Abstract
The proteolytically activated thrombin receptor (TR) is expressed by T lymphocytes, which suggests that thrombin may modulate T-cell activation at sites of hemostatic stress. We examined the relationship between TR function and T-cell activation in the Jurkat human T-cell line and in T-cell lines with defined defects in T-cell antigen receptor (TCR) function. Stimulation with thrombin or the synthetic TR peptide SFLLRN produced intracellular Ca2+ transients in Jurkat cells. As the concentration of TR agonist was increased, peak Ca2+ mobilization increased, but influx of extracellular Ca2+ decreased. TR signaling was enhanced in a TCR-negative Jurkat line and in T-cell lines deficient in the tyrosine kinase lck or the tyrosine phosphatase CD45, both of which are essential for normal TCR function. TCR cross-linking with anti-CD3 IgM desensitized TR signaling in Jurkat cells, but not in CD45-deficient cells. A proteinase-activated receptor (PAR-2)–specific agonist peptide, SLIGKV, produced small Ca2+ transients in both MEG-01 human megakaryocytic cells and Jurkat cells, but was less potent than the TR-specific agonist TFRIFD in both cell types. Like TR signaling, PAR-2 signaling was enhanced in TCR-negative or lck-deficient Jurkat clones. These findings provide evidence for functional cross-talk between proteolytically activated receptors and the TCR.
THROMBIN IS A serine protease that functions as a central enzyme in the coagulation cascade. Thrombin cleaves fibrinogen to form a fibrin clot, activates coagulation proteases, and stimulates platelet aggregation.1 Because of its potent procoagulant properties, thrombin is the target of several new antithrombotic agents being developed for use in patients with arterial and venous thrombosis.2 In addition to its role in coagulation, thrombin stimulates inflammatory and proliferative responses in a variety of target cells, including endothelial cells, smooth muscle cells, fibroblasts, and T lymphocytes, among others.3
Cellular activation by thrombin is mediated in part by a proteolytically activated, G protein-coupled thrombin receptor (TR).4-6 Activation signals are transduced through TR by a tethered ligand mechanism in which the amino terminal extracellular domain of the receptor is proteolytically cleaved by thrombin.4 Structure-function studies have shown that cleavage of TR is required for stimulation of signal transduction by thrombin and that TR activation signals can be generated in the absence of thrombin by synthetic TR peptides that correspond in sequence to the tethered ligand.7 Although there is evidence for the existence of additional thrombin receptors,8,9 the cloned TR appears to be a major signal transducing thrombin receptor present on human platelets9,10 and many other types of cells, including fibroblasts,5,11 smooth muscle cells,6,12 endothelial cells,13 osteoblasts,14 and T lymphocytes.15-19
Stimulation of T cells with thrombin or TR agonist peptides induces transient increases in intracellular [Ca2+], activation of protein kinase C, and phosphorylation of several proteins on tyrosine residues.16,19 TR stimulation also has been reported to potentiate distal T-cell activation events, including mitogenesis, production of interleukin-2, and expression of the activation antigen CD69, that are induced by cross-linking of the T-cell antigen receptor (TCR).15 19
More recently, a second proteinase-activated receptor (PAR-2) was identified.20 The mouse PAR-2 sequence has an overall 28% amino acid identity with mouse TR, but differs considerably in its amino-terminal domain.20,21 PAR-2 has been detected in several human cell lines,22-24 including T-lymphocyte lines.25 PAR-2 can be activated by nanomolar concentrations of trypsin, but not by thrombin at concentrations up to 100 nmol/L.20 Some TR agonist peptides cross-react with PAR-2.26 27
To characterize relationships between TR, PAR-2, and T-cell activation, we examined signal transduction in the Jurkat human T-cell line and in several mutant T-cell lines with defined defects in TCR signal transduction. Our results indicate that signal transduction through proteolytically activated receptors is modulated by the activation state of the TCR signaling pathway.
MATERIALS AND METHODS
Materials.Human thrombin was purchased from Enzyme Research Laboratories (South Bend, IN). The human TR peptide SFLLRN was purchased from Bachem California (Torrence, CA). The Xenopus TR peptide TFRIFD28 and the human PAR-2 peptide SLIGKV29 were synthesized by Macromolecular Resources (Ft Collins, CO). The anti-human TR monoclonal antibody (MoAb) ATAP230,31 was provided by Dr Lawrence Brass (University of Pennsylvania, Philadelphia, PA). The anti-CD3 MoAbs 23532 and OKT333 were provided by Dr Shu Man Fu (University of Virginia, Charlottesville, VA), and Dr Brian Link (University of Iowa, Iowa City, IA), respectively. Control MoAb MOPC195 and fluorescein isothiocyanate (FITC)-conjugated goat-antimouse IgG (γ specific) were purchased from Cappel Laboratories (Malvern, PA).
Cell lines.The human megakaryocytic cell line MEG-0134 was provided by Dr Philip Majerus (Washington University, St Louis, MO). The human promyelocytic cell line HL6035 was obtained from Dr Timothy Ley (Washington University). The human T-cell leukemia line Jurkat (clone E6-1); Jurkat variants J.RT-T3.5, J.CaM1/rep3, and J.CaM1/lck; and two subclones (HPB.45.1 and HPB.45.0) of the immature (CD4+/CD8+) human thymocytic cell line HPB.ALL were obtained from Dr Arthur Weiss (University of California, San Francisco, CA). J.RT-T3.5 is a radiation-induced Jurkat mutant that is surface TCR-negative.36 J.CaM1/rep3 and J.CaM1/lck are derived from J.CaM1,37 an ethyl methanesulfonate-induced Jurkat mutant that lacks lck expression, by transfection with empty vector or lck cDNA, respectively.38 J45.01 is a radiation-induced Jurkat mutant that is surface CD45 deficient.39 J45/CH11 is a clone of J45.01 transfected with an expression vector encoding a chimeric HLA-A2/CD45 protein containing the CD45 cytoplasmic domain.40 HPB.45.1 is a subclone of HBP.ALL that expresses normal levels of CD45. HPB.45.0 is a subclone of HPB.ALL that is CD45 deficient.41 All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2.0 mmol/L glutamine.
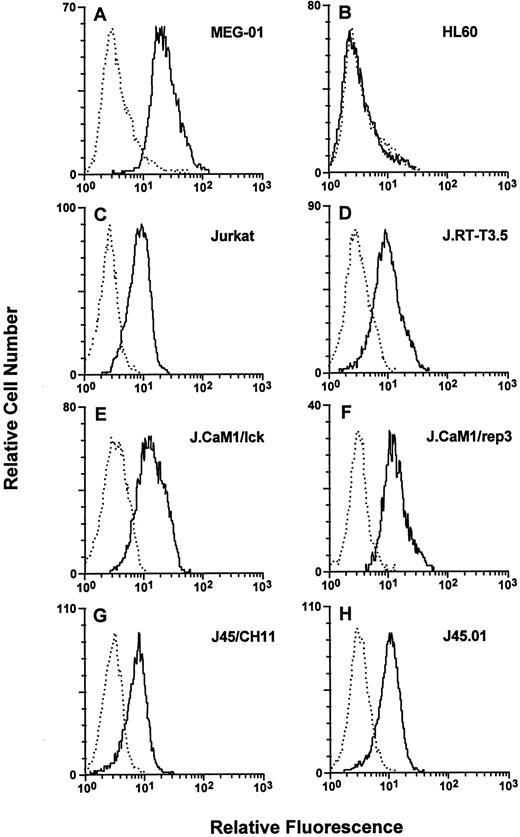
Cell-surface TR expression. Cells were stained with either the negative control MoAb MOPC195 (dotted lines) or the anti-human TR MoAb ATAP2 (solid lines), and flow cytometry was performed as described in the Materials and Methods. (A) MEG-01, (B) HL60, (C) Jurkat, (D) J.RT-T3.5 (TCR-negative), (E) J.CaM1/lck, (F) J.CaM1/rep3 (lck-deficient), (G) J45/CH11, and (H) J45.01 (CD45-deficient).
Cell-surface TR expression. Cells were stained with either the negative control MoAb MOPC195 (dotted lines) or the anti-human TR MoAb ATAP2 (solid lines), and flow cytometry was performed as described in the Materials and Methods. (A) MEG-01, (B) HL60, (C) Jurkat, (D) J.RT-T3.5 (TCR-negative), (E) J.CaM1/lck, (F) J.CaM1/rep3 (lck-deficient), (G) J45/CH11, and (H) J45.01 (CD45-deficient).
Flow cytometry.Cells were incubated with the indicated MoAbs followed by an FITC-conjugated goat-antimouse IgG secondary antibody and analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA) as described previously.42 Viable cells were gated by forward and side scatter and by propidium iodide staining.
Equilibrium binding.Direct equilibrium binding with 125I-labeled anti-TR MoAb ATAP2 was performed by a modification of methods described previously.31,43 ATAP2 IgG was radioiodinated in the University of Iowa Diabetes and Endocrinology Research Center using lactoperoxidase. Total binding was measured by incubating 106 cells for 60 minutes at 4°C in RPMI 1640, 50 mmol/L HEPES, pH 7.3, and 1.0% bovine albumin containing 0 to 25 μg/mL [125I]ATAP2, followed by sedimentation of cells through 40% sucrose to separate cell-bound from free IgG. Cell-associated [125I]ATAP2 was measured in a γ counter. Nonspecific binding was determined by adding a 100-fold excess of unlabeled ATAP2 IgG to the incubation mixture and also by performing binding studies with HL60 cells, which do not express TR.17 Specific binding of [125I]ATAP2 to MEG-01 cells was concentration-dependent and saturable, with a kd of 0.37 ± 0.23 μg/mL and Rmax of 130,000 ± 30,000 binding sites per cell (mean ± SD) determined by Scatchard analysis. This number of thrombin receptors per cell is comparable to that reported for other hematopoietic cell lines.31 For quantitative comparison of TR surface expression on mutant T-cell lines, specific binding was measured with each cell line using a saturating concentration (1.5 μg/mL) of [125I]ATAP2.
Quantitation of Cell-Surface TR Expression
| Cell Line . | [125I]ATAP2 Bound . | Surface TR . |
|---|---|---|
| . | (fmol/106 cells) . | (receptors/cell) . |
| HL60 | 0 ± 43 | 0 ± 26,000 |
| MEG-01 | 186 ± 21 | 112,000 ± 12,000 |
| Jurkat | 115 ± 38 | 69,000 ± 23,000 |
| JRT-T3.5 | 113 ± 24 | 68,000 ± 14,000 |
| J.CaM1/rep3 | 171 ± 27 | 103,000 ± 16,000 |
| J.CaM1/lck | 136 ± 34 | 82,000 ± 20,000 |
| J45.01 | 158 ± 29 | 95,000 ± 18,000 |
| J45/CH11 | 148 ± 39 | 89,000 ± 23,000 |
| Cell Line . | [125I]ATAP2 Bound . | Surface TR . |
|---|---|---|
| . | (fmol/106 cells) . | (receptors/cell) . |
| HL60 | 0 ± 43 | 0 ± 26,000 |
| MEG-01 | 186 ± 21 | 112,000 ± 12,000 |
| Jurkat | 115 ± 38 | 69,000 ± 23,000 |
| JRT-T3.5 | 113 ± 24 | 68,000 ± 14,000 |
| J.CaM1/rep3 | 171 ± 27 | 103,000 ± 16,000 |
| J.CaM1/lck | 136 ± 34 | 82,000 ± 20,000 |
| J45.01 | 158 ± 29 | 95,000 ± 18,000 |
| J45/CH11 | 148 ± 39 | 89,000 ± 23,000 |
Direct equilibrium binding of anti-TR MoAb ATAP2 was performed by incubating 106 cells with 125I-labeled ATAP2 for 60 minutes at 4°C. Values represent of mean ± SE of three to five separate determinations.
Ca2+ responses to TR agonists in Jurkat cells. Indo-1–loaded Jurkat cells were stimulated with thrombin or SFLLRN either in the absence (solid line) or the presence (dotted line) of 2.0 mmol/L EGTA, and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) 5 nmol/L thrombin, (B) 50 nmol/L thrombin, (C) 4 μmol/L SFLLRN, and (D) 40 μmol/L SFLLRN.
Ca2+ responses to TR agonists in Jurkat cells. Indo-1–loaded Jurkat cells were stimulated with thrombin or SFLLRN either in the absence (solid line) or the presence (dotted line) of 2.0 mmol/L EGTA, and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) 5 nmol/L thrombin, (B) 50 nmol/L thrombin, (C) 4 μmol/L SFLLRN, and (D) 40 μmol/L SFLLRN.
Measurement of intracellular [Ca2+].Cells were loaded with the pentaacetoxymethyl ester of Indo-1 (Molecular Probes, Eugene, OR) as described,44 washed, and resuspended at 6 × 106 cells/mL in 25 mmol/L HEPES, pH 7.4, 1.0 mmol/L Na2HPO4 , 125 mmol/L NaCl, 5.0 mmol/L KCl, 0.5 mmol/L MgCl2 , 1.0 mmol/L CaCl2 , 0.1% glucose, and 1.0 mg/mL bovine serum albumin. Continuous Indo-1 fluorescence was measured using a spectrofluorimeter (Spex Industries, Edison, NJ), with excitation at 334 nm and detection at 400 nm. The fluorimeter was calibrated for each determination by complete lysis with 0.1% Triton-X 100, followed by chelation of Ca2+ with 16 mmol/L EGTA. Increases in intracellular [Ca2+] were calculated using the formula [Ca2+]i = Kapp(R-Rmin) /(Rmax -R), where Kapp for Ca2+ binding to Indo-1 = 0.25 μmol/L.45
RESULTS
Cell-surface thrombin receptor expression.Cell-surface TR expression was detected by flow cytometry and quantitated by equilibrium binding using the MoAb ATAP2, which recognizes both cleaved and uncleaved forms of human TR.31 In agreement with previous studies,17 TR was not detected on the surface of the human promyelocytic cell line HL60, but strong TR surface expression was observed on the human megakaryocytic cell line MEG-01 (Fig 1 and Table 1). TR also was detected on the surface of Jurkat cells and on the Jurkat variants J.RT-T3.5, JCaM1/lck, JCaM1/rep3, J45/CH11, and J45.01. Jurkat cells expressed approximately 40% less surface TR than MEG-01 cells, and all of the Jurkat cell lines expressed similar levels of TR.
Thrombin receptor signal transduction.TR signal transduction was measured in Jurkat and MEG-01 cells using the calcium sensitive fluor Indo-1. In Jurkat cells, the addition of thrombin or the human TR peptide SFLLRN produced dose-dependent, transient increases in intracellular [Ca2+], with higher doses of thrombin or SFLLRN producing responses of higher peak amplitude and shorter duration (Fig 2). Chelation of extracellular Ca2+ with EGTA did not affect the peak amplitude of the Ca2+ transient, but shortened its duration in Jurkat cells stimulated with 5 nmol/L thrombin or 4 μmol/L SFLLRN (Fig 2A and C). Chelation of extracellular Ca2+ had little effect on the duration or amplitude of the calcium transient in Jurkat cells stimulated with 50 nmol/L thrombin or 40 μmol/L SFLLRN (Fig 2B and D). TR agonists also stimulated Ca2+ transients in MEG-01 cells (Fig 3). Compared with Jurkat cells, MEG-01 cells were more sensitive to low doses of thrombin and SFLLRN, and the Ca2+ transients were more sustained. Moreover, chelation of extracellular Ca2+ dramatically shortened the duration the Ca2+ transient in MEG-01 cells stimulated with either low or high concentrations of thrombin or SFLLRN. These findings indicate that Jurkat cells express functional TR and suggest that TR coupling to calcium influx differs between T cells and megakaryocytic cells.
Ca2+ responses to TR agonists in MEG-01 cells. Indo-1–loaded MEG-01 cells were stimulated with thrombin or SFLLRN either in the absence (solid line) or the presence (dotted line) of 2.0 mmol/L EGTA, and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) 5 nmol/L thrombin, (B) 50 nmol/L thrombin, (C) 4 μmol/L SFLLRN, and (D) 40 μmol/L SFLLRN.
Ca2+ responses to TR agonists in MEG-01 cells. Indo-1–loaded MEG-01 cells were stimulated with thrombin or SFLLRN either in the absence (solid line) or the presence (dotted line) of 2.0 mmol/L EGTA, and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) 5 nmol/L thrombin, (B) 50 nmol/L thrombin, (C) 4 μmol/L SFLLRN, and (D) 40 μmol/L SFLLRN.
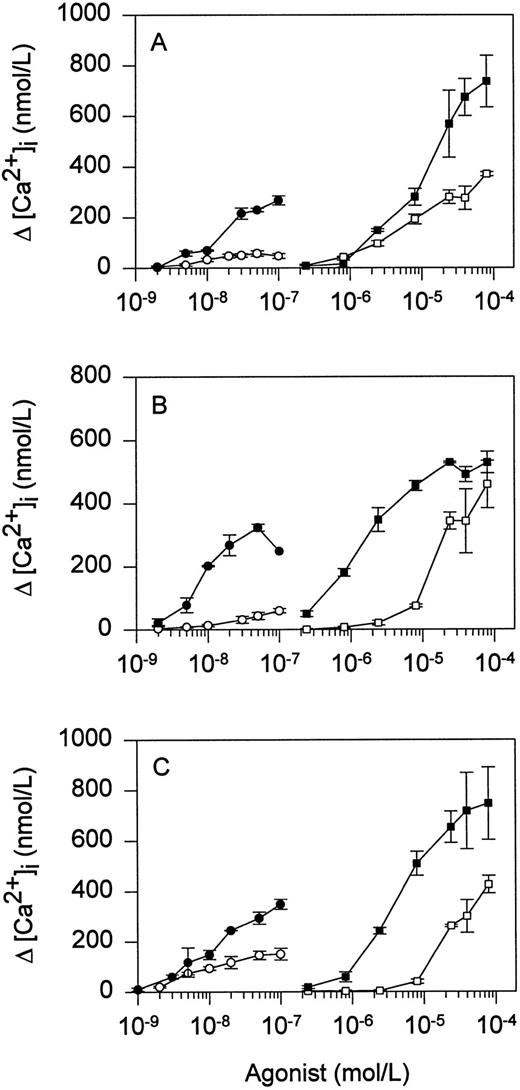
Thrombin receptor signaling in Jurkat mutants.To determine whether signal transduction through TR is altered in T cells lacking a functional TCR signaling pathway, we examined Ca2+ mobilization in response to TR agonists in the Jurkat variant J.RT-T3.5, which does not express TCR,36 and in two Jurkat variants with defined defects in protein tyrosine phosphorylation, JCaM1/rep3 and J45.01. J.CaM1/rep3 is deficient in the src family protein tyrosine kinase lck,46 and J45.01 is deficient in the protein tyrosine phosphatase CD45.39 Both lck and CD45 are essential for normal signal transduction through TCR.47 Compared with Jurkat, JRT3.5 exhibited greater increases in peak intracellular [Ca2+] in response to increasing concentrations of thrombin or SFLLRN (Fig 4A). Enhanced sensitivity to thrombin or SFLLRN also was observed in the lck-deficient cell line JCaM1/rep3 (Fig 4B) and the CD45-deficient cell line J45.01 (Fig 4C). Enhanced sensitivity to TR agonists was lost in cell lines JCaM1/lck and J45/CH11, in which TCR function had been reconstituted by stable transfection with lck or the phosphatase domain of CD45, respectively (Fig 4B and C). Thus, Jurkat TR signal transduction was sensitized in the absence of TCR or in the presence of a dysfunctional TCR signaling pathway.
Ca2+ responses of mutant Jurkat cell lines to TR agonists. Indo-1–loaded cells were stimulated with the indicated concentrations of thrombin (○, •) or SFLLRN (□, ▪). (A) Jurkat (open symbols) and J.RT-T3.5 (TCR-negative; solid symbols); (B) J.CaM1/lck (open symbols) and J.CaM1/rep3 (lck-deficient; solid symbols); and (C) J45/CH11 (open symbols) and J45.01 (CD45-deficient; solid symbols). Values represent the mean ± SD of triplicate determinations.
Ca2+ responses of mutant Jurkat cell lines to TR agonists. Indo-1–loaded cells were stimulated with the indicated concentrations of thrombin (○, •) or SFLLRN (□, ▪). (A) Jurkat (open symbols) and J.RT-T3.5 (TCR-negative; solid symbols); (B) J.CaM1/lck (open symbols) and J.CaM1/rep3 (lck-deficient; solid symbols); and (C) J45/CH11 (open symbols) and J45.01 (CD45-deficient; solid symbols). Values represent the mean ± SD of triplicate determinations.
To determine whether the correlation between TCR dysfunction and TR sensitization was unique to the Jurkat cell line, we compared responses to TR agonists in CD45+ and CD45− subclones of the human immature thymocytic cell line HPB.ALL. HPB.45.1 is a CD45+ subclone with a normal TCR signaling pathway, and HPB.45.0 is a CD45-deficient subclone with defective TCR signaling function.41 Like CD45-deficient Jurkat cells, CD45-deficient HPB.45.0 cells were hyperresponsive to thrombin (Fig 5). HPB.45.0 cells also showed enhanced responses to the TR agonist peptides TFRIFD (Fig 5) or SFLLRN (not shown).
Ca2+ responses of HPB.ALL subclones to TR agonists. CD45+ HPB.45.1 cells (□) or CD45-deficient HBP.45.0 cells (▨) were loaded with Indo-1 and stimulated with either 50 nmol/L thrombin or 120 μmol/L TFRIFD, and intracellular [Ca2+] was measured using a spectrofluorimeter. Values represent the mean ± SD of triplicate determinations.
Ca2+ responses of HPB.ALL subclones to TR agonists. CD45+ HPB.45.1 cells (□) or CD45-deficient HBP.45.0 cells (▨) were loaded with Indo-1 and stimulated with either 50 nmol/L thrombin or 120 μmol/L TFRIFD, and intracellular [Ca2+] was measured using a spectrofluorimeter. Values represent the mean ± SD of triplicate determinations.
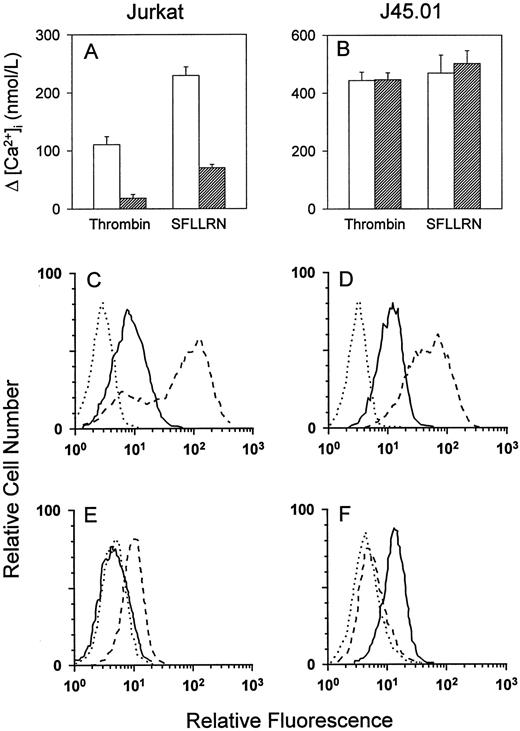
Effect of TCR activation on TR signal transduction.To determine the effect of TCR activation on TR signal transduction, Jurkat cells were incubated with the IgM MoAb 235, which cross-links the CD3 complex of the TCR. In agreement with Mari et al,19 incubation of Jurkat cells with anti-CD3 decreased subsequent responsiveness to thrombin or SFLLRN by approximately 70% to 80% in a Ca2+ mobilization assay (Fig 6A). Compared with unstimulated Jurkat cells (Fig 6C), Jurkat cells incubated with anti-CD3 exhibited decreased surface expression of both TCR and TR (Fig 6E). Desensitization to TR agonists and loss of cell-surface TR were observed after incubation of Jurkat cells with anti-CD3 for time periods as short as 15 minutes or as long as 16 hours. In contrast, incubation of CD45-deficient J45.01 cells with anti-CD3 did not affect responses to TR agonists (Fig 6B). Incubation with anti-CD3 stimulated internalization of TCR in J45.01 cells, but surface expression of TR did not change (Fig 6D and F). These findings suggest that TCR-mediated signal transduction, rather than merely TCR internalization, is necessary for desensitization of TR.
Effect of TCR stimulation on TR Ca2+ responses and cell-surface expression. Jurkat cells (A) or J45.01 cells (B) were incubated for 16 hours with either control medium (□) or the IgM anti-CD3 MoAb 235 (▨), and Ca2+ responses were measured in response to 50 nmol/L thrombin or 40 μmol/L SFLLRN. Values represent the mean ±SD of triplicate determinations. To measure effects of TCR stimulation on cell-surface expression, flow cytometry was performed using the negative control MoAb (dotted lines), the antihuman TR MoAb ATAP2 (solid lines), or the IgG anti-CD3 MoAb OKT3 (dashed lines). (C) Unstimulated Jurkat cells; (D) unstimulated J45.01 cells; (E) Jurkat cells stimulated with anti-CD3; and (F ) J45.01 cells stimulated with anti-CD3.
Effect of TCR stimulation on TR Ca2+ responses and cell-surface expression. Jurkat cells (A) or J45.01 cells (B) were incubated for 16 hours with either control medium (□) or the IgM anti-CD3 MoAb 235 (▨), and Ca2+ responses were measured in response to 50 nmol/L thrombin or 40 μmol/L SFLLRN. Values represent the mean ±SD of triplicate determinations. To measure effects of TCR stimulation on cell-surface expression, flow cytometry was performed using the negative control MoAb (dotted lines), the antihuman TR MoAb ATAP2 (solid lines), or the IgG anti-CD3 MoAb OKT3 (dashed lines). (C) Unstimulated Jurkat cells; (D) unstimulated J45.01 cells; (E) Jurkat cells stimulated with anti-CD3; and (F ) J45.01 cells stimulated with anti-CD3.
PAR-2 signaling in MEG-01 and Jurkat cells.In most of the Jurkat cell lines, SFLLRN consistently produced larger peak calcium transients than thrombin. This suggested that Jurkat cells may contain a second SFLLRN-responsive receptor such as PAR-2. To distinguish between responses mediated by TR and those mediated by PAR-2, Ca2+ mobilization assays were performed with the Xenopus TR peptide TFRIFD, which activates human TR but not PAR-2, or the human PAR-2 peptide SLIGKV, which activates PAR-2 but not TR.27 TFRIFD at 120 μmol/L produced Ca2+ transients in both MEG-01 and Jurkat cells (Fig 7A and B). SLIGKV at 120 μmol/L produced a small Ca2+ transient in MEG-01 cells (Fig 7A), but had a minimal effect on intracellular [Ca2+] in Jurkat cells (Fig 7B). Larger Ca2+ responses to both TFRIFD and SLIGKV were observed in TCR-negative (J.RT-T3.5) and lck-deficient (J.CaM1/rep3) Jurkat cell lines (Fig 7C and D). These results suggest that, like TR signaling, PAR-2 signaling is enhanced in T cells with a defective TCR pathway.
Ca2+ responses to specific PAR-2 and TR agonists. Indo-1–loaded cells were stimulated with either the PAR-2–specific agonist SLIGKV (120 μmol/L; solid line) or the TR-specific agonist TFRIFD (120 μmol/L; dotted line), and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) MEG-01; (B) Jurkat; (C) J.RT-T3.5 (TCR-negative); and (D) J.CaM1/rep3 (lck-deficient).
Ca2+ responses to specific PAR-2 and TR agonists. Indo-1–loaded cells were stimulated with either the PAR-2–specific agonist SLIGKV (120 μmol/L; solid line) or the TR-specific agonist TFRIFD (120 μmol/L; dotted line), and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) MEG-01; (B) Jurkat; (C) J.RT-T3.5 (TCR-negative); and (D) J.CaM1/rep3 (lck-deficient).
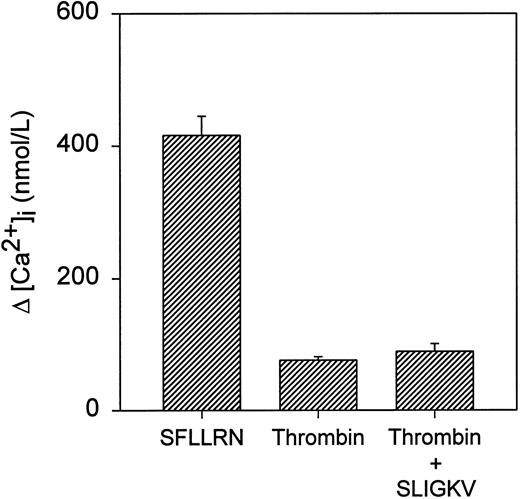
To directly test the possibility that the discrepancy between maximal calcium responses to SFLLRN and thrombin was caused by stimulation of PAR-2 by SFLLRN, Jurkat cells were stimulated simultaneously with 50 nmol/L thrombin and 120 μmol/L SLIGKV (Fig 8). There was no significant difference in the amplitude of the peak Ca2+ transient between cells stimulated with thrombin alone and those stimulated with both thrombin and SLIGKV. In each case, the peak response was markedly less than that seen after stimulation with 40 μmol/L SFLLRN. This raises the possibility that Jurkat cells may contain additional SFLLRN-responsive receptors other than TR and PAR-2.48
Ca2+ responses to TR and PAR-2 agonists. Indo-1–loaded Jurkat cells were stimulated with either 40 μmol/L SFLLRN or 50 nmol/L thrombin or simultaneously with 50 nmol/L thrombin and 120 mmol/L SLIGKV. Values represent the mean ± SD of triplicate determinations.
Ca2+ responses to TR and PAR-2 agonists. Indo-1–loaded Jurkat cells were stimulated with either 40 μmol/L SFLLRN or 50 nmol/L thrombin or simultaneously with 50 nmol/L thrombin and 120 mmol/L SLIGKV. Values represent the mean ± SD of triplicate determinations.
DISCUSSION
In addition to its role as a central enzyme in blood coagulation, thrombin functions as a potent agonist for a variety of target cells.3 Current models of thrombin function propose that thrombin generated in response to vascular injury or increased vascular permeability mediates the coordinated activation of hemostatic and proliferative processes during tissue repair.7 Recent demonstrations that T lymphocytes express TR15-19 suggest that thrombin may modulate T-cell activation at sites of hemostatic stress. In agreement with these earlier studies, we detected functional TR on the surface of the Jurkat human T-cell leukemia line.
Compared with MEG-01 human megakaryocytic cells, Jurkat cells expressed approximately 40% less surface TR and were less sensitive to stimulation with thrombin. Moreover, we observed different patterns of TR coupling to Ca2+ influx in Jurkat and MEG-01 cells. Stimulation of Jurkat cells with low concentrations of TR agonists (5 nmol/L thrombin or 4 μmol/L SFLLRN) produced measurable Ca2+ influx from the extracellular medium as well as Ca2+ mobilization from intracellular stores. Stimulation with 10-fold higher concentrations of thrombin or SFLLRN increased peak Ca2+ mobilization, but decreased Ca2+ influx. In comparison, MEG-01 cells exhibited sustained Ca2+ influx in response to stimulation with either low or high concentrations of TR agonists. Unlike previous studies in osteoblasts,14 we did not observe agonist-dependent differences in TR-stimulated Ca2+ influx in Jurkat cells, and Ca2+ influx was not inhibited by pretreatment with the serine/threonine phosphatase inhibitor calyculin A (not shown). This suggests that TR coupling to Ca2+ influx in Jurkat cells may be regulated by mechanisms other than receptor phosphorylation.
Our results with mutant T-cell lines provide evidence for functional cross-talk between the TR and TCR signaling pathways. TR Ca2+ signaling was enhanced in a TCR-negative cell line and in T-cell lines deficient in the protein tyrosine kinase lck or the protein tyrosine phosphatase CD45. Thus, unlike signal transduction through TCR, signal transduction through TR does not require lck or CD45. Conversely, TR signal transduction appears to be sensitized in cells lacking an intact TCR signaling pathway. The basal activation state of the TCR signaling pathway is thought to represent a dynamic equilibrium between positive and negative regulatory signals.47 Our observation that TR responsiveness is enhanced in cell lines lacking an intact TCR pathway implies that TR signaling is tonically inhibited by basal activation through the TCR pathway. This suggests that desensitized (tolerized) T cells may be more responsive to thrombin than resting T cells.
Maximal calcium mobilization in response to TR agonist peptides was somewhat lower in the lck-deficient cell line (JCaM1/rep3) than in TCR-deficient or CD45-deficient cell lines (Fig 4). Unlike the TCR- and CD45-deficient cell lines, the JCaM1 mutant retains a small amount of residual responsiveness to TCR stimulation.37 Therefore, the slightly lower peak [Ca2+]i response to TR agonists in this cell line may reflect a higher basal flux through the TCR signaling pathway. An alternative explanation for differential TR-mediated calcium responses in CD45-deficient and lck-deficient cell lines is that TR signaling may be regulated by src family kinases other than lck, which in turn may be subject to regulation by the CD45 phosphatase.
TR agonists have been reported to potentiate distal T-cell activation responses induced by TCR ligation,15 19 which suggests that effects of thrombin on T-cell activation may vary depending on the sequence in which T cells encounter TR and TCR agonists. Stimulation with thrombin before antigen presentation may enhance T-cell proliferation and interleukin-2 production, whereas stimulation with antigen before thrombin exposure may desensitize to TR stimulation, contributing to downregulation of the proliferative response.
TCR-generated signals may modulate signal transduction through TR by multiple mechanisms. In agreement with previous studies,19 we observed diminished TR signal transduction and decreased TR surface expression in Jurkat cells that had been activated by TCR cross-linking. This observation raised the possibility that enhanced sensitivity to TR agonists in cells with a defective TCR pathway may result from increased surface TR expression. However, we detected no significant differences in TR surface expression in cell lines with intact (Jurkat, JCaM1/lck, and J45/CH11) or dysfunctional (JRT3.5, JCaM1, and J45.01) TCR signaling pathways (Fig 1 and Table 1). This implies that the TCR pathway desensitizes TR signal transduction through mechanisms distinct from modulating TR surface expression. We also observed that TR signaling remained intact in CD45-deficient Jurkat cells after internalization of TCR with a cross-linking anti-CD3 MoAb. This observation provides evidence that signaling through TCR, rather than TCR internalization, is required for desensitization of TR.
The recent discovery that SFLLRN activates both TR and PAR-226,27 raised the possibility that differential responsiveness to thrombin and SFLLRN in Jurkat cells could be caused by stimulation of PAR-2 by SFLLRN. This possibility was tested using the PAR-2-specific agonist peptide SLIGKV.29 We found that SLIGKV produced small Ca2+ transients in MEG-01 cells, and also in several Jurkat clones, suggesting that these cells express both PAR-2 and TR. Responses to SLIGKV were likely mediated by PAR-2, rather than by cross-reactivity with TR, for the following reasons. (1) Using a Xenopus oocyte system, Blackhart et al27 found that SLIGKV, even at millimolar concentrations, did not activate human TR. (2) Compared with Ca2+ transients produced by TFRIFD, which develop immediately upon stimulation, Ca2+ transients produced by SLIGKV are delayed in onset and more gradual in slope (Fig 8). (3) Human PAR-2 mRNA has been detected by Northern hybridization in spleen and blood leukocytes29 and in Jurkat cells.25 Like TR, PAR-2 appears to be modulated by the TCR pathway, because responses to SLIGKV were enhanced in both TCR-negative and lck-deficient Jurkat clones.
The low levels of functional PAR-2 relative to TR in Jurkat and MEG-01 cells contrast with those reported in human keratinocytes, which respond better to PAR-2–specific agonists than to TR-specific agonists.26 These findings argue that PAR-2 activation is an unlikely explanation for the greater sensitivity of Jurkat cells to SFLLRN compared with thrombin. Enhanced responsiveness to SFLLRN in Jurkat cells also is unlikely to be caused by expression of PAR-3, a recently reported third member of the proteinase-activated receptor family, since PAR-3 does not respond to SFLLRN.48 However, we cannot exclude the possibility that Jurkat cells express other SFLLRN-sensitive receptors in addition to TR and PAR-2.48
In summary, this study shows that signal transduction through proteolytically activated receptors is influenced by the activation state of T cells. These observations support the model that thrombin provides a functional link between hemostasis and immune activation, two highly regulated processes that have coevolved in response to environmental stress.
ACKNOWLEDGMENT
The authors thank Dr Lawrence F. Brass for MoAbs to human TR and for several helpful suggestions.
Supported by the Office of Research and Development, Department of Veterans Affairs; National Institutes of Health Grants No. CA-56843, CA-56050, DK-25295, and HL-07344; American Heart Association Grant No. IA-95-GS-45; and the Carver Trust of the University of Iowa. G.A.K. is an established investigator of the American Heart Association.
Address reprint requests to Steven R. Lentz, MD, PhD, Department of Internal Medicine, C303 GH, The University of Iowa, Iowa City, IA 52242.

![Fig. 2. Ca2+ responses to TR agonists in Jurkat cells. Indo-1–loaded Jurkat cells were stimulated with thrombin or SFLLRN either in the absence (solid line) or the presence (dotted line) of 2.0 mmol/L EGTA, and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) 5 nmol/L thrombin, (B) 50 nmol/L thrombin, (C) 4 μmol/L SFLLRN, and (D) 40 μmol/L SFLLRN.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1893/4/m_bl_0018f2.jpeg?Expires=1769141311&Signature=1TtpEaYmAoAoEO9oPG6FFYhAsPP9vXFdlJ0tjBFK6DY0eRVhpQrCfpvfqJ2JjX9yAEKVf90f9BzLULPOXufIqUB9vL6mB~8cR2ZOqnyKTla4fwbsixb3h5WRr9mgByIwUR440vSptgBtec89f-D1kVY9sUypZIiiZQqMwtFhoWk-ks5uQcspRHArk~V2oPMMfDIgrdXzy97JQnStkf2vQfmndEqLAIN0kr~VJF-pQgv1nj6sh9vez~to4Tc2Bfs~bECai91dV8Sq6i-pC06tXzCyG1BRFDHCsvXtnNxMZvQ0n2f-eTnBW5M4OP0OMBfsTG77IKa79tMWLVq6SVGqtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Ca2+ responses to TR agonists in MEG-01 cells. Indo-1–loaded MEG-01 cells were stimulated with thrombin or SFLLRN either in the absence (solid line) or the presence (dotted line) of 2.0 mmol/L EGTA, and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) 5 nmol/L thrombin, (B) 50 nmol/L thrombin, (C) 4 μmol/L SFLLRN, and (D) 40 μmol/L SFLLRN.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1893/4/m_bl_0018f3.jpeg?Expires=1769141311&Signature=QNDGsaHX6BZ6hdA1JdeRtPCYEXMS8woOIqzVhy-PXjGupTrvXqK623nYaUJWPDWEw9n3Nuz0M2oABP49PIBLPaoyCbzZeSV4Fq13z4qLm4kNTLubF9zSVrQ971StmccgO0ImPPc8GUpm67iI7cb3kkULvZj1DzoqzAAgJtCmn9QkIXCTiftVRtzKvdvhhd-Q6MglEvYvaHP78ULdcBEbsfluRp-5w8~rO8-J4PCv-i6V5wXbooaxEd4rlCKg40BVKF9Kojj6BGw2fC3DIYkYi8FYSe~av~WfzISuDMyVk1z632N2OO1aMInbTj8W8H66w9zoaHuGWaPi5fMC1ZlOTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Ca2+ responses of HPB.ALL subclones to TR agonists. CD45+ HPB.45.1 cells (□) or CD45-deficient HBP.45.0 cells (▨) were loaded with Indo-1 and stimulated with either 50 nmol/L thrombin or 120 μmol/L TFRIFD, and intracellular [Ca2+] was measured using a spectrofluorimeter. Values represent the mean ± SD of triplicate determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1893/4/m_bl_0018f5.jpeg?Expires=1769141311&Signature=k3S5uNnmKkO91HoDAYqkSvLjQUYZ7~hfTfyrRtmHLm4qGoA8mSumqWoCMDLNbcikMG2TkZbRSAMK2kg1fw1D7a3BNlVU8cu-lCOVAT-FkX9oPvt5Eh4GYfhxwiNvwjVmb2QZ-NpCCQXf7wQcTIyeGgz57uYPyLKtjJFTSeGoZ51oFJz9H6LiJux2z19rN0nKDvAqgA1zTvAC5w1i2avt8-R8itIxf3KL0Z7HpNjb~SK9U26~eYVF7UdySHOCuKqwAFQvFY4SzmNj1rxpYgcCWBtpoLqbf3fYUeZ6zuFFcM2RcRcpdhifQgDzPfvKBY-qIr7B4~q9MbVayCNlqkqZJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Ca2+ responses to specific PAR-2 and TR agonists. Indo-1–loaded cells were stimulated with either the PAR-2–specific agonist SLIGKV (120 μmol/L; solid line) or the TR-specific agonist TFRIFD (120 μmol/L; dotted line), and intracellular [Ca2+] was measured using a spectrofluorimeter. (A) MEG-01; (B) Jurkat; (C) J.RT-T3.5 (TCR-negative); and (D) J.CaM1/rep3 (lck-deficient).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1893/4/m_bl_0018f7.jpeg?Expires=1769141311&Signature=QcaV3PQsP99mhkEsSGv2FtlPmo6lDL-reaCNrG-8YwnnQGRKsXICIBvTcKgqlJM5abbUaNEn4vsY08kIuP7AZJeqxGRa6vdzlXTLiJZ9sFw5ln7o0GRVq81cO7sS-SVzyL-kUGVnwl1IXWajViPwkzRleg7FPpou67b8r6UVS776DG~TgQenK6CIo3IyLwK5dmE4zYACaHI~ZG5iOLf70UVlVAzqT89k~hUfNBJVuYEcqZQwfaJ4f8Hdc3v0vLl3cxFuTaAshtS-dxN4Ssn0Jtoa0FlD-BTKGByK7fMjkmmOR1SqO9LvjMTfDqQBHMbLjScpJLNVkXXjjUmW01NzqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal