Abstract
The influence of the suspension pH (pHo ) on the transmembrane mobility of spin-labeled phospholipid analogues in the human red blood cell was investigated. The passive transverse diffusion of spin-labeled phospholipid analogues was independent of pHo in the investigated range (5.8 to 8.5). However, upon acidification to pHo 5.8, a significant decrease of the rapid adenosine triphosphate (ATP)-dependent inward movement of aminophospholipids was found at physiologic ionic concentration, whereas a change of pH from 7.4 to 8.5 did not affect this transport. Evidence is given that the intracellular pH affects the active transport of aminophospholipids but not the extracellular pH. Suppression of the ATP-dependent outside-inside redistribution of aminophospholipid analogues by low pH was reversible because original transport activity was re-established upon reneutralization. pH dependence of the active phospholipid transport was not caused by the spin-labeled reporter group or by depletion of intracellular ATP. Because the same influence of pH on aminophospholipid movement could be observed for resealed ghosts, constituents of the red blood cell cytoplasm do not mediate the influence of pH on the ATP-dependent inward movement of aminophospholipids.
BASIC KNOWLEDGE on the different pathways of the movement and the asymmetric distribution of phospholipids between the two halves of mammalian plasma membranes has been gained from the erythrocyte membrane. The passive transverse diffusion of phospholipids, the so-called flip-flop, is very slow, with half-times in the order of hours or even days.1 It is unspecific with respect to the phospholipid species as well as to the direction of movement between both bilayer halves. In contrast, the adenosine triphosphate (ATP)-dependent transport of aminophospholipids from the exoplasmic to the cytoplasmic monolayer that was described for human erythrocytes more than a decade ago by Seigneuret and Devaux2 is fast and highly specific for the aminophospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE). The half-times of the outward-inward redistribution of spin-labeled phospholipid analogues PS and PE are in the order of about 3 minutes and 30 minutes, respectively, at 37°C.3 By using those as well as fluorescent or radioactive-labeled lipid probes, this lipid-specific ATP-dependent movement in the plasma membrane has been confirmed for red blood cells4,5 and other mammalian cells,4,6-10 showing unambiguously that the observed phenomenon is not related to the reporter group, too.5,11 The ATP-dependent transport is supposed to be mediated by the aminophospholipid translocase. Although a significant amount of data is compatible with the existence of such a translocase in the plasma membrane of various eukaryotic cells, the identity of this membrane protein is not unequivocally established.1 12-18
Evidence has been given that the aminophospholipid translocase is important for the maintenance and, perhaps, for the origin of the transmembrane phospholipid asymmetry in erythrocyte membranes19-23 and, presumably, in other mammalian plasma membranes.9-11 This asymmetry is characterized by an enrichment of phosphatidylcholine (PC) and sphingomyelin (SM) in the exoplasmic leaflet, whereas aminophospholipids are preferentially (PE) or almost exclusively (PS) oriented to the cytoplasmic layer.24 The aminophospholipid translocase activity might be responsible for phenomena that have been ascribed to alterations of the asymmetric distribution of phospholipids, eg, as erythrocyte shape changes.2,25,26 Likewise, an impairment of the aminophospholipid translocase in aged human erythrocytes may cause the exposure of PS on the outer leaflet27,28 that could serve as a signal for recognition and, eventually, removal of those senescent cells from blood circulation.29 Indeed, it has been shown by several studies that red blood cells, upon incorporation of exogenous PS or exposure of endogenous PS in their outer leaflet, bind more readily to macrophages and are rapidly removed from blood circulation.28 30-33
Similarly, evidence has been provided that PS exposure on the exoplasmic leaflet may play a role in the shortened posttransfusion viability of stored red blood cells.34 Using spin-labeled phospholipid analogues, a continuous loss of the aminophospholipid translocase activity in stored red blood cells paralleled by a loss of the transmembrane asymmetry of analogues was found. Although the primary reason for the inhibitory effect on the aminophospholipid translocase activity was the ATP depletion, the investigators pointed out that other mechanisms may be involved as well that remain to be elucidated. It is already known that, during storage of red blood cells, several alterations occur such as the loss of membrane lipids35,36 and of the antioxidant potential.37 But alteration of physico-chemical parameters that may affect the aminophospholipid translocase activity also have to be considered. For example, the accumulation of lactate in the closed system during blood conservation is accompanied by a decrease of the intracellular and extracellular pH that may reach values as low as pH 5.8.38 39
In the present study we have investigated the influence of the suspension pH (pHo ) on the transverse movement of spin-labeled phospholipids. The redistribution of the analogues was measured by the back-exchange method of the analogues in the outer plasma membrane leaflet to bovine serum albumin (BSA).3 20 We found an inhibitory influence of an acidic intracellular pH (pHi ) on the ATP-dependent inward motion of the aminophospholipids PS and PE. The passive diffusion of the phospholipid analogues PS, PE, PC, and SM was unaffected.
MATERIALS AND METHODS
Media.The following media were used: phosphate-buffered saline (PBS) contained 150 mmol/L NaCl and 5.8 mmol/L Na2HPO4/NaH2PO4 , pH 7.4; HEPES-buffered solution (HBS), MES-buffered solution (MBS), and CHES-buffered solution (CBS) contained 120 mmol/L NaCl, 30 mmol/L sucrose, 5 mmol/L glucose, 1 mmol/L MgCl2 , and 20 mmol/L HEPES (pH 7.4), 20 mmol/L MES (pH 5.8), and 20 mmol/L CHES (pH 8.5), respectively. All substances were obtained from Sigma (Deisenhofen, Germany) if not otherwise stated.
Preparation of erythrocytes.Citrate-stabilized blood samples of healthy donors were purchased from the local blood bank (Berlin, Germany). Red blood cells were washed once in PBS (2,000g for 10 minutes at 4°C) to remove plasma and buffy coat. Subsequently, cells were washed in HBS and twice in HBS, MBS, and CBS, respectively, as indicated. For measurements of redistribution kinetics in isotonic medium of low ionic strength, cells were washed three times in HEPES (pH 7.4) or MES (5.8) containing 5 mmol/L NaCl, 280 mmol/L sucrose, and 5 mmol/L glucose. Before labeling, erythrocytes were incubated for 5 minutes in the respective buffer (25% hematocrit) at the desired temperature.
Preparation of resealed ghosts.Citrate-stabilized blood samples were washed twice in PBS at 2,000g (10 minutes at 4°C) and resuspended to a 50% hematocrit in 166 mmol/L NaCl. Resealed ghosts were prepared by hypotonic lysis as described by Schwoch and Passow.40 Briefly, lysis was performed at 4°C by dilution of 3 mL erythrocyte suspension (50% hematocrit) with 60 mL lysis buffer containing 1.2 mmol/L acetic acid, 4 mmol/L MgSO4 , and 0.5 mmol/L EGTA (pH 3.2). After 5 minutes, 1 mmol/L ATP, 10 mmol/L creatine phosphate, and 100 IU creatine kinase/mL (final concentrations) were added. For measurements of intracellular pH, fluorescein isothiocyanate (FITC)-Dextran was added to a final concentration of 0.1 mmol/L. Isotonicity was established by the addition of 7 mL of 10-fold concentrated HBS. The pH was readjusted to 7.4 by the addition of NaOH. Cells were resealed by incubation at 37°C for 25 minutes and collected by centrifugation (10 minutes of 20,000g at 4°C). Resealed ghosts were washed once in HBS and twice in HBS or MBS, respectively.
Spin-labeling and measurement of transverse lipid redistribution.Spin-labeled phospholipids were synthesized according to Fellmann et al.41 To diminish hydrolysis of phospholipid analogues, in particular that of PS, at 37°C, diisopropyl fluorophosphate (DFP; Aldrich, Steinheim, Germany) was added to the suspension of erythrocytes (final concentration, 5 mmol/L) before labeling.3,20 Because of the low degree of hydrolysis at 4°C, DFP could be omitted in experiments at that temperature. The spin-labeled phospholipids were incorporated into the erythrocyte plasma membrane and the amount of analogues in the outer monolayer was measured using the back-exchange method as described.3,20 Briefly, suitable amounts of analogues (corresponding to 1 mol% of endogenous phospholipids) in chloroform were dried under nitrogen and resuspended in 3 vol of the desired buffer. Subsequently, 4 vol of erythrocyte suspension (hematocrit 25%) were mixed with the analogue suspension and incubated at the experimental temperature, 37°C or 4°C. At regular intervals, 80 μL of the suspension was drawn and mixed with 20 μL of ice-cold buffer containing 10% fatty-acid free BSA. After 1 minute of incubation on ice, the suspension was centrifuged (13,000g for 2 minutes). Fifty microliters of the supernatant was drawn and mixed with 5 μL of 100 mmol/L potassium ferricyanide to reoxidize reduced lipid analogues. The amount of probe present in the supernatant corresponding to lipid analogues in the outer leaflet was estimated from the intensity of the electron paramagnetic resonance (EPR) spectrum measured by a Bruker ECS 106 (Bruker, Karlsruhe, Germany) or a Miniscope MS100 (Magnettech, Berlin, Germany) spectrometer. Intensities were corrected for hydrolysis as described previously.3 Hydrolysis of the spin-labeled analogues into lyso derivatives became obvious from the appearance of three narrow peaks superimposed on the anisotropic spectrum of BSA-bound spin labels. This narrow component corresponds to the cleaved short-chain, spin-labeled fatty acid that is water soluble. The accuracy of determination of analogues in the outer leaflet by the back-exchange assay is about 5%.
Kinetics of the fast redistribution of aminophospholipid analogues were fitted to a monoexponential equation by least square nonlinear regression (Sigma Plot).
pH changes before and during translocation assay.At the indicated time points, acidification (to pH 5.8) or neutralization (to pH 7.4) was performed by the addition of 1 vol MBS (pH 2.5) or HBS (pH 10.5), respectively. Subsequently, the suspension was centrifuged and 1 vol of the supernatant was discarded to readjust the cell concentration. To check any loss of spin-label, an aliquot before and after the pH switch was taken. After pelleting, cells were disrupted by freeze-thawing cycle. Then, EPR spectra were recorded in the presence of potassium ferricyanide (10 mmol/L) and the double integrals were compared.
ATP depletion and measurement of intracellular ATP content.ATP depletion was performed by incubating red blood cells (hematocrit 10%) for 15 minutes with 5 mmol/L iodoacetamide at 37°C according to Henseleit et al.42 Intracellular ATP content was determined by extraction of ATP from erythrocyte pellets obtained after BSA-extraction (see above) with 1% trichloroacetic acid and 2 mmol/L EDTA and was measured using a luciferin-luciferase assay (Colora, Lorch, Germany).
Measurement of pHi.Erythrocytes were washed as described (see preparation of erythrocytes above). Subsequently, cells were centrifuged at 13,000g for 5 minutes at 4°C. After aspiration of the supernatant, erythrocytes were lysed by 4 freeze-thaw cycles (−70°C for 15 minutes and 37°C for 10 minutes). The pH of the lysate was determined by a Micro pH electrode InLab 423 (Mettler-Toledo, Urdorf, Switzerland) at 4°C. The addition of Triton X-100 (0.5% vol/vol) did not affect the pH.
If not stated otherwise, the average ± standard error of estimate (with n being the number of independent experiments) is given.
RESULTS
Assessment of experimental conditions.A main prerequisite for studying the influence of the pH on redistribution of phospholipid analogues is the stability of the pH value in the erythrocyte suspension in the time course of the experiments. Therefore, we have examined the pH stability by variation of buffer and cell concentration at 37°C and 4°C, respectively. A concentration of 20 mmol/L of the desired buffer and a hematocrit of about 15% were found to ensure a pH stability of ΔpH ≤ ± 0.2. Under all conditions, hemolysis was less than 5% during the time period of our experiments (data not shown).
Incorporation of spin-labeled phospholipid analogues into the erythrocyte membrane was followed by the line shape changes of the EPR spectra as described.2 Independent of the buffer (CHES, HEPES, and MES, respectively) and the pH in the erythrocyte suspension, spin labels were rapidly incorporated into the outer membrane leaflet within less than 1 minute even at 4°C. No analogue remaining in the suspension medium could be detected. The efficient and fast extractability of the lipid analogues from the outer leaflet at various pH was proven by incubation of red blood cells with BSA (see the Materials and Methods) immediately after incorporation of phospholipid analogues. More than 95% of the analogues could be recovered in the supernatant after centrifugation. In accordance with that finding, no EPR signal could be detected in the pellet. We did not observe any influence of the pHo on the extraction procedure described. This verifies that the back-exchange technique using BSA is an appropriate assay for investigating the redistribution of spin-labeled analogues at different pHo values.
Transbilayer movement of spin-labeled aminophospholipid analogues at 37°C.We have measured the transbilayer movement of spin-labeled phospholipids from the outer to the inner leaflet by the back-exchange of the analogues in the outer leaflet to BSA. In the case of 1-palmitoyl-2-(4-doxylpentanoyl) (SL)-PS, redistribution at 37°C could only be measured in the presence of 5 mmol/L DFP to prevent hydrolysis.3 5 However, a decrease of cellular ATP at acidic pHo in the presence of DFP was frequently observed. In an experiment typical for that, the ATP level decreased from 1.1 mmol/L at pH 7.4 to 0.6 mmol/L at pH 5.8. In the case of SL-PE, we could monitor the inward redistribution up to 60 minutes after incorporation of the analogue into the outer layer because hydrolysis was ≤10% even in the absence of DFP. However, this time period was not sufficient to measure the steady-state transverse arrangement of SL-PE (see below).
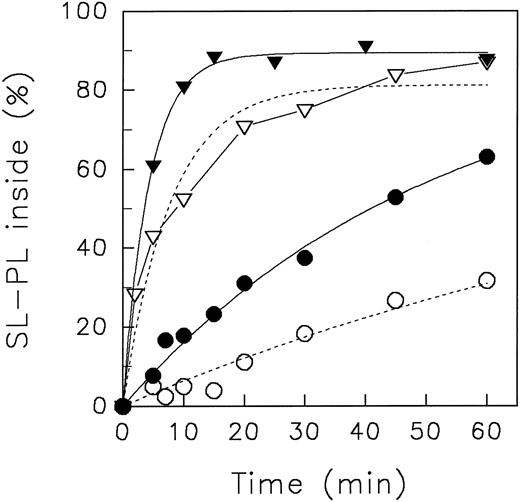
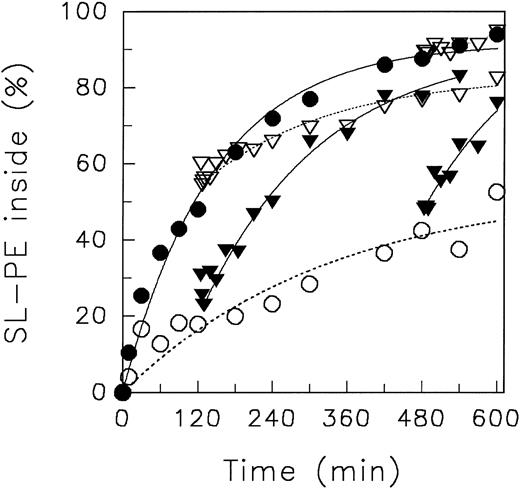
In Fig 1, representative experiments of the aminophospholipid redistribution at different pH values are shown. At pHo 7.4, SL-PS and SL-PE redistributed rapidly to the inner leaflet, as already described.3,27 We did not find any significant difference of the initial velocity of inward movement as well as of the equilibrium distribution of lipid analogues between pHo 7.4 and pHo 8.5 (not shown). However, in acidic medium (pHo 5.8), a significant reduction of the initial velocity of inward translocation of aminophospholipid analogues was observed. To quantify kinetics, we have fitted the data to a monoexponential equation (see Fig 1, lines). The initial velocity (given in percentage of analogues per minute) of aminophospholipid inward movement at neutral and at acidic pHo was for SL-PS 20.9 and 10.6 and for SL-PE 1.8 and 0.7, respectively. However, as can be seen, the monoexponential fit of the redistribution of SL-PS at pHo 5.8 does not describe the data well. Presumably, the decrease of intracellular ATP in the experimental time course affects the kinetics. But, deduced from the experimental data, we found no difference in the steady-state distribution of SL-PS between neutral and acidic pHo after 60 minutes of analogue incorporation. Nonlinear fitting suggested that the steady-state distribution of SL-PE decreased from 78% (pHo 7.4) to 53% (pHo 5.8) of analogues inside. Although the plateau of SL-PE at neutral pHo is in accordance with previous reports,3 27 the value for the plateaus at low pHo has to be treated with caution because it was derived only from regression.
Redistribution of spin-labeled phospholipid analogues SL-PE and SL-PS in human red blood cells at neutral (7.4) and acidic (5.8) pH, respectively, of the suspension medium at 37°C. Erythrocytes were incubated for 5 minutes at 37°C with (in the case of SL-PS) or without DFP. After incorporation of spin-labeled phospholipids, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). SL-PE: pH 5.8 (○), pH 7.4 (•). SL-PS: pH 5.8 (▿), pH 7.4 (▾). Curves were fitted by nonlinear regression to a monoexponential function (see the Materials and Methods). In the case of SL-PS redistribution at acidic pH, measured values were connected by straight lines (see Results).
Redistribution of spin-labeled phospholipid analogues SL-PE and SL-PS in human red blood cells at neutral (7.4) and acidic (5.8) pH, respectively, of the suspension medium at 37°C. Erythrocytes were incubated for 5 minutes at 37°C with (in the case of SL-PS) or without DFP. After incorporation of spin-labeled phospholipids, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). SL-PE: pH 5.8 (○), pH 7.4 (•). SL-PS: pH 5.8 (▿), pH 7.4 (▾). Curves were fitted by nonlinear regression to a monoexponential function (see the Materials and Methods). In the case of SL-PS redistribution at acidic pH, measured values were connected by straight lines (see Results).
Transbilayer movement of spin-labeled aminophospholipid analogues at 4°C.Further experiments were performed at 4°C because no hydrolysis of analogues was detected even in the absence of DFP and the intracellular ATP did not significantly decline in the experimental time course neither at acidic nor at basic pHo (data not shown). Importantly, at this temperature the aminophospholipid translocase activity is not abolished.2,27 43
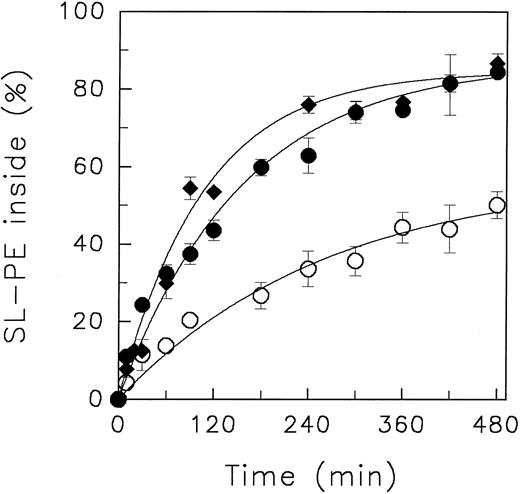
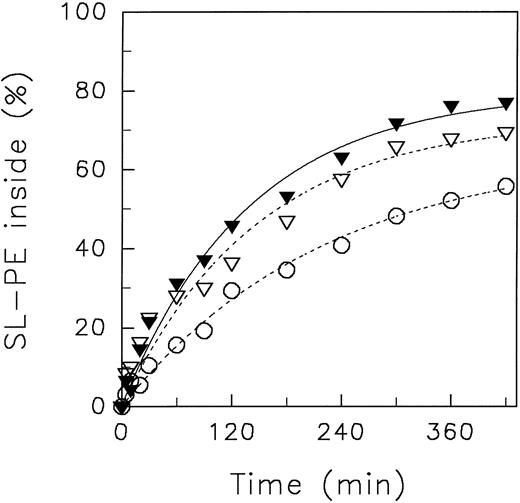
In Fig 2, the redistribution kinetics of SL-PE at different pHo values at 4°C are shown. For quantification, data were fitted to a monoexponential function.
Redistribution of the spin-labeled phospholipid analogue SL-PE in human red blood cells at various pH of the suspension medium at 4°C. After incorporation of SL-PE, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). pH 5.8 (○), pH 7.4 (•), pH 8.5 (♦). Data represent the average ± standard error of estimate with n = 4 (pH 7.4 and pH 5.8), except for those measured at pH 8.5 (average of 2 independent experiments). Curves were fitted by nonlinear regression to a monoexponential function.
Redistribution of the spin-labeled phospholipid analogue SL-PE in human red blood cells at various pH of the suspension medium at 4°C. After incorporation of SL-PE, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). pH 5.8 (○), pH 7.4 (•), pH 8.5 (♦). Data represent the average ± standard error of estimate with n = 4 (pH 7.4 and pH 5.8), except for those measured at pH 8.5 (average of 2 independent experiments). Curves were fitted by nonlinear regression to a monoexponential function.
Similar to the results obtained at 37°C, only minor differences of the transbilayer redistribution of both analogues between pHo 7.4 and pHo 8.5 were observed (Table 1). However, at pHo 5.8, the initial velocity of the inward redistribution significantly declined in comparison to the values obtained at pHo 7.4. The initial velocities for SL-PE decreased by a factor of about two (Table 1). A similar decline was found for SL-PS from 1.82 % analogues/min (n = 2: 1.80; 1.83) at pHo 7.4 to 0.91 (n = 2; 0.97; 0.84) at pHo 5.8 (redistribution curves of SL-PS not shown). This was accompanied by reduction of the pronounced asymmetric distribution of these phospholipid analogues. For example, the steady-state transverse distribution of SL-PE decreased from 87.0% ± 1.8% (pHo 7.4) to 57.8% ± 5.1% (pHo 5.8) (n = 4) of analogue oriented to the cytoplasmic leaflet.
The Initial Velocity vi (% Analogues/Min) and the Steady-State Distribution (% of PE Analogues on the Cytoplasmic Leaflet) of SL-PE Redistribution at Different pH at 4°C
| Extracellular pH . | pH 5.8 . | pH 7.4 . | pH 8.5 . |
|---|---|---|---|
| vi (% analogues/min) | 0.22 ± 0.03 (4)* | 0.57 ± 0.06 (4) | 0.79 ± 0.2 (2)† |
| Plateau (% of PE inside) | 57.8 ± 5.1* | 87.0 ± 1.8 | 84.6 ± 5.8† |
| Extracellular pH . | pH 5.8 . | pH 7.4 . | pH 8.5 . |
|---|---|---|---|
| vi (% analogues/min) | 0.22 ± 0.03 (4)* | 0.57 ± 0.06 (4) | 0.79 ± 0.2 (2)† |
| Plateau (% of PE inside) | 57.8 ± 5.1* | 87.0 ± 1.8 | 84.6 ± 5.8† |
Data were obtained by nonlinear regression of redistribution kinetics to a monoexponential function. The average ± standard error of estimate of n measurements (in parentheses) is given. Statistical significance between data was tested by the t-test for unpaired observations at a significance level of α = .05.
Significant with respect to values at pH 7.4.
Not significant with respect to values at pH 7.4.
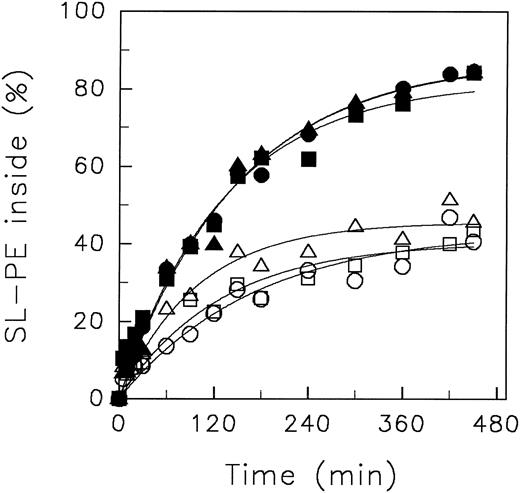
To exclude the possibility that the chemical nature of the different buffer systems is responsible for the observed results, we performed translocation experiments with SL-PE at neutral and acidic pH0 in (1) a mixture of MBS/HBS (each 10 mmol/L) and (2) PBS solution. As can be seen in Fig 3, almost identical kinetics of analogue redistribution were obtained at pHo 7.4 using different buffer systems. Moreover, at pHo 5.8, we found the same decline of SL-PE inward movement independent of the buffer.
Redistribution of the spin-labeled phospholipid analogue SL-PE in human red blood cells resuspended in buffer systems of different chemical nature. After incorporation of SL-PE, the redistribution kinetics were monitored (4°C) by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). pH 5.8: MBS (○), PBS (□), MBS/HBS (▵). pH 7.4: HBS (•), PBS (▪), MBS/HBS (▴). Curves were fitted by nonlinear regression to a monoexponential function.
Redistribution of the spin-labeled phospholipid analogue SL-PE in human red blood cells resuspended in buffer systems of different chemical nature. After incorporation of SL-PE, the redistribution kinetics were monitored (4°C) by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). pH 5.8: MBS (○), PBS (□), MBS/HBS (▵). pH 7.4: HBS (•), PBS (▪), MBS/HBS (▴). Curves were fitted by nonlinear regression to a monoexponential function.
Effect of pHo on the passive transverse movement of spin-labeled aminophospholipid analogues.Aminophospholipids can transverse the erythrocyte membrane (1) by the fast inward translocation mediated by the translocase and (2) by slow passive diffusion (see introduction above). To elucidate which of both processes is modified by acidic pH, we have depleted erythrocytes from ATP to inhibit the active translocation of aminophospholipids (see the Materials and Methods). The cellular ATP concentration was decreased from 1.15 mmol/L (control, n = 2) to 0.09 mmol/L (n = 2). Those conditions allow one to investigate the passive movement of SL-PS and SL-PE. In Fig 4, the passive transverse mobility of both aminophospholipid analogues at pHo 5.8 and at pHo 7.4 is shown (4°C). No pH-dependent effect on the redistribution kinetics of both analogues was observed. The time-dependence of the transmembrane redistribution of SL-PS is in the same order of that of SL-PE. But we note for SL-PS a slightly faster redistribution as compared with SL-PE. This could be ascribed to the residual amount of cellular ATP or by an inhomogeneous ATP-depletion of red blood cells allowing some active inward transport of SL-PS. It is known that the aminophospholipid translocase-mediated inward motion of PE is more sensitive to ATP depletion than that of PS.43
Redistribution of spin-labeled phospholipid analogues SL-PE and SL-PS in ATP-depleted human red blood cells at neutral and acidic pH, 7.4 and 5.8, respectively, of the suspension medium at 4°C. ATP depletion was performed by incubation of red blood cells in the presence of iodoacetamide. After incorporation of spin-labeled phospholipids, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA. SL-PE: pH 5.8 (○), pH 7.4 (•). SL-PS: pH 5.8 (▿), pH 7.4 (▾). For details, see the Materials and Methods. Curves were fitted by nonlinear regression to a monoexponential function.
Redistribution of spin-labeled phospholipid analogues SL-PE and SL-PS in ATP-depleted human red blood cells at neutral and acidic pH, 7.4 and 5.8, respectively, of the suspension medium at 4°C. ATP depletion was performed by incubation of red blood cells in the presence of iodoacetamide. After incorporation of spin-labeled phospholipids, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA. SL-PE: pH 5.8 (○), pH 7.4 (•). SL-PS: pH 5.8 (▿), pH 7.4 (▾). For details, see the Materials and Methods. Curves were fitted by nonlinear regression to a monoexponential function.
The kinetics of the aminophospholipid redistribution in ATP-depleted cells were similar to those of the analogue SL-PC that transverses the erythrocyte plasma membrane exclusively by passive mechanisms (see below).
Effect of pH on the passive transverse movement of SL-PC and SL-SM.Although we do not have any indication that ATP-depletion could diminish an influence of pH on passive diffusion, we have investigated at 4°C the passive transbilayer movement of SL-PC and SL-SM in erythrocytes that were not ATP depleted. It is already known that PC and SM are not recognized by the aminophospholipid translocase.2 3 We did not observe a dependence of the transmembrane passage of SL-PC on the pH (data not shown). Moreover, the kinetics of analogue redistribution was comparable to those of SL-PS and SL-PE in ATP-depleted cells. Furthermore, no effect of the pH of suspension medium on the passive redistribution of SL-SM was found (not shown). This confirms that the passive diffusion of spin-labeled phospholipids is not affected by the pH at least in the range investigated here. The same conclusion was obtained when performing those experiments at 37°C (not shown). From those investigations it becomes evident that acidification of the suspension medium affects the fast, ATP-dependent translocation of aminophospholipids but not their passive transbilayer movement.
Effect of changing pHo during translocation measurement.To investigate whether the decrease of the ATP-dependent translocation of aminophospholipids by acidic pH is reversible, we have changed the pH of the suspension medium during the redistribution kinetics of the spin-labeled analogue PE measured at 4°C (for details see the Materials and Methods). In a first experiment, the redistribution of SL-PE was observed at pHo 5.8 for 120 minutes (Fig 5). After switching the pHo to 7.4, an accelerated inward movement of the analogue was found comparable to that of SL-PE redistribution performed exclusively at neutral pHo (see above). A similar enhancement of SL-PE inward movement was established when the pHo was shifted from 5.8 to 7.4 after 480 minutes. Thus, the influence of pHo on the fast active translocation is reversible and, within the time resolution of our experimental approach, instantaneous. Moreover, the data indicate that the decrease of the fast inward translocation is not caused by an ATP depletion. In that case, we would not expect a fast reversibility of the pH effect.
Redistribution of the spin-labeled phospholipid analogue SL-PE in human red blood cells at various pH of the suspension medium at 4°C. In part of the experiments, the pH of suspension medium was changed during the translocation measurements. After incorporation of spin-labeled phospholipids, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). pH 5.8 (○), pH 7.4 (•), pH 7.4 → pH 5.8 (▿) at 120 minutes, pH 5.8 → pH 7.4 (▾) at 120 minutes, pH 7.4 → pH 5.8 (▿) at 480 minutes, and pH 5.8 → pH 7.4 (▾) at 480 minutes. Curves were fitted by nonlinear regression to a monoexponential function.
Redistribution of the spin-labeled phospholipid analogue SL-PE in human red blood cells at various pH of the suspension medium at 4°C. In part of the experiments, the pH of suspension medium was changed during the translocation measurements. After incorporation of spin-labeled phospholipids, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). pH 5.8 (○), pH 7.4 (•), pH 7.4 → pH 5.8 (▿) at 120 minutes, pH 5.8 → pH 7.4 (▾) at 120 minutes, pH 7.4 → pH 5.8 (▿) at 480 minutes, and pH 5.8 → pH 7.4 (▾) at 480 minutes. Curves were fitted by nonlinear regression to a monoexponential function.
To sustain that an alteration of the pH rapidly affects the translocation of aminophospholipids, we have measured the redistribution of SL-PE at neutral pH and shifted the pH of the cell suspension in the opposite direction, ie, from 7.4 to 5.8, after 120 minutes (Fig 5). Indeed, the inward movement of SL-PE immediately decreased and proceeded with a slope similar to that of SL-PE redistribution performed exclusively at this acidic pHo. Performing the same pHo switch (7.4 → 5.8) in the plateau phase of SL-PE redistribution (after 480 minutes), the steady-state distribution of the analogue was not affected during the following 2 hours. We note that those treatments did not increase the degree of hemolysis and did not cause any loss of lipid analogues (see the Materials and Methods).
Effect of changing pHo before translocation measurement.We have further confirmed the reversibility of the pH effect on the ATP-dependent inward motion of aminophospholipids by another set of experiments. For that purpose, we have preincubated red blood cells either at pHo 5.8 or at pHo 7.4, 4°C, for 4 hours. After resuspension of those cells at pHo 7.4, we have measured the transverse redistribution of SL-PE at 4°C (not shown). We found no significant difference of the initial velocity and the steady-state distribution of SL-PE inward motion neither between cells preincubated at various pH nor between preincubated and nonpreincubated cells.
Influence of the transmembrane potential and the intracellular pH on the redistribution of aminophospholipid analogues.The transmembrane potential (TMP) and the pHi of human erythrocytes depends on the pH of the suspension medium.44,45 Using an experimentally verified model,44-48 we have estimated that the TMP changed from about −0.3 mV to about 7.2 mV by shifting the pHo from 7.4 to 5.8 at physiologic ionic concentration (for details see Table 2 and the corresponding legend). Thus, one may ask whether the influence of acidic pHo on the redistribution of aminophospholipid analogues is mediated by an increase of the TMP. To test this notion, we have measured the redistribution of SL-PE in human erythrocyte membranes as a function of the TMP by variation of the extracellular ionic concentration at neutral pHo. The TMP of human red blood cells increases from about −0.3 mV to about 40 mV by decreasing the NaCl concentration from 120 mmol/L to 5 mmol/L (Table 2, pH 7.4). No significant influence of the ionic concentration of the suspension medium on the outside-inside movement neither of SL-PE nor of SL-PS was found (data not shown). Thus, we infer that an increase of the TMP does not affect the activity of the aminophospholipid translocase. Likewise, a decrease of the TMP does not affect the translocase activity because no significant difference of the redistribution of aminophospholipid analogues between pHo 7.4 and 8.5 was detected (see above). At such alkaline pH, the transmembrane potential is about −12 mV (Table 2).
The pHi and the TMP of Human Red Blood Cells as a Function of the pHo , Chloride [Cl−]o , and Sucrose Concentration
| [Cl−]o (mmol/L) . | [Sucrose]o (mmol/L) . | pH0 . | pHi (theoretical) . | pHi (experimental) . | TMP [mV] (theoretical) . |
|---|---|---|---|---|---|
| 120 | 30 | 8.5 | 8.3 | ND | −12.1 |
| 120 | 30 | 7.4 | 7.4 | 7.2; 7.1 | −0.3 |
| 120 | 30 | 5.8 | 5.7 | 5.9; 5.9 | 7.2 |
| 5 | 280 | 7.4 | 8.1 | 7.7; 7.8 | 39.4 |
| 5 | 280 | 5.8 | 6.8 | 6.6; 6.6 | 52.4 |
| [Cl−]o (mmol/L) . | [Sucrose]o (mmol/L) . | pH0 . | pHi (theoretical) . | pHi (experimental) . | TMP [mV] (theoretical) . |
|---|---|---|---|---|---|
| 120 | 30 | 8.5 | 8.3 | ND | −12.1 |
| 120 | 30 | 7.4 | 7.4 | 7.2; 7.1 | −0.3 |
| 120 | 30 | 5.8 | 5.7 | 5.9; 5.9 | 7.2 |
| 5 | 280 | 7.4 | 8.1 | 7.7; 7.8 | 39.4 |
| 5 | 280 | 5.8 | 6.8 | 6.6; 6.6 | 52.4 |
pHi and TMP were calculated by a model44,46,47 (for details, see Brumen et al46 and Glaser44,45 ) assuming a time-independent equilibrium of water, chloride, and pH across the red blood cell. By taking into account (1) the condition of electroneutrality, (2) the pH dependence of the hemoglobin charge, (3) the activity coefficients, and (4) the equilibration of the osmotic pressure π (Δπ = πo − πi = 0), the intracellular and extracellular concentrations of Cl− and H+are given by the Nernst equation. For calculation, we have assumed an intracellular Na+-K+ concentration of 146 mmol/L cell water, a hemoglobin concentration of 7 mmol/L cell water at a relative volume of the erythrocyte 1,45,64 and a temperature of 4°C. According to the composition of the suspension media used, the glucose concentration of 5 mmol/L and an ionic strength of the buffer concentration 30 mmol/L were taken into account for calculations. It has been shown that the model provides a faithful description of the measured TMP and pHi .44 47 The experimentally determined intracellular pH is given (T = 4°C; 2 independent experiments).
Abbreviation: ND, not determined.
To elucidate whether the extracellular or the cytosolic pH is responsible for the pH-dependent aminophospholipid redistribution across the erythrocyte membrane, we have investigated the transverse movement of SL-PE under conditions that allow one to lower the pHo of the suspension while keeping the intracellular pH close to neutral (for measurement of pHi see the Materials and Methods). At pHo 5.8 in a medium of low NaCl concentration (5 mmol/L), the intracellular pH is about 6.6, whereas at high NaCl concentration (120 mmol/L) the pHi is about 5.7 (Table 2). Note that, at 120 mmol/L NaCl and neutral pHo , the cytosolic pH is about 7.2 (Table 2). The experimentally determined values of the intracellular pH are in good agreement with those obtained from the model mentioned above (see Table 2).
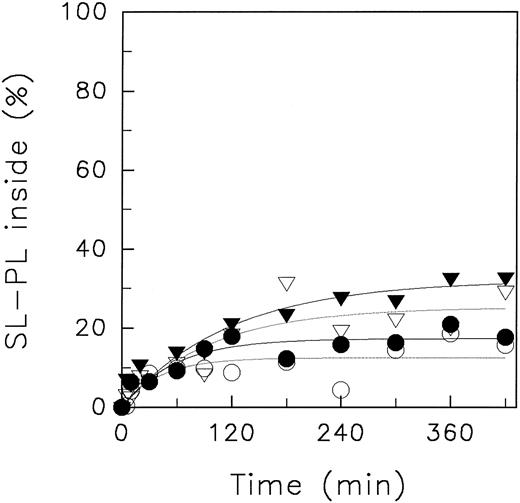
In Fig 6, the redistribution kinetics of SL-PE in media of different NaCl concentration at pHo 5.8 are presented (4°C). If the cytosolic pH was close to neutral (in medium of 5 mmol/L NaCl; pHi 6.6) the transbilayer movement of SL-PE (initial velocity, 0.50 % analogues/min) was only slightly diminished in comparison to that found for high ionic concentration and neutral pH of the suspension medium (see Fig 2). Thus, acidification of the suspension medium does not lead necessarily to a decrease of the aminophospholipid translocase activity. Only under conditions in which the intracellular lumen of erythrocytes became acidified (in medium of 120 mmol/L NaCl) did we find the already mentioned decrease of the ATP-dependent transport of SL-PE (initial velocity, 0.29 % analogues/min). For comparison, we have presented also the transverse redistribution of SL-PE in a suspension of low NaCl concentration but neutral pHo (Fig 6). In that case, we found only a slight increase of the initial velocity (0.58 % analogues/min). The cytosolic pH is about 7.8 under those conditions (Table 2). We conclude that the intracellular pH affects the fast inward translocation of aminophospholipids but not the extracellular pH. Moreover, the absence of a significant effect on the outside-inside movement of SL-PE confirms that the transmembrane potential is not a determinant of the aminophospholipid translocase activity. The TMP increases by about 45 mV when lowering the NaCl concentration of the suspension medium from 120 mmol/L to 5 mmol/L at pHo 5.8.
Redistribution of SL-PE in human erythrocytes suspended in media of high (120 mmol/L NaCl) or low ionic concentration (5 mmol/L NaCl) at neutral and acidic pH (4°C). After incorporation of SL-PE, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). High ionic concentration: pH 5.8 (○); low ionic concentration: pH 5.8 (▿), pH 7.4 (▾). Curves were fitted by nonlinear regression to a monoexponential function.
Redistribution of SL-PE in human erythrocytes suspended in media of high (120 mmol/L NaCl) or low ionic concentration (5 mmol/L NaCl) at neutral and acidic pH (4°C). After incorporation of SL-PE, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). High ionic concentration: pH 5.8 (○); low ionic concentration: pH 5.8 (▿), pH 7.4 (▾). Curves were fitted by nonlinear regression to a monoexponential function.
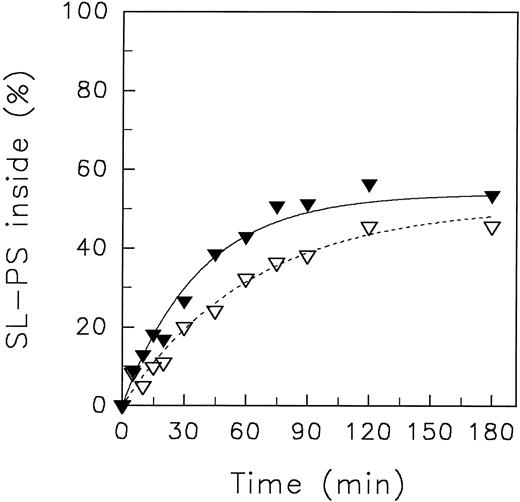
Transbilayer movement of spin-labeled phosphatidylserine in resealed ghosts at 4°C.To investigate whether the impairment of the aminophospholipid translocase activity at acidic pH is transposed by cytosolic components, we have measured the transverse redistribution of aminophospholipid analogues in resealed ghosts. The aminophospholipid translocase activity in resealed erythrocyte ghosts is preserved in the presence of ATP but reduced with respect to intact red blood cells by unknown reasons.20 Moreover, the intracellular ATP concentration of ghosts even in the presence of a regenerating system as used here can be maintained only for a limited time period. Therefore, we have limited these experiments only to SL-PS due to its higher affinity to the aminophospholipid translocase and, thus, its faster inward movement in comparison to SL-PE.43 Figure 7 shows the initial transverse redistribution of SL-PS at 4°C in resealed ghosts. Whereas at neutral pHo the initial velocity of SL-PS redistribution to the inner leaflet was 1.44 % analogue/min, it decreased by a factor of about 2 (0.79 % analogue/min) at pHo 5.8. Presumably, the lower steady-state plateau of SL-PS with respect to intact red blood cells could be caused by the lower aminophospholipid translocase activity.
Redistribution of the spin-labeled phospholipid analogue SL-PS in resealed erythrocyte ghosts at neutral and acidic pH of the suspension medium at 4°C. Resealed ghosts were prepared by hypotonic lysis as described by Schwoch and Passow.40 After incorporation of SL-PS, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). pH 5.8 (▿), pH 7.4 (▾). Curves were fitted by nonlinear regression to a monoexponential function.
Redistribution of the spin-labeled phospholipid analogue SL-PS in resealed erythrocyte ghosts at neutral and acidic pH of the suspension medium at 4°C. Resealed ghosts were prepared by hypotonic lysis as described by Schwoch and Passow.40 After incorporation of SL-PS, the redistribution kinetics were monitored by the back-exchange of analogues on the outer leaflet to BSA (see the Materials and Methods). pH 5.8 (▿), pH 7.4 (▾). Curves were fitted by nonlinear regression to a monoexponential function.
To measure the intracellular pH of resealed ghosts under those conditions, the fluorescence intensity of FITC-Dextran encapsulated into the ghost lumen was compared with those intensities of FITC-Dextran solved in buffer of various pH. We found that the intracellular pH was similar to the value of suspension medium at neutral and acidic pHo , respectively (data not shown).
DISCUSSION
In this study we have shown that acidification (pHo 5.8) of the suspension medium may provoke a decrease of the initial velocity of the inward movement as well as a loss of the asymmetric transverse arrangement of spin-labeled aminophospholipid analogues in erythrocyte membranes in comparison to neutral pHo (7.4). The reduction of the fast outside-inside redistribution of these analogues at acidic pH is caused by a decrease of the transverse lipid transport mediated by the aminophospholipid translocase. A further analysis showed that the cytosolic, but not the extracellular pH affects this ATP-dependent transbilayer movement. The influence of the acidic pH on the redistribution of aminophospholipids is completely reversible; the activity of the ATP-dependent translocation of aminophospholipid analogues was restored upon neutralization (pHo 7.4) of the suspension medium. The ATP-independent, passive movement of phospholipid analogues across the human erythrocyte membrane is not changed upon lowering the pHo. An increase of the pHo to 8.0 did not exert a substantial effect neither on the passive nor on the active transverse motion of phospholipids.
Recently, Gedde et al49 have investigated the influence of the suspension pH (between 5.5 and 9.0) on transverse equilibrium distribution of the radioactively labeled phospholipids dilauroylphosphatidylcholine (DLPC) and dilauroylphosphatidylserine (DLPS) at 37°C. Because of the presence of two long-chain fatty acid residues, these analogues reflect closely the transverse movement of the corresponding endogenous phospholipids. The investigators could not detect any alteration of the asymmetric lipid distribution in the pHo range investigated. This does not contradict our results because we observed at 37°C for the spin-labeled analogues SL-PS and SL-PC no significant influence of the suspension pH on the transverse equilibrium distribution. The coincidence in the steady-state distribution supports that the spin-labeled aminophospholipid analogues reflect very well the behavior of endogenous phospholipids that typically carry two long fatty acid residues.50 51 Although we did not see a change of the steady-state distribution of SL-PS at 37°C upon acidification, we found a significant reduction for SL-PE at 37°C and for both aminophospholipid analogues at 4°C. One may question whether the corresponding endogenous aminophospholipids rapidly adopt a similar altered asymmetric distribution upon acidification. This seems very unlikely, because our study suggests that the alteration of the steady-state distribution of aminophospholipids is a rather slow process. Indeed, the steady-state distribution of SL-PE at pHo 7.4 did not alter within 2 hours after switching the pHo to 5.8 (Fig 5). This can be explained by the fact that the passive outward movement of the phospholipid is very slow and that, although diminished, the activity of the aminophospholipid translocase is still present after acidification. Thus, a low pH induced loss of the transmembrane asymmetry of endogenous aminophospholipids is certainly very slow.
We have precluded experimentally several factors that could transpose the influence of the suspension pH on the ATP-dependent transverse redistribution of aminophospholipid. (1) The pH dependence is not caused by alterations of the intracellular ATP level. At 4°C, the low pH-mediated suppression of the active inward movement of aminophospholipid analogues was not paralleled by a decline of the intracellular ATP concentration. However, under certain conditions (37°C, presence of DFP) we found a decrease of the intracellular ATP concentration at acidic pHo. Thus, in addition, a low pH of the suspension can affect the aminophospholipid translocase activity also by a depletion of ATP. The reason for the depletion is unclear and is beyond the scope of this study. (2) An increase of the intracellular Ca2+ concentrations may cause an inhibition of the translocase activity52,53 as well as lipid scrambling.54,55 Because our experiments were performed in the absence of Ca2+ in the suspension media, an increase of the cytoplasmic Ca2+ concentration at low pHo is very unlikely. Our results on translocation of aminophospholipid analogues in resealed ghost membranes preclude any role of Ca2+. The intracellular lumen of those ghosts are depleted of Ca2+ because they were prepared in the presence of EGTA in Ca2+-free media. (3) The chemical nature and any pH-dependent property of the NO-moiety of our analogues cannot account for the observed phenomenon because we found also a reduction of the ATP-dependent inward movement of the aminophospholipid analogue C6-NBD-PS (J. Libera, unpublished results). This fluorescent analogue is also recognized by the aminophospholipid translocase.5 (4) The extracellular pH effects the transmembrane potential of erythrocytes (see Results). However, we could not find any evidence for an influence of the transmembrane potential on the inward movement of the aminophospholipid analogues. For example, an increase of the TMP by about 40 mV at pHo 7.4 did not affect the transverse redistribution of those probes; neither the initial velocity nor the steady-state distribution was affected. Recently, we have also shown that the passive movement of spin-labeled analogues (SL-PE, SL-PS, and SL-PC) across the erythrocyte membrane is not sensitive to alteration of the TMP.56 (5) Because a decrease of the NaCl concentration in the suspension from 120 to 5 mmol/L did not exert any influence on SL-PE redistribution, we have no indication that the surface potential of the outer leaflet is a significant determinant of the aminophospholipid translocase activity. (6) Because we observed a decline of the aminophospholipid translocase activity at pHo 5.8 also for resealed erythrocyte ghosts, we surmise that components of the cytoplasm in red blood cells are not involved in the pH-dependent suppression of the translocase activity.
Our results suggest that the intracellular pH affects the translocase-mediated inward movement of aminophospholipids. At the present stage of investigation it is not possible to present a conclusive model that explains the influence of pH on the activity of the aminophospholipid translocase. However, several hypotheses may be put forward. (1) A pH-dependent lateral rearrangement of the translocase has to be considered. For example, the formation of protein aggregates including the aminophospholipid translocase could affect the affinity of the translocase as well as the number of available binding sites for phospholipids. Several studies have shown that incubation of erythrocyte membranes at low pH causes an aggregation of integral membrane proteins, so-called intramembrane particles (IMP)57-59 as well as, of spectrin, the major constitutent of the erythrocyte membrane skeleton.59-61 (2) Protonation of ionizable amino acids of the translocase could modulate the affinity of the respective binding sites to ATP and/or to the aminophospholipids directly or by affecting the translocase conformation. It has been reported that the protonation of even a small number of ionizable residues can significantly decrease the stability of a native protein.62
The data presented could be of relevance for understanding the shortened posttransfusion viability of stored red blood cells. They suggest that exposure of aminophospholipids on the outer leaflet can be promoted at the temperature of storage (about 4°C) not only by ATP-depletion,34 but also by a decrease of the pH. The latter can be caused by an accumulation of lactate (see the introduction above). Albeit Geldwerth et al34 have shown that the pronounced asymmetric steady-state distribution of spin-labeled aminophospholipid analogues similar to those used by us is disturbed in stored red blood cells, they could not detect a modification of endogenous phospholipid organization. But the investigators pointed out that (1) the appearance of only a few percent of endogenous aminophospholipids, in particular phosphatidylserine, might be sufficient to alter cell surface properties and, thus, shorten posttransfusion viability of red blood cells due to recognition and uptake by macrophages; and (2) this low percentage of exposed aminophospholipids may not be detectable by the phospholipase assay used.
Finally, we want to emphasize that the influence of pH on the aminophospholipid translocase activity seems to depend also on the cell type. In a very recent study, Hanada and Pagano63 have shown that the ATP-dependent transmembrane movement of C6-NBD-PS in Chinese hamster ovary cells was stimulated by acidification of the cytoplasm. This suggests that cell-specific mechanisms for the regulation of the aminophospholipid translocase activity may exist that can be affected by the cytosolic pH.
ACKNOWLEDGMENT
The authors thank S. Schiller for the synthesis of spin-labeled analogues and B. Hillebrecht for technical assistance.
Supported by a grant from the Deutsche Forschungsgemeinschaft to P.M. (Mu 1017/1-3).
Address reprint requests to Andreas Herrmann, PhD, Humboldt-Universität zu Berlin, Mathematisch-Naturwissenschaftliche Fakultät I, Institut für Biologie/Biophysik, Invalidenstr. 43, D-10115 Berlin, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal