes, it has been shown previously that in developing mice lacking the EKLF gene, embryonic erythropoiesis was normal, but there was a deficiency of β-globin chains in fetal definitive erythroid cells.26,27 The resulting anemia led to prenatal death at 14 to 15 days postcoitum,26 27 precluding any analysis of the requirement for EKLF in adult erythropoiesis.
To circumvent this prenatal death and examine the role of EKLF in adult erythropoiesis, homozygous mutant (EKLF−/−) ES cells were generated by homologous recombination. By generating chimeric mice with these ES cells and analyzing their contribution to the reticulocyte and erythrocyte populations of chimeric adult animals, we were able to assess the ability of EKLF−/− cells to contribute to the reticulocyte and erythrocyte populations. In vitro differentiation of ES cells also provided us with an alternative source of pure EKLF−/− erythroid cells for molecular characterization.
EKLF−/− ES cells differentiate in vitro to form enucleated definitive red cells.Since in vitro differentiation of ES cells recapitulates the process of erythropoiesis in vivo,31,33,37EKLF−/− ES were differentiated in vitro to analyze the role of EKLF in the formation of definitive enucleated erythrocytes. The number and size of erythroid colonies derived from EKLF−/− ES cells were similar to those derived from wild-type ES cells and their gross colony morphology was similar, except that EKLF−/− erythroid colonies were less hemoglobinized. Both EKLF−/− and wild-type erythroid colonies were shown to contain enucleated erythroid cells, and RT-PCR analysis revealed an absence of embryonic εy- and ζ-globin mRNAs, confirming that these colonies contain definitive rather than primitive erythroid cells. However, embryonic βh1-globin mRNA was present at very low but detectable levels in EKLF−/− and EKLF+/−, but not EKLF+/+ erythroid colonies. This observation raises the possibility that EKLF may play a role in the silencing of the βh1-globin gene in definitive erythropoiesis. Other workers have shown that in EKLF−/− fetuses transgenic for the human β-globin locus, human β-globin expression is dramatically reduced or absent with an elevation of γ-globin gene expression.38 39 They proposed, in essence, that EKLF is important in stabilizing the interaction of the adult β-globin gene with the LCR over that of the fetal γ-globin gene, and that in the absence of EKLF, the decrease in transcriptional activity of the adult β-globin gene leads to impaired silencing of the γ-globin gene. Our observation on βh1 expression appears to be consistent with this interpretation. RT-PCR analysis also showed a 15-fold decrease in the ratio of adult β-globin/adult α-globin mRNA in EKLF−/− definitive erythroid cells, which presumably resulted in a deficiency of adult β-globin chains.
Together, these data suggest that EKLF−/− erythroid progenitors can be established and are able to proliferate to normal levels. In addition, EKLF−/− cells can contribute to the in vivo reticulocyte population to a limited extent. However, completion of the erythroid program is compromised by the low level of β-globin expression within these cells, which leads to globin chain imbalance, formation of numerous Heinz bodies, and decreased in vivo viability of the more mature erythrocytes. Our observations in the in vitro system are consistent with previous observations that definitive erythropoiesis is deficient in EKLF−/− mice at the fetal stage, resulting in severe anemia and prenatal death.26,27 Analysis of these EKLF−/− fetuses also showed that the level of adult β-globin mRNAs in their fetal livers was decreased by about 10-fold,26 27 which is consistent with our measurements in vitro.
EKLF−/− erythrocytes in vivo have a shortened life span, which can be ameliorated by human globin chains.To test the ability of EKLF −/− cells to contribute to red blood cell populations in vivo, EKLF−/− ES cells were used to generate chimeric mice. RT-PCR analysis of mRNAs extracted from red blood cells of the chimeric mice indicated that βmaj-and βmin-globin mRNAs, characteristic of the ES cell Hbbd haplotype, were present. However, analysis of β-globin chains and GPI-1 isozymes from the blood of the chimeric animals failed to detect the βmaj- and βmin-globin chains, or the GPI-1A isozyme, which would be produced in ES-derived erythrocytes. This suggests that the EKLF−/− cells are capable of participating in erythropoiesis through the reticulocyte stage, but that they do not survive in substantial numbers as mature erythrocytes, presumably because the presence of Heinz bodies results in their being quickly culled from circulation by the spleen.
To test whether the expression of human γ-globin chains could extend the life span of the EKLF−/− ES derived erythrocytes, a human γ-globin gene linked in cis to the β-globin locus control region was introduced into the EKLF−/− ES cells. The rationale was that the promoter of the human globin gene does not have an optimal binding site for EKLF , and presumably does not require EKLF for expression,20 and the γ-globin chains would combine with the excess α-globin chains and prevent the formation of inclusion bodies. In chimeric mice generated with the EKLF−/−, LCRγ ES cells, the genetically modified EKLF−/− ES cells were able to contribute to both the reticulocyte and mature erythrocyte fractions of the blood, suggesting that the γ-globin chains corrected, at least partially, the globin chain imbalance, increasing the life span of mutant erythrocytes. This supports the hypothesis that the major consequence of the lack of EKLF is a globin chain imbalance, leading to the reduced survival of mature erythrocytes.
In summary, our results show that EKLF is important for definitive erythropoiesis in the adult animal. Although it is not essential for the establishment of definitive erythroid progenitors, EKLF is necessary for maximal expression of the adult β-globin gene during terminal differentiation. Consequently, the lack of EKLF results in a deficiency of β-globin chains in adult erythrocytes and reduces their viability in the intact animal. Whether other, more subtle parameters of erythroid function and viability are also affected by lack of EKLF expression will be addressed by future studies.
Pure red cell aplasia in a lung-transplant patient under immunosuppressive treatment. Bone marrow aspiration shows giant megaloblastic proerythroblasts containing nuclear inclusion bodies and vacuolization of the cytoplasm with absence of other erythroid precursors. Parvovirus B19 genome was found by polymerase chain reaction analysis of the bone marrow and peripheral blood. (Courtesy of Ph. Beris, MD, and K. Samii, MD, Division d'Hématologie, Hôpitaux Universitaires de Genève, Geneva 14, Switzerland.)
Pure red cell aplasia in a lung-transplant patient under immunosuppressive treatment. Bone marrow aspiration shows giant megaloblastic proerythroblasts containing nuclear inclusion bodies and vacuolization of the cytoplasm with absence of other erythroid precursors. Parvovirus B19 genome was found by polymerase chain reaction analysis of the bone marrow and peripheral blood. (Courtesy of Ph. Beris, MD, and K. Samii, MD, Division d'Hématologie, Hôpitaux Universitaires de Genève, Geneva 14, Switzerland.)
ACKNOWLEDGMENT
We thank Adras Nagy for the R1 ES cell line, Tim Townes for the human LCRγ construct, and John Gilman for the TG(γ) mice.
Supported in part by National Institutes of Health (Bethesda, MD) Grant No. 5-27250 to F.C. and S.-K.L. was supported by a Special Fellowship from the Leukemia Society of America. J.J.B. is a Scholar of the Leukemia Society of America (New York, NY).
Address reprint requests to Frank Costantini, PhD, Department of Genetics and Development, 701 W 168th St, Columbia University, New York, NY 10032.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal