Abstract
Myeloma plasma cells constitute 10% to 90% of the total bone marrow cell count in patients with multiple myeloma (MM). These cells express a variety of cell surface markers, such as HLA-ABC and HLA-DR, and surface antigens that are necessary for professional antigen-presenting cells, including adhesion and costimulatory molecules. In this study, we examined the expression of major histocompatability complex (MHC) and costimulatory molecules on CD38(bright,++) plasma cells in bone marrow aspirates from eight MM patients. Small percentages of plasma cells expressed weak but detectable levels of HLA-DR, HLA-DQ, CD40, CD80, and CD86, which could be upregulated by interferon-γ (IFN-γ) and tumor necrosis factor-α. CD38++ plasma cell and CD38(dim,+) cells were sorted from freshly isolated bone marrow mononuclear cells and tested for their capacity to act as antigen-presenting cells. Indeed, both CD38++ plasma cells and CD38+ cells were able to stimulate allogeneic T cells and present the soluble antigens purified protein derivative and tetanus toxoid to autologous T cells. Recognition of the antigens led to T-cell proliferation and secretion of IFN-γ and was MHC class-I and -II restricted. Antigen processing and presentation by CD38++ and CD38+ cells were abolished by treatment of the cells with chloroquine. Hence, our study provides for the first time evidence that myeloma plasma cells may act as antigen-presenting cells. Further studies are warranted to examine in detail the molecules required for inducing T-cell stimulation.
MULTIPLE MYELOMA (MM) is a B-cell neoplasia characterized by clonal expansion of malignant plasma cells in the bone marrow. These plasma cells produce a monoclonal Ig that can be detected in the serum and/or urine.1 Plasma cells represent the terminal differentiation stage of B-cell development. In MM, plasma cells constitute at least 10% and can even reach greater than 90% of the total bone marrow cell count.2 The growth and differentiation of myeloma plasma cells are believed to be regulated by a functional interplay between the tumor cells and the bone marrow microenvironment mediated by cytokines such as interleukin-6 (IL-6) and by close cellular contact between myeloma cells and marrow stromal components (reviewed in Moscinski and Ballester3 and Klein et al4 ).
The immune system has a potential role in the regulation of MM.1 One important aspect is whether the bone marrow myeloma plasma cells can be recognized and regulated by tumor-specific T cells. Myeloma plasma cells may express a variety of cell surface markers, such as CD10, CD33, and HLA-DR.5,6 In addition, adhesion molecules such as CD44, CD56, CD54, and VLA-47-9 and signaling or costimulatory molecules CD40,10,11 CD28,11,12 and CD8012 13 were found on freshly isolated myeloma bone marrow plasma cells or myeloma-derived cell lines. Thus, based on the expression of the surface antigens, myeloma plasma cells may belong to the category of antigen-presenting cells.
Freshly isolated myeloma plasma cells are best characterized by the expression of cytoplasmic Ig and high densities of surface antigen CD38 (CD38bright,++).14,15 However, the myeloma cell phenotype and morphology appear to be heterogeneous, varying from early plasmablasts to mature plasma cells. It is well documented that the CD38++ fraction includes only plasma cells, whereas the CD38(dim,+) population may contain, among other cells, immature plasma cells.9 14-19 In this study, we first analyzed noninduced and cytokine-induced expression of major histocompatability complex (MHC) molecules and signalling or costimulatory antigens on CD38++ plasma cells from bone marrow aspirates of MM patients. Second, we investigated the antigen-presenting capacity of sorted CD38++ plasma cells and CD38+ cells from bone marrow mononuclear cells (BMMCs). Our results show that both CD38++ plasma cells and CD38+ cells were able to stimulate allogeneic peripheral blood mononuclear cells (PBMCs) and to present the soluble antigens purified protein derivative (PPD) and tetanus toxoid (TT) to autologous T cells.
MATERIALS AND METHODS
Cell preparation.Bone marrow aspirates were obtained from eight patients with MM (Table 1). BMMCs were isolated by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. The cells were washed twice and resuspended in culture medium (RPMI 1640) supplemented with L-glutamine (4 mmol/L), penicillin (100 IU/mL), streptomycin (100 μg/mL), and 10% heat-inactivated pooled serum from individuals with blood group ABRh+.
Patients' Characteristics and Percentage of CD38++ Plasma Cells Expressing Surface MHC (HLA), Signaling or Costimulatory Molecules, and CD19 Antigen
| Patient . | Age (yr)/Sex . | Clinical . | Monoclonal . | % PC . | % of CD38++ Plasma Cells Expressing . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | . | Stage . | Ig . | in BM . | DR . | DQ . | ABC . | CD40 . | CD80 . | CD86 . | CD19 . |
| 1 | 72/F | IIA | IgGλ | 42 | 11.6 | 23.3 | 99.5 | ND | ND | ND | ND |
| 2 | 72/F | IA | IgG1κ | 22 | 44.0 | 7.8 | 96.9 | 6.4 | 1.7 | 4.9 | 1.3 |
| 3 | 70/M | IIIB | IgG1κ | 70 | 13.4 | ND | 99.0 | 64.0 | 3.9 | 5.5 | 0.2 |
| 4 | 55/M | IA | IgG1κ | 28 | 10.0 | 20.1 | 99.8 | 3.6 | 9.7 | 20.0 | 0.7 |
| 5 | 67/M | IIA | IgAκ | 56 | 33.2 | 8.4 | 97.8 | 2.2 | 1.6 | 12.0 | 0.8 |
| 6 | 60/M | IIA | IgG1λ | 25 | 12.6 | 5.8 | 100 | 21.2 | 3.8 | 18.2 | 1.0 |
| 7 | 89/F | IA | IgG1κ | 19 | 22.7 | 7.7 | 98.0 | 3.5 | 2.0 | 11.0 | 1.0 |
| 8 | 66/M | IA | IgAκ | 18 | 9.2 | 10.0 | 96.9 | 24.3 | 3.5 | 15.4 | 0.3 |
| Median | 69/ — | — | — | 28 | 12.5 | 8.4 | 98.5 | 6.4 | 3.5 | 12.0 | 0.7 |
| Patient . | Age (yr)/Sex . | Clinical . | Monoclonal . | % PC . | % of CD38++ Plasma Cells Expressing . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | . | Stage . | Ig . | in BM . | DR . | DQ . | ABC . | CD40 . | CD80 . | CD86 . | CD19 . |
| 1 | 72/F | IIA | IgGλ | 42 | 11.6 | 23.3 | 99.5 | ND | ND | ND | ND |
| 2 | 72/F | IA | IgG1κ | 22 | 44.0 | 7.8 | 96.9 | 6.4 | 1.7 | 4.9 | 1.3 |
| 3 | 70/M | IIIB | IgG1κ | 70 | 13.4 | ND | 99.0 | 64.0 | 3.9 | 5.5 | 0.2 |
| 4 | 55/M | IA | IgG1κ | 28 | 10.0 | 20.1 | 99.8 | 3.6 | 9.7 | 20.0 | 0.7 |
| 5 | 67/M | IIA | IgAκ | 56 | 33.2 | 8.4 | 97.8 | 2.2 | 1.6 | 12.0 | 0.8 |
| 6 | 60/M | IIA | IgG1λ | 25 | 12.6 | 5.8 | 100 | 21.2 | 3.8 | 18.2 | 1.0 |
| 7 | 89/F | IA | IgG1κ | 19 | 22.7 | 7.7 | 98.0 | 3.5 | 2.0 | 11.0 | 1.0 |
| 8 | 66/M | IA | IgAκ | 18 | 9.2 | 10.0 | 96.9 | 24.3 | 3.5 | 15.4 | 0.3 |
| Median | 69/ — | — | — | 28 | 12.5 | 8.4 | 98.5 | 6.4 | 3.5 | 12.0 | 0.7 |
Abbreviations: PC, plasma cells; BM, bone marrow; ND, not done.
Sorting of CD38++ and CD38+ cells from BMMCs of an MM patient. BMMCs were stained with PE-conjugated CD38 MoAb and sorting was performed with a FACS Vantage flow cytometer.
Sorting of CD38++ and CD38+ cells from BMMCs of an MM patient. BMMCs were stained with PE-conjugated CD38 MoAb and sorting was performed with a FACS Vantage flow cytometer.
PBMCs were isolated from heparinized blood by Ficoll-Paque (Pharmacia) density gradient centrifugation and suspended in medium as described above.
T cells were isolated from PBMCs incubated on a Petri dish for 30 minutes (to obtain adherent cells) and the remaining monocytes were depleted using iron powder.20 The resulting cell population was incubated with anti-CD19 antibody-coated Dynabeads (M-450; Dynal A.S., Oslo, Norway) at a bead to target cell ratio of 50:1 to deplete B cells. CD3+ T cells in the monocyte-depleted and B-cell–depleted population were greater than 95%. CD14+ monocytes in adherent cells were greater than 60%.
Monoclonal antibodies (MoAbs) and flow cytometry analysis.For the identification of bone marrow plasma cells and their surface antigens, the following MoAbs were used: phycoerythrin (PE)-conjugated anti-CD38 (Leu-17; Becton Dickinson Immunocytometry Systems, Mountain View, CA) and fluorescein isothiocyanate-conjugated anti–HLA-DR, anti–HLA--DQ (Becton Dickinson), HLA-ABC (Chemicon International Inc, Temecula, CA), CD80 (MAB104; Immunotech, Marseille, France), CD86 (2331; Pharmingen, San Diego, CA), CD40 (5C3; Pharmingen), and CD19 (Becton Dickinson). BMMCs were stained with these antibodies and analyzed by a FACScan flow cytometer (Becton Dickinson). CD38++ plasma cells expressing the antigens were determined.
Bone marrow cell culture.For the study of inducible surface antigens on plasma cells, BMMCs were cultured in the absence or presence of 500 U/mL interferon-γ (IFN-γ), 500 U/mL tumor necrosis factor-α (TNF-α), or 10 ng/mL IL-6 (Genzyme Corp, Boston, MA) for 48 hours. After culture, cells were harvested, washed, and analyzed by FACScan for the expression of surface MHC class I and II molecules as well as CD80, CD86, and CD40 antigens on CD38++ plasma cells. Both the percentage of CD38++ plasma cells expressing the antigens and the level of expression of the antigen defined as mean fluorescence intensity (MFI) were determined.
Fluorescence-activated cell sorting (FACS) sorting of BMMCs into CD38++ and CD38+ populations.Cell sorting was performed with BMMCs from seven patients with MM. A total number of 20 to 30 × 106 BMMCs were stained with PE-conjugated anti-CD38 antibody at 4°C for 30 minutes. After staining, cells were washed twice and resuspended in 4 mL of phosphate-buffered saline. Sorting was performed on a FACS Vantage flow cytometer (Becton Dickinson). Cells were collected into tubes containing 1 mL of complete cell culture medium and 5 to 8 × 105 cells were collected into each tube. The cells were sorted into two fractions containing either CD38++ or CD38+ cells, resuspended in complete cell culture medium, and used for functional studies. Contamination of CD14+ monocytes and CD20+ B cells in the CD38++ population was undetectable (<0.1%).
Expression of MHC Class-I and -II and Signaling or Costimulatory Molecules on Noninduced and Cytokine-Induced Bone Marrow CD38++ Plasma Cells
| . | Noninduced . | IFN-γ–Induced . | TNF-α–Induced . | Interleukin-6–Induced . | |||
|---|---|---|---|---|---|---|---|
| . | MFI . | MFI . | Ratio* . | MFI . | Ratio* . | MFI . | Ratio* . |
| HLA-DR | 497 (417-518) | 1,019 (686-1,615) | 2.1 | 840 (320-1,219) | 1.7 | 540 (440-640) | 1.1 |
| HLA-DQ | 300 (270-340) | 716 (560-890) | 2.4 | 490 (402-550) | 1.6 | 334 (302-390) | 1.1 |
| HLA-ABC | 1,444 (1,217-2,124) | 3,719 (2,535-4,835) | 2.6 | 2,998 (1,332-5,001) | 2.1 | 2,077 (1,211-2,944) | 1.4 |
| CD40 | 175 (146-165) | 278 (125-280) | 1.6 | 176 (120-227) | 1.1 | 141 (120-160) | 0.8 |
| CD80 | 135 (107-163) | 286 (114-286) | 2.1 | 273 (92-437) | 2.0 | 142 (112-172) | 1.1 |
| CD86 | 180 (156-209) | 393 (226-407) | 2.2 | 246 (198-279) | 1.4 | 257 (229-285) | 1.4 |
| . | Noninduced . | IFN-γ–Induced . | TNF-α–Induced . | Interleukin-6–Induced . | |||
|---|---|---|---|---|---|---|---|
| . | MFI . | MFI . | Ratio* . | MFI . | Ratio* . | MFI . | Ratio* . |
| HLA-DR | 497 (417-518) | 1,019 (686-1,615) | 2.1 | 840 (320-1,219) | 1.7 | 540 (440-640) | 1.1 |
| HLA-DQ | 300 (270-340) | 716 (560-890) | 2.4 | 490 (402-550) | 1.6 | 334 (302-390) | 1.1 |
| HLA-ABC | 1,444 (1,217-2,124) | 3,719 (2,535-4,835) | 2.6 | 2,998 (1,332-5,001) | 2.1 | 2,077 (1,211-2,944) | 1.4 |
| CD40 | 175 (146-165) | 278 (125-280) | 1.6 | 176 (120-227) | 1.1 | 141 (120-160) | 0.8 |
| CD80 | 135 (107-163) | 286 (114-286) | 2.1 | 273 (92-437) | 2.0 | 142 (112-172) | 1.1 |
| CD86 | 180 (156-209) | 393 (226-407) | 2.2 | 246 (198-279) | 1.4 | 257 (229-285) | 1.4 |
Median and range (in parentheses) of MFI are shown. Data were obtained from experiments using BMMCs from four different MM patients.
Ratio was calculated from the median value of MFI from cytokine-induced cells divided by the median of MFI from noninduced cells.
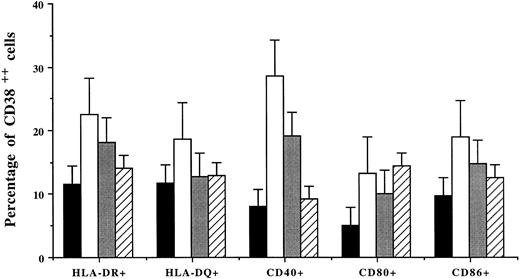
Percentages (mean ± SD of 4 patients) of CD38++ plasma cells expressing HLA-DR and HLA-DQ, CD40, and costimulatory molecules after incubation of BMMCs without (▪) or with the addition of IFN-γ (□), TNF-α (), or IL-6 (▨).
Percentages (mean ± SD of 4 patients) of CD38++ plasma cells expressing HLA-DR and HLA-DQ, CD40, and costimulatory molecules after incubation of BMMCs without (▪) or with the addition of IFN-γ (□), TNF-α (), or IL-6 (▨).
Cytoplasmic light chain staining.A total number of 1 × 105 cells from each sorted population was cytocentrifuged for 5 minutes at 450 rpm on slides and fixed with 70% acetone at −20°C for 10 minutes. After fixation, slides were washed in phosphate-buffered saline, incubated for 30 minutes with alkaline phosphatase-conjugated rabbit anti-κ or anti-λ MoAb (Sigma, St Louis, MO), and stained with substrate (fast red; Vector Laboratories, Burlingame, CA).
Allogeneic MLR.To test for T-cell stimulatory effect, CD38++, CD38+, unfractionated BMMCs or PBMCs were irradiated with 30 Gy from a 137Cs source and added in graded doses as stimulators for 1 × 105 allogeneic adult PBMC in 96-well round-bottomed microtitre plates (Nunc U96; Nunclon, Roskilde, Denmark). Proliferation was determined on day 6 with the addition of 1 μCi/well of 3H-thymidine (Amersham Life Science, Aylesbury, UK) 18 hours before harvest. The results are expressed as the mean counts per minute (cpm) of triplicate values.
Antigen-presentation assay.To test if sorted plasma cells were able to present the soluble antigens PPD and TT, 1 × 105 T cells were cultured with different numbers of autologous adherent cells (monocytes) or irradiated CD38++, CD38+, and BMMCs in the absence or presence of PPD (2.5 μg/mL) or TT (50 ng/mL). Antigen-induced T-cell stimulation was determined on day 3 by 3H-thymidine incorporation assay and was determined on day 2 by enumerating the number of IFN-γ–secreting cells using an enzyme-linked immunospot (ELISPOT) assay.21 22 The results are expressed as total number of IFN-γ–secreting cells/105 T cells.
MHC restriction.To study MHC molecules involved in allogeneic MLR and autologous antigen presentation, mouse MoAb against HLA-DR (IgG2b; Immunotech), HLA-ABC (IgG2b; Chemicon), and a mouse isotypic control IgG2b (Immunotech) were used. The MoAb or control IgG was added to cell cultures at a final concentration of 1 μg/mL for the whole incubation period.21 Results are expressed as the percentage of inhibition compared with cultures without the addition of anti-MHC MoAb or control IgG.
Chloroquine treatment.A lysosomotropic agent (chloroquine; Sigma) was used to inhibit antigen processing and presentation.23 The effect of the drug was evaluated by adding it, at a final concentration of 50 μmol/L, to cultures containing 1 × 105 CD38++ or CD38+ cells and 1 × 105 purified autologous T cells with the addition of PPD or concanavalin A (Con A; 20 μg/mL). Alternatively, CD38++ or CD38+ cells were incubated with or without PPD in the absence or presence of chloroquine (200 μmol/L) for 4 hours at 37°C and then used as antigen-presenting cells.
RESULTS
Morphology and cytoplasmic light chain of CD38++ and CD38+ cells.BMMCs from MM patients were sorted into CD38++ and CD38+ cells (Fig 1). Staining with May-Grünewald-Giemsa showed that 99% of CD38++ cells had plasma cell cytology, whereas CD38+ population contained plasma cells (∼10%), lymphocytes, plasmablasts, and other types of bone marrow cells. Because cytoplasmic Ig is a typical feature of plasma cells, staining of the sorted cells with antibodies against human light chains was performed on slides. CD38++ plasma and 10% of CD38+ cells were stained with antibody against the same light chain as the individual patient's monoclonal Ig (data not shown).
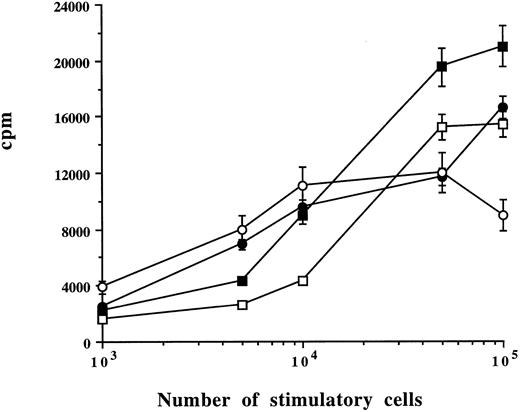
Capacity of myeloma plasma cells to stimulate alloreactive T cells (MLR). Allogeneic PBMCs were cultured with different numbers of CD38++ plasma cells (▪), CD38+ cells (□), BMMCs (•), or PBMCs (○) from one patient. Means ± SD were determined on triplicate values from two experiments. Cell proliferation was measured on day 6. Concanavalin A-induced cell activation in allogeneic PBMCs was 22,305 cpm.
Capacity of myeloma plasma cells to stimulate alloreactive T cells (MLR). Allogeneic PBMCs were cultured with different numbers of CD38++ plasma cells (▪), CD38+ cells (□), BMMCs (•), or PBMCs (○) from one patient. Means ± SD were determined on triplicate values from two experiments. Cell proliferation was measured on day 6. Concanavalin A-induced cell activation in allogeneic PBMCs was 22,305 cpm.
Induction of T-Cell Stimulation by Bone Marrow CD38++ Plasma Cells, CD38+ Cells, and Antigen-Presenting Cells
| APC . | MLR (cpm) . | Ag-Induced Cell Proliferation (cpm) . | No. of Ag-Induced IFN-γ–Secreting Cells . | |||||
|---|---|---|---|---|---|---|---|---|
| . | APC Only . | APC + AlloPBMCs . | No Ag . | PPD . | TT . | No Ag . | PPD . | TT . |
| CD38++ | 462 (304-786) | 14,753 (4,370-35,670) | 396 (313-554) | 1,770 (944-2,391) | 1,524 (1,314-2,140) | 5 (4-8) | 39 (26-40) | 33 (22-39) |
| CD38+ | 459 (242-870) | 10,451 (7,511-34,307) | 477 (367-898) | 1,527 (917-2,739) | 2,075 (2,007-2,122) | 6 (4-12) | 33 (17-43) | 25 (15-35) |
| BMMCs | 542 (260-623) | 11,600 (6,344-40,151) | 504 (359-980) | 1,570 (596-3,573) | 1,893 (688-2,391) | 5 (4-9) | 30 (25-37) | 22 (18-30) |
| PBMCs | 482 (170-526) | 11,989 (7,001-31,474) | ND | ND | ND | ND | ND | ND |
| Mø | ND | ND | 418 (376-772) | 1,455 (697-1,883) | 1,058 (1,010-1,485) | 6 (3-7) | 28 (17-43) | 27 (27-33) |
| No APCs | 422 (302-1,373)3-150 | 431 (275-732)3-151 | 537 (283-950)3-151 | 659 (560-680)3-151 | 5 (3-12)3-151 | 6 (4-14)3-151 | 7 (5-12)3-151 | |
| APC . | MLR (cpm) . | Ag-Induced Cell Proliferation (cpm) . | No. of Ag-Induced IFN-γ–Secreting Cells . | |||||
|---|---|---|---|---|---|---|---|---|
| . | APC Only . | APC + AlloPBMCs . | No Ag . | PPD . | TT . | No Ag . | PPD . | TT . |
| CD38++ | 462 (304-786) | 14,753 (4,370-35,670) | 396 (313-554) | 1,770 (944-2,391) | 1,524 (1,314-2,140) | 5 (4-8) | 39 (26-40) | 33 (22-39) |
| CD38+ | 459 (242-870) | 10,451 (7,511-34,307) | 477 (367-898) | 1,527 (917-2,739) | 2,075 (2,007-2,122) | 6 (4-12) | 33 (17-43) | 25 (15-35) |
| BMMCs | 542 (260-623) | 11,600 (6,344-40,151) | 504 (359-980) | 1,570 (596-3,573) | 1,893 (688-2,391) | 5 (4-9) | 30 (25-37) | 22 (18-30) |
| PBMCs | 482 (170-526) | 11,989 (7,001-31,474) | ND | ND | ND | ND | ND | ND |
| Mø | ND | ND | 418 (376-772) | 1,455 (697-1,883) | 1,058 (1,010-1,485) | 6 (3-7) | 28 (17-43) | 27 (27-33) |
| No APCs | 422 (302-1,373)3-150 | 431 (275-732)3-151 | 537 (283-950)3-151 | 659 (560-680)3-151 | 5 (3-12)3-151 | 6 (4-14)3-151 | 7 (5-12)3-151 | |
Median and range (in parentheses) are shown. The results were obtained from experiments using an optimal amount of antigen-presenting cells from five MM patients.
Abbreviations: Ag, antigen; APCs, antigen-presenting cells; AlloPBMCs, allogeneic PBMCs; ND, not done; Mø, monocytes.
Data obtained from cultures with allogeneic PBMCs only.
Data obtained from cultures with purified T cells only.
Phenotype of CD38++ plasma cells.A gate on CD38++ plasma cells was set and used to analysis their expression of other surface molecules. The percentages of bone marrow CD38++ plasma cells expressing MHC and costimulatory molecules varied between patients (Table 1). CD19+ plasma cells were very few, which is in accord with previous observation.7 24
Modulation of surface antigens by IFN-γ, TNF-α, and IL-6.BMMCs from four MM patients were cultured for 48 hours with or without the addition of IFN-γ, TNF-α, or IL-6. Noninduced bone marrow CD38++ plasma cells express high level of HLA-ABC, and a small percentage of the plasma cells expressed low but detectable level of HLA-DR and HLA-DQ and signaling or costimulatory antigens CD40, CD80, and CD86 (Table 2). After culture with IFN-γ for 48 hours, a greater than twofold increase in MFI of all the antigens, except CD40 (1.6-fold), was noted on CD38++ plasma cells. TNF-α, to a lesser extent, had similar effects (1.4- to 2.1-fold enhancement). The expression of HLA-ABC and CD86, but not other antigens, was slightly upregulated by IL-6 (Table 2).
Coculture of BMMCs with cytokines also increased the percentage of CD38++ plasma cells expressing MHC and costimulatory molecules (Fig 2). Among the cytokines, IFN-γ was most efficient: it induced an increased population of CD38++ plasma cells expressing HLA-DR (11.6% to 22.6%; 2.0-fold increase), HLA-DQ (11.8% to 18.7%; 1.6-fold), CD40 (8% to 28.6%; 3.6-fold), CD80 (5.1% to 13.3%; 2.6-fold), and CD86 (9.8% to 19.0%; 1.9-fold). TNF-α also had similar effects and induced 1.5- to 2.4-fold increases in cells expressing HLA-DR or costimulatory molecules. IL-6 induced a 2.8-fold increase in CD80+CD38++ plasma cells but had no effect on other cell populations (Fig 2).
Stimulatory capacity of CD38++ plasma cells and CD38+ cells (MLR).Sorted CD38++ plasma cells and CD38+ cells were compared with BMMCs and PBMCs for their capacity to stimulate alloreactive T cells. Different numbers of plasma cells, BMMCs, or PBMCs from the same patient were cultured with 1 × 105 of allogeneic PBMCs. As exemplified by the experiments in Fig 3, a dose-dependent response curve was obtained. A total of 1 × 105 CD38++ plasma cells, CD38+ cells, and BMMCs and 0.5 × 105 PBMCs induced a maximal response. Cultures with stimulatory cells or allogeneic PBMCs alone had a very low background count (data not shown). It is interesting to note that CD38++ plasma cells induced a stronger response than other cells (Fig 3 and Table 3). Similar results were reproduced with cells from the other four MM patients. The results obtained with the optimal number of stimulatory cells (105 CD38++ plasma cells, CD38+ cells, and BMMCs and 0.5 × 105 PBMCs) and control experiments from a total of five patients are summarized and shown in Table 3.
Presentation of the soluble antigens PPD and TT to autologous T cells.To evaluate the capacity of sorted plasma cells to present the soluble antigens PPD and TT, we compared CD38++ plasma cells, CD38+ cells, BMMCs, and monocytes (adherent cells) for their capacity to present the antigens to purified autologous T cells. Different numbers (1 × 103 to 1 × 105) of the putative antigen-presenting cells were cultured with 105 autologous T cells in the absence or presence of PPD or TT. Antigen-induced T-cell stimulation was measured by 3H-thymidine incorporation assay and by the ELISPOT assay to enumerate IFN-γ–secreting cells.21 22
As exemplified by the experiments in Fig 4, sorted CD38++ plasma cells, CD38+ cells, and unfractionated BMMCs could present PPD to T cells and induce T-cell stimulation in the similar way as monocytes. However, the number of antigen-presenting cells needed to induce the maximal proliferative response varied with the type of cells: 1 × 104 monocytes were sufficient, which is in line with our previous observation,20 whereas 1 × 105 of CD38++ plasma cells, CD38+ cells, or BMMCs were not yet sufficient to induce the maximal response (Fig 4A). This is also true when soluble antigen TT was used (data not shown). Similar results also were obtained by enumerating antigen-induced IFN-γ–secreting cells (Fig 4B). The T-cell stimulation was clearly antigen-dependent, because coculture of T cells with sorted plasma cells, BMMCs, or monocytes without the antigens yielded the same low counts (cpm or number of IFN-γ–secreting cells) as cultures with antigen-presenting cells or T cells alone (data not shown). We have repeated these experiments with sorted cells from the other four MM patients, and the results were reproducible. Table 3 shows the summarized data from the five patients obtained with the optimal number of antigen-presenting cells (1 × 105 CD38++, CD38+, and BMMCs and 1 × 104 monocytes) and control experiments.
Capacity of myeloma plasma cells to present antigen to T cells and induce T-cell stimulation. Different numbers of CD38++ plasma cells (▪), CD38+ cells (□), BMMCs (•), or monocytes (○) from one patient were cultured in the presence of PPD with autologous purified T cells. PPD-induced cell proliferation (A) or IFN-γ–secreting cells (B) were measured. Means ± SD were determined on triplicate values from two experiments.
Capacity of myeloma plasma cells to present antigen to T cells and induce T-cell stimulation. Different numbers of CD38++ plasma cells (▪), CD38+ cells (□), BMMCs (•), or monocytes (○) from one patient were cultured in the presence of PPD with autologous purified T cells. PPD-induced cell proliferation (A) or IFN-γ–secreting cells (B) were measured. Means ± SD were determined on triplicate values from two experiments.
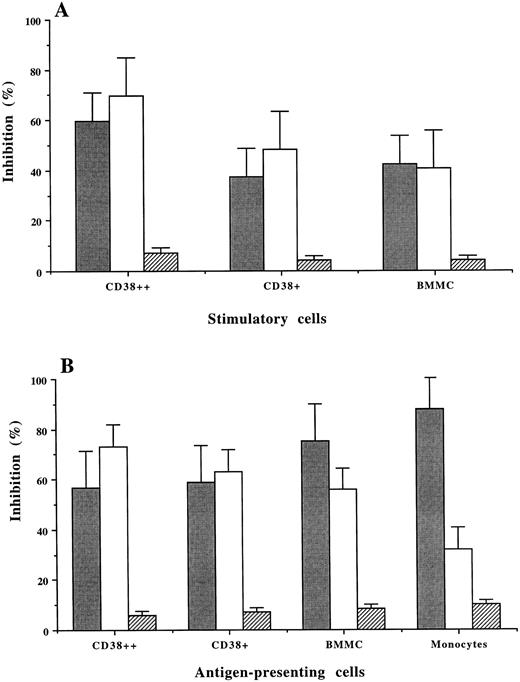
MHC restriction in MLR and antigen presentation by the plasma cells.Mouse MoAb against human MHC class I (HLA-ABC) or class II (HLA-DR) were used to inhibit allogeneic MLR and PPD-induced T-cell stimulation. Although both MoAb showed inhibitory effects, MoAb against HLA-ABC resulted in a higher percentage of inhibition than MoAb against HLA-DR on CD38++ and CD38+ cell-induced allogeneic (MLR; Fig 5A) and autologous T-cell stimulation (PPD-induced; Fig 5B). When BMMCs and, especially, monocytes were used as the antigen-presenting cells, PPD-induced T-cell stimulation was inhibited predominantly by anti-HLA-DR antibody (75% and 85%, respectively), which is consistent with our previous observation.20 The unspecific inhibition by mouse isotypic control IgG was 4% to 10%.
MHC restriction in allogeneic MLR (A) and PPD-induced T-cell stimulation (B) using CD38++ plasma cells, CD38+ cells, BMMCs, and monocytes as antigen-presenting cells. The percentage (mean ± SD of 3 experiments) of inhibition induced by MoAb against HLA-DR (), HLA-ABC (□), and a mouse control IgG (▨) is shown.
MHC restriction in allogeneic MLR (A) and PPD-induced T-cell stimulation (B) using CD38++ plasma cells, CD38+ cells, BMMCs, and monocytes as antigen-presenting cells. The percentage (mean ± SD of 3 experiments) of inhibition induced by MoAb against HLA-DR (), HLA-ABC (□), and a mouse control IgG (▨) is shown.
Effect of chloroquine.PPD-induced T-cell stimulation was totally abolished by the presence of chloroquine in the culture (Table 4), whereas cell activation induced by Con A was not affected (data not shown). An inhibitory effect was also observed when the drug was present during the pulse of CD38++ with PPD or when chloroquine-pretreated cells were used as antigen-presenting cells (Table 4). Similar results were obtained with CD38+ cells (data not shown).
Effect of Chloroquine on Antigen Processing and Presentation by CD38++ Plasma Cells
| Experiments . | Chloroquine . | No Antigen . | PPD . | Inhibition (%) . |
|---|---|---|---|---|
| Presence in cultures | − | 320 ± 110 | 2,320 ± 430 | |
| + | 298 ± 122 | 302 ± 116 | 100 | |
| During pulse of plasma cells with PPD | − | 240 ± 88 | 1,504 ± 340 | |
| + | 280 ± 110 | 380 ± 122 | 92 | |
| Pretreatment of plasma cells | − | 250 ± 98 | 1,882 ± 240 | |
| + | 220 ± 112 | 579 ± 125 | 78 |
| Experiments . | Chloroquine . | No Antigen . | PPD . | Inhibition (%) . |
|---|---|---|---|---|
| Presence in cultures | − | 320 ± 110 | 2,320 ± 430 | |
| + | 298 ± 122 | 302 ± 116 | 100 | |
| During pulse of plasma cells with PPD | − | 240 ± 88 | 1,504 ± 340 | |
| + | 280 ± 110 | 380 ± 122 | 92 | |
| Pretreatment of plasma cells | − | 250 ± 98 | 1,882 ± 240 | |
| + | 220 ± 112 | 579 ± 125 | 78 |
PPD-induced T-cell proliferation was measured on day 6. Means ± SD were determined on triplicate values of two experiments.
DISCUSSION
This study describes the capacity of freshly isolated bone marrow plasma cells from patients with MM to act as antigen-presenting cells. First, we analyzed the MHC (HLA-DR, HLA-DQ, and HLA-ABC) and signaling or costimulatory molecules (CD40, CD80, and CD86) on CD38++ plasma cells and found that these molecules could be upregulated by cytokines such as IFN-γ and TNF-α. Second, we showed that sorted CD38++ plasma cells and CD38+ cells were able to induce a strong allogeneic T-cell stimulation and to present PPD and TT to autologous T cells. Thus, our study provides evidence that myeloma plasma cells can act as fully functional antigen-presenting cells in vitro. Whether the cytokine-induced upregulation of the expression of the molecules on plasma cells may affect their capacity to stimulate alloreactive T cells and present antigens to T cells are currently under investigation.
Generally, stimulated macrophages, activated B cells, and dendritic cells are the professional antigen-presenting cells. They are required for the initiation of antigen-specific reactions, because they express high levels of MHC molecules and adhesion and costimulatory antigens (reviewed in Germain25 and Thompson26 ). Antigen presentation to CD4+ and CD8+ T cells depends on the constitutive or induced expression of MHC class II or I, and triggering of T-cell stimulation requires the presence of costimulatory antigens on antigen-presenting cell surface.25,26 The most important costimulatory molecules identified so far are CD80 (B7-1) and CD86 (B7-2). CD80 is expressed by the professional antigen-presenting cells mentioned above, whereas CD86 is expressed by unstimulated antigen-presenting cells (resting B cells and monocytes or macrophages).27-29
There is increasing evidence that cells not normally capable of antigen presentation may acquire this function after induction by inflammatory cytokines such as IFN-γ.30,31 Myeloma plasma cells may belong to this category. First, myeloma plasma cells express MHC class I and some if not all HLA-DR and HLA-DQ (Duperray et al,5 Elsässer et al,6 and the present study), as well as adhesion molecules such as CD44, CD56, CD54, and VLA-4.7-9 Second, myeloma cells may also express costimulatory molecules. It is evident from these studies that most of the myeloma-derived cell lines were CD80+.12,13 A weak expression of CD80 was detected on freshly isolated myeloma plasma cells from 9% of MM patients,13 which is in line with our results. In addition, the present study also showed that 12% of CD38++ plasma cells expressed CD86. Third, we have shown that the expression of MHC and the costimulatory molecules as well as the percentage of the positive cells could be upregulated by IFN-γ and TNF-α. Hence, myeloma plasma cells, similar to resting B cells and unstimulated monocytes or macrophages, may fulfill the phenotypic requirements as antigen-presenting cells.
Myeloma plasma cells also behaved functionally as antigen-presenting cells in vitro. Sorted CD38++ plasma cells were able to induce a strong allogeneic T-cell stimulation, as compared with CD38+ cells, BMMCs, and PBMCs from the same patient. They presented the recall antigens PPD and TT to autologous T cells and induced T-cell stimulation, which was abolished by the treatment of CD38++ or CD38+ cells with a lysosomotropic agent (chloroquine). It was evident that MHC molecules, especially class-I antigens, were involved in these responses, because MoAb against HLA-ABC had higher inhibitory effects than antibody against HLA-DR. This is different from the results obtained using monocytes or macrophages to present PPD to autologous T cells, which was mainly MHC class-II restricted and led to stimulation of CD4+ T cells.20 It can be explained by the high level expression of class-I, but not class-II, antigens on all the plasma cells. The costimulatory molecules should also be involved in these T-cell responses. Nevertheless, studies suggest that the activation of naive T cells is critically dependent on costimulatory signals, whereas memory T cells are less dependent on them for activation.26 32 Considering that the plasma cells stimulated allogeneic adult, not cord, T cells and presented antigens to antigen-primed or memory T cells (secondary responses), the low expression of CD80 on the cells is not conflicting with the ability of plasma cells to do so in the present study. On the other hand, CD86 was present on 12% of plasma cells, and both CD80 and CD86 expression could be upregulated by cytokines. Thus, it is reasonable that the plasma cells may be able to provide necessary costimulatory signals to the T cells.
This study indicates that myeloma plasma cells have the capacity to act as antigen-presenting cells and can thus be recognized by T cells in vitro. It also suggests that T cells, especially tumor-specific T cells, may play a role in regulation of the growth and differentiation of myeloma cells in vivo. Idiotypes present on the monoclonal Ig have been considered as the best defined tumor-specific antigen in MM. Thus, idiotype-specific T cells that have been shown in the patients20-22 are one, if not the only, type of tumor-specific T cells that may interact with the myeloma plasma cells. If idiotype-specific T cells are of the CD8+ cytotoxic cells, as we have shown occasionally in some of MM patients, the interaction between them may lead to killing of such antigen-presenting cells.33-35 However, some questions remain. Does such interaction and killing take place in vivo? If they do, why is there still a large number of tumor cells in the patients? One of the possible explanations is that the tumor cells do not express sufficient costimulatory molecules on the surface and, as a result, are not able to prime antitumor immunity, especially the cytotoxic T-cell response. Instead, T-cell tolerance may be induced.36 The other possibilities may include that the function of T cells is deficient, such as loss or downregulation of T-cell receptor ζ chain that have been found in other tumor systems,37,38 as well as the presence of tumor-specific type-II T cells22 that may suppress the function of type-I cells by cytokines such as interleukin-10.39 Nevertheless, the understanding of the antigen-presentation capacity of myeloma plasma cells may help to develop tumor-specific immunotherapy in MM.
ACKNOWLEDGMENT
The authors thank Ingrid Eriksson, Wen He, and Doina Anton for the skillful laboratory assistance; Marcello Toro for sorting of the cells; Dr Anja Porwit for help in the morphologic study; and Dr Fredrik Celsing for providing patients' samples.
Supported by grants from the Swedish Cancer Society, the Cancer Society in Stockholm, the Swedish Society of Medicine, and the Karolinska Institute Foundations.
Address reprint requests to Qing Yi, MD, PhD, Immunological Research Laboratory, Department of Medicine, Karolinska Hospital, S-171 76 Stockholm, Sweden.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal