Abstract
IDEC-C2B8 is a chimeric monoclonal antibody (MoAb) directed against the B-cell–specific antigen CD20 expressed on non-Hodgkin's lymphomas (NHL). The MoAb mediates complement and antibody-dependent cell-mediated cytotoxicity and has direct antiproliferative effects against malignant B-cell lines in vitro. Phase I trials of single doses up to 500 mg/m2 and 4 weekly doses of 375 mg/m2 showed clinical responses with no dose-limiting toxicity. We conducted a phase II, multicenter study evaluating four weekly infusions of 375 mg/m2 IDEC-C2B8 in patients with relapsed low-grade or follicular NHL (Working Formulation groups A-D). Patients were monitored for adverse events, antibody pharmacokinetics, and clinical response. Thirty-seven patients with a median age of 58 years (range, 29 to 81 years) were treated. All patients had relapsed after chemotherapy (median of 2 prior regimens) and 54% had failed aggressive chemotherapy. Infusional side effects (grade 1-2) consisting of mild fever, chills, respiratory symptoms, and occasionally hypotension were observed mostly with the initial antibody infusion and were rare with subsequent doses. Peripheral blood B-cell depletion occurred rapidly, with recovery beginning 6 months posttreatment. There were no significant changes in mean IgG levels and infections were not increased over what would be expected in this population. Clinical remissions were observed in 17 patients (3 complete remissions and 14 partial remissions), yielding an intent to treat response rate of 46%. The onset of these tumor responses was as soon as 1 month posttreatment and reached a maximum by 4 months posttreatment. In the 17 responders, the median time to progression was 10.2 months (5 patients exceeding 20 months). Likelihood of tumor response was associated with a follicular histology, with the ability to sustain a high serum level of antibody after the first infusion, and with a longer duration of remission to prior chemotherapy. One patient developed a detectable but not quantifiable immune response to the antibody that had no clinical significance. IDEC-C2B8 in a dose of 375 mg/m2 weekly for 4 weeks has antitumor activity in patients with relapsed low-grade or follicular NHL. Results with this brief, outpatient treatment compare favorably with results with standard chemotherapy, and IDEC-C2B8 has a better safety profile. Further studies evaluating IDEC-C2B8 in other types of lymphoma either alone or combined with chemotherapy are warranted.
THE TREATMENT OF cancer with agents specific for the tumor cell with sparing of the host has long been a goal of oncology. Unfortunately, treatment approaches using chemotherapy and radiotherapy have increased host toxicity to obtain antitumor activity. The development of monoclonal antibodies (MoAbs) provided hope that tumor-targeted therapy would one day play a role in the treatment of cancer. Indeed, promising results have been presented in several areas; however, these treatments have often proved technically difficult, produced disappointing efficacy, and were often not broadly applicable to patients with a given malignancy. The treatment of patients with lymphoma is an exception. Patients with advanced stage or relapsed low-grade non-Hodgkin's lymphoma (NHL) are not curable using conventional approaches and are usually treated with combination chemotherapy regimens of increasing intensity as needed to reduce disease and palliate symptoms.1 Aggressive approaches using high-dose therapy and autologous marrow support may provide maximal tumor reduction; however, there is currently no plateau on the failure-free survival curve.2 New treatment modalities and approaches that provide tumor reduction with less toxicity are needed.
MoAbs against a variety of tumor surface antigens have shown activity in the treatment of patients with low-grade NHL. Unmodified murine MoAbs have included custom, patient-specific anti-idiotype MoAbs,3-6 anti-CD20 MoAbs,7 and antibodies against CD19 and CD22 armed with immunotoxins.8-10 Anti-CD20 MoAbs radiolabeled with 131-I or 90-Y in low dose11-13 and in high dose with stem cell support14 15 have also demonstrated significant antitumor activity in the treatment of patients with relapsed disease, but have encountered dose-limiting toxicities and are limited by bone marrow involvement.
IDEC-C2B8 is a chimeric anti-CD20 MoAb containing human IgG1 and κ constant regions with murine variable regions.16 It binds the CD20 antigen with high affinity (5 × 10−9 mol/L). Because of the human constant Ig regions, the chimeric antibody efficiently kills CD20+ cells in vitro by augmented complement-mediated lysis and participates in antibody-dependent cell-mediated cytotoxicity (ADCC) using human complement and immune effector cells.16 In some NHL cell lines, the binding of the antibody inhibits proliferation and directly induces apoptosis.17 In patients with lymphoma, the administration of single doses up to 500 mg/m2 was not associated with any dose limiting toxicity. Rapid binding to and depletion of CD20+ normal B cells and tumor cells in the peripheral blood and bone marrow was observed, and tumor cells in lymph node biopsies obtained 2 weeks after antibody therapy showed that the chimeric MoAb bound to CD20 antigen sites.18 The treatment was well tolerated, causing only minimal infusion-related symptoms. Tumor responses (2 partial remissions [PR] lasting 8.1 and 8.6 months and 5 minor responses lasting 0.9 to 6 months) were observed in 7 of 9 patients treated with single doses of at least 100 mg/m2. Repeated weekly infusion for 4 weeks of doses of 125, 250, or 375 mg/m2 in 20 patients with relapsed lymphoma has also recently been reported.19 The treatment was well tolerated and 6 of 18 evaluable patients had a PR with no dose-limiting toxicity identified. The 375 mg/m2 for 4 weeks dose was selected for phase II evaluation. We report here on the clinical results obtained in the treatment of 37 patients with relapsed low-grade or follicular lymphoma.
MATERIALS AND METHODS
Study design. This was a phase II, open-label, single-arm, multicenter trial evaluating four intravenous infusions of the chimeric monoclonal anti-CD20 antibody IDEC-C2B8 at a dose of 375 mg/m2 in patients with relapsed low-grade NHL. Study objectives included the evaluation of safety, dose-limiting toxicities, pharmacokinetics, immune response, and clinical activity. Patients received a single infusion weekly, and completed the 4 antibody infusions in 22 days.
Antibody. IDEC-C2B8 is a chimeric MoAb containing human IgG1 (κ) heavy and light chain constant regions and murine variable regions from the murine antihuman CD20 MoAb IDEC-2B8 (murine IgG1, κ). The chimeric antibody was produced in vitro from a transfected Chinese hamster ovary (CHO) cell line.16 The antibody is specific for the human CD20 antigen, a 35-kD phosphoprotein present on normal and malignant B cells, but not on other tissues.20 The antibody was produced and supplied by IDEC Pharmaceuticals Corp (San Diego, CA).
Patient population and sample size. Adults with histologically confirmed, relapsed B-cell lymphoma expressing the CD20 antigen were eligible, as were nonresponders to first chemotherapy. All patients met the following criteria: expected survival of ≥3 months with no serious nonmalignant disease; prestudy performance status of 0, 1, or 2 on the World Health Organization (WHO) scale; seronegative for human immunodeficiency virus (HIV) and hepatitis B surface antigen; serum IgG level of ≥600 mg/dL; hemoglobin level of ≥8.0 g/dL; white blood cell count of ≥3.0 × 103/μL; absolute granulocyte count of ≥1.5 × 103/μL; platelet count of ≥75 × 103/μL; bilirubin level of less than 1.5 mg/dL; alkaline phosphatase level of less than 2× normal; aspartata amino transferase (AST) level of less than 2× normal; and serum creatinine level of less than 2.0 mg/dL. Patients tested negative for human antimurine antibody (HAMA).
The 375 mg/m2 dose was identified from a phase I dose-escalation trial evaluating 125, 250, and 375 mg/m2 infusions for 4 weeks.19 To determine clinical efficacy, a total of 37 patients were treated at the 375 mg/m2 dose and form the basis of this report.
Study sites. Patients were treated at seven sites in the United States. All patients signed an informed consent approved by the local Institutional Review Boards at Stanford University Medical Center (Palo Alto, CA), San Diego Regional Cancer Center (San Diego, CA), Scripps Memorial Hospitals (La Jolla and Encinitas, CA), Fox Chase Cancer Center (Philadelphia, PA), University of New Mexico Cancer Center (Albuquerque, NM), Henry Ford Hospital (Detroit, MI), or Markey Cancer Center (Lexington, KY). Patients were enrolled and treated between August 12, 1993 and September 13, 1994.
Pharmacokinetic sampling and HAMA/HACA evaluation. Serum samples were obtained pretreatment and at scheduled follow-up evaluations to determine anti-CD20 MoAb levels and to analyze for evidence of human antichimeric antibody (HACA) and HAMA immune response. Extended pharmacokinetic sampling was performed during the treatment course in selected patients whose schedules permitted. This included samples during the weeks 1 and 4 of treatment, including before infusion, postinfusion, and 24, 48, 72, and 96 hours postinfusion, as well as preinfusion and postinfusion samples from weeks 2 and 3. Serum pharmacokinetics were determined using frozen serum samples on microtiter plates coated with polyclonal goat anti–IDEC-2B8 antibody. Serum samples were serially diluted and washed, and bound IDEC-C2B8 was detected using labeled goat antihuman IgG and compared with a standard curve obtained using IDEC-C2B8 diluted into normal human serum. The assay has a lower limit of quantification of 0.5 μg/mL of the chimeric antibody.
HAMA was detected using microtiter plates coated with murine IgG. Serum samples were serially diluted on the plate and washed. Any bound human Ig was then detected using labeled goat antihuman Ig. HACA was detected by incubating serum samples on plates coated with IDEC-C2B8. Human antibody bound to the microtiter wells was then detected using biotin–IDEC-C2B8 followed by avidin-horseradish peroxidase and the substrate 2, 2-azinobis (3-ethylbenzthiazoline sulfonic acid) (ABTS). The minimum quantifiable level for these assays was 100 ng/mL (detectable to 5 ng/mL).
Patient monitoring and efficacy evaluation. Patients were monitored for safety and for clinical antitumor effect with regular medical histories, physical examinations, and laboratory studies including complete blood count and differential, chemistry panel, quantitative serum Igs, serum complement, serum anti-CD20 levels (HACA and HAMA), and urinalysis performed at baseline; at weeks 1, 2, 3, and 4; and at specified intervals to a maximum of 4 years. Toxicity was evaluated using the National Cancer Institute's Adult Toxicity Criteria. Peripheral blood was analyzed by flow cytometry to determine lymphocyte subsets at baseline, week 1, months 1, and 3 and at 3-month intervals for 1 year. Chest x-ray was performed at baseline and at 1 and 3 months after the completion of IDEC-C2B8 therapy. Computed tomography scans of the neck, chest, abdomen, and pelvis, as well as physical examination and measurement of palpable tumor masses or lymph nodes was conducted at baseline, at 1 and 3 months after treatment, and at 3-month intervals for 1 year and then yearly for 4 years. Tumor lesions were serially measured by physical exam and radiologic procedures. PR were defined as a decrease of greater than 50% in the total sum of the products of the bidimensional measurements. Complete remissions (CR) were defined as the disappearance of all disease. Stable disease was defined by no significant change in tumor measurements without progression over the period of observation. Remissions (PR and CR) were confirmed on a second complete evaluation at least 28 days later. Disease progression was determined by a 25% increase in measurable disease or the appearance of any new lesion. Time to progression was measured from date of first antibody infusion to first date when progressive disease was documented.
RESULTS
Patient characteristics. Thirty-seven patients were assigned to receive treatment at the phase II dose level of 375 mg/m2 for 4 weeks, the highest dose used in the dose escalation phase I portion of the trial. Patient characteristics at diagnosis and prior lymphoma therapy for all 37 patients are detailed in Table 1. The median age was 58 years, with a range of 29 to 81 years. All but 1 patient had an initial diagnosis of a low-grade or follicular histology (Working Formulation [WF ] groups A-D), and 76% were diagnosed with stage III or IV disease. At the time of antibody therapy, 17 patients (46%) had bone marrow involvement, 10 patients (27%) had an elevated lactate dehydrogenase (LDH) level, and 10 patients (27%) had at least one lesion greater than or equal to 7 cm. All patients had relapsed after standard chemotherapy with a median of 2 prior regimens (range, 1 to 5). Forty-four percent of patients had failed to respond to an initial aggressive combination chemotherapy regimen such as CHOP, PROMACE-CYTABOM, or MACOP-B. Fifty-six percent of patients were initially treated with a single agent (25%) or combination therapy with an alkylating agent and prednisone. Most patients (81%) had a CR or PR to initial chemotherapy with a median duration of 12 months. Relapsed disease was treated with a variety of agents including radiotherapy, single-agent, and aggressive combination chemotherapy including high-dose therapy supported with autologous stem cells (3 patients). Eighty-six percent of patients had responded to their most recent treatment with a CR (41%) or PR (45%) with a median duration of 12 months (20 patients with duration of <6 months). Treatment was administered at a median of 4.5 years from initial diagnosis (range, 0.8 to 13.3 years) and a median of 10.2 months from last therapy. Two patients had previously been treated in a phase I single-dose study using IDEC-C2B8, one of whom achieved a PR lasting 8 months.
Patient Characteristics (N = 37)
| . | No. . | % or Range . |
|---|---|---|
| Age | 58 | 29-81 |
| Sex | ||
| Female | 16 | 43 |
| Male | 21 | 57 |
| Histologic grade* | ||
| Low | 34 | 92 |
| A | 4 | 11 |
| B | 21 | 57 |
| C | 9 | 24 |
| Intermediate | 3 | 8 |
| D | 2 | 5 |
| G | 1 | 3 |
| Stage at diagnosis | ||
| I | 5 | 14 |
| II | 2 | 5 |
| III | 10 | 27 |
| IV | 18 | 49 |
| Unknown | 2 | 5 |
| Prior Therapy | ||
| Chemotherapy | 37 | 100 |
| Median no. of regimens | 2 | 1-5 |
| Time from last Rx median (mo) | 10.2 | 0.9-70.4 |
| Radiotherapy | 13 | 35 |
| ABMT | 3 | 8 |
| All therapy | 37 | 100 |
| Median no. | 2 | 1-8 |
| Time from last Rx, median (mo) | 7.2 | 0.9-67.9 |
| First chemotherapy | ||
| Single agent | 9 | 25 |
| COP or CP | 11 | 31 |
| Aggressive combination | 17 | 44 |
| Response to first chemotherapy | ||
| CR or PR | 29 | 81 |
| Duration, median (mo) | 12 | |
| No response | 5 | 14 |
| Unknown | 2 | 5 |
| Response to last therapy | ||
| CP or PR | 25 | 86 |
| Duration, median (mo) | 12 | |
| Unknown | 2 | 5 |
| . | No. . | % or Range . |
|---|---|---|
| Age | 58 | 29-81 |
| Sex | ||
| Female | 16 | 43 |
| Male | 21 | 57 |
| Histologic grade* | ||
| Low | 34 | 92 |
| A | 4 | 11 |
| B | 21 | 57 |
| C | 9 | 24 |
| Intermediate | 3 | 8 |
| D | 2 | 5 |
| G | 1 | 3 |
| Stage at diagnosis | ||
| I | 5 | 14 |
| II | 2 | 5 |
| III | 10 | 27 |
| IV | 18 | 49 |
| Unknown | 2 | 5 |
| Prior Therapy | ||
| Chemotherapy | 37 | 100 |
| Median no. of regimens | 2 | 1-5 |
| Time from last Rx median (mo) | 10.2 | 0.9-70.4 |
| Radiotherapy | 13 | 35 |
| ABMT | 3 | 8 |
| All therapy | 37 | 100 |
| Median no. | 2 | 1-8 |
| Time from last Rx, median (mo) | 7.2 | 0.9-67.9 |
| First chemotherapy | ||
| Single agent | 9 | 25 |
| COP or CP | 11 | 31 |
| Aggressive combination | 17 | 44 |
| Response to first chemotherapy | ||
| CR or PR | 29 | 81 |
| Duration, median (mo) | 12 | |
| No response | 5 | 14 |
| Unknown | 2 | 5 |
| Response to last therapy | ||
| CP or PR | 25 | 86 |
| Duration, median (mo) | 12 | |
| Unknown | 2 | 5 |
Histologic group based on Working Formulation.
Antibody infusions. IDEC-C2B8 infusions were administered in an outpatient setting, usually through peripheral intravenous catheters. Patients were often premedicated for the first antibody infusion with oral diphenhydramine and acetaminophen. The total dose of antibody was diluted into normal saline (generally 1 mg/mL) and administered intravenously by infusion at an initial rate of 50 mg/h, escalating every 30 minutes in the absence of side effects to a maximum of 200 mg/h.
The average dose of antibody administered during each infusion was 711 mg (range, 562 to 825 mg), with a cumulative dose over 4 infusions of 2,730 mg (maximum cumulative dose of 3,300 mg). The initial antibody infusion duration averaged 5.6 hours (range, 1.5 to 12.7 hours). In the absence of symptoms, subsequent infusions started at 200 mg/h with an average duration of infusion of 4.5, 4.4, and 4.2 hours for doses number 2, 3, and 4, respectively.
Adverse events. The most common adverse events thought to be related to treatment are detailed in Table 2. Thirty-two of the 37 patients experienced adverse events during the course of the trial; 6 patients experienced 12 grade 3 or 4 adverse events (9 reported as related to treatment). The most frequent infusion-related events included grade 1 or 2 fever (73% of patients), chills (38%), nausea (19%), asthenia (16%), headache (16%), and a sensation of tongue or throat swelling (11%). Hypotension occurred in 6 patients and was limited to grade 1 in 3 cases and grade 2 in 3 cases. Treatment-related side effects were seen most frequently with the initial antibody infusion (129 of 192 [67%]) and dramatically decreased during the second, third, and fourth infusions. Adverse events were managed by the administration of diphenhydramine or acetaminophen as indicated and by temporarily halting the antibody infusions until the symptoms abated. The infusions could in general then be restarted with minimal further toxicity. Two patients were withdrawn from study after receiving only the first of 4 planned antibody infusions (described in detail below).
Observed Adverse Events
| . | N (%) . | Events (%) . |
|---|---|---|
| Any adverse event | 32 (86.5) | 192 (100) |
| Body as a whole | ||
| Fever | 27 (73.0) | 55 (28.6) |
| Chills | 14 (37.8) | 14 (7.3) |
| Asthenia | 6 (16.2) | 6 (3.1) |
| Headache | 6 (16.2) | 11 (5.7) |
| Cardiovascular system | ||
| Hypotension | 6 (16.2) | 6 (3.1) |
| Digestive system | ||
| Nausea | 7 (18.9) | 7 (3.6) |
| Vomiting | 4 (10.8) | 4 (2.1) |
| Dyspepsia | 2 (5.4) | 2 (1.0) |
| Blood and lymphatics | ||
| Thrombocytopenia | 7 (18.9) | 7 (3.6) |
| Neutropenia | 3 (8.1) | 5 (2.6) |
| Anemia | 2 (5.4) | 3 (1.6) |
| Coagulation disorder | 2 (5.4) | 2 (1.0) |
| Leukopenia | 1 (2.7) | 4 (2.1) |
| Nervous system | ||
| Vasodilation | 1 (2.7) | 3 (1.6) |
| Respiratory system | ||
| Rhinitis | 6 (16.2) | 6 (3.1) |
| Laryngismus | 4 (10.8) | 4 (2.1) |
| Chest pain | 2 (5.4) | 2 (1.0) |
| Skin and appendages | ||
| Rash | 5 (13.5) | 5 (2.6) |
| Night sweats | 3 (8.1) | 3 (1.6) |
| Urticaria | 3 (8.1) | 4 (2.1) |
| Pruritus | 2 (5.4) | 3 (1.6) |
| Special senses | ||
| Lacrimation disorder | 5 (13.5) | 5 (2.6) |
| . | N (%) . | Events (%) . |
|---|---|---|
| Any adverse event | 32 (86.5) | 192 (100) |
| Body as a whole | ||
| Fever | 27 (73.0) | 55 (28.6) |
| Chills | 14 (37.8) | 14 (7.3) |
| Asthenia | 6 (16.2) | 6 (3.1) |
| Headache | 6 (16.2) | 11 (5.7) |
| Cardiovascular system | ||
| Hypotension | 6 (16.2) | 6 (3.1) |
| Digestive system | ||
| Nausea | 7 (18.9) | 7 (3.6) |
| Vomiting | 4 (10.8) | 4 (2.1) |
| Dyspepsia | 2 (5.4) | 2 (1.0) |
| Blood and lymphatics | ||
| Thrombocytopenia | 7 (18.9) | 7 (3.6) |
| Neutropenia | 3 (8.1) | 5 (2.6) |
| Anemia | 2 (5.4) | 3 (1.6) |
| Coagulation disorder | 2 (5.4) | 2 (1.0) |
| Leukopenia | 1 (2.7) | 4 (2.1) |
| Nervous system | ||
| Vasodilation | 1 (2.7) | 3 (1.6) |
| Respiratory system | ||
| Rhinitis | 6 (16.2) | 6 (3.1) |
| Laryngismus | 4 (10.8) | 4 (2.1) |
| Chest pain | 2 (5.4) | 2 (1.0) |
| Skin and appendages | ||
| Rash | 5 (13.5) | 5 (2.6) |
| Night sweats | 3 (8.1) | 3 (1.6) |
| Urticaria | 3 (8.1) | 4 (2.1) |
| Pruritus | 2 (5.4) | 3 (1.6) |
| Special senses | ||
| Lacrimation disorder | 5 (13.5) | 5 (2.6) |
Included are events reported as possibly or probably related or of unknown relationship to study treatment.
Abbreviations: N, number of patients; Events, number of events.
Grade 3 and grade 4 adverse events included thrombocytopenia and anemia in 1 patient, abdominal pain and constipation in 1 patient, transient neutropenia at 4 months and 9 months in 2 patients, myocardial infarction in 1 patient, and malignant pleural effusion with dyspnea and traumatic hip fracture 3 weeks after antibody therapy in 1 patient. Two of the 37 patients did not complete the 4 planned antibody infusions. The first patient, who had small lymphocytic lymphoma, had previously been treated with a single infusion of IDEC-C2B8 in an earlier trial and had experienced transient grade 3 thrombocytopenia. Within 24 hours of the first infusion of 375 mg/m2 antibody, the platelet count again decreased from a baseline value of 93,000/μL to a nadir of 19,000/μL. This was associated with marked elevation in LDH level (629 to 2660 U/L), a decrease in hemoglobin level from 12.6 to 7.8 g/dL, and an elevation in uric acid to 10.5 mg/dL. She received outpatient treatment with allopurinol, prednisone, and a single platelet transfusion. Evaluation for disseminated intravascular coagulation was nondiagnostic and the patient improved (counts returned to above baseline) by day 31. The second patient, a 63-year-old man with follicular small cleaved cell NHL, had a prior history of hypertension, hypercholesterolemia, and atherosclerotic cardiovascular disease, including a prior myocardial infarction (4 months earlier); developed anginal pain within 30 minutes of initiation of his first IDEC-C2B8 infusion; and received nitroglycerin with resolution of symptoms. The antibody infusion was then completed with stable vital signs. However, the patient was hospitalized 5 days later with a myocardial infarction and was removed from study. An occluded right coronary artery was found and the patient underwent a surgical coronary artery bypass procedure.
Hematologic and chemistry laboratory effects. A total of 18 patients had some clinically significant hematologic nadir after antibody therapy and 11 of these patients had bone marrow involvement by their lymphoma. Eleven patients sustained decreases in hemoglobin or hematocrit of greater than 1.5 g or 3%, respectively. Eight patients had decreases in platelet counts to less than 100,000/μL and 10 patients had transient reductions in white blood cell count or absolute granulocyte count of less than 3,000/μL or 1,500/μL, respectively. In general, these effects were mild and patients spontaneously recovered without intervention. Two patients developed late onset isolated neutropenia of unclear etiology at 4 and 10 months after therapy. Both instances were transient and resolved within 1 month.
No clinically significant changes in chemistry laboratory values were noted.
Reported infections. A total of 9 patients (11 infections) were reported to have acquired an infection during the course of the treatment or follow-up. Infections during the first 60 days of antibody therapy included viral upper respiratory infections in 3 patients, localized skin infections in 3 patients, and herpes zoster and otitis media in 1 patient each. Reported late infections, occurring 7 to 12 months after antibody therapy, included pneumonia, impetigo, and sinusitis. All infections were grade 1 or 2 and resolved without complications after appropriate therapy.
Depletion of peripheral blood B cells. Peripheral blood lymphocytes were evaluated by flow cytometry at baseline, 72 hours after the first infusion, and at 1, 2, 6, 9, and 12 months posttreatment. The percentage and absolute number of cells expressing the CD19 and CD20 antigens was determined. Treatment with the chimeric anti-CD20 antibody rapidly and effectively depleted B cells from the peripheral blood circulation as detected by the unrelated anti-CD19 B-cell antibody. In some patients, baseline CD20 cell counts were elevated (due to the presence of circulating lymphoma cells). Most patients had a rapid decrease in the relative percentage and absolute number of CD19+ cells by day 4. This level remained nearly undetectable until approximately 6 months posttreatment, followed by slow gradual recovery.
Serum Ig and complement levels. The CD20 antigen is expressed on early B cells but not upon differentiation to antibody secreting plasma cell. Because of the possibility that the elimination of normal B cells and plasma cell progenitors could affect antibody production, serum Ig (IgG, IgA, and IgM) levels were quantitated at baseline and throughout the study. Mean serum Ig levels remained stable; however, individual patients experienced transient decreases in some subsets. Among these 37 patients, 4, 4, and 5 patients experienced more than a 20% decline in IgG, IgA, or IgM levels, respectively (total of 10 patients with 3 patients having decreases in both IgG and IgA levels).
No clinically significant change in serum complement levels was observed.
Evaluation of immune response (HAMA/HACA). Of the 37 patients evaluated, there were no quantifiable HAMA or HACA titers identified. One patient (with a PR) had a detectable, but not a quantifiable (<100 ng/mL) HACA titer 7 months posttreatment. There was no toxicity related to this immune response.
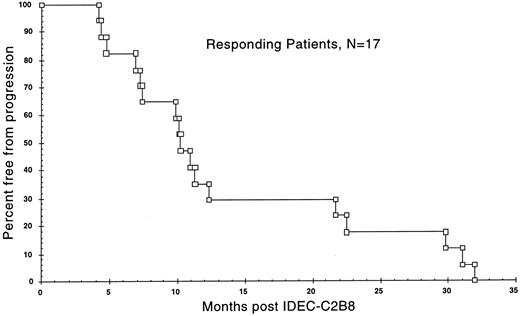
Clinical response. Clinical responses (CR or PR) were observed in 17 of the 37 patients (46%) and are detailed in Table 3. Two patients were withdrawn from the study for adverse events after receiving a single infusion of the antibody and 1 patient had a high-grade B-cell malignancy. Thus, 34 patients completed all four antibody infusions and were considered evaluable for response. All 34 had a low-grade or follicular histology. Three of the 34 patients (9%) had a CR and 14 of 34 (41%) had a PR for an overall response rate for evaluable patients of 50%. An additional 8 patients had some evidence of tumor response (>20% reduction), but did not qualify as responders (PR or CR). Thus, 25 of the 34 evaluable patients (74%) had evidence of antitumor effect. Eleven patients (32%) had stable disease (SD) and 6 patients (18%) were judged to have progressive disease (PD). The median time to onset of clinical response for the 17 responders was 50 days (range, 7 to 112 days). Measured tumor masses continued to regress, with the maximum response in sentinel tumor lesions occurring 3 to 4 months after antibody therapy. The median time to progression for the clinical responders was 10.2 months, with 5 patients exceeding 20 months and 2 patients exceeding 30 months. The median duration of response was 8.6 months. A Kaplan-Meier survival curve for the time to progression is shown in Fig 1 for the clinical responders.
Summary of Clinical Response
| Patient Group . | N . | CR (%) . | PR (%) . | CR and PR (%) . | 95% CI . |
|---|---|---|---|---|---|
| . | . | . | . | . | (CR and PR) . |
| Intent to treat | 37 | 3 (8) | 14 (38) | 17 (46) | 30-62% |
| Evaluable3-150 | 34 | 3 (9) | 14 (41) | 17 (50) | 33-67% |
| Patient Group . | N . | CR (%) . | PR (%) . | CR and PR (%) . | 95% CI . |
|---|---|---|---|---|---|
| . | . | . | . | . | (CR and PR) . |
| Intent to treat | 37 | 3 (8) | 14 (38) | 17 (46) | 30-62% |
| Evaluable3-150 | 34 | 3 (9) | 14 (41) | 17 (50) | 33-67% |
Abbreviation: CI, confidence interval.
Patients had low-grade or follicular lymphoma and completed four antibody infusions.
Kaplan-Meier graph of the time to progression from the date of initial treatment with the first dose of IDEC-C2B8 until documented tumor relapse or progression in the 17 clinical responders.
Kaplan-Meier graph of the time to progression from the date of initial treatment with the first dose of IDEC-C2B8 until documented tumor relapse or progression in the 17 clinical responders.
The majority of the patients had follicular histologies. Of 21 patients with follicular small cleaved histology (WF group B), there were 11 PR and 2 CR for a response rate of 62%. Of 9 patients with follicular mixed small and large cell histology (WF group C), there was 1 CR and 3 PR (44%). One of 2 patients with follicular large cell lymphoma (group D) had a PR. The response rate appears similar among the follicular histologies. In contrast, none of 4 patients with small lymphocytic lymphoma (1 with mantle cell histology) had a clinical remission.
There was no clear influence of type of prior therapy on the likelihood of clinical response to antibody treatment. Two of three patients who had been treated previously with high-dose therapy and autologous stem cell rescue achieved a PR to antibody therapy with times to disease progression of 4.2 and 21.7 months. Twenty patients had extranodal disease and two showed a CR and seven showed a PR to antibody therapy. There was a correlation observed between the duration of response to last chemotherapy and response to treatment with IDEC-C2B8 (P = .05; Fisher's exact test). Twenty of the 37 patients had a duration of response to last chemotherapy of 6 months or less. Of these 20, 6 (30%) responded to chimeric MoAb therapy. Seventeen of the 37 patients had a duration of response to last chemotherapy of more than 6 months. Of these 17, 11 (65%) responded to IDEC-C2B8.
Antibody pharmacokinetics. At the 375 mg/m2 dose used in this trial, all studied patients exhibited detectable circulating antibody throughout the treatment period. Preinfusion (doses 2 through 4) antibody concentrations ranged from 0.8 to 517.7 μg/mL and postinfusion levels ranged from 107.8 to 962.7 μg/mL. Five patients consistently had preinfusion antibody levels at or less than 10 μg/mL, suggesting that they did not reach steady state or cleared the antibody at a faster rate. Most patients exhibited increasing preinfusion antibody concentrations with each subsequent infusion.
The Wilcoxon rank sum test was used to examine the relationship between the preinfusion and postinfusion chimeric antibody concentrations and the clinical response. A correlation was observed between clinical response and the median values of IDEC-C2B8 serum levels before the second infusion (P = .029), with responders having a median of 82.7 μg/mL (range, 1.2 to 125.3 μg/mL) versus nonresponders with a median of 21.9 μg/mL (range, 1.1 to 99.0 μg/mL). Detailed pharmacokinetic sampling was performed on 9 patients, with a mean Cmax of 500.5 ± 135.2 μg/mL (range, 201.4 to 663.0 μg/mL) and serum antibody half-life values from 12.7 to 370.8 hours (mean T1/2 of 225.9 ± 102.7 hours).
DISCUSSION
IDEC-C2B8 represents a new treatment for patients with recurrent low-grade or follicular NHL. This therapy has a response rate in evaluable patients of 50% and a time to progression of 10.2 months. The treatment consists of four intravenous infusions administered over a 22-day period in the outpatient setting with minimal toxicity. This compares favorably with other agents currently in use for the treatment of patients with relapsed disease. These results have recently been confirmed in preliminary reports in a separate phase II/III multicenter trial in more than 150 patients.21
The adverse events observed in this trial were predominately infusion related and generally consisted of mild grade 1 or 2 symptoms of fever, chills, rigor, rash, nausea, and, rarely, mild respiratory symptoms or hypotension. All of these side effects were transient and did not interfere with the completion of the planned antibody infusion. Two patients were removed from study after a single dose of the antibody; 1 patient developed thrombocytopenia and an elevated LDH and a second patient with known cardiac disease experienced chest pain and was admitted 1 week later with a myocardial infarction. All other patients completed the planned 4 weekly infusions. The etiology of the observed infusion related symptoms is not completely understood, but may represent the rapid depletion of B cells and lysis in the reticuloendothelial systems of the lung, liver, and spleen. This constellation of symptoms has been seen in other studies using murine MoAbs.22 The marked decrease in infusional side effects noted with subsequent infusions likely reflects the elimination of most circulating B lymphocytes, the saturation of antigenic sites with the first infusion, and persistent antibody serum levels present at the time of the subsequent antibody infusions.
As expected, normal B cells were rapidly depleted from the peripheral blood of nearly all patients and remained depleted until nearly 6 months posttreatment, followed by a slow recovery. Despite this depletion of B cells, there was minimal change in serum Ig levels and no increase in the frequency or severity of infectious complications. The infections noted in this trial are common to patients with relapsed B-cell lymphoma and are not clearly related to treatment with the antibody. Prolonged B-cell depletion is common to other chemotherapy treatments and after BMT. The type and risk of infection with MoAb treatment appears less than that reported with administration of some of the newer purine analogues (fludarabine and 2-CDA) that are associated with significant depletion of the CD4+ T-cell population.
The patients included in this trial are typical of those represented in the general population and of low-grade patients studied in the Working Formulation Lymphoma Classification Project.23 The International Prognostic Index (IPI) originally developed for aggressive NHL uses 5 prognostic factors, including age greater than 60 years; stage III/IV disease; performance status greater than 1; elevated LDH level; and extranodal sites greater than 1.24 It has been applied to patients with low-grade NHL and permits prognostic stratification into risk groups.25 Using data from presentation, we were able to determine the IPI classification in 32 of the 37 patients included in this trial. As expected, in patients with low-grade NHL, the majority of patients were in the low (41%) and low-intermediate (27%) prognostic groups, followed by those in intermediate/high (16%), high (3%), and unknown (14%) groups. There was no statistically significant correlation (Fisher's exact test, P = .31) between the risk groups and response to treatment with IDEC-C2B8. However, there was a correlation observed between the duration of response to last chemotherapy and the response to IDEC-C2B8. Patients with less than a 6-month response to last chemotherapy had a 30% response rate, compared with a 65% response rate for patients with last duration of remission of greater than 6 months. In contrast with the experience with some MoAbs, antitumor activity was observed in patients with bulky disease and in poor prognostic patients who had relapsed after anthracycline-containing regimens or after high-dose therapy with stem cell support. Tumor responses were noted in patients with bulky lymph nodes, splenomegaly, extranodal masses, and lymphoma in blood and bone marrow.
A correlation was also observed between the level of IDEC-C2B8 present in the serum before infusion number 2 and the response to MoAb therapy. Whether this is due to differences in tumor burden or to differences in intrinsic clearance of the MoAb is unknown. All 17 clinical remissions occurred in patients with a follicular histology (WF groups B-D), although the number of patients with other histologies was small. In contrast, none of the 4 patients with small lymphocytic lymphoma (WF group A) responded. These patients had heterogenous disease, including 1 patient with mantle cell histology. Although patients with chronic lymphocytic leukemia (CLL) were excluded from this trial (based on the presence of >5,000 lymphocytes/μL for this histologic subgroup), it is possible that the decreased response rate in this subgroup was due to a lower expression of the CD20 surface antigen that has been observed in cases of CLL.
The method of tumor cell killing by the MoAb is not completely understood and likely involves several mechanisms. The chimeric MoAb was engineered to contain human IgG1 constant regions to augment immune-mediated antitumor activity using human complement and human ADCC effector cells.16 In vitro, the antibody clearly exhibits much greater activity than the parent murine IgG1 MoAb in these assays. However, in this clinical trial, there were no significant changes in serum complement levels observed after the MoAb treatments. In addition, in earlier phase I trials, tumor biopsies 2 weeks posttreatment showed antibody bound to tumor cells in patients thought to be immunocompetent who did not have a clinical remission.18 The CD20 molecule is thought to aggregate on the cell surface and create or control a Ca2+ channel that is important in initiation and progression through the cell cycle.26 Antibodies binding to CD20 may directly inhibit the cell cycle.27 Recent data using IDEC-C2B8 have shown that the antibody inhibits proliferation and directly induces apoptosis in some B-cell NHL lines in vitro.17 The degree to which this additional antitumor mechanism contributes to the observed clinical remissions is unknown and is actively being evaluated in the laboratory.
The optimal treatment for patients with advanced-stage or relapsed low-grade NHL remains unsettled. Diverse options, from watch and wait to treatment with aggressive chemotherapy, are frequently used.1 Although most patients initially respond to a variety of chemotherapeutic regimens with a PR or CR, all will eventually relapse and require additional treatment with decreasing response rates and decreasing durations of remission. Aggressive combination chemotherapy produces higher CR rates, and high-dose therapy with stem cell or bone marrow support may result in longer durations of remission. Unfortunately, most trials of such aggressive therapies have shown continued risk of relapse without a convincing plateau on the failure-free survival curve.2 An exception may be allogenic BMT, but this treatment is restricted to younger patients with an appropriate donor and carries a high risk of morbidity and mortality.28 Most patients undergo a series of treatments with increasing toxicity and decreasing bone marrow reserve until they ultimately become refractory to treatment and die of disease progression or from complications of treatment.
IDEC-C2B8 chimeric anti-CD20 MoAb presents the opportunity to obtain meaningful tumor reductions with minimal toxicity in patients with relapsed low-grade NHL. Retreatment is possible and early experience suggests that a majority of patients with a prior response may respond to retreatment. Fludarabine has also been studied in patients with relapsed disease, with an overall response rate of approximately 40%.29,30 Time to treatment failure was 4.6 months in one study.31 Treatment complications from fludarabine included infections or fever of unknown origin in 62% of patients, bone marrow suppression, and opportunistic infections. Similar responses have been observed with cladribine; however, marked immunosuppression and hematologic toxicity was noted.32 33
To date, experience using IDEC-C2B8 has been predominately in patients with relapsed measurable disease. A clinical trial combining IDEC-C2B8 with 6 cycles of CHOP chemotherapy in newly diagnosed patients has recently been completed. Early evaluation of this experience suggests that this combination resulted in a PR or CR in all patients and in the clearance of polymerase chain reaction-detectable cells from the peripheral blood and bone marrow in 7 of 8 patients with a positive pretreatment assay.34 It remains to be determined if this will ultimately decrease the risk of relapse or prolong disease-free survival. Additional areas that should be investigated using this new agent include (1) extended and repeated dosing regimens, (2) combination with or after standard chemotherapy, (3) use as an in vivo B-cell purging agent before collection of bone marrow or stem cells for high-dose therapies, (4) evaluation in other B-cell histologies, and (5) lymphomas arising in association with immunodeficiency such as acquired immunodeficiency syndrome or organ transplantation.
In conclusion, this experience using an engineered chimeric MoAb finally provides practical substance to the promise of antibody therapy of cancer that was envisioned nearly 100 years ago by Ehrlich.35
R.L. is a American Cancer Society Clinical Research Professor. D.G.M. and T.D. were supported by a Clinical Associate Physician grant from the General Clinical Research Center. This study was supported by a clinical grant from IDEC Pharmaceuticals Corp.
Address reprint requests to David G. Maloney, MD, PhD, Fred Hutchinson Cancer Research Center, M-385, 1124 Columbia St, Seattle, WA 98104.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal