Abstract
CD44 is expressed in various isoforms on numerous cell types and tissues during embryogenesis and in the mature organism. CD44 may also be involved in tumor growth. To study the multiple roles of CD44, we abolished expression of all known isoforms of CD44 in mice by targeting exons encoding the invariant N-terminus region of the molecule. Surprisingly, mice were born in Mendelian ratio without any obvious developmental or neurological deficits. Hematological impairment was evidenced by altered tissue distribution of myeloid progenitors with increased levels of colony-forming unit–granulocyte-macrophage (CFU-GM) in bone marrow and reduced numbers of CFU-GM in spleen. Fetal liver colony-forming unit–spleen and granulocyte colony-stimulating factor mobilization assays, together with reduced CFU-GM in peripheral blood, suggested that progenitor egress from bone marrow was defective. In what was either a compensatory response to CD44 deficiency or an immunoregulatory defect, mice also developed exaggerated granuloma responses to Cryotosporidium parvum infection. Finally, tumor studies showed that SV40-transformed CD44-deficient fibroblasts were highly tumorigenic in nude mice, whereas reintroduction of CD44s expression into these fibroblasts resulted in a dramatic inhibition of tumor growth.
CD44 IS A HYALURONAN-binding surface receptor present on lymphocytes and other cells of hematopoietic and nonhematopoietic origins. CD44 is implicated in a variety of immunological functions, such as vascular extravasation and T-cell costimulation, although broad tissue expression of CD44 isoforms and their upregulation at various stages during development has also suggested a variety of other important nonimmunological roles. There is evidence that CD44 may be involved in tumor metastasis,1-3 and high levels of CD44 expression on embryonic cells has suggested a role in embryogenesis.4-6 During critical stages of morphogenesis, CD44 is expressed at high levels in developing heart, somites, and condensing limb-bud mesenchyme. In these tissues hyaluronate has been found to regulate morphogenetic events,4-6 increasing the possibility that CD44 might play a critical role during embryogenesis.
Various reports have implicated CD44 in the development and function of the immune system. Hematopoietic cells express CD44 in a temporally regulated manner.7-11 CD44 expression has been reported on pluripotent stem cells,12 although it has not been detected on hematopoietic progenitors from embryonic yolk sac.13 Relatively high expression is also seen early in T- and B-cell development.11,12,14-16 T and B cells also upregulate CD44 after antigen receptor stimulation or mitogen activation.2,17,18 Antibody studies have indicated that CD44 may be involved in lymphocyte extravasation across endothelial vessels and homing into peripheral organs.19-22 CD44 has been reported to have a costimulatory function on T cells in vitro after crosslinking with anti–CD-2 or anti-CD3 and anti-CD44 antibodies.23-27 However, in the case of anti-CD3 crosslinking, some investigators failed to show CD44 costimulation and even found inhibitory effects with anti-CD44 antibodies (reviewed in detail by Lesley et al14 ). Activation through CD44 has also been reported to trigger lytic function of cytotoxic T cells28,29 and enhance natural killer (NK) cell activity.30-32 High expression levels of CD44 have been used as a marker for memory cells,33-35 which may reflect a role for CD44 in lymphocyte apoptosis.36-38 Delayed-type hypersensitivity responses have also been shown to be reduced by anti-CD44 antibody treatment, possibly the result of reduced lymphocyte extravasation into the skin.39
CD44 may mediate adhesion between bone marrow (BM) progenitor cells and stromal cells,7,9 which express high levels of the CD44 ligand, fibronectin. The interaction with stromal cells is important for survival and differentiation of hematopoietic progenitors. This has been shown using monoclonal antibodies (MoAbs) against CD44 in Dexter-type long-term BM cultures, in which the antibodies have been shown to prevent emergence of myeloid cells.7
CD44 isoforms are encoded by a single gene containing 19 or 20 exons, 12 of which can be differentially spliced. All known membrane-associated CD44 isoforms have 5 constant exons at the amino terminus followed by 10 alternatively spliced exons, 2 constant membrane-proximal coding exons, 1 constant transmembrane-coding exon, and 2 potentially alternatively spliced exons coding for cytoplasmic domains. The great variability in splicing leads to at least 18 different CD44 isoforms,40,41 some preferentially expressed in certain cells of mesenchymal and neuroectodermal origin, including keratinocytes and fibroblasts42-44 and hematopoietic cells, astrocytes, and glial cells,45-48 respectively. Independently cloned by several groups, CD44H, also called CD44s, is the most common isoform and the major receptor for hyaluronic acid (HA) on hematopoietic cells and fibroblasts.49-53
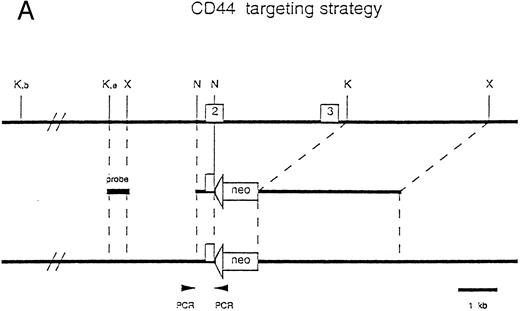
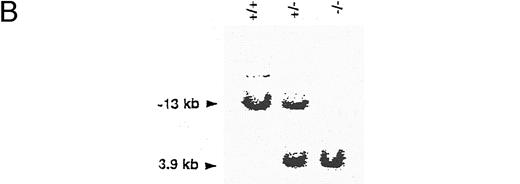
Generation of CD44-deficient mice. (A) Restriction map of the CD44 genome fragment used to generate the targeting vector. Open boxes represent exons 2 and 3. 5′ flanking probe is designated as probe. Targeting vector replaces 3′ end of exon 2 and the entire exon 3 with a neomycin resistance gene in an antisense orientation containing several stop codons for all reading frames. Map of predicted homologous hybridization results in a 3.9-kb gene-targeted fragment compared with a 13-kb wild-type Kpn I fragment in C57BL/6J. (Abbreviations: N, Nco I; X, Xba I; K, Kpn I; K, e, Kpn I restriction sites in 129J mouse strain (missing in C57BL/6J mice), K, b, Kpn I restriction sites in C57BL/6 mice.) (B) Southern blot of homozygous-, heterozygous-mutant, and wild-type mice. Genotypes are indicated at the top of each lane: +/+, wild-type; +/−, heterozygous mutant; and −/−, homozygous mutant.
Generation of CD44-deficient mice. (A) Restriction map of the CD44 genome fragment used to generate the targeting vector. Open boxes represent exons 2 and 3. 5′ flanking probe is designated as probe. Targeting vector replaces 3′ end of exon 2 and the entire exon 3 with a neomycin resistance gene in an antisense orientation containing several stop codons for all reading frames. Map of predicted homologous hybridization results in a 3.9-kb gene-targeted fragment compared with a 13-kb wild-type Kpn I fragment in C57BL/6J. (Abbreviations: N, Nco I; X, Xba I; K, Kpn I; K, e, Kpn I restriction sites in 129J mouse strain (missing in C57BL/6J mice), K, b, Kpn I restriction sites in C57BL/6 mice.) (B) Southern blot of homozygous-, heterozygous-mutant, and wild-type mice. Genotypes are indicated at the top of each lane: +/+, wild-type; +/−, heterozygous mutant; and −/−, homozygous mutant.
CD44 binding capacity for HA correlates with the level of cell activation and can require phosphorylation of one of two cytoplasmic serine residues or CD44 dimerization.54,55 All known membrane-bound CD44 molecules contain a serine-threonine–rich “mucin-like domain” located between the amino-terminal HA-binding motif and the transmembrane region that can be highly O-linked glycosylated. Another element encoded by the two invariant exons are four serine-glycine residues, believed to be potential sites for proteoglycan substitutions.56-60 More recent data show that the majority of chondroitin- and heparin-sulfate modifications require the presence of the variable exon v3.56 58
Since it was reported that an alternative spliced form of CD44 could confer metastatic capacity to a rat pancreatic carcinoma cell line,3 a large amount of work has been published with regard to the role of CD44 in tumor progression and metastasis.61-64 Therefore, we decided to investigate the tumorigenicity of SV40 large T-transformed fibroblast derived from CD44-deficient mice. We report here that these cells are significantly more tumorigenic than CD44s-containing fibroblasts. Taken together, our investigations of gene-targeted mice implicate the CD44 molecule as a regulator of hematopoietic progenitor distribution and of immune responses to granuloma-inducing bacteria. We also provide unequivocal findings of CD44 involvement in tumor growth.
MATERIALS AND METHODS
Generation of the CD44 mutant mice.The targeting construct was generated as follows: A polymerase chain reaction (PCR)-amplified probe containing CD44 exons 2 and 3 was used to screen a 129J-l DashII genomic library (kindly provided by Dr Janet Rossant, Toronto, Canada). Five phage clones were selected and partially mapped. One of these clones, containing 15 kb of genomic CD44 sequence including exons 2 and 3, was transferred into pBlue script (BS-SK; Stratagene, La Jolla, CA) for mapping and partial sequencing. The 3′ region of exon 2 and the complete exon 3 were replaced with pMC1-Neomycin (pMC1-neo; Stratagene). The 5′ short arm consisted of a 600-bp Nco I-fragment of exon 2 containing its first 122-bp (of a total of 160 bp) blunt end ligated to pMC1-neo. The pMC1 neo fragment used was originally isolated with Xho I and HindIII and contains a polyadenylation signal. For the 3′ long arm, a 6-kb intronic region 3′ of exon 3 isolated with Kpn I digestion was chosen. It should be noted that pMC1-neo was inserted in the antisense orientation, which leads to several stop codons in all three reading frames. The construct was linearized at the 5′ end of the short arm using the BS-SK restriction site Not I, and 25 μg digested DNA was electroporated into 2 × 106 E14 embryonic stem cells (Bio-Rad Gene Pulser, 340 V, 250 μF; Bio-Rad Laboratories, Hercules, CA). Colonies resistant to G418 were screened for homologous recombination by PCR as described.65 The following primers were used: upstream of the targeting vector, 5′-TGG TCT TGA ACT CAG GTG ATC CTC-3′ and 5′-TAT CAG GAC ATA GCG TTG GCT ACC C-3′. The PCR amplification conditions were 5 minutes at 94°C, 1 minute at 94°C, 30 seconds at 64°C, and 2 minutes at 72°C for 35 cycles.
Recombinant colonies were confirmed by Southern blotting and injected into 3.5-day-old C57Bl/6J blastocysts, which were then transferred into 2.5-day pseudopregnant CD1 foster mothers as previously described.65-67 Chimeras were backcrossed into C57Bl/6J or CB6F1 mice (both from Jackson Laboratories, Bar Harbor, ME), and agouti offspring were screened by Southern blot analysis.
Southern blot analysis to identify targeted clones and to genotype mice.Individual embryonic stem cells (ES) clones and mouse tails were lysed in 100 mmol/L NaCl, 10 mmol/L Tris/HCl pH 8.0, 1 mmol/L EDTA, 1% sodium dodecyl sulfate (SDS), 150 μg/mL proteinase K (Sigma, St Louis, MO), 1 mg/mL self-digested Pronase E (Sigma) overnight at 37°C. Debris was removed by rapid centrifugation. Samples were extracted in phenol-chloroform and DNA was isolated by ethanol precipitation. Genomic DNA (10 μg) was digested with KpnI, resolved on 0.8% agarose gels, and transferred to nylon membranes (Hybond N+) by using standard transfer procedures. Prehybridization and hybridization were performed in 0.5 mol/L sodium phosphate buffer (pH 7.0), 7% SDS, 1 mmol/L EDTA, 1% bovine serum albumin (BSA), and 100 μg/mL salmon sperm DNA at 65°C. Probes were radiolabeled with 32P-dCPT by using the Multiprime DNA Labeling System (Amersham, Arlington Heights, IL) following the manufacturer′s instructions. Filters were washed twice in 50 mmol/L sodium phosphate buffer (pH 7.0), 1% SDS, 1 mmol/L EDTA, 0.5% BSA, and twice without BSA at 65°C. Washed filters were exposed on phosphorimager screens and analyzed on a phosphorimager analyzer (Molecular Dynamics, Sunnyvale, CA). A 500-bp KpnI fragment upstream of exon 2 was used as a flanking probe (Fig 1). For further confirmation, DNA from all ES cell clones was digested with several restriction enzymes and hybridized to probes for the neomycin coding region.
Flow cytometric analyses.The following MoAbs were used: anti-CD44 phycoerythrin (PE; clone IM7; Pharmingen, San Diego, CA), anti–CD44-PE (clone KM 201; Southern Biotechnology, Birmingham, AL), CD44 clone KM81 and clone KM114 (both obtained from American Type Culture Collection, Rockville, MD), anti–CD4-fluorescein isothiocyanate (FITC; clone YTS 191.1.2; Cedarlane Laboratories Ltd, Hornby, Ontario), anti –CD8-PE (clone YTS 169.4; Cedarlane), B220 (clone RA3-6B2; Pharmingen), and anti-CD3 (clone 1452C11; Pharmingen).
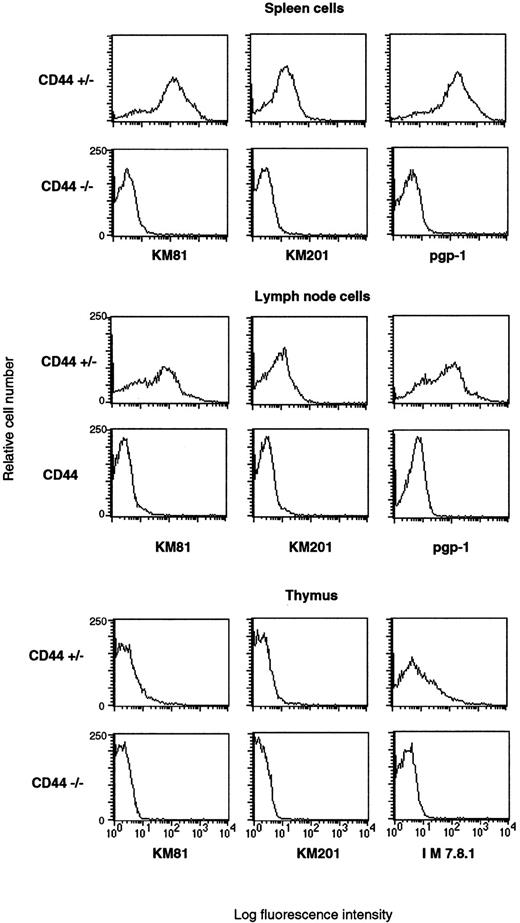
Spleen, lymph node, and thymus cells from CD44 gene mutated mice lack surface expression of CD44. Lymph node cells, spleen cells, and thymocytes were stained with anti-CD44 KM81 supernatants and secondary labeled with PE-conjugated goat antirat, PE-conjugated anti-CD44 KM201, or PE-conjugated anti-CD44 IM 7.8.1 (pgp-1). Mice with homozygous disruptions in the CD44 gene show no detectable surface expression. FCM data were collected for viable lymphocytes according to side scatter (SSC) and foreward scatter (FSC).
Spleen, lymph node, and thymus cells from CD44 gene mutated mice lack surface expression of CD44. Lymph node cells, spleen cells, and thymocytes were stained with anti-CD44 KM81 supernatants and secondary labeled with PE-conjugated goat antirat, PE-conjugated anti-CD44 KM201, or PE-conjugated anti-CD44 IM 7.8.1 (pgp-1). Mice with homozygous disruptions in the CD44 gene show no detectable surface expression. FCM data were collected for viable lymphocytes according to side scatter (SSC) and foreward scatter (FSC).
Thymus, spleen, and lymph node (LN) suspensions were prepared according to standard procedures. Cells (5 × 105) were incubated in fluorescence-activated cell sorting (FACS) staining buffer (2% fetal calf serum [FCS], 10 mmol/L EDTA, 15 mmol/L NaN3 in phosphate buffered saline [PBS]) with MoAbs for 30 minutes at 4°C. Cells were washed twice in staining buffer, resuspended in PBS and fixed with 1% paraformaldehyde. Cells stained with unlabeled antibodies were washed, incubated with a secondary FITC- or PE-conjugated goat antirat or antihamster antiserum (Southern Biotechnology), and resuspended and fixed as described previously. A total of 10,000 gated viable lymphocytes were analyzed using a FACScan flow cytometer and Lysis II software program (Becton Dickinson, San Jose, CA). Windows for analysis of granulocytes and lymphocytes were set using side scatter and forward scatter.
Fetal liver colony-forming unit-spleen (CFU-S) assay.CD44−/− females were crossed with CD44+/− male mice, and the females were checked for vaginal plaques. At day 13 of pregnancy, fetal liver cells from individual embryos were prepared according to standard procedures. Fetal liver cells were injected intravenously (IV) into 8-week-old C57BL/6J female mice that had been previously irradiated (850 rads). Four individual recipients for every fetal liver were injected with 2.3 × 104 cells resuspended in 300 μL PBS. Remaining cells were cultured and stained with KM201 for CD44 expression. Control mice were injected with PBS alone. Twelve days after transfer, injected recipients were sacrificed and the spleens transferred into fixing buffer (75% vol picric acid solution, 25% vol formaldehyde solution, and 5% vol acetic acid solution). Colonies were counted using a magnifying glass. The results were expressed as an average of the number of spleen colonies in mice injected with fetal liver cells from CD44+/− or CD44−/− mice.
Colony assay.CD44+/− and CD44−/− mice (matched for age and sex) were injected subcutaneously twice daily on days 0 to 5 with granulocyte colony-stimulating factor (G-CSF ) from Amgen (125 μg/kg body weight) diluted in PBS. Control mice received PBS only. At day 7, peripheral blood (PB), BM, and spleen cells were isolated according to standard procedures. Leukocytes were counted individually in these tissues and then pooled. Three-fold dilutions of pooled BM, spleen, and PB leukocytes (BM and PB, 104 to 106 cells; spleen, 104 to 3 × 105 white blood cells) were resuspended in Iscove's modified Dulbecco's medium supplemented with fetal bovine serum, 2-mercaptoethanol (2-ME) and either recombinant interleukin-3 (IL-3), erythropoietin (EPO), macrophage colony-stimulating factor (M-CSF ), or supernatant containing IL-1, IL-3, IL-11, erythropoietin (Epo), transferrin, cystine, HEPES, insulin, lipids, and 5637. Cells were plated in 1% methylcellulose as quadruplicates and incubated at 37°C in 5% CO2. Total colony numbers were counted at day 8. Differential counts were taken 10 days after plating. Discrimination of colonies was based on colony morphology and color. Colony-forming unit–granulocyte-macrophage (CFU-GM) was measured at day 5, 3 hours after the last G-CSF and PBS treatments.
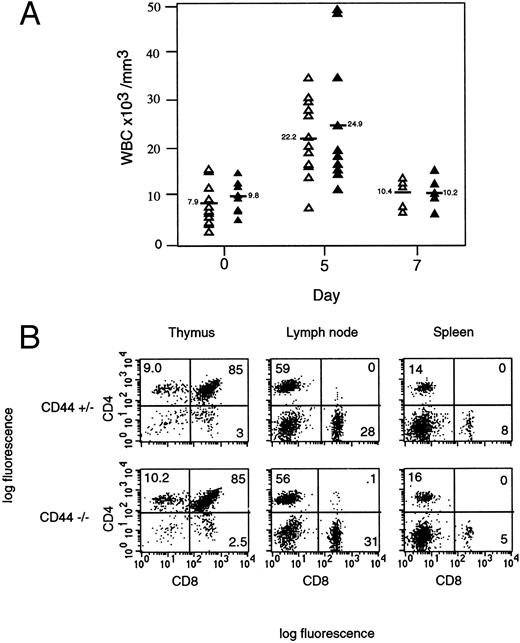
Normal leukocyte and T-cell numbers. (A) White blood cell (WBC) counts are unaltered in CD44−/− mice. Mice were injected twice daily for 4 days with G-CSF. Control mice were injected with PBS, indicated as day 0. Mice were sacrificed at days 5 and 7 after the start of G-CSF treatment, and peripheral blood was collected individually by heart bleeding. White blood cells were analyzed with a Coulter counter (Coulter, Miami, FL) calibrated for mice cells. Results from individual mice are shown. CD44−/−, open triangles and CD44+/−, closed triangles. Average white blood cells are indicated with bars and values. (B) Normal T-cell subsets in thymus, lymph nodes, and spleen. Different organs were used to examine T cells from CD44+/+, CD44+/−, and CD44−/− mice. Thymocytes, lymph node, and spleen cells were stained with PE-conjugated anti-CD8 and FITC-conjugated anti-CD4. No detectable difference was seen in total T-cell number or CD4/CD8 ratio in these organs. FCM data were collected for viable lymphocytes according to SSC and FSC.
Normal leukocyte and T-cell numbers. (A) White blood cell (WBC) counts are unaltered in CD44−/− mice. Mice were injected twice daily for 4 days with G-CSF. Control mice were injected with PBS, indicated as day 0. Mice were sacrificed at days 5 and 7 after the start of G-CSF treatment, and peripheral blood was collected individually by heart bleeding. White blood cells were analyzed with a Coulter counter (Coulter, Miami, FL) calibrated for mice cells. Results from individual mice are shown. CD44−/−, open triangles and CD44+/−, closed triangles. Average white blood cells are indicated with bars and values. (B) Normal T-cell subsets in thymus, lymph nodes, and spleen. Different organs were used to examine T cells from CD44+/+, CD44+/−, and CD44−/− mice. Thymocytes, lymph node, and spleen cells were stained with PE-conjugated anti-CD8 and FITC-conjugated anti-CD4. No detectable difference was seen in total T-cell number or CD4/CD8 ratio in these organs. FCM data were collected for viable lymphocytes according to SSC and FSC.
Overall Reduction of Colonies Formed by Spleen-Derived Hematopoietic Progenitor Cells in CD44-Deficient Mice
| . | . | PBS . | G-CSF . |
|---|---|---|---|
| Spleen (colonies/105 cells) | CD44+/− | 13.25 ± 2.75 | 203.50 ± 11.00 |
| CD44−/− | 7.25 ± 2.63 | 113.00 ± 12.40 | |
| (P = .02) | (P < .001) | ||
| Bone marrow (colonies/104 cells) | CD44+/− | 27.25 ± 4.92 | 22.75 ± 4.99 |
| CD44−/− | 30.75 ± 5.85 | 33.00 ± 9.90 | |
| (P = .395) | (P = .022) | ||
| Peripheral blood (colonies/105 cells) | CD44+/− | — | 10.82 ± 4.32 |
| CD44−/− | — | 4.16 ± 5.46 | |
| (P = .149) |
| . | . | PBS . | G-CSF . |
|---|---|---|---|
| Spleen (colonies/105 cells) | CD44+/− | 13.25 ± 2.75 | 203.50 ± 11.00 |
| CD44−/− | 7.25 ± 2.63 | 113.00 ± 12.40 | |
| (P = .02) | (P < .001) | ||
| Bone marrow (colonies/104 cells) | CD44+/− | 27.25 ± 4.92 | 22.75 ± 4.99 |
| CD44−/− | 30.75 ± 5.85 | 33.00 ± 9.90 | |
| (P = .395) | (P = .022) | ||
| Peripheral blood (colonies/105 cells) | CD44+/− | — | 10.82 ± 4.32 |
| CD44−/− | — | 4.16 ± 5.46 | |
| (P = .149) |
CD44 influences number/mobilization of hematopoietic progenitor cells. Mice were treated with G-CSF (125 μg/kg body weight) or PBS twice daily for 4 days. At day 7 spleen, bone marrow, and peripheral blood cells were collected. Colonies were grown in supplemented Iscove's Modified Dulbecco's Medium 1% methylcellulose. Colonies were counted at day 8 after plating. Values are presented as mean ± standard error for quadruplicate cultures. Significance was calculated by Student's t-test.
Abbreviations: PBS, phosphate buffered saline; G-CSF, granulocyte colony-stimulating factor.
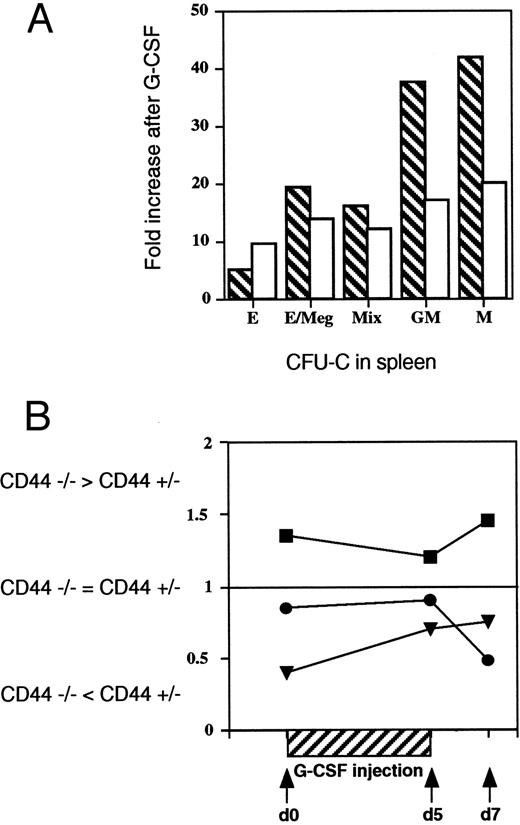
Impaired distribution of CFU-GM and CFU-M. (A) Decreased spleen-derived CFU-GM and CFU-M colonies in CD44−/− mice. The specific kinds of CFU-C in the spleens of G-CSF treated and untreated CD44−/− and +/− mice were characterized and counted separately for each mouse to determine the numbers of CFU-E/BFU-E, CFU-E/Meg, CFU-G, CFU-M, and CFU-GM. The bar graph shows reduced increases of CFU-GM and CFU-M in CD44−/− (open) compared with CF44+/− (crosshatched) mice. Increases represent (specific colony) ratios for G-CSF-treated and -untreated animals. (B) Altered tissue distribution of CSF-GM colonies in CD44−/− mice after G-CSF treatment. GM-CSF colony number derived from bone marrow (BM), spleen, and peripheral blood of CD44−/− and CD44+/− mice were compared at the indicated time points after the start of G-CSF treatment. The quotient of CFU-GM colonies from CD44−/− and CD44+/− were given for BM (closed squares), spleen (closed triangles), and PB (closed circles). Similar data were obtained from four independent tests with pools of two to five mice per group/experiment. The data show that at all times examined, CD44−/− mice have fewer progenitors in the spleen than +/− control littermates (ie, ratio < 1). Conversely, CD44−/− mutants have elevated numbers of CFU-GM progenitors in the bone marrow compared with +/− control mice (ie, ratio < 1).
Impaired distribution of CFU-GM and CFU-M. (A) Decreased spleen-derived CFU-GM and CFU-M colonies in CD44−/− mice. The specific kinds of CFU-C in the spleens of G-CSF treated and untreated CD44−/− and +/− mice were characterized and counted separately for each mouse to determine the numbers of CFU-E/BFU-E, CFU-E/Meg, CFU-G, CFU-M, and CFU-GM. The bar graph shows reduced increases of CFU-GM and CFU-M in CD44−/− (open) compared with CF44+/− (crosshatched) mice. Increases represent (specific colony) ratios for G-CSF-treated and -untreated animals. (B) Altered tissue distribution of CSF-GM colonies in CD44−/− mice after G-CSF treatment. GM-CSF colony number derived from bone marrow (BM), spleen, and peripheral blood of CD44−/− and CD44+/− mice were compared at the indicated time points after the start of G-CSF treatment. The quotient of CFU-GM colonies from CD44−/− and CD44+/− were given for BM (closed squares), spleen (closed triangles), and PB (closed circles). Similar data were obtained from four independent tests with pools of two to five mice per group/experiment. The data show that at all times examined, CD44−/− mice have fewer progenitors in the spleen than +/− control littermates (ie, ratio < 1). Conversely, CD44−/− mutants have elevated numbers of CFU-GM progenitors in the bone marrow compared with +/− control mice (ie, ratio < 1).
T-cell stimulation assays.LN and spleen cell suspensions were prepared according to standard procedures. Spleen cells were enriched for T cells using enrichment columns (R&D Systems, Minneapolis, MN). LN or T-cell enriched splenocytes were resuspended in HL-1 (Hycor, Irvine, CA) media supplemented with 2% heat inactivated FCS. Cells (1 × 105) were placed in flat-bottomed 96-well plates to which phorbol 12-myristate 13-acetate (PMA)-calcium ionophore (250 ng/mL calcium ionophore and 12.5 ng/mL PMA) had been added. Proliferation in response to soluble anti-CD3 (clone 145 2C11; Pharmingen; 10, 5, 2.5, 1.25, 0.6, 0.3, and 0.15 μg/mL), Con A (5, 2, 1, 0.5, and 0.25 μg/mL), staphylococcal enterotoxin B (SEB) (S4881; Sigma), and IL-2 was tested by using a range of concentrations and time points over several days. For T-cell receptor (TCR) crosslinking, 96-well flat-bottomed plates were coated overnight with 10 μg purified goat antihamster IgG serum. Plates were washed twice before incubation with anti-CD3 (KT3; rat IgG). Cells were harvested at intervals (2 to 6 days) after a 6- to 12-hour pulse with 1 μCi of [3H]thymidine per well. Proliferation was measured by 3H-thymidine incorporation using a Matrix 96-Beta-counter (Canberra Packard, Meriden, CT).
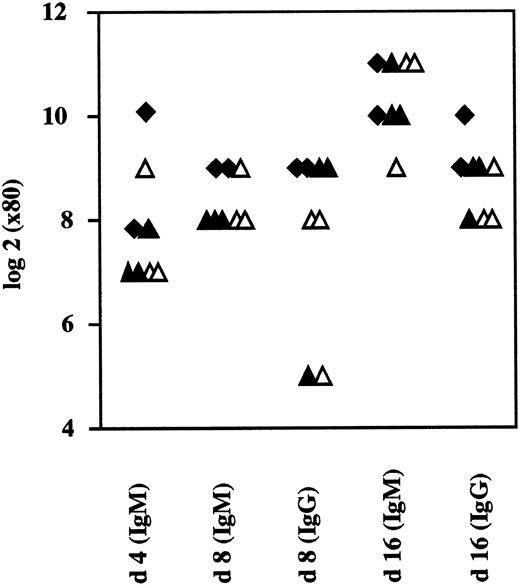
Normal antivirus IgM and IgG responses in CD44−/− mice. Mice were immunized with VSV (2 × 106 pfu IV) and analyzed for neutralizing antibodies in the serum. No differences were seen for neutralizing IgM or IgM between CD44−/−, +/−2, or +/+ mice. (closed diamonds, +/+; closed triangles, +/−; open triangles, −/−).
Normal antivirus IgM and IgG responses in CD44−/− mice. Mice were immunized with VSV (2 × 106 pfu IV) and analyzed for neutralizing antibodies in the serum. No differences were seen for neutralizing IgM or IgM between CD44−/−, +/−2, or +/+ mice. (closed diamonds, +/+; closed triangles, +/−; open triangles, −/−).
Mixed lymphocyte reaction.T cells from LNs and spleens of CD44+/+, CD44+/−, and CD44−/− mice (representing a mixture of 129J and C57BL/6 backgrounds, both H-2b) were prepared as described previously and used as responder cells. Spleen cells from C57BL/6 (H2b), B10BR (H2k), BALB/c (H-2d), BM1 (B6.C H2bm1/ByJ), and BM12 (B6.C H2bm12/khEG) to be used as stimulator cells were prepared according to standard procedures. Irradiated (2000R) responder cells (1 × 105/well) and stimulator cells (1 × 105/well) were cultured together in HL-1 media supplemented with 2% heat-inactivated FCS. Cells were monitored for blast formation and pulsed overnight with 1 μCi of [3H] thymidine per well at various intervals (4 to 6 days). Analyses were performed as described previously.
Virus-specific Ig response.Mice were immunized with vesicular stomatitis virus serotype New Jersey (VSV-NJ; 2 × 106 plaque forming units [pfu] IV). Blood was taken from tail veins on days 4, 8, and 16. Sera were tested as previously described.68 Briefly, sera were 40-fold diluted in supplemented Dulbecco's minimal essential medium (DMEM) followed by heat inactivation for 30 minutes at 56°C. Serial twofold dilutions were mixed with equal volumes of virus dilutions containing 500 pfu/mL. The mixtures were incubated at 37°C, 5% CO2 for 90 minutes. A total of 100 μL of each serum-virus mixture was transferred onto Vero cell monolayers in 96-well plates and incubated at 37°C for 1 hour. The monolayers were overlayed with 100 μL DMEM containing 5% FCS and 1% methylcellulose. After an additional incubation at 37°C for 24 hours, the overlays were removed and the monolayer fixed and stained with 0.5% crystal violet. The highest dilution of serum that reduced the number of plaques by 50% was taken as the titer. To determine IgG titers, undiluted serum was pretreated with an equal volume of 0.1 mol/L 2-ME in saline, which eliminates IgM while preserving IgG.69
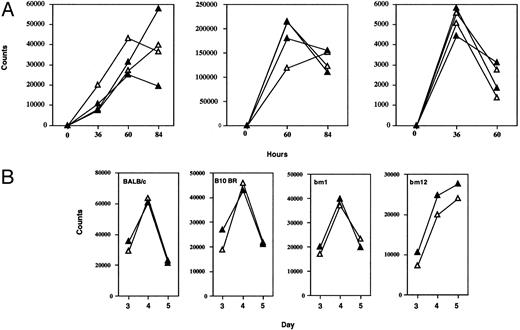
Lymphocyte function in mutant mice. (A) Proliferation assay. T-cell enriched splenocytes and lymph node cell suspensions were prepared and stimulated with Con A (2.5 μg/mL), soluble hamster antimouse CD3 (10 μg/mL), or PMA (125 μg/mL) + Ca Ionophore (50 μL/mL). At indicated time points, proliferating cells were pulsed with 1 μCi 3H-thymidine and cultured overnight. Data represent average 3H-uptake of quadruplicates (closed triangles, +/−; open triangles, −/−). Similar results were obtained in three independent experiments. (B) MLRs were performed by mixing irradiated stimulator spleen cells (1 × 105) from C57BL/6, BALB/c, B10.BR, BM1, or BM12 mice with responder T-cell enriched splenocytes and lymph node cells (1 × 105) harvested from +/− (closed triangles) or −/− (open triangles) mice. Each line represents an individual mouse. (Results are shown for days 3, 4, and 5; similar results were obtained in three independent tests when littermates were used as responders.)
Lymphocyte function in mutant mice. (A) Proliferation assay. T-cell enriched splenocytes and lymph node cell suspensions were prepared and stimulated with Con A (2.5 μg/mL), soluble hamster antimouse CD3 (10 μg/mL), or PMA (125 μg/mL) + Ca Ionophore (50 μL/mL). At indicated time points, proliferating cells were pulsed with 1 μCi 3H-thymidine and cultured overnight. Data represent average 3H-uptake of quadruplicates (closed triangles, +/−; open triangles, −/−). Similar results were obtained in three independent experiments. (B) MLRs were performed by mixing irradiated stimulator spleen cells (1 × 105) from C57BL/6, BALB/c, B10.BR, BM1, or BM12 mice with responder T-cell enriched splenocytes and lymph node cells (1 × 105) harvested from +/− (closed triangles) or −/− (open triangles) mice. Each line represents an individual mouse. (Results are shown for days 3, 4, and 5; similar results were obtained in three independent tests when littermates were used as responders.)
Primary ex vivo cytotoxicity assay.VSV grown in baby hamster kidney (BHK) cells was kindly provided by Dr Lud Prevec (McMaster University, Hamilton, Ontario, Canada). Mice were infected with VSV (2 × 106 pfu, IV) and spleens were removed after 6 or 8 days. Spleen cells were incubated for 5 hours with 51Cr-labeled EL-4 (H-2b) target cells, which had been infected with VSV. EL-4 cells were infected with VSV for 2 hours (15 pfu per cell) before the assay. Specific lysis was calculated as (experimental release) − (spontaneous release)/(total release-spontaneous release) × 100%.
Secondary response against VSV.Mice were immunized IV with VSV (2 × 106 pfu, Indiana strain). After 40 and 80 days, spleen cells were restimulated with specific antigen by adding VSV nuclear protein class-1–binding peptide (50 mmol/L) to bulk cultures as previously described.70 After 5 days, in vitro cultures were harvested and tested for specific cytotoxicity against VSV-infected EL-4 (H-2b) target cells. Specific lysis was calculated as described previously.
Lymphocytic choriomeningitis virus (LCMV) footpad assay and secondary LCMV challenge.Mice were injected with 30 μL containing 100 pfu LCMV-Armstrong strain into one hind footpad. Swelling of the footpad was measured daily or every other day with a spring-loaded caliper.
Mice were infected IV with 2,000 pfu LCMV-Armstrong. At day 80 the same animals were injected intracerebrally with 100 pfu LCMV-Armstrong. Mice were monitored daily for signs of choriomeningitis and lethality.
Contact hypersensitivity assay.Contact hypersensitivity was elicited in mice using 2,4 dinitrofluorobenzene (DNFB). In brief, 6- to 8-week-old mice were immunized on shaved abdomens with 50 μL 0.5% DNFB in 4:1 acetone/olive oil at day 0 and day 1. At day 5 these mice were challenged with 10 μL 0.2% DNFB in 4:1 acetone/olive oil on the inner and outer side of one ear. Ear thickness was measured 12, 24, 36, and 48 hours after reexposure and compared with the untreated ear.
Corynebacterium parvum.CD44−/− and wild-type control mice (C57BL/6) between 10 and 15 weeks of age were used to study C parvum-induced granuloma formation. Granulomas were induced by a single IV injection of 7.5 mg/kg of heat-killed C parvum (Ribi Immunochem Research, Hamilton, MT). Mice were sacrificed and analyzed 2 weeks later. Liver and spleen were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Granulomas were counted in the liver at ×200 magnification in 10 randomly marked microscopic fields. To quantify granuloma size, the number of cells per granuloma was counted. Results are expressed as the mean ± standard deviation; comparisons for significance were performed using Student's t-test.
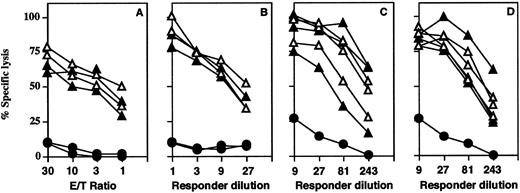
CTL responses are normal in CD44−/− mice. (A) CD44+/− (closed triangles) and CD44−/− (open triangles) mice were infected IV with VSV (2 × 106 pfu). VSV-infected syngeneic (EL-4) cells were labeled with 51Cr-label and used as targets. Closed circles represent noninfected control CD44+/+ mice. Specific lysis of noninfected control targets less than 5%; spontaneous lysis of all targets less than 25%. (B-D) Secondary restimulation against VSV is normal in CD−/− mice. Splenocytes from mice immunized with VSV (2 × 106 pfu) on day 40 (B) or 80 (C and D) were restimulated with VSV nuclear protein class-1–binding peptide for 5 days in the absence (B and C) or presence (D) of ConA supernatant. Specific cytotoxicity against VSV-infected EL-4 (H-2b) target cells was comparable with heterozygous control mice. Specific lysis of noninfected EL-4 cells is indicated by closed circles and was below 10% for all effector cells used. Spontaneous release was below 20% for both targets.
CTL responses are normal in CD44−/− mice. (A) CD44+/− (closed triangles) and CD44−/− (open triangles) mice were infected IV with VSV (2 × 106 pfu). VSV-infected syngeneic (EL-4) cells were labeled with 51Cr-label and used as targets. Closed circles represent noninfected control CD44+/+ mice. Specific lysis of noninfected control targets less than 5%; spontaneous lysis of all targets less than 25%. (B-D) Secondary restimulation against VSV is normal in CD−/− mice. Splenocytes from mice immunized with VSV (2 × 106 pfu) on day 40 (B) or 80 (C and D) were restimulated with VSV nuclear protein class-1–binding peptide for 5 days in the absence (B and C) or presence (D) of ConA supernatant. Specific cytotoxicity against VSV-infected EL-4 (H-2b) target cells was comparable with heterozygous control mice. Specific lysis of noninfected EL-4 cells is indicated by closed circles and was below 10% for all effector cells used. Spontaneous release was below 20% for both targets.
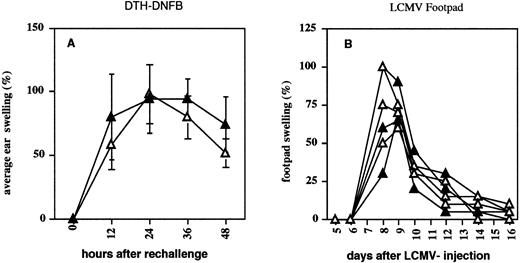
CD4- and CD8-mediated DTH response in CD44−/− mice. Four CD44+/− (closed triangles) and four CD44−/− (open triangles) mice were immunized (A) on shaved abdomen and rechallenged on one ear with DNFB. Ear thickness is shown as a percentage of swelling compared with the untreated ear. (B) CD44+/− and CD44−/− mice were infected with 100 pfu LCMV at day 0, and footpad swelling was measured every day or every other day. Swelling is given as percent increase compared with footpad size before infection.
CD4- and CD8-mediated DTH response in CD44−/− mice. Four CD44+/− (closed triangles) and four CD44−/− (open triangles) mice were immunized (A) on shaved abdomen and rechallenged on one ear with DNFB. Ear thickness is shown as a percentage of swelling compared with the untreated ear. (B) CD44+/− and CD44−/− mice were infected with 100 pfu LCMV at day 0, and footpad swelling was measured every day or every other day. Swelling is given as percent increase compared with footpad size before infection.
Transfection and transformation of primary fibroblasts from CD44-deficient mice.Primary fibroblasts derived from mouse embryos were transfected with pSV3-neo (a plasmid containing the SV40 large T antigen) using lipofectin (GIBCO, Grand Island, NY). Transformed cells that survived in tissue culture after crisis were pooled and expanded. SV40 large T-transformed fibroblasts were infected with the murine stem cell virus (MSCV) defective retrovirus71 carrying the full length mouse CD44s.72 Infected cells were selected in 150 mg/mL of Hygromycin B. Hygromycin B-resistant clones were pooled but also individual clones were isolated by using cloning cylinders. Cell lysates were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blot analysis was performed, as previously described, by using the KM201 antimouse CD44 MoAb.
Tumorigenicity assay.BALB/c nude mice were injected subcutaneously with 5 × 106 cells (5 mice/cell line). After 2 months the mice were sacrificed and tumors measured using a caliper. Tumors were fixed in formalin and paraffin embedded sections and immunostained as previously described73 by using an anti-SV40 large T antibody (Oncogene Science, Uniondale, NY) and the anti-CD44 antibody KM201. The 96-well plates were covered with 0.5 mg/mL of hyaluronic and incubated overnight at 40°C. Plates were washed with PBS and adhesion assay was performed as previously described74 in the presence or absence of anti-CD44 antibody KM201.
RESULTS
Generation of CD44-deficient mice.Exons 2 and 3 of the CD44 gene were disrupted by an antisense neomycin (neo) gene expression vector (Fig 1A). Colonies of G418-resistant embryonic stem cells were screened for homologous recombination events by the PCR, and positive clones were confirmed by genomic Southern blot analysis using a flanking probe upstream of the targeting vector. ES cell lines were also probed with neomycin using multiple restriction enzymes (data not shown). Five of the six PCR-positive colonies, which had a homologous recombination event and showed a single neo integration, were injected into blastocysts of C57BL/6 mice. The resulting chimeras were backcrossed into C57BL/6J mice. Germline transmission of the mutation in all five ES cell lines was documented by Southern blotting (Fig 1B). Heterozygous offspring were bred to obtain homozygous gene-targeted mice and further backcrossed into C57BL/6J. To confirm the absence of CD44 expression in gene-targeted mice, we examined the surface levels of CD44 on wild-type, heterozygous, and knock-out mice. Immunofluorescence analysis using different MoAbs against murine CD44 was performed on leukocytes obtained from different organs. All MoAbs tested (IM7, KM81, KM114, and KM201) confirmed the predicted absence of CD44 expression (Fig 2), whereas a small reduction in CD44 expression levels was seen between in heterozygous mice (data not shown). Heterozygous and homozygous mutant animals were born in Mendelian ratio, survived, grew normally, and were fertile. Animals remained healthy when maintained under specific pathogen-free conditions.
Enlarged Number and Size of Granulomas Induced by Corynebacterium parvum in CD44−/− Mice
| Genotype . | Number . | Size . |
|---|---|---|
| CD44+/− | 17.8 ± 9.7 | 25.2 ± 1.5 |
| CD44−/− | 30.5 ± 9.0 | 36.5 ± 3.7 |
| (P < .03) | (P < .001) |
| Genotype . | Number . | Size . |
|---|---|---|
| CD44+/− | 17.8 ± 9.7 | 25.2 ± 1.5 |
| CD44−/− | 30.5 ± 9.0 | 36.5 ± 3.7 |
| (P < .03) | (P < .001) |
CD44 influences the formation of C parvum-induced granulomas. CD44-deficient and wild-type mice (n = 6) were injected with 7.5 mg/kg of heat-killed C parvum. Mice were sacrificed 14 days after injection, and liver sections were analyzed for granuloma formation. Significance was calculated by Student's t-test.
Normal organ morphology and unaltered leukocyte numbers/lymphocyte subsets.Six- to 8-week-old mice did not show any gross abnormalities in size, cellularity, or tissue architecture of major organ systems. CD44−/− mice showed normal hematopoiesis, and white blood cell counts were comparable to controls both before and after treatment with G-CSF (Fig 3A). Analysis of lymphocyte subsets in spleen and LNs showed normal distribution and numbers of CD4+ and C8+ T cells. CD44 is normally expressed on prothymocytes12,75 and thymocytes, thus CD44 deficiency might be expected to influence thymocyte ontogeny.76-78 However, in CD44 mutant mice CD4+ and CD8+ thymocytes showed normal total numbers and subset populations (Fig 3B).
CD44 is important for CFU-GM emigration from BM.CFU-S progenitors were quantitated in fetal liver of CD44−/− mice to analyze the homing of myeloid progenitor cells to fetal liver as well as to study the influence of the CD44 deficiency on CFU-S survival and proliferation. Fetal liver cells from five CD44+/− and three CD44−/− 13.5-day embryos were injected IV into 8-week-old C57BL/6J females previously irradiated with 850R and spleen colonies enumerated 12 days after transfer. No significant differences were found in the number of fetal liver cells or spleen colonies (CD44+/−, 5.28 ± 2.32; CD44−/−, 6.04 ± 2.56). Similar results were obtained when the numbers of CFU-S d12 were determined by using cells from day 14.5 embryos (data not shown). Nonetheless, other studies have suggested that interactions between hematopoietic progenitors and stromal cells are critical for normal maturation, survival, and egress of progenitors from BM79,80 and that these may involve CD44.7 Thus, we also examined BM, PB, and spleen in mutant mice for hematopoietic colony forming cells and for the ability to mobilize colonies after G-CSF treatment. CD44-deficient mice showed reduced total numbers of CFUs in spleen and PB (Table 1). CFU-GM and CFU-macrophage (CFU-M) colonies were significantly reduced in the spleen (Fig 4A). The number of CFU-erythroid/burst-forming unit-erythroid (BFU-E), CFU-erythroid/Meg, and CFU-Mix from spleen was unchanged in mutant mice compared with heterozygous controls. Conversely, the total number of colonies, as well as CFU-GM, were elevated in the BM of CD44−/− mice compared with CD44+/− control animals.
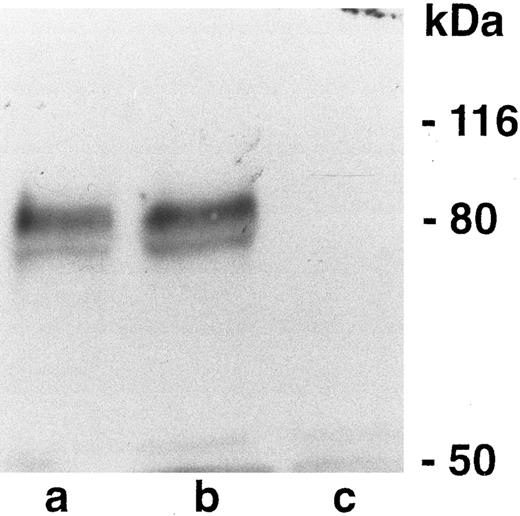
Western blot analysis of CD44 in CD44-negative and -positive fibroblasts. (a) CD44 positive (pooled clones). (b) CD44 positive (single clone). (c) CD44 negative. Numbers on the right represent molecular weight markers.
Western blot analysis of CD44 in CD44-negative and -positive fibroblasts. (a) CD44 positive (pooled clones). (b) CD44 positive (single clone). (c) CD44 negative. Numbers on the right represent molecular weight markers.
CD44-Transfected Fibroblasts Show Enhanced Binding to Hyaluronic Acid
| Cells . | Attachment (OD)3-150 . | |
|---|---|---|
| Anti-CD44 antibody . | − . | + . |
| CD44− | 0.309 | 0.327 |
| Vector alone | 0.234 | 0.211 |
| CD44+ (pooled) | 0.721 | 0.323 |
| CD44+ (single clone) | 0.947 | 0.320 |
| Cells . | Attachment (OD)3-150 . | |
|---|---|---|
| Anti-CD44 antibody . | − . | + . |
| CD44− | 0.309 | 0.327 |
| Vector alone | 0.234 | 0.211 |
| CD44+ (pooled) | 0.721 | 0.323 |
| CD44+ (single clone) | 0.947 | 0.320 |
Abbreviation: OD, optical density.
Results represent the average of triplicates. Standard deviation was <20%.
BALB/c Nude Mice Were Injected Subcutaneously With 5 × 106 Cells (5 mice/cell line)
| Cells . | Experiment 1 (tumor size mm3) . | Experiment 2 (tumor size mm3) . |
|---|---|---|
| CD44− | 510; 486; 1296; 1181; 726 | 445; 1008; 405; 405; 1183 |
| Vector alone (CD44−) | 710; 384; 910; 343; 520 | ND |
| CD44+ (pooled) | 22; 13; 22; 0; 0 | 12; 24; 0; 0; 0 |
| CD44+ (single clone) | 16; 13; 0; 0; 0 | 18; 10; 10; 18; 0 |
| Cells . | Experiment 1 (tumor size mm3) . | Experiment 2 (tumor size mm3) . |
|---|---|---|
| CD44− | 510; 486; 1296; 1181; 726 | 445; 1008; 405; 405; 1183 |
| Vector alone (CD44−) | 710; 384; 910; 343; 520 | ND |
| CD44+ (pooled) | 22; 13; 22; 0; 0 | 12; 24; 0; 0; 0 |
| CD44+ (single clone) | 16; 13; 0; 0; 0 | 18; 10; 10; 18; 0 |
Abbreviation: ND, not determined.
After 2 months mice were sacrificed and tumors measured using a caliper. CD44− tumor cells have significant growth advantage in vivo. The original cell lines were derived from the intestines of CD44-deficient embryos after transformation with SV40 large T antigen. Transformed CD44−/− fibroblasts were transfected with CD44s or vector alone. The CD44-deficient embryos were obtained from breeding of homozygous-deficient mice (129J × C57BL/6; both H2-b).
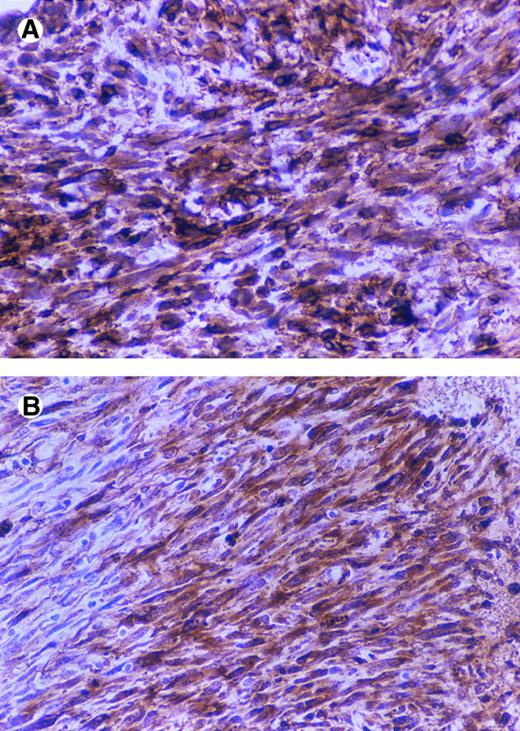
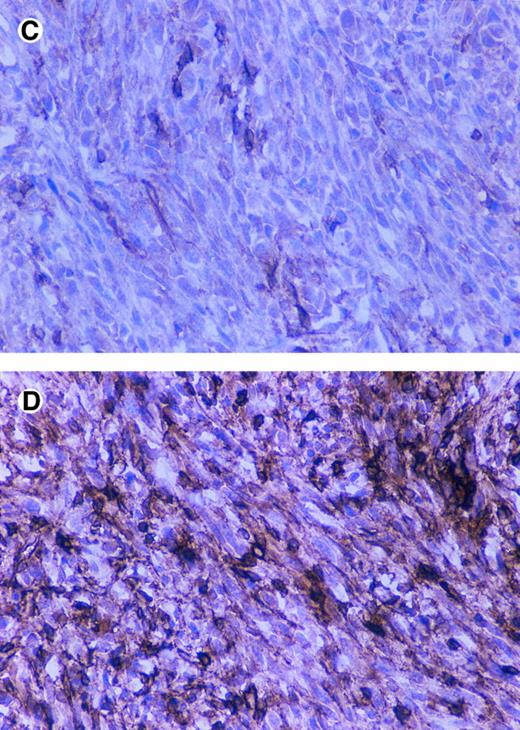
Immunostaining of CD44 and SV40 large T in tumors. BALB/c nude mice were injected subcutaneously with 5 × 106 cells (5 mice/cell line) and after 2 months were sacrificed and tumors were fixed in formalin and paraffin embedded sections and immunostained by using an anti-SV40 large T antibody and the anti-CD44 antibody KM201.CD44-positive tumor (A and B), CD44-negative tumor (C and D), anti-CD44 (A and C), and anti-SV40 large T (B and D).
Immunostaining of CD44 and SV40 large T in tumors. BALB/c nude mice were injected subcutaneously with 5 × 106 cells (5 mice/cell line) and after 2 months were sacrificed and tumors were fixed in formalin and paraffin embedded sections and immunostained by using an anti-SV40 large T antibody and the anti-CD44 antibody KM201.CD44-positive tumor (A and B), CD44-negative tumor (C and D), anti-CD44 (A and C), and anti-SV40 large T (B and D).
To further explore this result, CD44+/− and CD44−/− mice were treated with G-CSF twice daily for 5 days, and CFU-GM and CFU-M assays were performed at day 5 and day 7 (Fig 4B). The total number of colonies, as well as the number of CFU-GM detected in the spleen after mobilization were again significantly reduced in mice lacking CD44, whereas the number of all other tested hematopoietic progenitors from spleen and BM were comparable with numbers found in CD44+/− mice. Similar results were seen in four independent experiments in which organs of 2 to 5 animals were pooled.
Normal Ig levels and responses to VSV infection.Ig subclass irregularities have been shown to reflect general dysfunction of the immune system. Moreover, because CD44 expression fluctuates widely during B-cell development, it is conceivable that CD44 might play a role in T:B cell cooperation and in Ig class switching. We investigated whether the absence of CD44 affected the level of serum Ig in mice. We found mutant mice housed under pathogen-free conditions to have normal total serum Ig levels and normal levels of the various subclasses (IgM, IgG1, IgG2, IgG2a, IgG2b, IgG3, IgE, and IgA; data not shown). This suggested that B cells undergo normal class switching under these conditions and that cognate T:B interactions were not critically altered by an absence of CD44. B-cell IgM responses against VSV do not require T helper cells, although IgG responses require T help and, thus, sufficient T:B cell function and interaction. As a result, analysis of responses to VSV infections can provide information about both Th-dependent and Th-independent B-cell function. We injected mice IV with VSV and analyzed serum samples 4, 8, and 16 days later for specific IgM and IgG levels. No differences in VSV neutralizing IgM and IgG antibodies were detectable between CD44 heterozygous and homozygous mutant animals (Fig 5). Moreover, after immunization with ovalbumin and keyhole limpet hemocyanin (KLH) in the presence of incomplete or complete Freund's adjuvant, no differences in specific primary and secondary IgM, IgG, and IgG1 immune responses were detectable at various time points (data not shown).
T-cell proliferative responses, cytotoxic T-cell function (CTL), secondary restimulation, and immunological memory.CD44 has been proposed as a possible T-cell costimulatory molecule.14,23-27 81 We examined whether T cells from CD44-deficient mice had normal proliferative potential (Fig 6A). In vitro lymphocyte proliferative responses to soluble anti-CD3 antibodies, crosslinked anti-CD3 antibodies, SEB and ConA showed no differences between CD44+/+, CD44+/−, and CD44−/− mice, even when suboptimal doses of anti-CD3, Con A, and SEB were used (data not shown). Proliferation assays were also used to test allogeneic T-cell responses in mixed lymphocyte reactions (MLRs). Responder LN cells from CD44−/− mice (H2b) showed normal proliferation responses to BALB/c (H2d), B10 BR (H-2k), BM1 (H2bm1), and BM12 (H2bm12) stimulator cells (Fig 6B). Additionally, we examined the capacity of CD44-deficient spleen cells to act as stimulators in MLRs. No differences in proliferation of BALB/c (H2d), B10 BR (H2k), BM1, and BM12 derived responders were detectable against CD44+/− or CD44−/− stimulators (data not shown).
CD44 was initially reported to be a molecule centrally involved in NK and CTL killer function.28-32 Mice were injected IV with 2 × 106 pfu VSV 6 days before analysis. Spleens from infected mice were removed and splenocytes incubated with 51Cr labeled target cells infected with VSV. Contrary to expectations from previous reports, splenocytes from CD44−/− mice were found to mount normal ex vivo CTL responses to VSV (Fig 7A). CD44−/− mice also had normal survival after secondary intracerebral rechallenge with LCMV (data not shown).
Additionally, CD44 expression is widely accepted as a marker for lymphocyte memory cells,33-35 thus we used a VSV virus assay to assess the possible importance of CD44 in the development of memory cells. Splenocytes isolated after 40 (Fig 7B) and 80 days (Fig 7C and D) from VSV-infected mice were restimulated with VSV nuclear protein class-1–binding peptide in culture for 5 days. Splenocytes derived from CD44 mutant mice showed a specific cytotoxicity against VSV-infected EL-4 (H-2b) target cells that was comparable with heterozygous control mice.
CD44−/− mice mount delayed-type hypersensitivity (DTH) responses.Anti-CD44 MoAb treatment has been reported to reduce DTH inflammatory responses in mice.39 DTH responses require T-cell priming in draining LNs and migration of T cells to skin. Eight- to 12-week-old mice (C57BL/6Jx129J) were immunized twice with DNFB and rechallenged after 5 days on one ear. After 12, 24, 36, and 48 hours, ear thickness was measured and compared with the untreated ear. Homozygous-deficient mice when compared with heterozygous littermate controls showed a minor reduction of ear swelling, although the difference was not statistically significant (Fig 8A). Swelling reactions were also normal in mice after footpad infection with LCMV (Fig 8B).
Exaggerated granuloma response to C parvum. C parvum is an intracellular parasite that normally induces granuloma formation in tissues of infected animals. Moreover, cell-mediated immunity and granuloma formation are likely critical to recovery from C parvum infection.82 83 We injected heat-killed C parvum to specifically assess granuloma responses of mutant mice to C parvum. CD44-deficient mice developed a statistically significant 1.71-fold greater number of granulomas compared with wild-type controls. Granulomas were also larger in mutant mice (Table 2).
CD44 regulates tumorigenicity of fibroblasts.To study the potential role of CD44 in malignant transformation, we transfected primary fibroblasts derived from the intestine of CD44-deficient mice with the SV40 large T antigen. To compare the effect of CD44 in the same genetic background, CD44-positive fibroblasts were generated by infecting the SV40 large T-transformed fibroblasts with a defective retrovirus carrying the full length mouse CD44s cDNA. Anti-CD44 MoAb detects a band of the expected molecular weight in the pooled infected cells and in an independently derived single clone (Fig 9), which is not seen in a pooled population of CD44-negative fibroblasts infected with a retrovirus control; moreover, CD44-transfected fibroblasts showed significantly better attachment to HA compared with CD44−/− cells (Table 3). We did not detect any obvious differences in the morphology or in the in vitro growth properties between CD44-negative and -positive fibroblasts. CD44-negative fibroblasts gave rise to large subcutaneous tumors that forced us to sacrifice mice after 2 months. However, the CD44-positive fibroblasts gave rise to very small tumors over the same period of time (Table 4). Tumor sections were stained for SV40 large T antigen and CD44. The small tumors derived from CD44-containing fibroblasts retained CD44 expression, and both CD44-positive and -negative tumors expressed similar levels of SV40 large T antigen (Fig 10). Histologically both tumors were similar, although CD44-negative tumor cells showed more cellular pleomorphism and had a higher mitotic index.
DISCUSSION
The expression of CD44 molecules is modulated during the development and activation of a large variety of cell and tissue types. Given the extensive expression of CD44 during embryogenesis, we anticipated that mice lacking CD44 might die during embryonic stages of development or that they might manifest some form of developmental defects.4-6 To our surprise, CD44-deficient mice backcrossed into C57BL/6 were born healthy in a Mendelian ratio and developed normally. No gross deficits in neurological function or behavior were apparent.
During hematopoiesis, CD44 is highly expressed on early myeloid cells and downregulated during commitment to granulopoiesis.11 In addition, it has been shown that in BM cells sorted into CD44high and CD44low populations, only the CD44high expressing cells are able to form CFU-GM colonies.8 The presence of CD44 MoAbs in Dexter-type long-term BM cultures was shown to prevent the emergence of myeloid cells, most likely the result of blocked attachment and interaction between stromal cells and hematopoietic progenitors.7 More recently, it has been shown that anti-CD44 antibodies as well as antibodies against VLA-4 (alpha 4/beta 1-integrins) can block the formation of cell adhesion to fibronectin, an important molecule on BM stromal cells involved in the attachment of hematopoietic progenitors.9 However, CD44-deficient mice do not show a significant reduction in the total numbers of white blood cells or granulocytes in the PB. Furthermore, there was no significant difference in the number of hematopoietic progenitor cells in the fetal liver of CD44−/− mice. Finally, there was normal engraftment of CD44-deficient fetal liver cells into the spleen of wild-type recipients, indicated by comparable numbers of fetal liver-derived CFU-S colonies.
CD44-deficient mice showed a defect in the distribution of granulocyte-macrophage progenitor cells between BM and spleen. There were significantly lower numbers of CFU-GM colonies in the spleen as well as in the PB. Interestingly, this phenotype became more prominent after stimulation with G-CSF. At the same time, whereas CFU-GM were reduced from PB and spleen, the numbers of these progenitor cells were elevated from BM of mutant animals. Thus, despite reduced numbers of GM progenitors in blood and spleen, the accumulation in BM leads us to the conclusion that CD44 deficiency probably causes an altered distribution of GM progenitors rather than a general defect in maturation. An earlier report suggested that galactosyl and mannosyl structures on the surface of CFU-GM might be involved in interactions between CFU-GM and marrow stromal cells.84 These interactions may include CD44 and be important in determining the egress of CFU-GM from BM.
Although the maturation of CFU-GM in BM appears not to require stimulation through the CD44 receptor, such signaling may be required for the normal release or distribution of cytokines in spleen or PB. Three lines of evidence support a role for CD44 in regulating cytokine release and distribution. (1) It has been shown that stimulation through the CD44 receptor can induce production of a number of different growth factors, including M-CSF, tumor necrosis factor-α (TNFα), interferon-γ (IFN-γ), insulin-like growth factor-1, and TNFβ.85-88 (2) Certain cytokines (ie, MIP-1β, β-FGF, and HB-EGF) bind glycosaminoglycans, increasing the possibility that glycosaminoglycans on CD44 might compete with cytokines and, therefore, regulate cytokine binding to surrounding cells. (3) CD44 may immobilize cytokines directly on the cell surface and present them to other cell types.58,89 90 It is conceivable that CD44 may be involved in either the production or delivery of cytokines that could be important in the regulation of cell:cell interaction and in the tissue distribution of CFU-GM progenitors.
CD44 is expressed on erythropoietic progenitors. Kansas et al11 reported high levels of CD44 on early progenitor cells capable of erythroid development and observed CD44 downregulation during erythroid differentiation. More recently, Ghaffari et al10 found that the majority of BM cells capable of differentiating into BFU-E were CD44+, although expression levels were not high and were not downregulated during differentiation. In our experiments, we did not see a significant difference in the number of erythrocytes (red blood cells) or BFU-E colonies in CD44-deficient animals compared with controls. As a consequence, we cannot provide any evidence that would suggest a critical role for CD44 in erythroid development.
Fetal or BM thymocyte progenitors display a fluctuating expression of CD44 during thymocyte ontogeny.12,76-78 It has been reported that the binding of prothymocytes to the thymus endothelial cells is mediated through the interaction of CD44 and that anti-CD44 antibodies can block colonization of the thymus.14,75 Thus, we had reasonable expectations that CD44-deficient mice might have abnormal numbers of thymocytes or thymocyte subsets. No such defects were seen in CD44-deficient mice. However, in same report by Patel et al,75 it was found that, in addition to CD44, β-1 integrins on immature T cells interact with fibronectin on endothelium; clearly this interaction may compensate for a lack of CD44 during T-cell development. It is still possible that undetectable defects in thymocyte-positive or -negative selection might become obvious when the CD44 mutation is introduced into TCR transgenic backgrounds, where highly exaggerated frequencies of specific T cells can be subjected to positive or negative selection in vivo.91
CD44 has been reported to be costimulatory in combination with CD2 and/or the TCR complex (CD3), although other studies have not observed costimulation with CD44/CD3 and have reported inhibitory effects after CD44 stimulation.24,92,93 In addition, CD44 has been found to augment CD28 costimulation in T cells, most likely in an IL-2–independent fashion.94 Nonetheless, activation of T cells is probably triggered for the most part by TCR interactions with the antigen-major histocompatibility complex (MHC), perhaps in combination with binding of the CD4/CD8 coreceptors and CD28. Thus, we were not surprised to see normal T-cell proliferative responses to various mitogenic stimuli, such as anti-CD3, PMA-Ca ionophore, SEB, and allogeneic cells. In our mutant mice, we could not detect a major costimulatory role for CD44, although such a function could well be compensated by other molecules, especially CD28.
The invariant chain (Ii) can be postranslationally modified by the addition of chondroitin sulfate (Ii-CS) before it associates with class II MHC molecules. CD44 binds Ii-CS, appears to augment the accessory function of Ii-CS, and may be involved in class II-dependent allogeneic and mitogenic T-cell responses.95 In MLRs using CD44−/− (C57BL/6 background) splenocytes as stimulators, we found no significant defect in the proliferation of T-cell responders from BALB/c (H2d), B10BR (H2k), BM1(H2bm1), or BM12(H2bm12) mice.
Further reports have suggested a role for CD44 in CTL, which may reflect the ability of CD44 to enhance IFN-γ, TNFα, and TNFβ production. After infection with VSV and LCMV, we saw no reduction in CTL in CD44-deficient mice. In vitro and in vivo results 40 and 80 days postinfection also suggested that CD44 did not have a critical role in generating T- and B-memory lymphocytes. We further investigated CD4 and CD8 T-cell function using DTH tests with DNFB and LCMV. These reactions require migration of antigen presenting cells into draining LNs, recirculation of CD4 and CD8 T lymphocytes into LNs, and lymphocyte proliferation and migration into skin or footpads. DTH was not significantly reduced in CD44 mutant mice, indicating that other adhesion molecules (ie, selectins and β-2 integrins) play a greater role in DTH responses.96,97 Moreover, the ability of T cells to regulate and help B cells appeared to be normal in CD44-deficient mice, as evidenced by normal levels of all Ig classes and subclasses in untreated mice (data not shown), as well as normal Th-dependent IgG responses to VSV and KLH. It should be noted that the peripheral T cells in C57BL/6 mice have lower levels of CD44 compared with the BALB/c mouse strain, which express the CD44.2 and CD44.1 alleles, respectively.34,76 98-101 T cells in the C57BL/6 strain may use molecules that functionally compensate for lower CD44 expression. To investigate this, at least ten backcrosses into BALB/c background are necessary. This is currently under way.
The granuloma is the central defense mechanism against intracellular bacteria: The failure to develop adequate granulomatous lesions that entrap the bacteria results in susceptibility to disease while overproduction of granulomas is pathogenic.102 Both αβ and γδ T cells surround granulomatous lesion103,104 and supply the IFN-γ needed to activate macrophage bactericidal activity. Thus, properly regulated granuloma responses require optimally coordinated responses between CD4 and CD8 T lymphocytes and appropriate Th1- versus Th2-type phenotypes and involve a host of other leukocyte and cytokine activity.104-108 Because CD44-deficient mice showed increased number and size granulomatous lesions after challenge with heat-killed C parvum, a defect appears to exist in the coordinate orchestration of this immune response critical to the control of many intracellular pathogens. Thus, the defect may reflect minor deficits in T-cell function in addition to possible dysfunctions in monocytes and macrophages, any or all of which might alter the granuloma responses.
Initial studies reported that high levels of CD44 correlated with aggressive phenotypes in several kinds of tumors and that it might be involved in metastasis.17,109-114 In different reports, it appeared that CD44 correlated with less malignant tumor phenotypes. For example, CD44 expression in neuroblastoma was found to be a marker of good prognosis.115,116 It has been thought that these apparently conflicting reports may have reflected the expression of different CD44 isoforms, which may have either antitumor or tumor-promoting effects. Similar to our results, CD44s expression is reduced in colorectal tumors compared with normal colonic cells, whereas reintroduction of this molecule in colorectal tumor cells was found to reduce tumorigenicity.74 More recently, however, studies of human epidermal skin tumors find that neither qualitative nor quantitative changes in CD44 splice-variant expression correlate with invasive or metastatic potential. Rather, the report suggests that CD44 isoform expression varies with the degree of tumor differentiation.117 Correlation between the stage of tumor differentiation and CD44 expression has been reported elsewhere.118-120 The mechanism by which CD44s inhibits tumorigenicity has not been deciphered, but our results seem to indicate that this mechanism only operates in vivo. Because it has been reported that the engagement of CD44 can induce the release of cytokines,121 it is tempting to speculate that in fibroblasts this might alter the interaction of tumor cells and surrounding stroma and result in an inhibitory effect on tumor growth.
Overall, we were surprised about the moderate phenotype of the mice lacking CD44, particularly in regard to embryogenesis. Reports about stimulation, proliferation and other effects modulated by anti-CD44 antibody treatment might be characteristic of the in vitro nature of the assays, especially when complete antibodies were used, or might reflect nonspecific effects of antibody treatment, such as capping of other crucial surface molecules or even killing of CD44 expressing cells, effects that are possibly very useful in the treatment of disease. On the other hand, it is possible that there is some compensation by other adhesion molecules like selectins and lectins in the absence of CD44122,123 and overlapping functions, especially mediated by other hyaluronan-binding proteins like neurocan, versican, aggrecan, link protein, TSG6, and RHAMM with structural similarity to CD44.124-126
ACKNOWLEDGMENT
The authors thank S. Jacob and D. Siderovsky for their careful readings of the manuscript.
Supported by grants from the Medical Research Council of Canada, the National Cancer Institute of Canada, and the National Science and Engineering Research Council of Canada. R.S. was supported by the Dr Mildred-Scheel Stifting, Germany; T.M.K. was supported by the Swiss National Science foundation; T.M. was supported by the Medical Research Council of Canada; D.D.H. was supported by the National Institutes of Health and the Veterans Affairs Career Development and Merit Review program.
Address reprint requests to Tak W. Mak, PhD, Departments of Immunology and Medical Biophysics, 500 Sherbourne St, University of Toronto, Toronto, Ontario M4X 1K9, Canada.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal