Abstract
Dendritic cells are attractive candidates for vaccine-based immunotherapy because of their potential to function as natural adjuvants for poorly immunogenic proteins derived from tumors or microbes. In this study, we evaluated the feasibility and consequences of introducing foreign genetic material by retroviral vectors into dendritic cell progenitors. Proliferating human bone marrow and cord blood CD34+ cells were infected by retroviral vectors encoding the murine CD2 surface antigen. Mean transduction efficiency in dendritic cells was 11.5% from bone marrow and 21.2% from cord blood progenitors. Transduced or untransduced dendritic cell progeny expressed comparable levels of HLA-DR, CD83, CD1a, CD80, CD86, S100, and p55 antigens. Granulocytes, macrophages, and dendritic cells were equally represented among the transduced and mock-transduced cells, thus showing no apparent alteration in the differentiation of transduced CD34+ precursors. The T-cell stimulatory capacity of retrovirally modified and purified mCD2-positive allogeneic or nominal antigen-pulsed autologous dendritic cells was comparable with that of untransduced dendritic cells. Human CD34+ dendritic cell progenitors can therefore be efficiently transduced using retroviral vectors and can differentiate into potent immunostimulatory dendritic cells without compromising their T-cell stimulatory capacity or the expression of critical costimulatory molecules and phenotypic markers. These results support ongoing efforts to develop genetically modified dendritic cells for immunotherapy.
IMMUNIZATION requires the coupled introduction of antigen with adjuvant to attain an optimal inflammatory reaction. Dendritic cells are the most potent naturally occurring T-cell stimulating accessory cells and have been termed nature's adjuvant.1 When loaded with antigens that are accessible to class I or class II major histocompatibility complex (MHC) molecules, dendritic cells can prime resting or naı̈ve T cells and generate memory T-cell responses in vitro and in vivo without additional exogenous adjuvant.2-7
Dendritic cells are bone marrow-derived leukocytes8-10 that express high levels of antigen-presenting major histocompatibility complex products (class I and II MHC) as well as accessory molecules that mediate T-cell binding and costimulation.11-13 Dendritic cells develop from CD34+ progenitors in cord blood,14,15 bone marrow,16-18 and cytokine mobilized peripheral blood.19 Human dendritic cells develop along a nonlymphoid or myeloid pathway supported by granulocyte-macrophage colony-stimulating factor (GM-CSF ),14-18 and at least some dendritic cells share a downstream bipotential intermediate precursor with macrophages.20 21
The induction of antigen-specific T-cell responses by antigen-pulsed dendritic cells has provided support for the potential use of dendritic cells in clinical immunotherapy.3-7,22,23 In certain models, the immune responses induced regressions of established small tumors.6,24 Tumor regression has also been observed after vaccination with dendritic cells pulsed with lymphoma-specific idiotypes.25
Immunization protocols have used either synthetic peptides or intact antigens such as whole virus or tumor cell extracts to pulse dendritic cells exogenously. Generation of MHC class I-restricted CD8+ cytotoxic T lymphocytes (CTLs) by synthetic CTL epitopes available for widespread clinical use may be limited by the availability of known immunogenic peptide sequences. Unfractionated acid-eluted tumor peptides or extracted tumor mRNA may bypass the need for peptide identification.6 23 However, this approach carries a potentially greater risk of eliciting T-cell responses against several normal tissue antigens.
Another strategy is based on the genetic modification of antigen-presenting cells (APCs) to establish long-term expression of a cDNA that encodes an immunogenic antigen. These may provide cytoplasmic degradation products, some of which could serve as CTL epitopes. Such genetically modified dendritic cells may generate multiple CTL epitopes available for endogenous processing and presentation by the transduced cells' own MHC molecules. In this study, we report that both bone marrow- and cord blood-derived dendritic cells can in fact be transduced by replication-defective retroviral vectors. We also present phenotypic and functional analyses of genetically modified dendritic cells showing that retroviral transduction of dendritic cells does not alter their specialized accessory properties.
MATERIALS AND METHODS
Isolation of human CD34+ bone marrow and cord blood progenitors. CD34+ cells were isolated by positive selection using an immunomagnetic bead system17 from normal bone marrow and cord blood, according to Institutional Review Board-approved protocols.
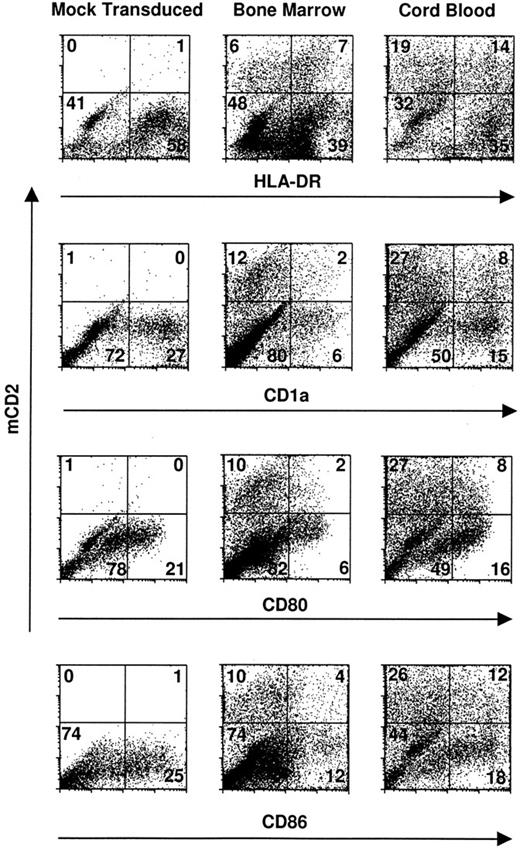
Transduced dendritic cells express critical costimulatory molecules and surface markers. CD34+ bone marrow and cord blood progenitors were cultured in complete IMDM-20% FCS with c-kit-ligand, GM-CSF, and TNFα. Proliferating cells were transduced on day 3 of culture by cocultivation for 24 hours and were recultured in original media with fresh cytokines for an additional 8 to 10 days. Two-color cytofluorographic evaluation was performed with FITC-conjugated rat antimurine CD2 and the indicated PE-conjugated murine antihuman MoAbs. Mock-transduced cells were cocultivated with an empty packaging cell line and are represented here by those derived from cord blood CD34+ cells. The percentage of total gated events is indicated in each quadrant.
Transduced dendritic cells express critical costimulatory molecules and surface markers. CD34+ bone marrow and cord blood progenitors were cultured in complete IMDM-20% FCS with c-kit-ligand, GM-CSF, and TNFα. Proliferating cells were transduced on day 3 of culture by cocultivation for 24 hours and were recultured in original media with fresh cytokines for an additional 8 to 10 days. Two-color cytofluorographic evaluation was performed with FITC-conjugated rat antimurine CD2 and the indicated PE-conjugated murine antihuman MoAbs. Mock-transduced cells were cocultivated with an empty packaging cell line and are represented here by those derived from cord blood CD34+ cells. The percentage of total gated events is indicated in each quadrant.
Culture media and cytokines. CD34+ bone marrow or cord blood progenitors were cultured in complete Iscove's modified Dulbecco's medium (IMDM)-20% fetal calf serum (FCS) as previously reported17,20 in the presence of exogenous cytokines as indicated. The recombinant human cytokines were c-kit-ligand (KL; or stem cell factor [SCF ]; R&D Systems, Minneapolis, MN) at 20 ng/mL, GM-CSF (Immunex Corp, Seattle, WA) at 1,000 IU/mL, tumor necrosis factor α (TNFα; R&D Systems) at 5 ng/mL to 10 ng/mL, and flt-3 ligand (R&D Systems and Immunex Corp) at 100 ng/mL, respectively. Functional assays for evaluating the genetically modified progeny used complete RPMI 1640-10% heat-inactivated pooled human serum, as previously reported.17,18 20
Retroviral construct. The amphotropic producer cell line encoding murine CD226 was derived from the ψ-Crip packaging cell line.27 In this vector, the wild-type murine CD2 molecule is under the transcriptional control of the Moloney murine leukemia virus long terminal repeat.26,28 Viral titration was performed as described on NIH 3T3 and A549 cells,29 30 yielding a titer of 1.6 × 105 and 1.1 × 105 infectious particles/mL, respectively.
Retroviral transduction of dendritic cell progenitors. CD34+ cells were cultured in complete IMDM-20% FCS at an initial concentration of 2 × 105 cells/5 mL in 35-mm tissue culture wells (#25810-6; Corning Costar Corp, Cambridge, MA) supplemented with c-kit-ligand, GM-CSF, and TNFα, with or without flt-3 ligand. The expanding cells were harvested on day 3 of culture and resuspended in 2 mL/well of 2× fresh cytokine-replenished medium. The cells from each original well were transferred to a single corresponding 35-mm tissue culture well that had been seeded 6 hours earlier with the ψ-Crip packaging cell line (3,000 rad 137Cs) that secretes the murine CD2 retroviral construct (final volume, 4 mL). The cells were cocultured for 24 hours in the presence of polybrene (Sigma, St Louis, MO) at 5 μg/mL.
Mean Fluorescence Index of Surface Epitopes Compared Between mCD2-Tranduced and Mock-Transduced Dendritic Cells
| . | Isotype Control . | HLA-DR . | CD1a . | CD80 . | CD86 . |
|---|---|---|---|---|---|
| BM mCD2+ | 9 | 1,155 | 894 | 470 | 394 |
| BM mCD2− | 9 | 1,125 | 915 | 450 | 346 |
| CB mCD2+ | 10 | 2,805 | 2,451 | 553 | 408 |
| CB mCD2− | 10 | 3,037 | 1,939 | 473 | 370 |
| . | Isotype Control . | HLA-DR . | CD1a . | CD80 . | CD86 . |
|---|---|---|---|---|---|
| BM mCD2+ | 9 | 1,155 | 894 | 470 | 394 |
| BM mCD2− | 9 | 1,125 | 915 | 450 | 346 |
| CB mCD2+ | 10 | 2,805 | 2,451 | 553 | 408 |
| CB mCD2− | 10 | 3,037 | 1,939 | 473 | 370 |
Bone marrow (BM) and cord blood (CB) CD34+ cells were cultured in parallel and expanded in IMDM supplemented with 20% FCS, c-kit-ligand, fit-3 ligand, GM-CSF, and TNFα for 12 days. Retroviral transduction was performed during day 3 of CD34+ expansion by coculture with mCD2 encoding ψ-Crip retroviral producers. Cytofluorographic analysis of day-12 nonadherent progeny was performed by dual staining with FITC-conjugated rat antimouse-CD2 and the indicated PE-conjugated test antibodies. All events in both FITC (FL1) and PE (FL2) channels were recorded and analyzed for log10 fluorescence. The mean fluorescence index (MFI) in the PE channel was calculated by the Consort 30 software (Hewlett Packard) after quadrants were set according to background staining by isotype controls.
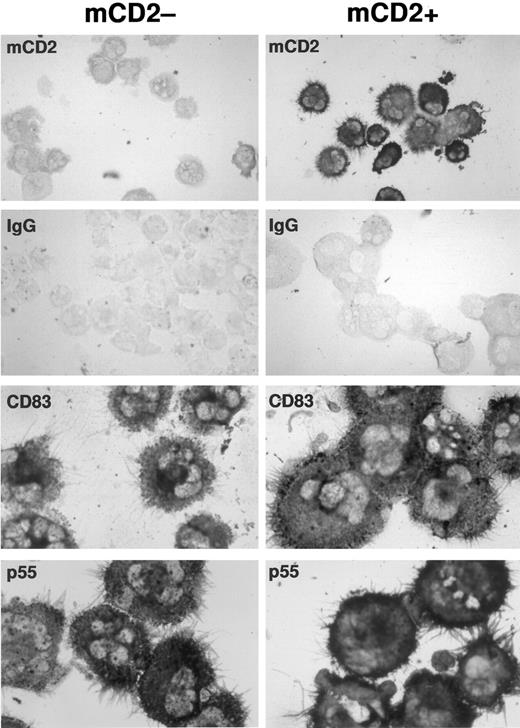
Transduced and mock-transduced CD34+ progenitors differentiate into dendritic cells expressing typical morphology and dendritic cell markers CD83 and p55. CD34+ cord blood progenitors were cultured in complete IMDM-20% FCS with c-kit-ligand, GM-CSF, and TNFα and transduced on day 3 of culture. Cells were recultured after transduction in original media with replenished cytokines for an additional 8 to 10 days. Washed cells were cytofluorographically sorted into two populations based on the presence or absence of surface mCD2 and were then processed for immunostaining. To enrich for dendritic cells only, plastic nonadherent cells showing high forward and side scatter properties were collected. Expression of murine CD2, p55, and CD83 was detected by immunocytochemistry on cytocentrifuged, acetone-fixed cells using a standard alkaline phosphatase-based staining method. The mock-transduced population served as its own control for murine CD2 staining in the transduced population. An IgG isotype control for CD83 and p55 staining is illustrated for each of the mock-transduced and transduced populations. CD40 and S100 staining were also comparable in each population (not shown).
Transduced and mock-transduced CD34+ progenitors differentiate into dendritic cells expressing typical morphology and dendritic cell markers CD83 and p55. CD34+ cord blood progenitors were cultured in complete IMDM-20% FCS with c-kit-ligand, GM-CSF, and TNFα and transduced on day 3 of culture. Cells were recultured after transduction in original media with replenished cytokines for an additional 8 to 10 days. Washed cells were cytofluorographically sorted into two populations based on the presence or absence of surface mCD2 and were then processed for immunostaining. To enrich for dendritic cells only, plastic nonadherent cells showing high forward and side scatter properties were collected. Expression of murine CD2, p55, and CD83 was detected by immunocytochemistry on cytocentrifuged, acetone-fixed cells using a standard alkaline phosphatase-based staining method. The mock-transduced population served as its own control for murine CD2 staining in the transduced population. An IgG isotype control for CD83 and p55 staining is illustrated for each of the mock-transduced and transduced populations. CD40 and S100 staining were also comparable in each population (not shown).
Coculture was terminated upon removal of target cells by gentle swirling and pipetting to avoid detachment of the viral packaging cell line. The target cells were recultured in fresh cytokine-replenished medium. If plastic adherent packaging cells were noted in the transferred cultures, cell transfer was repeated on the following day. Mock-transduced control cells were handled in an identical manner, apart from coculture with an empty ψ-Crip packaging cell line.
All of these prestimulation, coculture, and reculture conditions were based on pilot experiments in which retroviral transduction efficiency was optimized. Cultures were split and replenished with fresh cytokines only if cells reached confluency or the medium became acidic.
Retroviral transduction efficiency was assessed 7 to 10 days after the termination of coculture or 10 to 13 days after the initiation of CD34+ cultures in cytokines. All cultures were maintained at 37°C in humidified 5% CO2 .
Monoclonal antibodies (MoAbs), cell phenotype analysis, and cytochemical staining. Fluorescein isothiocyanate (FITC)-conjugated rat antimurine CD2 (Pharmingen, San Diego, CA) was used to assess expression of the transfected murine cDNA. The following murine antihuman MoAbs were used: HLA-DR, CD80 (both from Becton Dickinson [BD], San Jose, CA); CD86 (Pharmingen); CD1a (Coulter Immunology, Miami, FL); and CD83 (Coulter). The MoAbs were phycoerythrin (PE)-conjugated, or PE-conjugated F(ab)′2 goat anti mouse IgG (TAGO, Burlingame, CA) was used as a second-step reagent for indirect staining. PerCP-conjugated anti-HLA-DR (BD) was used for three-color cytofluorographic evaluations. Stained cells were analyzed by cytofluorography on a FACScan (BD) and were compared with background staining by isotype controls. Expression of S100 (Dako, Carpinteria, CA), CD40 (Serotec, Harlan Bioproducts for Science, Inc, Indianapolis, IN), p5531 (generous gift of Dr Erik Langhoff, Boston, MA), and CD83 were detected by immunocytochemistry on sorted cytocentrifuged preparations using a standard alkaline phosphatase-based staining method (Vector Blue; Vector Laboratories, Burlingame, CA).
Peripheral blood mononuclear cells (PBMC) and enrichment of blood dendritic cells, monocytes, and T lymphocytes. Mononuclear cells were prepared from buffy coats according to previously published procedures to obtain primary leukocyte subpopulations of T cells, macrophages, and dendritic cells.12 32-34
Allogeneic mixed leukocyte reaction (MLR) and autologous T-cell proliferation assays. APCs (blood dendritic cells, blood macrophages, and marrow-derived CD34+ progeny) were added in graded doses to triplicate wells, each containing 1 × 105 allogeneic or autologous T cells in round-bottomed, 96-microwell tissue culture plates. APCs expanded from CD34+ precursors were thoroughly washed free of cytokines before addition to T cells and were tested either as a bulk transduced population or after cytofluorographic sorting (FACStar Plus; BD) of transduced (mCD2+) or mock-transduced (mCD2−) dendritic cells. Candidate dendritic cells were identified either by positive staining for CD1a and/or by gating cells with high forward and side scatter after depletion of plastic adherent macrophages. APCs were irradiated with 1,500 rad [137Cs] before the addition of T cells. Tetanus toxoid (Massachusetts State Public Health Laboratories, Jamaica Plains, MA) at 17.5 LF/mL final concentration and SEA bacterial superantigen (Toxin Tech, Sarasota, FL) at 5 ng/mL final concentration were added where indicated. Cultures were pulsed during approximately the last 8 to 12 hours of culture with 1 μCi of 3H-thymidine (3HTdR; New England Nuclear, Boston, MA) per well approximately 72 hours from the start of culture with superantigen or approximately 96 to 120 hours from the start of allogeneic MLRs or tetanus toxoid presentation. The amount of 3HTdR incorporated by the responder T cells was plotted against the dose of stimulator cells as a measure of stimulatory capacity. Responses have been reported as the mean cpm ± SD of triplicates. Wells containing only T cells or only APCs (1,500 rad 137Cs) always incorporated less than 500 cpm 3HTdR.
RESULTS
Cord blood and bone marrow CD34+ progenitors undergoing dendritic cell differentiation are efficiently transduced by retroviral vectors. Cytokine-prestimulated cord blood and bone marrow CD34+ cells were cocultivated on a packaging cell line that produces amphotropic retroviral particles. Transduction efficiency and stability of the construct were monitored cytofluorographically 1 week to 10 days after retroviral infection of the target cells. Dual staining was performed using anti-mCD2-FITC antibody and PE-conjugated antibodies specific for surface epitopes broadly expressed by nonlymphoid cells, eg, CD13, CD14, and HLA-DR, along with more selective markers for CD34+-derived dendritic cells like CD83, CD1a, CD80, and CD86.
The mean transduction efficiency was 11.5% (range, 6.6% to 18% in 5 independent experiments) for bone marrow and 21.2% (range, 14.5% to 35% in 5 independent experiments) for cord blood specimens in the CD1a-expressing populations (Fig 1). Because CD14+ monocytes may also coexpress CD1a when cultured in GM-CSF,35 additional markers were evaluated in parallel to corroborate transduction of dendritic cells per se. Candidate dendritic cells, gated for HLA-DR, CD80 (B7-1), and/or CD86 (B7-2), were concordant with CD1a+ cells with respect to transduction; and both dendritic cell and nondendritic cell progeny were transduced in comparable proportions (Fig 1).
To determine the respective percentages of transduced CD14+/HLA-DR+ macrophages versus CD14−/CD1a+/HLA-DR+ dendritic cells versus CD14+/HLA-DR− granulocytes, two- or three-color cytofluorographic analyses were performed. There was no skewing of differentiation toward one or another subset after retroviral transduction, compared with what has previously been reported in this system17 (Fig 1). Cocultivation of CD34+ cells with the retroviral packaging line also had no effect on the expansion of these progenitors compared with mock-transduced cells (data not shown).
Transduced dendritic cells express normal levels of accessory molecules and characteristic surface markers. To determine whether retroviral infection or expression of the mCD2 marker affected phenotypic differentiation of dendritic cells, the transduced and untransduced populations were compared cytofluorographically. Mean channel fluorescence for CD1a, class II MHC, and essential T-cell accessory ligands expressed on dendritic cells, like CD80 and CD86, were comparable between gated populations of transduced versus untransduced cells (Table 1). Immunocytochemical stains for CD83, p55, CD40, and S100 antigens on sort-purified populations of transduced versus untransduced cells also confirmed comparable phenotype and morphology (Fig 2; CD40 and S100 not shown).
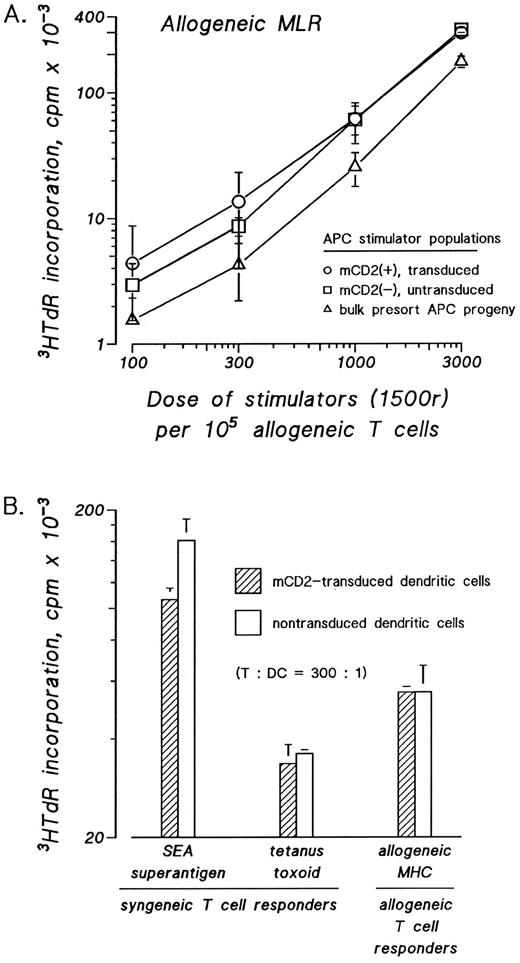
Transduced dendritic cells have normal T-cell stimulatory capacity in presenting allogeneic, nominal, and bacterial superantigens. The effects of retroviral transduction on dendritic cell accessory function were assessed in three functional assays. Dendritic cells were sorted according to presence or absence of surface mCD2 and assayed in graded doses as stimulatory APCs. The T-cell stimulatory capacity of mCD2 expressing dendritic cells was compared with mock-transduced dendritic cells and to the bulk myeloid progeny from which the mCD2+ and mCD2− DCs were sorted. Whether dendritic cells were tested as stimulators in the allogeneic MLR (Fig 3A and B) or as autologous T-cell stimulators pulsed with bacterial superantigen, eg, SEA (Fig 3B) or tetanus toxoid (Fig 3B), the measured 3HTdR incorporation by the stimulated T cells was not significantly affected by the presence or absence of the transduced mCD2 surface antigen on the stimulatory dendritic cells.
Transduced and mock-transduced progeny of cord blood and bone marrow CD34+ cells show similarly potent stimulatory function as accessory cells for resting T-cell responses. CD34+ cells were cultured in IMDM-20% FCS with c-kit-ligand, GM-CSF, and TNFα. Proliferating cells were transduced on day 3 of culture by cocultivation for 24 hours and were recultured in fresh medium with replenished cytokines for an additional 8 to 10 days. Nonadherent cells were stained with FITC-anti-mCD2 and candidate dendritic cells were cytofluorographically sorted according to the presence or absence of surface mCD2. Sorted cells were used in graded doses as stimulators in the allogeneic (A and B) mixed leukocyte reaction (MLR) or as stimulators of autologous T cells after pulsing with SEA superantigen or tetanus toxoid antigen (B). Cord blood progeny are evaluated in (A) and bone marrow progeny are evaluated in (B). The amount of 3HTdR incorporated by the responder T cells was plotted against the dose of stimulator cells as a measure of stimulatory capacity.
Transduced and mock-transduced progeny of cord blood and bone marrow CD34+ cells show similarly potent stimulatory function as accessory cells for resting T-cell responses. CD34+ cells were cultured in IMDM-20% FCS with c-kit-ligand, GM-CSF, and TNFα. Proliferating cells were transduced on day 3 of culture by cocultivation for 24 hours and were recultured in fresh medium with replenished cytokines for an additional 8 to 10 days. Nonadherent cells were stained with FITC-anti-mCD2 and candidate dendritic cells were cytofluorographically sorted according to the presence or absence of surface mCD2. Sorted cells were used in graded doses as stimulators in the allogeneic (A and B) mixed leukocyte reaction (MLR) or as stimulators of autologous T cells after pulsing with SEA superantigen or tetanus toxoid antigen (B). Cord blood progeny are evaluated in (A) and bone marrow progeny are evaluated in (B). The amount of 3HTdR incorporated by the responder T cells was plotted against the dose of stimulator cells as a measure of stimulatory capacity.
DISCUSSION
There are several gene delivery systems that may be applicable to dendritic cells. Retroviral vectors introduce genetic information into the chromosomal DNA of target cells that is further transmitted in the progeny and may be expressed until cell death. However, retroviral vectors require target cells to undergo cell division to integrate.36-38 In contrast, adenoviral vectors or nonviral vectors may be used for gene transfer in nondividing cells,39 40 but their utility may be hampered by the immunogenicity and/or transient gene expression inherent in these approaches.
In this study, we have shown that proliferating CD34+ dendritic cell progenitors from both bone marrow and cord blood can be successfully transduced by retroviral vectors carrying a foreign gene without altering differentiation into dendritic cells. We have shown that transduced dendritic cells preserve expression of critical accessory molecules and phenotypic markers. Most importantly, we have established that purified transduced dendritic cells exhibit no functional perturbations in antigen presentation and T-cell stimulation caused by the integration and stable expression of the replication-incompetent retrovirus. We have used a coculture method in these experiments that usually supports significantly higher transduction efficiency than that achieved with cell-free viral stocks. Although we have only achieved transduction efficiencies of 10% to 30%, this may still be sufficient to evoke clinically relevant T-cell responses. After retroviral transduction of CD34+ progenitors, mixed myeloid populations emerged in the presence of GM-CSF, TNFα, and c-kit-ligand, with or without flt-3-ligand, that included transduced granulocytes, macrophages, and dendritic cells in similar proportions as among the mock-transduced cells. Using amphotropic retroviral particles, we observed an almost twofold higher transduction efficiency with cord blood than with bone marrow. This finding is in accordance with a previously published report demonstrating a similar ratio of transduced long-term culture-initiating cell (LTC-IC)–derived colonies.41
Other groups have reported ex vivo gene transfer into human dendritic cells developing from CD34+ precursors42,43 or from nondividing peripheral blood mononuclear cells40,44 using retroviral and/or nonretroviral approaches. Aicher et al44 described retroviral transduction of peripheral blood monocyte-derived cells that were exposed to viral supernatant over a period of 3 days while undergoing differentiation toward dendritic cells in the presence of GM-CSF and interleukin-4 (IL-4). The reported mean transduction efficiency of 51% is peculiarly high for a retroviral construct in view of the fact that peripheral blood precursors of dendritic cells are nonproliferating.45 46
Henderson et al42 demonstrated expression of a retrovirally introduced epithelial mucin antigen in CD1a+ cells derived from transduced cord blood CD34+ precursors. Using centrifuged viral supernatant, approximately 13.5% mean transduction was reported. Mucin-expressing dendritic cells surprisingly elicited stronger allogeneic T-cell responses than their untransduced counterparts.42
Recently, Reeves et al43 have reported transduction of melan A/MART-1 into peripheral blood CD34+ precursors that differentiate into CD1a+ and CD86+ candidate dendritic cells in the presence of c-kit-ligand, GM-CSF, and TNFα. Notably, these progeny presented the transduced Melan A/MART-1, as determined by interferon γ release from IL-2–activated tumor-infiltrating lymphocyte (TIL) lines in all three patients tested. However, retroviral infection and stable gene expression were not shown. It is therefore possible that antigen released by transduced cells was captured and presented by nontransduced macrophages and/or dendritic cells.43
Ex vivo transduction of dendritic cells may be achieved by nonretroviral approaches. A recent report compared recombinant adenovirus constructs with various nonviral methods, including lipofection, electroporation, and CaPO4 precipitation regarding their potential to transduce peripheral blood monocyte-derived dendritic cells.40 None of the physical methods tested yielded efficient transduction, in contrast to the recombinant adenovirus construct that infected greater than 95% of the targets at a very high multiplicity of infection, ie, 1,000:1 or higher.40 The expression level of costimulatory T-cell ligands was assessed, but the CD80 costimulatory molecule was oddly absent in all conditions. The unexpected boost of allogeneic T-cell proliferation induced by transduced stimulators was attributed to expression of adenoviral proteins encoded in the vector's backbone. The consequences, either positive or negative, of using such immunogenic vectors in repeated vaccinations remains to be established.
With the capacity to generate large numbers of dendritic cells, in contrast to their trace numbers in the steady state, enormous enthusiasm has developed for the application of dendritic cells as immunotherapeutic adjuvants in a variety of settings. Culture conditions may be further optimized, including the possible use of additional cytokines with as yet unrecognized activities in dendritic cell development. Moreover, the efficacy of alternatives to fetal calf serum which support dendritic cell poiesis, foster the production of viral stocks, and avoid presentation of xenogeneic protein antigens, should all be established in this system. Our results nevertheless support ongoing efforts to develop genetically modified dendritic cells for immunotherapy. We report efficient and stable antigen expression by ex vivo retroviral transduction in human dendritic cells using the mCD2 surface marker as a model, and we have shown that transduced dendritic cells retain the expected phenotypic and functional properties. Future studies will identify the relative merits and limitations of gene-modified versus antigen-pulsed dendritic cells, with respect to the generation of potent antigen-specific helper and CTL responses.
ACKNOWLEDGMENT
The authors appreciate the expert technical assistance of Frank Isdell and Judy Adams, as well as the secretarial assistance of Jacqueline Chiappetta and Nancy Anton. We thank the physicians and staff of the Department of Obstetrics and Gynecology, especially Drs Terri Edersheim, J. Milton Hutson, and Lisa Kump (The New York Hospital-Cornell University Medical College) for their help in obtaining the cord blood samples for these studies. We also acknowledge the physicians and staff of the Allogeneic Bone Marrow Transplantation Service, Memorial Sloan-Kettering Cancer Center, for their assistance in procuring the bone marrow samples.
Supported by Grants No. K08-AI-01254 (P.S.), P01-CA-59350 (M.S.), P01-CA-23766 (J.W.Y.), and R01-AI-26875 (J.W.Y.) from the National Institutes of Health and by the McDonnell Foundation for Molecular Medicine (M.S.).
Address reprint requests to Paul Szabolcs, MD, Box 537, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal