Abstract
Interleukin-12 (IL-12) is a key regulator of cell-mediated immunity that has therapeutic potential in cancer and infectious disease. In a previous Phase 1 dose escalation study of a single test dose of recombinant human IL-12 (rhIL-12) followed 14 days later by cycles of five consecutive daily intravenous injections every 3 weeks, we showed that a dose level up to 500 ng/kg could be administered with acceptable levels of safety. Based on these results, a Phase 2 study was conducted. In the Phase 2 study, however, administration of rhIL-12 at this same dose level resulted in severe toxicities with some patients unable to tolerate more than two successive doses. Of the 17 patients receiving rhIL-12 in the Phase 2 study, 12 patients were hospitalized and two patients died. A thorough scientific investigation to determine the cause of this unexpected toxicity failed to identify any difference in the drug products used or the patient populations enrolled in the Phase 1 and Phase 2 studies that could have accounted for the profound difference in toxicity. The focus of the investigation therefore shifted to the schedule of rhIL-12 administration. We determined that a single injection of rhIL-12 2 weeks before consecutive dosing included in the Phase 1 study, but not in the schedule of administration in the Phase 2 study, has a profound abrogating effect on IL-12–induced interferon-γ (IFN-γ) production and toxicity. This observation of schedule-dependent toxicity of IL-12 has been verified in mice, as well as nonhuman primates. In this regard, a single injection of IL-12 before consecutive daily dosing protected mice and cynomolgus monkeys from acute toxicity including mortality and was associated with an attenuated IFN-γ response. Because of this unique biologic response, careful attention to the schedule of administration is required to assure safe and effective clinical development of this highly promising cytokine.
INTERLEUKIN-12 (IL-12) is a novel heterodimeric cytokine originally cloned from an Epstein-Barr virus (EBV)- transformed cell line after an activity that enhanced natural killer (NK) cell killing and interferon-γ (IFN-γ) production was identified in the conditioned medium.1 The availability of recombinant IL-12 has allowed extensive evaluation of the biologic activities of both the human and murine proteins. IL-12 has subsequently been shown to increase IFN-γ production from NK and T cells, enhance NK/lymphokine-activated killer (LAK) cell cytotoxicity, and promote T-helper cell type 1 (Th1) immune responses both in vitro and in vivo (reviewed in Trinchieri,2 Brunda,3 and Wolf et al4 ). Such activities have formed the basis for many of the preclinical studies with recombinant murine IL-12 (rmIL-12), which have helped identify the therapeutic potential of this cytokine. Specifically, rmIL-12 has been shown to possess potent antitumor and antimetastatic activity in a variety of murine tumor systems.5-9 In general, the antitumor effects of IL-12 are dose-dependent and are apparent in mice with well-established tumors. In addition to both promoting the regression of primary tumors and inhibiting the formation of metastases, mice treated with rmIL-12 have been shown to develop a long lasting protective antitumor immune response.6,7 Although the mechanisms that mediate tumor regression are not completely understood, experiments in mice deficient in T and NK cells, as well as in vivo cell depletion studies, implicate a combination of both direct and indirect T-cell effector mechanisms, with IL-12–induced secretion of IFN-γ clearly an integral component of this activity.9
More recently, recombinant human IL-12 (rhIL-12) has been administered to nonhuman primates with biologic activity observed over a wide dose range.10,11 Treatment with rhIL-12 results in both a transient decrease in circulating leukocytes, as well as reversible thrombocytopenia and anemia. Similar changes in peripheral hematology have been reported in mice following rmIL-12 administration at dosages that maintain marked antitumor activity. In addition to the hematopoietic effects described, rhIL-12 administration to nonhuman primates has been shown to increase plasma neopterin concentrations, a surrogate marker for IFN-γ.11 In general, rhIL-12 has been well tolerated in nonhuman primates with biologic activity observed at doses well below the maximum tolerated dose (MTD).
The promotion of cytolytic activity of T and NK cells, induction of IFN-γ, and potent antitumor activity observed in preclinical models have formed the scientific foundation for initiation of clinical trials with rhIL-12 in patients with advanced malignancy. A Phase 1 open-label, nonrandomized, dose-escalation study was performed to establish the safety and tolerance of rhIL-12 administered intravenously.12 The first cycle of rhIL-12 administration was given as a single injection followed by 13 days of rest to determine the pharmacokinetics and pharmacodynamic parameters of rhIL-12. Subsequent cycles consisted of daily intravenous rhIL-12 administration at the same dose for 5 consecutive days followed by an additional 16 days of rest. After establishing the MTD of 500 ng/kg for intravenous administration using this regimen, a Phase 2 open-label, nonrandomized multicenter study in patients with advanced renal cell carcinoma was undertaken. The primary objective of this study was to determine the tumor response following rhIL-12 treatment at the MTD with multiple cycles of treatment. rhIL-12 was administered intravenously five times per week for 1 week every 3 weeks without the single injection test dose of rhIL-12 2 weeks before consecutive dosing. In marked contrast to the Phase 1 experience, treatment with rhIL-12 in this study was associated with unexpected serious adverse events. We conducted a thorough scientific investigation to determine the possible cause of this unexpected toxicity by comparing the drug product used, the clinical and laboratory results of the patient populations enrolled, and the schedule of rhIL-12 administration that could have accounted for the profound difference in toxicity between the Phase 1 and Phase 2 studies. To specifically investigate the relationship between the schedule of administration and IL-12–associated toxicity, studies were also performed in both C3H/Hej mice and nonhuman primates. The results from these studies show that prior exposure to IL-12 protects against the toxicity and is associated with an attenuated IFN-γ response following subsequent daily IL-12 administration.
MATERIALS AND METHODS
Clinical study design.The study design and patient characteristics for the Phase 1 study have been previously described.12 The Phase 2 study was an open-label, nonrandomized, multicenter outpatient study of rhIL-12 administered by intravenous injection to patients with advanced renal cell carcinoma conducted at four institutions. Eligibility criteria at the time of entry included the following: (1) age ≥18 years with a histologic diagnosis of advanced renal cell carcinoma with measurable evidence of residual, recurrent, or metastatic disease; (2) life expectancy of at least 12 weeks; (3) adequate hematopoietic, renal, and hepatic function; (4) Karnofsky performance status index ≥70; (5) absence of brain metastases or clinically significant central nervous system, cardiovascular, pulmonary, infectious, and autoimmune disease; (6) an interval of at least 2 weeks since prior surgery or radiation therapy, 4 weeks since prior chemotherapy or biologic therapy, and 6 weeks since prior IL-2 or nitrosoureas; (7) negative serology for hepatitis B or human immunodeficiency virus (HIV) disease; (8) lack of concurrent steroid use; (9) lack of prior rhIL-12 exposure; and (10) written informed consent. This study was approved by the appropriate institutional review boards and conducted according to the principles expressed in the Declaration of Helsinki.
rhIL-12 was administered by intravenous injection into a rapidly flowing intravenous line. All patients were scheduled to receive 500 ng/kg daily for 5 consecutive days every 3 weeks. Adverse experiences were assessed according to the National Cancer Institute Common Toxicity Criteria Scale13 with certain modifications. If a patient experienced unacceptable toxicities, then the dose administered for subsequent cycles would be reduced. Serum samples were obtained before treatment and daily for most patients and assayed for IL-12 concentrations by a chemiluminescent-based enzyme-linked immunosorbent assay (ELISA)14 and for IFN-γ by a commercially available ELISA assay (Endogen, Inc, Woburn, MA). Serum neopterin concentrations were determined by radioimmunoassay (Nichols Institute Reference Laboratories, San Juan Capistrano, CA). One patient was electively hospitalized for the first 24 hours after the initial dose of rhIL-12 to obtain serum for detailed pharmacokinetics analysis. Samples were obtained immediately before the first dose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 10, 12, 14, and 24 hours postdrug administration. The 24-hour postdose sample was obtained before the second dose of rhIL-12.
Cytokine administration in mice.Female C3H/Hej mice were purchased from The Jackson Laboratories (Bar Harbor, ME), housed five per cage, and fed standard rodent chow diet with water ad libitum. Mice received a single subcutaneous injection of rmIL-12 (0.5 or 1 μg) or vehicle 7 or 14 days before receiving daily administration of rmIL-12 at the same dose (days 0 to 5). Mice were weighed on day 0 before initiating rmIL-12 treatment and then again on day 5 after the last dose of rmIL-12. Mice were also observed for gross signs of toxicity and monitored daily for survival.
Analysis of cytokine production during consecutive rmIL-12 administration.C3H/Hej mice received a single injection of rmIL-12 (0.5 μg) or vehicle on day −7 followed by consecutive dosing with 0.5 μg of rmIL-12 on days 0 to 5. During the period of daily administration, mice from each group were anesthetized with methoxyflurane and blood collected by cardiac puncture for analysis of serum IFN-γ and tumor necrosis factor-α (TNF-α) by ELISA (Endogen, Inc).
Lymph nodes (popliteal, axillary, and brachial) were isolated from rmIL-12 or vehicle pretreated mice during the period of consecutive rmIL-12 administration. Single cell suspensions were prepared and cultured at a density of 5 × 106 cells/mL in RPMI-1640 containing 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT), 5 × 10-5 mol/L 2-mercaptoethanol, 100 μg/mL streptomycin, and 100 U/mL penicillin. Cells were stimulated with rmIL-12 (0.1 or 1 ng/mL) or concanavalin A (Con A) (0.5 μg/mL) for 48 hours. IFN-γ and IL-10 concentrations in the supernatant were determined by ELISA.
Coadministration of a neutralizing antibody against murine IFN-γ.C3H/Hej mice received six consecutive subcutaneous injections of rmIL-12 at a dose of 0.5 μg per mouse. Anti–IFN-γ antibody (XMG1.2, Endogen) or an isotype control antibody were administered by intraperitoneal injection on days 0 to 5 (250 μg/mouse).
rhIL-12 schedule-dependent toxicity in nonhuman primates.Groups of four male cynomolgus monkeys received a single injection of rhIL-12 (1 or 10 μg/kg) or diluent followed by 13 days of rest and subsequent daily dosing for 5 consecutive days. Animals were monitored daily for clinical signs of toxicity. IFN-γ concentrations were measured by ELISA.
Statistical analysis.Statistical analyses of the changes in serum levels of IFN-γ in mice were performed using an unpaired Student's t-test.
RESULTS
Patient characteristics and clinical experience.Table 1 lists the patient characteristics for the 17 patients enrolled in the Phase 2 study. The patients enrolled in the Phase 1 and Phase 2 protocols were similar with respect to age, race, and performance status. For example, the mean age of patients in the Phase 2 study was 57.1 years (range, 23 to 74) compared with 52.0 years (range, 25 to 76) in the Phase 1 study. In addition, 50% and 100% of patients had renal cell carcinoma in the Phase 1 and Phase 2 studies, respectively. Furthermore, all of the patients had a baseline Karnofsky performance status index of 80% or greater in the Phase 1 and Phase 2 studies. The baseline cancer status was similar in that all patients had metastatic disease. Fourteen of the 17 patients in the Phase 2 study and four of the six patients with renal cell carcinoma in the Phase 1 study treated at the 500 ng/kg dose level had received prior IL-2 therapy.
Phase 2 Study Patient Characteristics
| Total no. of patients | 17 |
| Mean age (range) | 57.1 yr (23-74) |
| Gender (male/female) | 13/4 |
| Performance status (Karnofsky) | |
| 100% | 10 |
| 90% | 0 |
| 80% | 7 |
| Tumor type | |
| Renal cell cancer | 17 |
| Prior therapy | |
| IL-2 | 14 |
| Surgery | 17 |
| Total no. of patients | 17 |
| Mean age (range) | 57.1 yr (23-74) |
| Gender (male/female) | 13/4 |
| Performance status (Karnofsky) | |
| 100% | 10 |
| 90% | 0 |
| 80% | 7 |
| Tumor type | |
| Renal cell cancer | 17 |
| Prior therapy | |
| IL-2 | 14 |
| Surgery | 17 |
All 17 patients in the Phase 2 study received a daily intravenous injection of rhIL-12 at 500 ng/kg, which was determined to be the MTD in the Phase 1 study. In marked contrast to the Phase 1 trial, most patients in the Phase 2 study experienced serious adverse events during the first cycle with some patients unable to tolerate more than two successive doses of rhIL-12. The clinical study was halted immediately and no patient entered a second cycle. Of the 17 patients receiving rhIL-12, 12 were hospitalized. Patients treated with rhIL-12 in the Phase 2 study had a substantially greater percentage of grade 3 (severe toxicity), or grade 4 (life-threatening toxicity) adverse experiences when compared with patients in the Phase 1 study. Both clinical adverse experiences and laboratory abnormalities were seen in many organ systems (Table 2). For example, grade 3 or 4 fatigue, dyspnea, stomatitis, leukopenia, hyperbilirubinemia, elevations in transaminases, and thrombocytopenia occurred in greater than 20% of patients treated in the Phase 2 study. In contrast, only grade 3 or 4 leukopenia and hyperbilirubinemia occurred in greater than 20% of patients treated in the Phase 1 study (Table 2).
Number of Patients Treated With rhIL-12 at 500 ng/kg Intravenously With Grade 3 (severe) or Grade 4 (life-threatening) Adverse Experiences
| Adverse Experience . | Phase 2 . | Phase 1 . |
|---|---|---|
| . | (n = 17) (%) . | (n = 12) (%) . |
| Clinical | ||
| Fatigue | 6 (35) | 0 |
| Dyspnea | 5 (29) | 0 |
| Stomatitis | 4 (24) | 0 |
| Acidosis | 3 (18) | 0 |
| Gastrointestinal hemorrhage | 2 (12) | 2 (17) |
| Laboratory | ||
| Leukopenia | 11 (65) | 12 (100) |
| Hyperbilirubinemia | 8 (47) | 6 (50) |
| Elevated AST | 8 (47) | 2 (17) |
| Elevated ALT | 6 (35) | 1 (8) |
| Thrombocytopenia | 4 (24) | 0 |
| Elevated creatinine | 3 (18) | 0 |
| Elevated alkaline phosphatase | 2 (12) | 2 (17) |
| Adverse Experience . | Phase 2 . | Phase 1 . |
|---|---|---|
| . | (n = 17) (%) . | (n = 12) (%) . |
| Clinical | ||
| Fatigue | 6 (35) | 0 |
| Dyspnea | 5 (29) | 0 |
| Stomatitis | 4 (24) | 0 |
| Acidosis | 3 (18) | 0 |
| Gastrointestinal hemorrhage | 2 (12) | 2 (17) |
| Laboratory | ||
| Leukopenia | 11 (65) | 12 (100) |
| Hyperbilirubinemia | 8 (47) | 6 (50) |
| Elevated AST | 8 (47) | 2 (17) |
| Elevated ALT | 6 (35) | 1 (8) |
| Thrombocytopenia | 4 (24) | 0 |
| Elevated creatinine | 3 (18) | 0 |
| Elevated alkaline phosphatase | 2 (12) | 2 (17) |
Adverse experiences that occurred in greater than 15% of patients are listed.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Two patient deaths occurred during the Phase 2 study that were determined to be related to study drug. A 65-year-old man with previously untreated advanced renal cell carcinoma with pulmonary, hepatic, and lymph node metastasis received rhIL-12 for 5 days. On study day 9, the patient had a lower gastrointestinal bleeding episode. The following day, the patient had a second bleeding episode, became hypotensive, and died. Postmortem examination showed hemorrhagic ulceration in the large intestine. A 57-year-old man with severe peripheral vascular disease and metastastic renal cell carcinoma progressed after receiving IL-2. He was treated with rhIL-12 for 3 days and on study day 4 developed multiorgan failure with acidosis, respiratory failure requiring ventilatory support, sepsis-like syndrome, liver failure, renal failure requiring dialysis, and gastrointestinal bleeding. After 3 weeks, intensive life support was withdrawn and death occurred on study day 23. Postmortem examination showed necrotizing aspiration pneumonia and diffuse hemorrhagic colitis.
An objective tumor response was observed in a 61-year-old man with advanced renal cell cancer and pulmonary hepatic and retroperitoneal metastases previously treated with IL-2. The patient developed atrial flutter after receiving three doses of rhIL-12, and further therapy was discontinued. The patient had a partial response with decreases in tumor masses in the lung, liver, and soft tissue lasting 12 months.
Although a minor change occurred in the manufacture of the rhIL-12 drug product used in the Phase 2 study compared with the drug product used in the Phase 1 study, no significant differences in the biochemical or in vitro biologic characteristics between the drug products used were evident when analyzed by amino acid composition, N-terminus sequencing, reduced and nonreduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), size exclusion chromatography, isoelectric focusing, peptide mapping, carbohydrate mapping, phytohemagglutinin (PHA) blast proliferation assay, and peripheral blood lymphocyte IFN-γ induction assay (data not shown). It was concluded that a difference in drug product could not account for the profound toxicity difference in the Phase 1 and 2 studies.
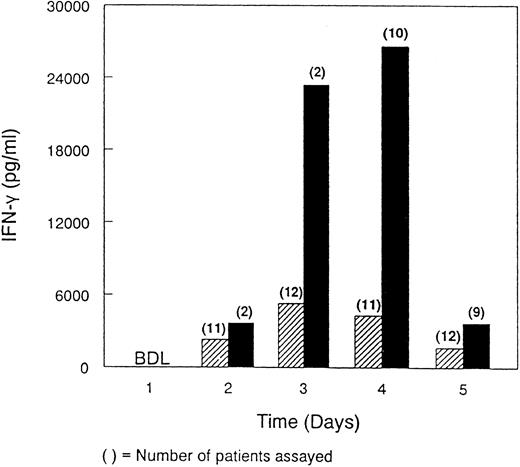
A comparison of the immunopharmacologic profile of rhIL-12 focusing on IFN-γ was also attempted. However, because of the trial design and unexpected toxicity observed in the Phase 2 study, only limited data were available making a direct comparison difficult. Analysis of the samples obtained from one patient in the Phase 2 trial showed a comparable pharmacokinetic profile for rhIL-12 to that observed in six patients with renal cell carcinoma in the Phase 1 trial in the 500 ng/kg cohort (data not shown). In the Phase 2 study, the mean IFN-γ levels measured from the available samples were substantially higher than those observed in the Phase 1 study despite the apparent comparable pharmacokinetic profile (Fig 1). TNF-α could not be detected by ELISA in serum samples obtained from either the Phase 1 or Phase 2 studies at any of the time points examined.
Comparison of serum IFN-γ concentrations in patients in the Phase 1 and Phase 2 studies. Serum IFN-γ concentrations were determined for 12 patients receiving 500 ng/kg rhIL-12 intravenously daily for 5 consecutive days in the Phase 1 study (▨) and for 10 patients who were scheduled to receive rhIL-12 at 500 ng/kg intravenously for 5 consecutive days in the Phase 2 study (▪). Serum samples were obtained immediately before the first dose and daily before subsequent rhIL-12 administration. The number of patients in the Phase 2 study receiving 2, 3, 4, or 5 days of rhIL-12 treatment were 1, 10, 2, and 4, respectively. BDL, below detectable levels.
Comparison of serum IFN-γ concentrations in patients in the Phase 1 and Phase 2 studies. Serum IFN-γ concentrations were determined for 12 patients receiving 500 ng/kg rhIL-12 intravenously daily for 5 consecutive days in the Phase 1 study (▨) and for 10 patients who were scheduled to receive rhIL-12 at 500 ng/kg intravenously for 5 consecutive days in the Phase 2 study (▪). Serum samples were obtained immediately before the first dose and daily before subsequent rhIL-12 administration. The number of patients in the Phase 2 study receiving 2, 3, 4, or 5 days of rhIL-12 treatment were 1, 10, 2, and 4, respectively. BDL, below detectable levels.
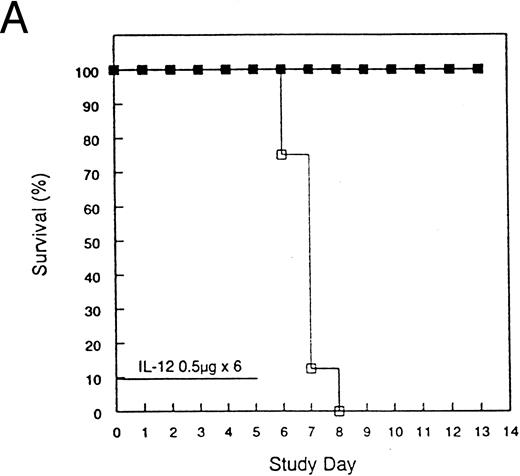
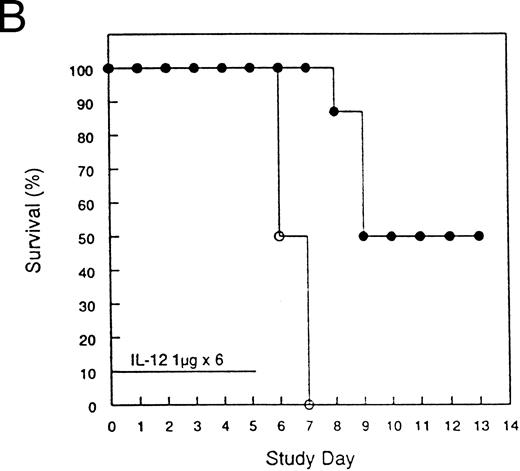
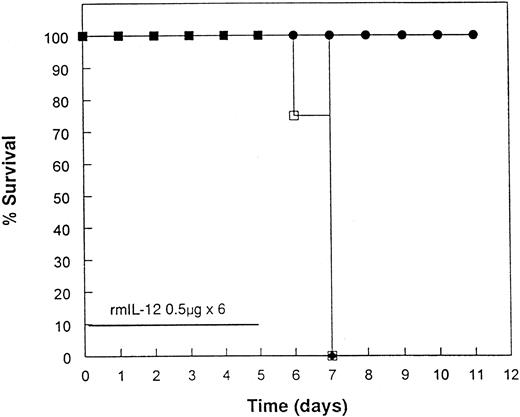
Protection from acute rmIL-12 toxicity in mice by pretreatment with rmIL-12.To investigate further the toxicities observed in the clinical trial, several experiments were performed in mice. Administration of 0.5 or 1 μg of rmIL-12 to C3H/Hej mice for 6 consecutive days resulted in 100% mortality by day 8 (Fig 2). During the course of rmIL-12 administration, mice displayed signs of severe gastrointestinal toxicity with diarrhea and substantial weight loss. Of great interest, a single injection of rmIL-12 (0.5 μg) 1 week before consecutive dosing prevented the toxicity associated with subsequent rmIL-12 treatment with no deaths observed (Fig 2A). This finding has been reproduced in three separate experiments. At the 1 μg dose, the protective effect of pretreating mice with rmIL-12 was more variable with survival following subsequent rmIL-12 administration ranging from 50% (Fig 2B) to 100% protection (data not shown). Pretreatment with rmIL-12 2 weeks before consecutive administration of rmIL-12 had no effect on survival at either dose tested (data not shown).
Pretreatment of mice with rmIL-12 protects against subsequent rmIL-12–induced toxicity. Female C3H/Hej mice received a single subcutaneous injection of rmIL-12 (0.5 or 1 μg) or vehicle 7 days before consecutive dosing with rmIL-12 at the same dose (days 0 to 5). The percent survival of vehicle and rmIL-12 primed mice receiving either 0.5 μg (A) or 1 μg (B) of rmIL-12 is shown (n = 8 per treatment group). The results are representative of three separate experiments. (A) (□), Vehicle day −7; (▪), rmIL-12 day −7. (B) (○), Vehicle day −7; (•), rmIL-12 day −7.
Pretreatment of mice with rmIL-12 protects against subsequent rmIL-12–induced toxicity. Female C3H/Hej mice received a single subcutaneous injection of rmIL-12 (0.5 or 1 μg) or vehicle 7 days before consecutive dosing with rmIL-12 at the same dose (days 0 to 5). The percent survival of vehicle and rmIL-12 primed mice receiving either 0.5 μg (A) or 1 μg (B) of rmIL-12 is shown (n = 8 per treatment group). The results are representative of three separate experiments. (A) (□), Vehicle day −7; (▪), rmIL-12 day −7. (B) (○), Vehicle day −7; (•), rmIL-12 day −7.
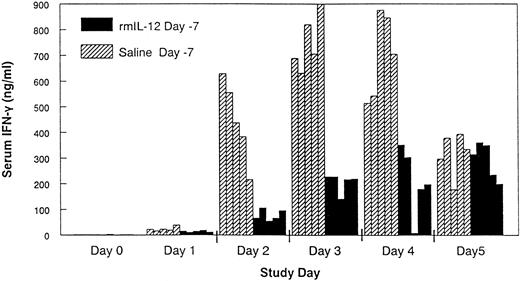
Changes in serum IFN-γ and TNF-α in control and rmIL-12 pretreated mice.Daily administration of rmIL-12 (0.5 μg) to control C3H/Hej mice resulted in a rapid increase in serum IFN-γ that was apparent as early as day 1 and peaked between days 3 and 4 (Fig 3). In mice that had been pretreated with rmIL-12, the IFN-γ response during subsequent rmIL-12 dosing was substantially attenuated on days 2 through 4 when compared with serum levels of IFN-γ in the control mice (P < .01). TNF-α could not be detected in the serum either during the period of the rmIL-12 administration or at later time points when control mice displayed signs of severe rmIL-12–induced toxicity.
Pretreatment with rmIL-12 attenuates the IFN-γ response following subsequent rmIL-12 administration. Mice received a single injection of either rmIL-12 (0.5 μg) or vehicle 7 days before daily rmIL-12 administration (days 0 to 5). Serum was collected by cardiac puncture during the period of daily rmIL-12 administration and IFN-γ measured by standard ELISA techniques. Individual mouse IFN-γ measurements are shown. Comparable results were seen in a separate experiment.
Pretreatment with rmIL-12 attenuates the IFN-γ response following subsequent rmIL-12 administration. Mice received a single injection of either rmIL-12 (0.5 μg) or vehicle 7 days before daily rmIL-12 administration (days 0 to 5). Serum was collected by cardiac puncture during the period of daily rmIL-12 administration and IFN-γ measured by standard ELISA techniques. Individual mouse IFN-γ measurements are shown. Comparable results were seen in a separate experiment.
In vitro cytokine production from control and rmIL-12 pretreated mice.To further characterize the cytokine response in mice pretreated with rmIL-12, lymph node cells (LNC) were isolated during the period of consecutive dosing and stimulated in vitro for measurement of IFN-γ and IL-10. In these assays, in vitro stimulation of LNC from vehicle pretreated mice with rmIL-12 or the T-cell mitogen Con A resulted in a marked increase in IFN-γ secretion (Fig 4A). The magnitude of this in vitro response increased during the period of rmIL-12 administration and mirrored the change in serum IFN-γ. In contrast, comparable cultures of LNC isolated from rmIL-12 pretreated mice secreted less IFN-γ following in vitro stimulation with either rmIL-12 or Con A (Fig 4B). Further cytokine analysis from the same cultures showed that in addition to an attenuation of IFN-γ secretion, LNC from rmIL-12 pretreated mice secreted less IL-10 (data not shown).
In vitro IFN-γ production from lymph node cells isolated during the period of rmIL-12 treatment. Mice received a single injection of either vehicle (A) or rmIL-12 (0.5 μg) (B) 7 days before daily rmIL-12 administration (days 0 to 5). Lymph nodes (popliteal, axillary, and brachial) were harvested during the period of daily rmIL-12 administration for in vitro cytokine assays. The cells were stimulated with either rmIL-12 (0.1 [] or 1 ng/mL [▪]) or Con A (0.5 μg/mL, ▨) as described in the Materials and Methods section. IFN-γ secretion was measured by a commercially available ELISA (Endogen). (▨), Control.
In vitro IFN-γ production from lymph node cells isolated during the period of rmIL-12 treatment. Mice received a single injection of either vehicle (A) or rmIL-12 (0.5 μg) (B) 7 days before daily rmIL-12 administration (days 0 to 5). Lymph nodes (popliteal, axillary, and brachial) were harvested during the period of daily rmIL-12 administration for in vitro cytokine assays. The cells were stimulated with either rmIL-12 (0.1 [] or 1 ng/mL [▪]) or Con A (0.5 μg/mL, ▨) as described in the Materials and Methods section. IFN-γ secretion was measured by a commercially available ELISA (Endogen). (▨), Control.
To determine if IFN-γ is a mediator of the acute toxicity of rmIL-12, C3H/Hej mice received daily administration of rmIL-12 (0.5 μg for 6 days) with or without treatment with a neutralizing antibody to murine IFN-γ. Consistent with the previous results, mice treated with rmIL-12 and a control antibody developed signs of severe toxicity with 100% mortality observed by day 7 (Fig 5). In contrast, mice that received rmIL-12 and a neutralizing antibody to murine IFN-γ showed no signs of acute toxicity of weight loss or diarrhea with no deaths observed in this treatment group.
Antibodies against IFN-γ abrogate rmIL-12–induced toxicity. C3H/Hej mice were injected subcutaneously with rmIL-12 (0.5 μg) for 6 consecutive days. Anti-IFN–γ antibody (XMG1.2, •) or an isotype control (□) was administered by intraperitoneal injection on days 0 to 5 at a dose of 250 μg per mouse (n = 4). (♦), Rat IgG.
Antibodies against IFN-γ abrogate rmIL-12–induced toxicity. C3H/Hej mice were injected subcutaneously with rmIL-12 (0.5 μg) for 6 consecutive days. Anti-IFN–γ antibody (XMG1.2, •) or an isotype control (□) was administered by intraperitoneal injection on days 0 to 5 at a dose of 250 μg per mouse (n = 4). (♦), Rat IgG.
Demonstration of rhIL-12 schedule-dependent toxicity in cynomolgus monkeys.To further investigate the effects observed in humans and mice, experiments were performed in nonhuman primates. Groups of four male monkeys received a single intravenous injection of rhIL-12 or diluent, which was followed by 13 days of rest and subsequent daily dosing with rhIL-12 (1 or 10 μg/kg/d) for 5 consecutive days. Both antemortem and postmortem findings identified greater toxicity in animals that had not received the single injection of rhIL-12 before daily treatment. Clinical signs of toxicity were especially evident in animals receiving the higher dosage of rhIL-12 without pretreatment including fatigue, anorexia, and episodes of emesis and diarrhea. The clinical signs of toxicity in this group were also accompanied by the presence of gastric congestion, ulcerative colitis and ileitis, lymphadenitis of the cecal Peyer's patches, and increased abdominal fluid. Consistent with the clinical and murine data, the ameliorated toxicity in rhIL-12 pretreated cynomolgus monkeys was associated with an attenuated IFN-γ response during consecutive rhIL-12 treatment (Table 3).
Comparison of Serum IFN-γ Concentrations in Control and rhIL-12 Pretreated Cynomolgus Monkeys During Consecutive rhIL-12 Administration
| rhIL-12 Dose . | Predose (Day-14) . | IFN-γ (Day 4), pg/mL . |
|---|---|---|
| 1 μg/kg/d | Vehicle | 793 ± 386 |
| rhIL-12 | <30 | |
| 10 μg/kg/d | Vehicle | 1,483 ± 882 |
| rhIL-12 | <30 |
| rhIL-12 Dose . | Predose (Day-14) . | IFN-γ (Day 4), pg/mL . |
|---|---|---|
| 1 μg/kg/d | Vehicle | 793 ± 386 |
| rhIL-12 | <30 | |
| 10 μg/kg/d | Vehicle | 1,483 ± 882 |
| rhIL-12 | <30 |
IFN-γ concentrations expressed as pg/mL ± standard error (SE).
DISCUSSION
IL-12 is a novel heterodimeric cytokine that is produced predominantly by macrophages and other antigen presenting cells and serves as a key regulator of cell-mediated immunity. Based on the results from a Phase 1 study that established an MTD of 500 ng/kg for rhIL-12,12 a multicenter Phase 2 trial was initiated with rhIL-12 administered at this dose level. In marked contrast to the Phase 1 trial, patients in the Phase 2 study experienced serious adverse events with no patient entering the second cycle. The adverse events involved multiple organ systems and were associated with two patient deaths. No differences in the patient populations enrolled in the Phase 1 and Phase 2 studies were identified that could have accounted for the profound difference in toxicity. The focus of the investigation therefore shifted to the schedule of rhIL-12 administration, which had been modified slightly, such that patients in the Phase 2 study received daily rhIL-12 dosing without the single injection of rhIL-12 2 weeks earlier that was employed in the Phase 1 study. Analysis of the limited serum samples available from the Phase 2 study indicated a more profound increase in serum IFN-γ following rhIL-12 administration than that observed for the Phase 1 patients treated with the same dose of rhIL-12. This difference was evident despite the fact that the pharmacokinetics profile for rhIL-12 in the Phase 2 study fell within the range of that observed for the Phase 1 study. The constitutional, cardiac, renal, hematopoietic, hepatic, and neurologic toxicities observed in the Phase 2 study were similar to those dose-limiting toxicities observed in previously reported Phase 1 and 2 studies with IFN-γ.15-24 For example, prolonged administration of IFN-γ by intravenous infusion was associated with constitutional side effects such as fever, chills, and fatigue. In addition, transient myelosuppression and elevation of liver function tests were common. Neurologic side effects consisted of lethargy, somnolence, confusion, tremor, and akathesia. Lastly, hypotension, electrocardiogram (ECG) changes, arrhythmias, and renal insufficiency were also described in clinical studies of IFN-γ. These findings suggest that the toxicity profile observed in the Phase 2 studies of rhIL-12 may be attributable in part to prolonged circulating levels of IFN-γ. In this regard, the mean IFN-γ levels measured in the Phase 2 study on days 3 and 4 were substantially greater than levels obtained during a 24-hour infusion of IFN-γ.17
IL-12 treatment was associated with toxicity of the gastrointestinal tract. For example, patients treated with rhIL-12 in the Phase 2 study experienced stomatitis, gastrointestinal bleeding, and colitis. Furthermore, exposure to high-dose levels of IL-12 in mice resulted in histopathologic evidence of gastrointestinal toxicity with diarrhea and weight loss. Moreover, cynomolgus monkeys treated with rhIL-12 exhibited decreased food intake, weight loss, and diarrhea. Recently, a novel murine model of hapten-induced chronic intestinal inflammation with severe diarrhea, weight loss, rectal prolapse, and colonic infiltration of CD4+ T cells has been described.25 Treatment with monoclonal anti-IL–12 antibodies was associated with significant improvement in the hapten-induced colitis. Taken together, these data suggest an important role of IL-12 in inflammatory gastrointestinal disease.
The relationship between the schedule of administration and IL-12–associated toxicity was evaluated in C3H/Hej mice using the recombinant murine protein. C3H/Hej mice were chosen because this strain is highly sensitive to rmIL-12–mediated toxicity. Extensive analysis confirmed that a single injection of rmIL-12 1 week before starting consecutive daily treatment with rmIL-12 has a profound and unexpected abrogating effect on toxicity. Consistent with the clinical data, protection from toxicity was associated with an attenuated IFN-γ response during subsequent daily rmIL-12 administration. Although IL-12 has weak stimulatory activity for TNF-α secretion,26 TNF-α was not detected at any time during the course of the experiment in either control or rmIL-12 pretreated mice.
The serum cytokine data was further substantiated by in vitro analysis of IFN-γ production from LNC isolated during the period of daily rmIL-12 administration. Consistent with the serum data, the production of IFN-γ following in vitro stimulation with rmIL-12 was substantially attenuated in rmIL-12 pretreated mice compared with comparable cultures of LNC isolated from control animals. As rmIL-12 has been shown to increase IL-10 mRNA in naive mice27 and IL-10 is a potent inhibitor of many of the actions of IL-12,28 we had hypothesized that pretreatment with rmIL-12 may result in a compensatory increase in IL-10 secretion. This does not appear to be the case as the in vitro production of IL-10 from isolated LNC was also attenuated by rmIL-12 pretreatment. Therefore, although the results are consistent with IL-10 mRNA expression, overproduction of this potentially suppressive cytokine does not appear to be the cause of the attenuated IFN-γ response. However, as IL-12 has been shown to be synergistic with IL-2, TNF-α, and B7 for IFN-γ secretion, it is conceivable that the attenuated production of IFN-γ in rmIL-12 pretreated mice is due to subtle changes in one or more of these synergistic factors. It is unlikely, however, that the attenuated response is due solely to changes in the expression of the IL-12 receptor as IFN-γ production in response to the T-cell mitogen Con A was also diminished by IL-12 pretreatment. In addition, many of the biologic responses to IL-12 were unaffected by prior exposure to IL-12 (see below), which further argues against downregulation of the IL-12 receptor as the sole mechanism for diminished IFN-γ production and toxicity.
To provide additional evidence for a schedule-dependent toxicity, a study was performed in cynomolgus monkeys using the same schedules as in the Phase 1 and 2 clinical trials. rhIL-12 was evaluated at dosages of 1 and 10 μg/kg/d based on previous toxicological studies, which indicated that 10 μg/kg/d approached the maximum tolerated dose in the monkey, while 1 μg/kg/d was a minimally toxic dose. Clinical signs of toxicity were less apparent in animals that had been pretreated with rhIL-12 2 weeks before daily treatment at both dose levels of rhIL-12. Protection from toxicity in cynomolgus monkeys was also associated with an attenuated IFN-γ response during the period of daily rhIL-12 treatment.
Collectively, the results show that a single injection of IL-12 has a profound abrogating effect on subsequent IL-12–induced toxicity that is associated with a reduction in both Th1 (IFN-γ) and Th2 (IL-10) cytokine production. It should be stressed, however, that not all of the biologic responses to IL-12 were altered by pretreatment. Most notably, changes in the concentration of serum neopterin, a surrogate marker for immune activation, observed in the Phase 1 trial were not affected by multiple cycles of rhIL-12 administration. Although IFN-γ is the principal mediator of neopterin production from cultured monocytes, IL-1, IL-2, and TNF-α are all capable of stimulating the release of neopterin from unfractionated peripheral blood mononuclear cells (PBMC).29 The continued production of neopterin suggests, therefore, that immunologic activation is not diminished during subsequent cycles of rhIL-12, however, it is conceivable that this response is independent of circulating IFN-γ. Finally, pretreatment with IL-12 did not ameliorate the thrombocytopenia observed during subsequent administration of IL-12 to either mice or humans. This observation has greater significance as the IL-12–induced thrombocytopenia observed in mice has been shown to be independent of secondary IFN-γ secretion. Thus, although toxicity is clearly abrogated, the magnitude of many of the biologic responses associated with IL-12 administration are not attenuated by IL-12 pretreatment.
Although the kinetics of protection following a single injection of IL-12 differ between mice and primates (1 and 2 weeks, respectively), the immunopharmacologic profile is remarkably similar with a reduction in IFN-γ secretion associated with the diminished toxicity. To date, the mechanism for this attenuated response remains undefined, however, a causative relationship between IFN-γ and the acute IL-12–induced toxicity in mice was shown using neutralizing antibodies to murine IFN-γ. Although we cannot rule out additional mediators of toxicity, histologic examination of control mice showed lesions in the gastrointestinal tract consistent with the pathology reported for IFN-γ.30 TNF-α was not detected in the serum in mice or man, however, locally produced TNF-α could contribute to the IL-12 toxicity given the synergistic interactions observed for IFN-γ and TNF-α.31,32 It is noteworthy that desensitization to TNF-α toxicity has been reported in mice, although the kinetics of this response is both rapid (0.5 to 1 hours) and short-lived (24 to 48 hours).33 This is in marked contrast to the response observed for IL-12 where a single injection is sufficient to abrogate the toxicity associated with daily administration of IL-12 either 1 or 2 weeks later in mice and humans, respectively.
In summary, our studies clearly show an unexpected and profound schedule-dependent toxicity after administration of IL-12 to mice, nonhuman primates, and man. In all instances, the diminished toxicity appears to be associated with an attenuated IFN-γ response following subsequent IL-12 administration, a finding that is consistent with the ability of neutralizing antibodies against IFN-γ to protect mice from acute rmIL-12–associated toxicity. Despite extensive in vitro analysis, the mechanism for the attenuated production of IFN-γ remains obscure, but does not appear to be due to an overproduction of the Th2-associated cytokine, IL-10. Although the toxicity of IL-12 has been shown to be schedule dependent, the impact of these findings on efficacy needs to be carefully evaluated, particularly the antitumor efficacy where IFN-γ has been shown to be closely linked to IL-12 activity. However, it should be noted that substantial increases in circulating IFN-γ were still detected following daily administration of IL-12 even after prior exposure to this cytokine. If the levels of IFN-γ are sufficient to maintain maximum biologic activity, the use of a single pretreatment injection of IL-12 may enable dose escalation in the absence of toxicity without loss of efficacy. Because of this unique schedule-dependent biologic phenomenon, careful attention to dose and especially schedule has been required to assure safe and effective clinical development of this highly promising cytokine. Indeed, additional clinical studies of rhIL-12 have been conducted and several hundred subjects have been safely treated with rhIL-12 (Genetics Institute, Inc, unpublished data).
ACKNOWLEDGMENT
We thank Sharon Hunter and Mark Collins for technical assistance, Dr Pamela Garzone and Michelle DeCoste for pharmacokinetics analyses, Drs Vic VanCleave and Abbie Celniker for bioanalytical support, and Dr Page Bouchard for helpful discussions.
Supported in part by Grant No. MOI-RR-00054 from the Division of Research Resources, National Institutes of Health, Bethesda, MD, to M.B.A.
Address reprint requests to John L. Ryan, PhD, MD, Genetics Institute, 87 Cambridge Park Dr, Cambridge, MA 02140.

![Fig. 4. In vitro IFN-γ production from lymph node cells isolated during the period of rmIL-12 treatment. Mice received a single injection of either vehicle (A) or rmIL-12 (0.5 μg) (B) 7 days before daily rmIL-12 administration (days 0 to 5). Lymph nodes (popliteal, axillary, and brachial) were harvested during the period of daily rmIL-12 administration for in vitro cytokine assays. The cells were stimulated with either rmIL-12 (0.1 [] or 1 ng/mL [▪]) or Con A (0.5 μg/mL, ▨) as described in the Materials and Methods section. IFN-γ secretion was measured by a commercially available ELISA (Endogen). (▨), Control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/7/10.1182_blood.v90.7.2541/3/m_bl_0034f4.jpeg?Expires=1765926324&Signature=Bk-cuLe7Vs4DkgjxB2t~iQEy2CsdfzFwJmgvIl00qN36qSrHYl2dcgXDhoXIqWmVh4kFVjXVRiSnibA2SO1emYrEmd7Yolrj8xrU6PcGqWapn2kbQWlomRiVFAEY4opZQP2p6HpWdt12Oj5aQ3yePKnD8FTlZFZ5tHNFTJjBoqLzSzf2ue7AGRYTs2DPO9rXgz0NoJs1rwPs5t2a5NTKQ5-PG1HonzuJCe3AQxQVgprH1Jc5XTDNgRc-xKd1mh2S~FI~zIAWvP6O6mfkEAVkmWJAroHmr8pL8dmt6i70ndjFSxAJUfHb~m9CRXUovSAhdo~yUTbkhHh6zztCsW3iWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal