Abstract
Intrauterine transfusion (IUT) therapy is the treatment of choice in severe hemolytic disease of the fetus. This treatment automatically implies the introduction of alloantigens in the fetal circulation, which might potentially influence the unprimed fetal immune system. The present study provides evidence that the fetal immune system is indeed prone to modulations of the T-cell receptor BV (TCRBV) repertoire as a result of IUT treatment. Most notably, IUT therapy affects the composition of the CD4+ repertoire, whereas this effect may be obscured in the CD8+ subset. The CD8+ subset was found to be influenced by alterations of the TCRBV repertoire both in IUT patients and controls, suggesting that modulations in this subset could be the result of developmental influences. A more detailed analysis on the composition of the individual TCRBV families was performed by evaluating the distribution of the complementarity determining region 3 (CDR3) size lengths of [32P]-radiolabeled TCRBV transcripts. Using this technique, referred to as spectratyping, only marginal changes were observed in the CD4+ and CD8+ subset during the course of treatment and gestational development of both IUT-treated patients and controls. Therefore, the alterations in the overall TCRBV repertoire were of a quantitative rather than a qualitative nature. To evaluate whether the observed alterations in TCRBV usage-frequencies were a reflection of an allo-reactive response, a primed lymphocyte test (PLT) was performed in 3 IUT-treated patients. We observed that IUT, performed as early as 23 weeks of gestation, may induce the establishment of memory T cells against the IUT donor. However, there was no association between the observed changes in TCRBV repertoire and the magnitude of the secondary allo-reactive response.
DESPITE THE USE of Rhesus(D) immunoprophylaxis and matching for this antigen in blood transfusion therapy, approximately 1% of the Rhesus(D)-negative fertile women develop antibodies to erythrocyte antigens.1 Pregnancies complicated by severe hemolytic disease can be successfully treated with IUT therapy. From a technical point of view, IUT has become an established procedure, resulting in a survival of approximately 90% of affected fetuses.2 3
Under physiological circumstances, the development of the fetal immune system occurs in an almost sterile and allo-antigen–free environment. Throughout gestation, the fetus is mainly confronted with both self and also selected maternal antigens. Therefore, it would not be unreasonable to assume that the introduction of allo-antigens into the fetal circulation by intrauterine transfusion (IUT) might have an impact on the fetal immune system. However, the immunologic consequences of IUT treatment have, until now, not been investigated in great detail.
Blood transfusions are known to have potent immunomodulatory properties. For instance, administration of an HLA-(B),DR–matched blood transfusion before transplantation can result in an increased allograft survival.4-7 This toleration effect might be the result of induction of anergy, suppression, or deletion of allo-reactive T cells.8 It has been suggested that these transfusions result in cytotoxic T-lymphocyte nonresponsiveness to donor antigens9 and can result in the absence of several T-cell receptor BV (TCRBV) families.10 Because tolerance is easier to induce in neonates than in adults,11 12 the effect of allogeneic blood transfusion might be even more pronounced in the fetus.
IUT treatment offers a unique model to simultaneously investigate the effect of blood transfusion on the fetal immune repertoire and the allogeneic response of an unprimed immune system in vivo. Direct access to the umbilical cord under ultrasound guidance has made it possible to obtain peripheral blood of the fetus at different time points in gestation.2 3
To evaluate semiquantitative influences of IUT therapy on the fetal CD4+ and CD8+ TCRBV repertoire, the polymerase chain reaction (PCR) technique with 25 TCRBV family-specific oligonucleotide primers was used to determine the TCRBV gene element usage both before and after IUT treatment in 5 fetuses. Changes in the distribution of complementary determining region 3 (CDR3) length of the TCRs during the course of therapy were analyzed using spectratyping.13 The results were compared with 5 patients who underwent fetal blood sampling (FBS) but did not receive IUT therapy. In 3 IUT-treated patients, the magnitude of the alloresponse against several IUT donors was determined by a primed lymphocyte test (PLT) in cord blood (CB), obtained after delivery.
MATERIALS AND METHODS
IUT Patients
The use of patient material (IUT-treated patients and controls) for research purposes was approved by the Commission of Medical Ethics of the University Hospital Leiden (protocol P244/94).
Patient and transfusion characteristics are shown in Table 1. Four fetuses (patients no. 1 through 4) received IUT therapy with unrelated donor erythrocytes for treatment of severe hemolytic disease caused by Rhesus(D) antibodies. The other fetus (patient no. 5) received maternal platelets for management of fetal allo-immune thrombocytopenia, which was induced by maternal antibodies directed against a paternal private antigen. The mother of this fetus received a high dose of intravenous gamma globulin (1 g/kg/wk) for 9 weeks (from 26 to 34 weeks of gestation). Before the initiation of the IUT procedure, the mothers of IUT patients received premedication, consisting of 75 mg pethidine, 25 mg phenergan, and 50 mg indomethacin, administered 30 minutes before the procedure. Pancuronium (dose depended on gestational age) was administered to the fetus to reduce fetal movement. This study included one pair of dizygotic twins, which were HLA nonidentical and of different gender (patients no. 1 and 2). Identification of the two fetuses before FBS and IUT was predicted by ultrasound.
Patient Characteristics and Correlation Coefficient Values of the Overall Changes in the TCRBV Repertoire of Fetuses Undergoing Diagnostic FBS or IUT
| Patient No. . | Diagnosis . | No. of IUTs . | First FBS* . | Second Analysis* . | Birth* . | R2 CD4 . | R2 CD8 . |
|---|---|---|---|---|---|---|---|
| IUT patients | |||||||
| 1. | Hemolytic disease | 5 | 21 | 33 (IUT5) | 34 | .141 | .655 |
| 2. | Hemolytic disease | 4 | 21 | 32 (IUT4) | 34 | .348 | .769 |
| 3. | Hemolytic diseae | 2 | 31 | 37 birth | 37 | .851 | .539 |
| 4. | Hemolytic disease | 2 | 32 | 39 birth | 39 | .193 | .169 |
| 5. | Allo-immune thrombocytopenia | 2 | 28 | 34 (IUT3) | 34 | .522 | .903 |
| FBS patients | |||||||
| 6. | Growth retardation | — | 30 | 40 | 40 | .940 | .468 |
| 7. | Congenital heart disease | — | 22 | 39† | 38 | .965 | .522 |
| 8. | Growth retardation | — | 31 | 38‡‡ | 35 | .871 | .779 |
| 9. | Dandy Walker | — | 34 | 37 | 37 | .867 | .901 |
| 10. | XO mosaic | — | 20 | 40 | 40 | .851 | .742 |
| Patient No. . | Diagnosis . | No. of IUTs . | First FBS* . | Second Analysis* . | Birth* . | R2 CD4 . | R2 CD8 . |
|---|---|---|---|---|---|---|---|
| IUT patients | |||||||
| 1. | Hemolytic disease | 5 | 21 | 33 (IUT5) | 34 | .141 | .655 |
| 2. | Hemolytic disease | 4 | 21 | 32 (IUT4) | 34 | .348 | .769 |
| 3. | Hemolytic diseae | 2 | 31 | 37 birth | 37 | .851 | .539 |
| 4. | Hemolytic disease | 2 | 32 | 39 birth | 39 | .193 | .169 |
| 5. | Allo-immune thrombocytopenia | 2 | 28 | 34 (IUT3) | 34 | .522 | .903 |
| FBS patients | |||||||
| 6. | Growth retardation | — | 30 | 40 | 40 | .940 | .468 |
| 7. | Congenital heart disease | — | 22 | 39† | 38 | .965 | .522 |
| 8. | Growth retardation | — | 31 | 38‡‡ | 35 | .871 | .779 |
| 9. | Dandy Walker | — | 34 | 37 | 37 | .867 | .901 |
| 10. | XO mosaic | — | 20 | 40 | 40 | .851 | .742 |
Given in weeks of gestation.
Peripheral blood was obtained 1 week after birth for analysis of the TCRBV repertoire.
Peripheral blood was obtained 3 weeks after birth for analysis of the TCRBV repertoire.
Controls
Fetal blood was obtained from 5 fetuses of other patients (patients no. 6 through 10) for prenatal diagnosis (Table 1). In 2 patients, FBS was performed to determine the possible cause of growth retardation in utero (patients no. 6 and 8). Patient no. 6 was born at 40 weeks and had a weight of 2,675 g. This neonate left the hospital in good health shortly after birth. The other infant (patient no. 8) was born at 35 weeks of gestation, had a weight of 890 g, and showed signs of dysmaturity. This child left the hospital in good condition 12 weeks after delivery. Patient no. 10 underwent an FBS procedure to confirm an XO mosaicism that was observed after amniocentesis. However, this diagnosis could not be confirmed in fetal blood, in cord blood at delivery, or in placental tissue. This infant left the hospital in good condition directly after birth. Ultrasound imaging showed multiple congenital heart defects in patient no. 7 and corrective heart surgery was successfully performed 3 weeks after birth. Patients no. 7 and 8 received two blood transfusions after birth for correction of anemia induced by laboratory testing. Patient no. 9 had a Dandy Walker malformation and died shortly after birth due to severe neurological impairment. FBS in control patients failed to show chromosomal aberrations or any other abnormalities that could explain the respective medical conditions. The FBS procedure in the control patients was performed without administration of premedication to the mothers. Furthermore, these fetuses did not receive medication to prevent fetal movement.
Preparation of IUT
Intrauterine red blood cell (RBC) transfusions.The IUTs were prepared from fresh (<24 hours) donor erythrocytes and were compatible with maternal erythrocyte antibodies. Donor blood was collected in citrate-phosphate-dextrose solution. Erythrocytes were filtered after buffycoat removal and contained less than 2 × 106 leukocytes per unit. The hematocrit was adjusted to approximately 0.85 L/L by using 0.9% saline. Donor blood was irradiated with 25 Gy and administered within 3 hours after preparation.
Intrauterine platelet transfusions.Maternal platelets were collected using a cell separator (COBE, Lakewood, CA). After centrifugation, maternal plasma was removed and platelets (containing an average of 50 × 106 leukocytes) were resuspended in 50-mL citrate-phosphate-dextrose plasma from a male, blood group AB donor. Maternal platelets were irradiated with 25 Gy and administered within 2 hours after preparation.
Collection of the Samples
Fetal blood was obtained by FBS in IUT patients before the onset of the transfusion. Immediately after birth, umbilical cord blood was collected before the delivery of the placenta. If this was not possible (patients no. 7 and 8), then peripheral blood was obtained after birth by venapuncture. All samples were collected in heparinized tubes, and 1 mL was used for TCRBV analysis. Mononuclear cells were isolated by Ficoll-Hypaque density gradient sedimentation within 12 hours after collection. The cells were then frozen in liquid nitrogen and stored at −170°C before fluorescence-activated cell sorting (FACS).
FACS
After thawing, mononuclear cells were washed twice with Hanks' balanced salt solution (GIBCO, Paisley, UK) and subsequently stained with fluorescein isothiocyanate-conjugated anti-CD4 and phycoerythrin-conjugated anti-CD8 (Becton Dickinson, San Jose, CA) monoclonal antibody for 30 minutes at 4°C. Cells were washed twice in Hanks' medium and resuspended in 50% RPMI 1640/50% fetal calf serum. CD4+ and CD8+ cells were separated using FACS (Becton Dickinson, Mountain View, CA) and collected in 50% RPMI1640/50% fetal calf serum. Cells were then washed twice in phosphate-buffered saline at 4°C.
RNA Isolation, cDNA Synthesis, and PCR Amplification
Analysis of the TCRBV was performed using a semiquantitative PCR as described previously.14 15 RNA was isolated from the sorted cells using the RNAzol method (Cinna/Biotecx Laboratories, Inc, Houston, TX), dissolved in 25 μL of distilled water, and stored at −80°C. Five micrograms of RNA was reversed-transcribed into first-strand cDNA using the Riboclone cDNA synthesis system (Promega, Madison, WI). This cDNA was first quantified with control primers and diluted with distilled H2O to an amount in which 1 μL per reaction resulted in PCR products in log phase for each primer used. The TCRBV repertoire was analyzed using 25 TCRBV-specific primers identifying 24 different TCRBV families. For each PCR, 1 μL of cDNA was used and added to a mixture of 20 pmol of 3′ antisense TCRBC primer, 0.5 mmol/L of each dNTP, 10 mmol/L Tris HCl (pH 8.4), 50 mmol/L KCl, 4 mmol/L MgCl2 , 0.06 mg/mL bovine serum albumin, and 2.5 U AmpliTaq DNA polymerase (Perkin/Elmer, Roche Molecular Systems, Inc, Branchurg, NJ) and 20 pmol of each TCRBV-specific 5′ sense primer in total volume of 100 μL. Twenty-five to 35 PCR cycles were performed in a Thermocycler 60 (Biomed Instruments, Fullerton, CA), depending on the amount of cDNA. The PCR cycles consisted of a 95°C denaturation, a 55°C primer annealing, and a 72°C extension step for 1 minute each. A 5′ sense primer specific for the constant region of the TCR was used as an internal control. As a negative control, a PCR without template was performed to exclude contamination. Five microliters of the PCR-amplified products was size fractionated on a 1% agarose gel and subsequently transferred to a nylon filter (Hybond N+; Amersham International plc, Little Chalfont, Buckinghamshire, UK) for Southern analysis. The TCRβ chain-specific sequences were detected by hybridization with a [32P]-radiolabeled TCRBC-specific probe. The amount of each individual TCRBV PCR product was determined using either autoradiography on Kodak XAR films (Eastman Kodak, Rochester, NY) and laser densitometry (LKB 2220-020, Ultrascan XL; Pharmacia LUB Biotechnology, Uppsala, Sweden) or phosphor-imaging (PhosphorImager 445SI; Molecular Dynamics, Sunnyvale, CA). All PCR amplifications were performed in duplicate with correlation coefficients (R2 ) of >.95 between the two analyses.
Spectratyping
Spectratyping of the different TCRBV families was determined according to the methodology described by Gorski et al.13 Briefly, 1 μL of cDNA was used for each PCR. PCR conditions were comparable to those described above, with the exception that 20 pmol of a 3′ antisense primer was used for each reaction that was end labeled with γ-[32P]-ATP. PCR consisted of 30 to 40 amplification cycles, including a 94°C denaturation, a 58°C primer annealing, and a 72°C extension step for 1 minute each. Before transfer of the radioactive PCR products to a prewarmed 6% acrylamide-urea sequencing gel (Ultrapure Sequagel-6; National Diagnostics, Atlanta, GA), samples were boiled for 5 minutes. Gels were run for 2.5 to 3.5 hours and subsequently dried. The spectratypes were visualized by autoradiography and phosphor-imaging.
Validation of the Procedure
PCR analysis with HLA-A– and HLA-B–specific probes, followed by allele-specific DNA-typing, showed that cord blood of the IUT treated patients used for analysis was not contaminated with donor leukocytes.
PLT
The proliferative alloresponses of 3 IUT-treated patients were determined against 8 original IUT donors using PLT. Briefly, 5 × 104 responder cells were cultured with 5 × 104 irradiated stimulators (30 Gy) in 96-well round-bottomed microtiter plates (Costar, Cambridge, MA). Cultures were incubated for 3 days at 37°C in humidified air containing 5% CO2 . Cells were subsequently pulsed overnight with [3H]-thymidine and then harvested with an automated cell harvester. Simultaneously, the responses of the IUT patients were tested against individuals who were either HLA-DR–matched or mismatched with the IUT donors. The responses of the IUT patients against the different stimulators were compared with responses of donors that were HLA-DR matched with the IUT patients and tested against the same set of stimulators. In the same test, responder cells of both IUT patients and HLA-DR–matched donors were incubated with autologous irradiated stimulators. The proliferative response was assessed by measuring [3H]-thymidine incorporation using scintillation counting. The stimulation index (SI) is calculated by dividing the counts per minute measured when stimulator and responder cells were cultured together by the sum of the counts per minute when the stimulator and responder cells were cultured separately.
Statistical Analysis
Overall changes in the TCRBV gene usage frequencies between the two time points of analysis were determined by correlation coefficients calculation (R2 ). An R2 value of 1.0 resembles a perfect correlation between the first time point of analysis and the second time point, whereas an R2 value of .0 resembles no correlation between the two time points. Differences in the usage frequencies of individual TCRBV genes in CD4+ as well as CD8+ T-cell subsets between controls and IUT-treated patients were determined by the two-tailed unpaired Student's t-test. When the standard deviations in the IUT-treated patients and controls were not equal, the nonparametric Mann-Whitney U test was used. Results were also corrected for the number of parameters analyzed by the method of Edwards.16 PLT responses were compared with the two-tailed paired or unpaired Student's t-test. For all statistical analyses, a P value of <.05 was considered significant.
RESULTS
To determine whether IUT therapy had any influence on the TCRB repertoire, we compared the composition of the TCRBV repertoire, both before and after IUT treatment, in 5 IUT patients using a semiquantitative PCR and spectratyping. To exclude developmental influences, similar experiments were performed in 5 control patients. In all patients, the first FBS was used as a reference. In IUT-treated patients, the first FBS was performed before the onset of transfusion therapy to determine the fetal hematocrit or platelet count. On average, the first FBS was performed at 26.6 weeks in the IUT group compared with 27.4 weeks in the control group (Table 1). The composition of the TCRBV repertoire of this CB sample was compared with a sample obtained at a second time point. For 3 IUT patients (nos. 1, 2, and 5), we compared the first FBS with a CB sample before the last IUT. This was performed to exclude possible contamination with donor leukocytes, because the interval between the last IUT and delivery was less than 2 weeks. The second time point of measurement was at 35 weeks of gestation, on average in the IUT group, compared with 38.8 weeks in the control group (Table 1). The median interval between the two measurements was 7 weeks for the IUT group and 10 weeks for the control group.
Semiquantitative Analysis of the TCRBV Gene Usage Frequencies Using PCR
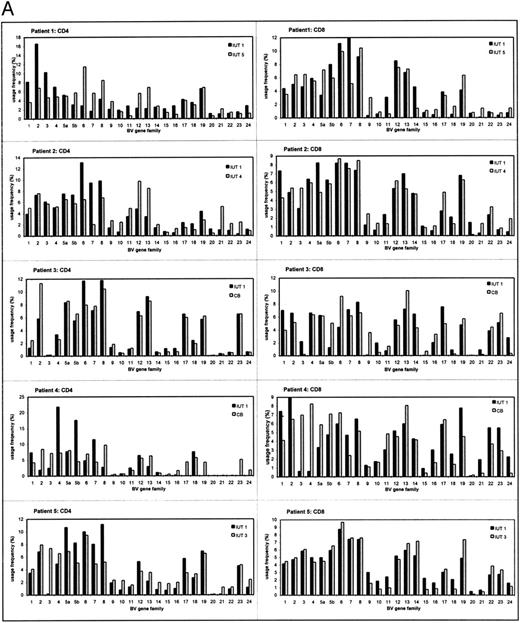
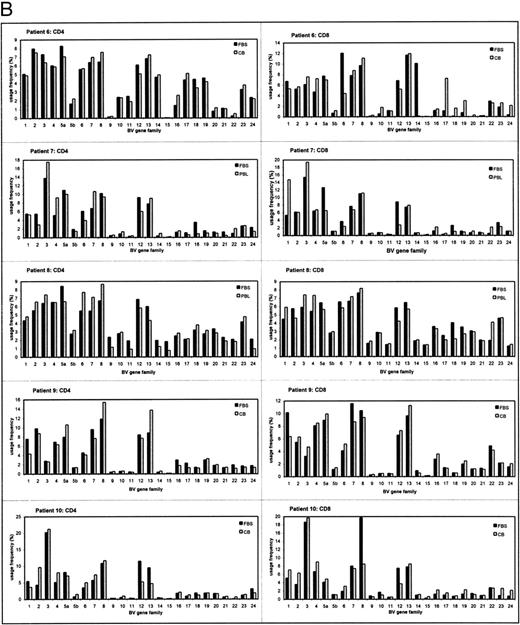
The TCRBV repertoires of CD4+ and CD8+ T-cell subsets were analyzed separately because they display different usage profiles.14,17 18 The individual TCRBV repertoires in IUT-treated patients and controls are shown in Fig 1A and B. Each individual patient showed a distinct usage pattern of the various TCRBV gene elements in the CD4+ and CD8+ subsets. All TCRBV gene elements were used for each individual patient both in IUT-treated patients and controls, except for patient no. 4 (Fig 1A). In this patient, several TCRBV gene segments remained undetectable in the CD4+ subset at 32 weeks of gestation (TCRBV 17 and 19 through 24). However, most of these genes were detected in cord blood after delivery, although the usage frequencies of TCRBV 20 through 22 remained low in this patient.
The TCRBV gene usage in CD4+ and CD8+ T cells of IUT patients (A) and controls (B) at two different time points of gestation. The usage frequency of each individual TCRBV gene element was given as a percentage of the total TCRBV expression. CB, cord blood (at delivery).
The TCRBV gene usage in CD4+ and CD8+ T cells of IUT patients (A) and controls (B) at two different time points of gestation. The usage frequency of each individual TCRBV gene element was given as a percentage of the total TCRBV expression. CB, cord blood (at delivery).
To determine changes in the overall CD4+ and CD8+ TCRBV repertoire, correlation coefficient calculations (R2 ) of the first FBS and a sample at a second time point of analysis were determined (Table 1). The results showed that, in 4 of 5 IUT-treated patients, significant changes occurred in the TCRBV repertoire of the CD4+ subset (mean R2 = .301). In 1 patient with hemolytic disease, the overall CD4+ TCRBV repertoire was relatively unaffected after IUT therapy (R2 = .851) and was therefore comparable to the control patients. In the control group, the CD4+ subset remained fairly stable during fetal development (mean R2 = .899). Alterations in the overall usage frequencies of TCRBV families within the CD8+ subset were observed both in IUT-treated patients (mean R2 = .607) and controls (mean R2 = .682). Although the control group was quite heterogeneous as regards the indication for FBS, similar patterns in the overall CD4+ and CD8+ TCRBV repertoire were observed. Statistical analysis showed that IUT therapy did not specifically induce changes in some TCRBV families. The alterations in the TCRBV repertoire were the result of increased and/or decreased usage frequency of several TCRBV families and seemed to be specific for each individual. Similar results were observed in the CD8+ subset, both of IUT-treated patients and controls.
Close examination of our results also showed differences in TCRBV usage patterns of IUT patients before the onset of transfusion therapy when compared with controls. Although these differences were observed in several TCRBV families, the statistical significance disappeared after multiparameter analysis. This was due to loss of statistical power in this limited group of patients (data not shown). However, the gene usage of TCRBV 19 in the CD8+ subset of the first FBS was significantly increased (uncorrected P value = .0013, corrected P value = .032) in all of the IUT-treated patients (mean, 5.9%) when compared with the controls (mean, 1.9%).
Spectratyping of the TCRBV Repertoire
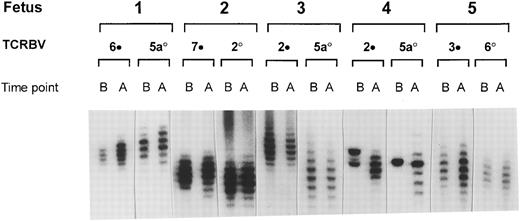
We have used the technique of spectratyping to investigate whether the observed semiquantitative changes of the TCRBV repertoire in the CD4+ subset of the IUT-treated patients also resulted in qualitative alterations in the distribution of CDR3 size lengths of the [32P] -radiolabeled TCRBV transcripts.13 In Fig 2, the distribution of the CDR3 size lengths of the TCRBV families that showed the most pronounced modulations of the TCRBV gene usage frequencies are displayed in conjunction with the least affected families. These results show that, in general, the observed expansions or contractions of TCRBV gene usage, as determined by semiquantitative PCR analysis (Fig 1A and B), in the CD4+ subset did not result in alterations of the respective spectratypes. One exception was patient no. 4, in whom major changes in the distribution of the CDR3 lengths were noted during the course of IUT treatment. Before IUT treatment, this patient showed a very restricted TCRBV repertoire that differed from all other patients in that they showed a more or less binomial distribution of all TCRBV products. These results were comparable with the semiquantitative PCR, showing a restoration to a more or less normal distribution pattern after the course of IUT treatment. Both in the CD4+ and CD8+ subset of the other IUT-treated patients and controls, the individual TCRBV families remained relatively unchanged and showed only marginal changes during gestation (data not shown).
Radioactive spectratype of selected CD4+ TCRBV families of IUT-treated patients. Selection was based on the most (•) and least (○) pronounced alterations during the course of IUT treatment. PCR products were analyzed on a phosphor-imager before (B) and after (A) the start of treatment.
Radioactive spectratype of selected CD4+ TCRBV families of IUT-treated patients. Selection was based on the most (•) and least (○) pronounced alterations during the course of IUT treatment. PCR products were analyzed on a phosphor-imager before (B) and after (A) the start of treatment.
Proliferative Responses to the Original IUT Donors
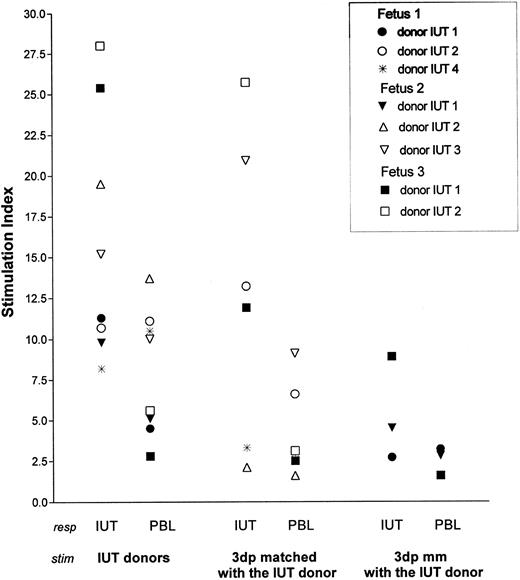
Finally, we determined if these molecular changes in the CD4 subset of IUT-treated patients were a reflection of an allo-reactive response against the IUT donor. Of 3 IUT-treated patients (patients no. 1, 2, and 3), sufficient numbers of CB mononuclear cells were available to perform such extended studies. Because no pretransfusion samples of these fetuses were available, proliferative responses against the IUT donors were compared with the responses of healthy nontransfused adult donors that were HLA-DR–matched with the IUT-treated patients (Fig 3). Both IUT patients and HLA-DR–matched responders were also tested against autologous mononuclear cells and third-party stimulators that were either HLA-DR matched or mismatched with the IUT donor. Clear proliferative responses of all IUT patients were observed against their original IUT donors (mean SI = 16.0). Responses of the IUT patients against stimulators mismatched with the IUT donors were much lower (mean SI = 5.4). Establishment of a statistical significance was not possible due to the limited number of individuals (mismatched with the IUT donor) tested. In all but 2 cases (fetus no. 1 against donors no. 2 and 4), the responses were higher than those of the HLA-DR–matched responders against the same set of IUT donors (P = .045). In 3 cases, the SI of IUT patients was 75% higher when compared with the HLA-DR–matched controls (patient no. 2 against the second IUT donor and patient no. 3 against both IUT donors). Patients no. 2 and 3 showed higher responses to all IUT donors when compared with HLA-DR–matched control responders. Patient no. 2 showed a memory response against the second IUT donor administered at 23 weeks of gestation. To determine if these responses were HLA class II specific, the allo-reactive responses against third-party individuals (that were HLA-DR matched with the original IUT donor) were determined (SI = 12.85). These responses were comparable to the allo-reactive capacity against the original IUT donor (P = .356), although in individual cases the responses were clearly lower. The spontaneous responses of IUT patients (average, 1,048 counts per minute; range, 425 to 2,168 counts per minute) were higher then those of the HLA-DR–matched controls (average, 349 counts per minute; range, 250 to 426 counts per minute) but was corrected for in the calculation of the SI.
IUT patients (IUT) and donors that were HLA-DR matched with these patients (PBL) were used as responders (resp ) in the PLT. The original IUT donors and third-party individuals that were HLA-DR matched (3dp matched) and completely mismatched (3dp mm) with the original IUT donors were used as stimulators (stim ). HLA-DR typing: Fetus 1. DR 1, 16(2); PBL, DR 1, 15(2); IUT donor, 1 •. DR 1, 2 ○. DR11 (5), 4*. DR 3, 4; 3dp matched, 2 ○. DR 5, 4*. DR 3, 4; 3dp mm, • DR 6, 8. Fetus 2. DR 1, 7; PBL, DR 1, 7; IUT donor, 1 ▾. DR 1, 2 ▵. DR 11(5), 3 ▿. DR 6, 8; 3 dp matched, 2 ▵. DR 5, 3 ▿. DR 6, 8; 3dp mm, ▾ DR 3, 4. Fetus 3. DR 1, 4; PBL, DR 1, 4; IUT donor, 1 ▪. DR 6, 7, 2 □. DR 2, 4; 3dp matched, 1 ▪. DR 6, 7, 2 □. DR 2, 4; 3dp mm, ▪ DR 3, 8.
IUT patients (IUT) and donors that were HLA-DR matched with these patients (PBL) were used as responders (resp ) in the PLT. The original IUT donors and third-party individuals that were HLA-DR matched (3dp matched) and completely mismatched (3dp mm) with the original IUT donors were used as stimulators (stim ). HLA-DR typing: Fetus 1. DR 1, 16(2); PBL, DR 1, 15(2); IUT donor, 1 •. DR 1, 2 ○. DR11 (5), 4*. DR 3, 4; 3dp matched, 2 ○. DR 5, 4*. DR 3, 4; 3dp mm, • DR 6, 8. Fetus 2. DR 1, 7; PBL, DR 1, 7; IUT donor, 1 ▾. DR 1, 2 ▵. DR 11(5), 3 ▿. DR 6, 8; 3 dp matched, 2 ▵. DR 5, 3 ▿. DR 6, 8; 3dp mm, ▾ DR 3, 4. Fetus 3. DR 1, 4; PBL, DR 1, 4; IUT donor, 1 ▪. DR 6, 7, 2 □. DR 2, 4; 3dp matched, 1 ▪. DR 6, 7, 2 □. DR 2, 4; 3dp mm, ▪ DR 3, 8.
DISCUSSION
In vivo exposure to allo-antigens can profoundly change the composition of the TCRBV repertoire.10,19,20 For example, heart transplantation patients showed quantitative changes of several TCRBV families in peripheral blood, just before a rejection crisis.20 Munson et al10 reported that transfusions with HLA-B,DR–shared donor blood can result in complete deletions of one or more TCRBV families. In vitro experiments have shown a restricted but heterogeneous TCRBV gene usage of the allo-reactive T cells.21-23
The present study provides evidence that the fetal immune system is prone to modulations of the T-cell compartment. This conclusion is derived from the observation that 4 of 5 IUT patients showed changes of the TCRBV gene usage frequencies in the CD4+ subset during the course of therapy. In 1 IUT patient, this subset remained quite stable and was therefore comparable to controls. The control patients showed a fairly stable overall composition of the CD4+ subset during gestation, at least from 21 weeks of gestation onwards, despite the different indications for FBS in this group of patients. The most likely cause of induction of the changes in CD4+ subset of the TCRBV repertoire of the IUT-treated patients is the transfusion blood from random donors that contains multiple nonself proteins. Although IUT blood was filtered before use, it still contained a relatively large number of donor leukocytes on which HLA antigens are expressed. We did not find evidence that IUT treatment resulted in a major contraction of one or more TCRBV families, despite the fact that all IUT treated patients received at least one HLA-DR–shared blood transfusion.
In contrast, the CD8+ TCRBV repertoire of both IUT patients and controls changed considerably during gestation. These alterations most likely reflect maturation of this T-cell compartment during fetal development and might have masked influences in this subset induced by IUT therapy. Contrary to the CD8+ subset, the composition of the CD4+ TCRBV repertoire seems to be completed early in gestation, because this subset was relatively unaffected during gestation in the control group.
Although the observed changes in the TCRBV repertoire were probably the result of exposure to allo-antigens, the influence of hemolytic disease of the fetus, resulting in severe anemia, cannot be ruled out. It has been observed that fetal anemia has an influence on the leukocyte counts. The degree of fetal anemia is associated with a corresponding decrease in total leukocyte, lymphocyte (T and B cells), natural killer (NK), and monocyte count.24 25 In this study, we only observed a statistically significant increase in TCRBV 19 in the eventual group of IUT patients before the onset of IUT therapy when compared with the controls.
To provide a more detailed insight into the nature of the alterations, we analyzed the distribution of CDR3 lengths using spectratyping. Surprisingly, no major changes in the composition of TCRBV families were observed in the CD4+ and CD8+ subset of IUT patients and controls. The individual TCRBV families showed a more or less binomial distribution in both subsets at the two different time points of analysis. With the exception of 1 IUT-treated patient (patient no. 4), the composition of the TCRBV repertoire of the first FBS was even more restricted than was expected on the basis of the TCRBV gene usage pattern. However, after the course of IUT treatment, the repertoire showed an almost normal distribution. Because we could not observe any significant qualitative changes in the CDR3 length of even the most severe quantitatively affected TCRBV families, the observed changes in the CD4+ T-cell compartment were therefore merely of a quantitative nature. The explanation behind this phenomenon remains speculative. However, these results are in agreement with a study in which the effect of blood transfusion on TCRBV repertoire has been evaluated.26 In 1 patient who received an HLA-B,DR–shared transfusion, a clear increase in the usage frequency of TCRBV 21 was observed by semiquantitative PCR. Subsequent spectratyping showed no changes in the distribution of the CDR3 lengths. This was in contrast to the monoclonal and oligoclonal expansion observed in the CD8+ subset of several transfused patients that resulted in altered banding patterns (unpublished results).
Because the procedure of FBS was comparable with IUT-treated patients and controls, the stress induced by the procedure cannot explain the observed differences in the TCRBV repertoire of the CD4+ subset. Furthermore, this procedure does not result in any increase in stress indicators such as cortisol or β-endorphin levels.27
The distinctive character of the fetal allo-immune response has been acknowledged by several investigators.28-35 CB is phenotypically immature. For instance, the T-cell subset is composed almost exclusively of unprimed naive CD45RA+ T cells, in contrast to adults expressing this phenotype in 50% of the T cells.36,37 Moreover, all allo-reactive CB T cells are of the CD45RA+ phenotype, whereas more than 50% of the adult allo-responsive T cells express CD45RO.38 Several investigators have proposed that the immature phenotype of CB leukocytes might explain the reduced graft-versus-host disease after CB transplantation.39,40 On the other hand, it has also been shown that CB is capable of inducing strong NK and lymphokine-activated killer (LAK) activity to various tumor cells after stimulation with interleukin-2 (IL-2)31,34,36,41 and IL-12.33
The proliferative capacity of CB to allo-antigens is still controversial. Some investigators claim that CB is functionally immature, resulting in a diminished allo-proliferative response to allo-antigens when compared with adults.31,34,36,42 This is in contrast with the observations that the allo-proliferative response of CB equals, or is sometimes even superior to, those of adults.28,30,32,43 Other investigators observed that this allo-reactive capacity is lost during the first few weeks or months after delivery, reaching adult levels again at approximately 2 years of age.30 These observations, in combination with alterations in the TCRBV repertoire after IUT therapy, encouraged us to investigate the proliferative capacity of CB of IUT patients against their original donors. These results indicated that CB showed a higher proliferative response against allo-antigens when compared with adults, but CB cells also showed evidence of generating a memory response against donor antigens after in vivo exposure. Priming occurred as early as 23 weeks of gestation. This seems to be in contrast with previous in vitro observations that CB can produce a strong primary immune response against allogeneic stimulators but exhibits a reduced proliferation capacity upon restimulation.43 However, in vitro and in vivo priming might not be comparable. First, not all IUT resulted in the establishment of a memory response. Immunization might therefore depend on the IUT unit and/or individual patient. The number of patients analyzed in this study was too small to determine the influence of HLA-sharing between donor and recipient and the magnitude of the allo-reactive response. Secondly, IUT treatment implicates changes in the normal development of the fetal immune system by the introduction of allogeneic donor antigens. We have already observed that IUT can result in an induction of a more mature T-cell phenotype in some patients, including an increase of CD45RO-expressing T cells.44 This premature maturation of the T-cell compartment might be responsible for the allo-reactive response comparable to that of adults.
Patient no. 3, with the highest SI against both original IUT donors, showed the least affected CD4+ TCRBV repertoire. These results are in agreement with earlier observations that there is no correlation between alterations in the TCRBV repertoire and allo-reactive capacity after blood transfusion.26 Because IUT therapy can seemingly influence the TCRBV repertoire and allo-reactive response independently of each other, they probably represent two different entities prone to modulations by blood transfusion.
To fully comprehend the influence of IUT treatment on the T-cell compartment, extended studies on the allo-reactive capacity of the IUT patients have to be performed. These might include determination of the phenotypic profile of the allo-reactive T cells in IUT patients, the effect on the cytolytic allo-reactive response and cytokine profiles released by allo-reactive fetal T cells.
ACKNOWLEDGMENT
The authors thank Dr E. Goulmy, Dr Frans Claas, Dr David Sherr, and Dr G.C. Beverstock for critically reading the manuscript; Jenny Verdoes and Annemiek van Rooden for collecting the cord blood samples; Maarten van der Keur and Arie van der Marel for FACsorting and analysis; and Jacqueline Anholts for her expert technical assistance.
Supported in part by the Red Cross Blood Bank Leidsenhage, The Netherlands.
Results were presented in part at the second meeting of the European Haematology Association, Paris, France, May 29-June 1, 1996.
Address reprint requests to Henk E. Viëtor, MD, Department of Immunohematology and Blood Bank, University Hospital Leiden, Bldg 1, E3-Q, PO Box 9600, 2300 RC Leiden, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal