Abstract
The Caenorhabditis elegans cell death gene, Ced-3, encodes a protein homologous to mammalian interleukin-1β–converting enzyme (ICE), a cysteine protease implicated in programmed cell death (PCD). CPP32, also known as Yama, apopain, and Caspase-3, is a member of this family, has substrate specificities similar to Ced-3, and has been shown to have an active role in PCD. Evidence suggests that these proteases act downstream of inhibitors of PCD such as Bcl-2 and Bcl-xL , which are frequently expressed in Reed-Sternberg (RS) cells of Hodgkin's disease (HD). To date there have been no studies examining the role of the ICE/Ced-3 family of proteins, in particular CPP32, in HD. We examined 24 cases of HD with a classical immunophenotype and 6 cases of nodular lymphocyte predominant HD (NLPHD) for the expression of CPP32 in the RS cells and lymphohistiocytic (L&H) cells as detected by immunohistochemistry. Twenty two of 24 cases (92%) of HD expressed the protein in the RS cells, whereas the L&H cells in all 6 cases of NLPHD lacked expression of CPP32. These results provide further evidence that NLPHD is a phenotypically different disease distinct from classical forms of HD. The differential expression of the cell death protein CPP32 may be an important factor contributing to the apparently different clinical behaviour of NLPHD in contrast to classical HD. The lack of expression of CPP32 in NLPHD shares similarities with low-grade B-cell non-Hodgkin's lymphomas and may explain their common clinical course. Further studies are required to elucidate the significance of CPP32 in HD.
PROGRAMMED CELL death (PCD), or apoptosis, plays a critical role in tissue homeostasis and is of fundamental importance for biological processes ranging from embryogenesis to the development of the immune system.1,2 A family of mammalian homologues of the nematode Caenorhabditis elegans cell death protein, Ced-3, have been recently discovered to have an integral role in PCD.3-5 These mammalian proteins encode novel cysteine proteases with homology to the interleukin-1β–converting enzyme (ICE).6,7 To date, at least 10 members of the ICE/Ced-3 family of cysteine proteases have been identified in humans,5,8-13 most of which induce apoptosis when overexpressed in mammalian cells. Among the ICE/Ced-3 family proteases identified in humans thus far, CPP32, also known as Yama (after a Hindu god of death), apopain, and Caspase-3, is probably best correlated with PCD.2-12 Proteolytic cleavage at a particular aspartic acid residue of the 32-kD inactive zymogen gives rise to two active subunits with molecular masses of 17 to 20 kD and 10 to 12 kD.11,12 These assemble to form an enzymatically active heterotetramer, whereas each subunit alone is ineffective.6 Activation of CPP32 has been described in a number of settings in which apoptosis occurs, such as T cells stimulated through Fas (APO 1/CD 95).14-16 Furthermore, antiapoptotic viral proteins, such as crm A from cowpox, have been found to bind to and inhibit CPP32.17
Although Hodgkin's disease (HD) is a well-defined clinical entity, the pathogenesis of HD and the origin of the Reed-Sternberg (RS) cell remains a pathological enigma. The distinction between classical HD and nodular lymphocyte predominant HD (NLPHD) is important, because the latter disease may be treated differently in many centers, particularly when it presents as a limited stage disease. Distinct phenotypic differences between classical HD and NLPHD are well described.18 The proposal that NLPHD is clinically distinct from classical HD is perhaps more controversial, but recent data suggest that NLPHD is characterized by a favorable overall survival with a tendency for late recurrences.19-23 This clinical course is not typically seen in classical forms of HD, but is more akin to low-grade B-cell non-Hodgkin's lymphomas (NHLs).20
We examined 24 cases of HD having a classical immunophenotype and 6 cases of NLPHD with respect to the expression of CPP32 as detected by immunohistochemistry. We sought to determine if this cell death protein is expressed both in RS and lymphohistiocytic (L&H) cells, thus providing possible insights into the pathogenesis of HD.
MATERIALS AND METHODS
Patient selection.We selected 30 cases of HD from the files of the British Columbia Cancer Agency. The morphological subclassication of these cases included 13 with nodular sclerosis HD (NSHD), 7 with mixed cellularity HD (MCHD), 3 with lymphocyte-rich classical HD (LRHD), 1 with lymphocyte-depleted HD (LDHD), and 6 with NLPHD. Diagnoses were established using routine criteria based on lymph node biopsy histology and paraffin section immunohistochemical assessment.
Assessment of morphology.Three micron tissue sections were cut from B5 and formalin-fixed material and stained with hematoxylin and eosin. Immunoperoxidase staining on sections of lymph node (CD3, CD15, CD20, CD30, CD45, CD57, and epithelial membrane antigen [EMA]) was performed by using the streptavidin-biotin complex method, with microwave antigen retrieval and trypsin pretreatment used as necessary. The chromogen used was diaminobenzidine. The diagnosis of HD was established by finding RS cells and their variants with a classical immunophenotype (CD15/CD30 positive) in the appropriate background of plasma cells, eosinophils, and histiocytes. NLPHD was diagnosed by finding the typical macronodular morphology and L&H cells with the appropriate immunophenotype (CD20/CD45 positive).
Immunohistochemistry for CPP32.By using recombinant human CPP32 protein, a highly specific polyclonal rabbit antiserum was prepared and was shown by immunoblotting to react with both the inactive 32-kD CPP32 zymogen and the 17-kD active subunit.11,12 Three micron tissue sections were stained using 0.1% (vol/vol) anti-CPP32 antiserum. The chromogen used was diaminobenzidine. Nuclei were counterstained with hematoxylin. In all cases strongly CPP32-positive normal cells within the lymph node served as internal controls and allowed comparison for assessing the intensity of staining. The specificity of the CPP32 results was confirmed by preadsorbing the anti-CPP32 antiserum with recombinant CPP32 before performing the procedure.11 12 B5 was determined to be the optimal fixative for performing CPP32 immunostaining.
RESULTS
RS cells or L&H cells showing cytoplasmic staining, whether weak or strong, were regarded as positive. Cases were determined to be negative when the diagnostic cells were completely negative or when less than 5% of these cells were positive. The latter cases uniformly showed very weak cytoplasmic staining if any immunostaining was present.
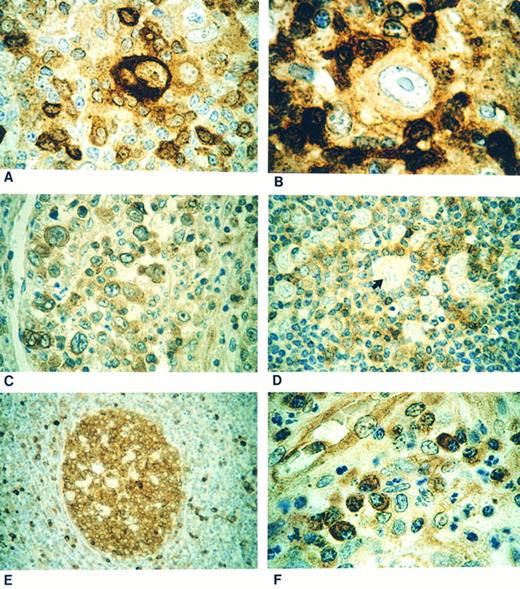
The RS cells and their variants were positive (Fig 1A and C) in 22 of 24 (92%) cases of HD with a classical immunophenotype. In all the cases of NSHD,13 LRHD,3 and LDHD1 and in 5 of 7 MCHD cases, the RS cells were positive for CPP32. In the two remaining cases of MCHD the RS cells were negative (Fig 1B). In all 6 cases of NLPHD the L&H cells were negative (Fig 1D) for immunostaining with CPP32 (Table 1).
(A) Classical Reed-Sternberg (RS) cell showing strong cytoplasmic CPP32 immunostaining. (B) Case showing a mononuclear variant of RS cell negative for CPP32 immunostaining. (C) Nodular sclerosis Hodgkin's disease showing positive CPP32 immunostaining of lacunar cells. (D) Negative staining lymphohistiocytic cell (arrow) in a case of nodular lymphocyte predominant Hodgkin's disease. (E) Strong CPP32-positive immunostaining of the germinal center of a secondary lymphoid follicle with negative staining of mantle zone cells. (F ) Plasma cells showing strong cytoplasmic positivity for CPP32 immunostaining.
(A) Classical Reed-Sternberg (RS) cell showing strong cytoplasmic CPP32 immunostaining. (B) Case showing a mononuclear variant of RS cell negative for CPP32 immunostaining. (C) Nodular sclerosis Hodgkin's disease showing positive CPP32 immunostaining of lacunar cells. (D) Negative staining lymphohistiocytic cell (arrow) in a case of nodular lymphocyte predominant Hodgkin's disease. (E) Strong CPP32-positive immunostaining of the germinal center of a secondary lymphoid follicle with negative staining of mantle zone cells. (F ) Plasma cells showing strong cytoplasmic positivity for CPP32 immunostaining.
Results of CPP32 Immunostaining
| Hodgkin's Disease Subtype . | CPP32 Positive . | CPP32 Negative . |
|---|---|---|
| NSHD | 13 | 0 |
| MCHD | 5 | 2 |
| LDHD | 1 | 0 |
| LRHD | 3 | 0 |
| NLPHD | 0 | 6 |
| Hodgkin's Disease Subtype . | CPP32 Positive . | CPP32 Negative . |
|---|---|---|
| NSHD | 13 | 0 |
| MCHD | 5 | 2 |
| LDHD | 1 | 0 |
| LRHD | 3 | 0 |
| NLPHD | 0 | 6 |
Abbreviations: NSHD, nodular sclerosis Hodgkin's disease; MCHD, mixed cellularity Hodgkin's disease; LDHD, lymphocyte-depleted Hodgkin's disease; LRHD, lymphocyte-rich classical Hodgkin's disease; NLPHD, nodular lymphocyte-predominant Hodgkin's disease.
The lymphocytes within secondary follicles (Fig 1E) and plasma cells (Fig 1F ) were strongly positive for CPP32 and served as positive internal controls. Histiocytes showed variable CPP32 staining intensity, ranging from negative to weakly positive, but were easily distinguished cytologically from L&H cells of NLPHD. Primary follicles and the cells of the mantle zone (Fig 1E) were immunonegative consistent with previous findings.12 During the course of the study we found that B5 was the fixative of choice for performing CPP32 immunostaining. Three cases (one NLPHD and two HD), in which only formalin-fixed tissue was available for immunostaining, showed significantly weaker reactivity but were still considered interpretable based on CPP32 immunostaining of normal cells.
DISCUSSION
Studies of the Bcl-2 family of apoptosis regulating proteins has improved our understanding of the pathobiology of follicular lymphomas (FL) and is now leading to the development of potentially new experimental treatment strategies.24 Previous studies have detailed the expression of apoptosis-related proteins including Bcl-2, Bax, and Bcl-x in HD.25-30 These studies have shown variable expression of Bcl-225-29 but frequent expression of the proapoptotic protein Bax28 and the antiapoptotic family member Bcl-xL .28-30 However, these studies were unable to distinguish between the two forms of Bcl-x; Bcl-xL (death repressing) and Bcl-xS (death promoting).27-29 Other types of death-repressor and death-promoting proteins have not been studied extensively in HD. Before this report there had been no studies examining the role of ICE/Ced-3 family proteases, in particular Caspase 3/CPP32/Yama, in HD.
Unlike the prototype cysteine protease ICE, CPP32/Yama and Ced-3 cleave the death substrate poly(ADP-ribose) polymerase at an Asp site to generate the 89-kD and ∼110-kD signature apoptotic fragments, an event known to occur essentially universally during PCD.6,8 Recently, it has been shown that the anti-apoptotic protein Bcl-2 and its homologue Bcl-xL can prevent the processing and activation of CPP32.31-35 Thus, Bcl-2 and Bcl-xL function upstream of CPP32,36 providing a biochemical connection between the Bcl-2 family and the ICE/Ced-3 family of proteases. The finding of an inverse immunostaining pattern of Bcl-2 and CPP32 in follicular small-cleaved cell lymphoma also suggests an important relationship between these two families of proteins.12 Finally, physical interactions of bcl-2 family proteins and the caspases have been shown through investigations of the Ced-4 protein.37-39
One caveat regarding interpretation of the immunohistochemical staining is the inability to exclude the possibility that anti-CPP32 antibody may have preferential reactivity for either the unprocessed or processed forms of CPP32 in fixed tissue sections, because of differences in the epitopes displayed by the active and inactive forms of the protease or because of differences in accessibility of epitopes caused by association of CPP32 with other proteins within the cell.11,12 The polyclonal antiserum used in this study reacts with the processed and unprocessed forms of CPP32 in Western blots and importantly shows no differences in immunostaining of cell lines induced to undergo apoptosis.11 Further immunostaining studies have suggested that the CPP32 epitopes recognized by the polyclonal antiserum are not masked by other proteins within the cell and are in keeping with distinct differences in CPP32 protein expression in NLPHD versus classical forms of HD (John C. Reed, unpublished observations). However, it remains unclear whether differential expression of CPP32 is caused by differences in the relative rates of transcription, mRNA levels or stability, protein half-life, or other steps involved in the regulation of gene expression. The mechanisms underlying the control of the in vivo expression of CPP32 in the histological subtypes of HD may be important to the further understanding of these entities but will prove difficult because of the paucity of neoplastic cells present in the involved tissues.
This study showed significantly different staining patterns between NLPHD and the other forms of HD having a classical immunophenotype. The L&H cells in all six cases of NLPHD were negative for CPP32, providing additional evidence that NLPHD represents a distinct disease from the other forms of HD. The absence of CPP32 immunostaining of the L&H cells is similar to the phenotype of many indolent B-cell NHLs. Although both indolent B-cell NHLs and NLPHD are very responsive to treatment, late relapse and transformation to diffuse large B-cell lymphoma are sequelae that characterize these disorders.19-23 Many low grade B-cell lymphomas express high levels of Bcl-2 protein and express only low levels of CPP32, yet the cells are prone to apoptotic death after treatment. This paradox of treatment responsiveness and incurability is difficult to explain, but may be caused by activation of alternative pathways brought about by DNA damage after exposure to chemotherapeutic agents. Activation of a p53-dependent pathway could upregulate expression of Bax, overcoming the antiapoptotic effect of Bcl-2 protein and, thus, translate into a good initial response to treatment.40 In NLPHD, the L&H cells typically do not express Bcl-2 protein, and as shown in this study, also fail to express CPP32. The predicted net effect appears to correlate with clinical outcome in NLPHD, but the role of other Bcl-2–related family members in NLPHD is unknown. Nonetheless, expression of CPP32 in NLPHD is different from classical HD, which may account for some of the clinical differences between these two disorders.
Expression of CPP32 was detected in 92% of HD having a classical immunophenotype, which increases the possibility that this protein may be involved in the pathogenesis of HD or may influence the response to treatment. In a study by Brousset et al,28 it was found that 43 of 48 (89%) of Bax-positive cases also expressed either Bcl-2 or Mcl-1. Although we did not test for the expression of these proteins, taken together the results suggest that CPP32 may be an important downstream regulator allowing progression of the PCD cascade during chemotherapy or radiotherapy. These preliminary observations suggest that a downstream protease involved in apoptotic cell death is differentially expressed in the malignant cells of classical HD versus NLPHD. Although it can be hypothesized that CPP32 expression may be an important predictor of therapeutic responsiveness, additional studies of large patient cohorts will be required to determine if CPP32 protein expression is an independant predictor of clinical outcome in HD.
ACKNOWLEDGMENT
We thank Guy Salvesen for providing the CPP32 cDNA.
Supported in part by the National Cancer Institute (CA-60421).
Address reprint requests to Randy D. Gascoyne, MD, British Columbia Cancer Agency, Department of Pathology, 600 W 10th Ave, Vancouver, British Columbia, V5Z 4E6, Canada.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal