Abstract
Multiple hematopoietic cytokines can stimulate granulopoiesis; however, their relative importance in vivo and mechanisms of action remain unclear. We recently reported that granulocyte colony-stimulating factor receptor (G-CSFR)-deficient mice have a severe quantitative defect in granulopoiesis despite which phenotypically normal neutrophils were still detected. These results confirmed a role for the G-CSFR as a major regulator of granulopoiesis in vivo, but also indicated that G-CSFR independent mechanisms of granulopoiesis must exist. To explore the role of interleukin-6 (IL-6) in granulopoiesis, we generated IL-6 × G-CSFR doubly deficient mice. The additional loss of IL-6 significantly worsened the neutropenia present in young adult G-CSFR–deficient mice; moreover, exogenous IL-6 stimulated granulopoiesis in vivo in the absence of G-CSFR signals. Near normal numbers of myeloid progenitors were detected in the bone marrow of IL-6 × G-CSFR–deficient mice and their ability to terminally differentiate into mature neutrophils was observed. These results indicate that IL-6 is an independent regulator of granulopoiesis in vivo and show that neither G-CSFR or IL-6 signals are required for the commitment of multipotential progenitors to the myeloid lineage or for their terminal differentiation.
GRANULOPOIESIS is the process whereby mature granulocytes are produced from a small number of pluripotent hematopoietic stem cells. Granulopoiesis has two important features: an enormous productive capacity with approximately 120 billion granulocytes produced daily in humans just to maintain homeostasis, and the ability to increase this production rapidly in response to stresses such as infections. Granulopoiesis is regulated in part by the actions of multiple hematopoietic cytokines, including granulocyte colony-stimulating factor (G-CSF ), granulocyte-macrophage colony-stimulating factor (GM-CSF ), interleukin-3 (IL-3), IL-6, stem cell factor (SCF ), and macrophage colony-stimulating factor (M-CSF ). The relative importance of individual cytokines to the regulation of granulopoiesis in vivo and their mechanisms of actions are incompletely defined.
The ability of G-CSF to stimulate granulopoiesis is well established. In fact, G-CSF is widely used to ameliorate neutropenia in a variety of clinical settings.1 The biologic effects of G-CSF are thought to be mediated through its interaction with the granulocyte colony-stimulating factor receptor (G-CSFR), a member of the cytokine receptor superfamily.2 To define further the role of G-CSF in the control of granulopoiesis, we recently generated G-CSFR–deficient mice.3 Similar to G-CSF (cytokine)-deficient mice,4 G-CSFR–deficient mice have chronic neutropenia with a uniform decrease in myeloid cells in the bone marrow. No accumulation of immature granulocytic cells in the bone marrow was observed suggesting that the residual granulocytic precursors present in these mice were able to differentiate normally into mature neutrophils. In agreement with this conclusion, the residual neutrophils present in G-CSFR–deficient mice appeared phenotypically normal as assessed by morphology, expression of myeloperoxidase, Gr-1, and CDllb, and by their ability to emigrate in response to intraperitoneal thioglycollate. These observations confirmed a role for the G-CSFR as a major regulator of granulopoiesis in vivo, but also indicated that G-CSFR independent mechanisms of granulopoiesis must exist.
We postulated that because multiple cytokines besides G-CSF can stimulate granulopoiesis, one or more of these cytokines may be stimulating the residual granulopoiesis in G-CSFR–deficient mice. Mice deficient for both G-CSF × GM-CSF have recently been generated.5 Peripheral blood granulocyte and monocyte counts in these mice were similar to G-CSF–deficient mice suggesting that GM-CSF is not solely responsible for the residual granulopoiesis in G-CSF–deficient mice. Recent data suggest that IL-6 may play an important role in the regulation of granulopoiesis. First, the administration of rIL-6 to mice induces an increase in peripheral neutrophil counts.6 Second, although IL-6–deficient mice have normal steady-state granulopoiesis,7 they are unable to mount a neutrophilic response to Listeria monocytogenes7 or Candida albicans8 infections. Third, the G-CSFR and IL-6 receptor complex initiate similar signal transduction cascades.9,10 Indeed, the G-CSFR and gp130 (the signal transduction subunit of the IL-6 receptor complex) share significant homology within their cytoplasmic domains. Finally, we recently have shown that in standard methylcellulose cultures, both IL-6 and G-CSF predominantly stimulate the same type of progenitor, namely the colony-forming unit-granulocyte (CFU-G) progenitor.3
To explore further the contribution of IL-6 to the regulation of granulopoiesis, we generated G-CSFR × IL-6 doubly deficient mice by intercrossing G-CSFR–deficient mice with IL-6–deficient mice. We show that the loss of IL-6 in G-CSFR–deficient mice leads to a further significant reduction in the number of mature neutrophils in peripheral blood or bone marrow. Interestingly, the number of myeloid progenitors responsive to IL-3, GM-CSF, or IL-6 in the bone marrow was nearly normal. Further, we show that IL-6 × G-CSFR–deficient progenitors are able to differentiate into mature neutrophils in vitro in response to either IL-3 or GM-CSF. These results confirm a role for IL-6 as a major regulator of granulopoiesis in vivo, but they also show that neither IL-6 or G-CSFR signals are required for the commitment of multipotential progenitors to the granulocyte lineage nor for the terminal differentiation of these progenitors to mature neutrophils.
MATERIALS AND METHODS
Mice.IL-6–deficient mice (outbred C57BL/6 × 129 Sv) were originally produced in the laboratory of Georges Köhler11 and were obtained through the Induced Mutant Resource at Jackson Laboratories (Bar Harbor, ME). The G-CSFR–deficient mice (outbred C57BL/6 × 129 Sv) were generated in our laboratory as follows. The production of chimeric mice using a single RW4 ES clone containing a targeted null mutation of the G-CSFR has been described previously.3 The chimeric mice were intercrossed with 129 SvJ mice and their progeny genotyped (see below) to identify heterozygous G-CSFR mutant mice on a 129 Sv genetic background; these mice were then intercrossed with C57BL/6 mice to generate heterozygous G-CSFR mutant mice on a C57BL/6 × 129 Sv genetic background. All mice were housed in a specific-pathogen free environment and examined daily by veterinary staff for signs of illness.
Southern blot analysis.Genomic DNA was isolated from the tails of mice, digested with Pst-1, resolved on 0.8% agarose gels, transferred to nitrocellulose filters, and hybridized with 32P-labeled probes. Probe G (see Fig 1) was generated as follows. Murine genomic DNA was amplified by polymerase chain reaction (PCR) using a G-CSFR intron 1 forward primer (5′-ACCTCAGACCATTCCCCAAC-3′) and a exon 2 reverse primer (5′-CTGCCTGGTGAACTTGGATGAGTT-3′). The resulting 1.6-kb amplicon was digested with Pst-1 and probe G (a 340-bp DNA fragment) was isolated. Probe 6 (a 450-bp DNA fragment) was generated by PCR amplification of murine genomic DNA using a murine IL-6 exon 1 forward primer (5′-TGAGACTGGGGATGTCTGTA-3′) and a exon 2 reverse primer (5′-AGAAGGCAACTGGATGGAAG-3′).
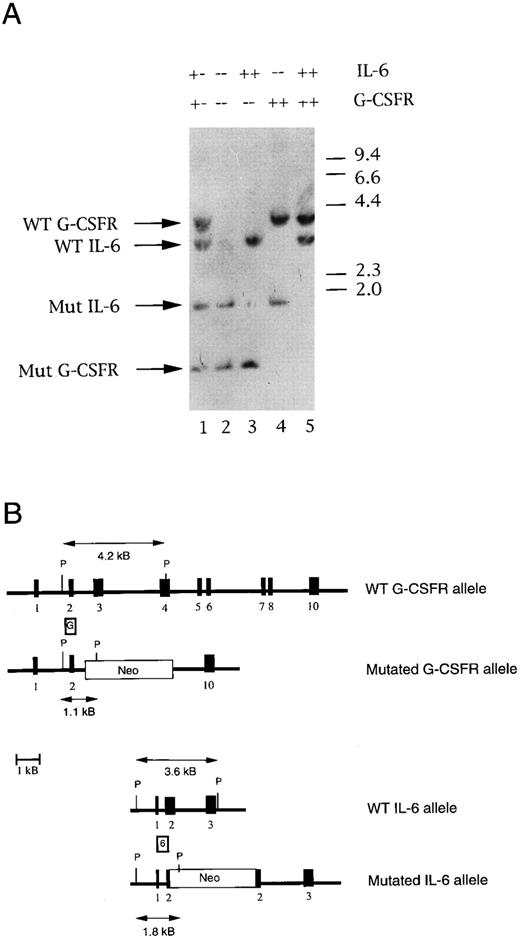
Genotyping of IL-6 × G-CSFR–deficient mice. (A) Representative Southern blot analysis of Pst1-digested genomic tail DNA isolated from the progeny of IL-6 × G-CSFR double heterozygous mice. The filter was hybridized simultaneously with probe G and probe 6. A 4.2-kb or 1.1-kb band is detected from G-CSFR wild-type or mutant alleles, respectively. A 3.6-kb or 1.8-kb band is detected from IL-6 wild-type or mutant alleles, respectively. (B) Structure of the targeted mutations. The positions of probe G and probe 6 are shown. The structure of the wild-type and mutant IL-6 alleles were derived from Kopf et al.11
Genotyping of IL-6 × G-CSFR–deficient mice. (A) Representative Southern blot analysis of Pst1-digested genomic tail DNA isolated from the progeny of IL-6 × G-CSFR double heterozygous mice. The filter was hybridized simultaneously with probe G and probe 6. A 4.2-kb or 1.1-kb band is detected from G-CSFR wild-type or mutant alleles, respectively. A 3.6-kb or 1.8-kb band is detected from IL-6 wild-type or mutant alleles, respectively. (B) Structure of the targeted mutations. The positions of probe G and probe 6 are shown. The structure of the wild-type and mutant IL-6 alleles were derived from Kopf et al.11
Enzyme-linked immunosorbent assay (ELISA).Serum IL-6 levels were measured using a commercial quantitative sandwich immunoassay (R&D systems, San Diego, CA) as per manufacturer's recommendations. The detectable level of IL-6 in mouse serum in this assay is 15.6 pg/mL. Eight sex-matched 5- to 8-week-old mice of each genotype were analyzed.
Peripheral blood and bone marrow analysis.Blood was obtained by retro-orbital venous plexus sampling in polypropylene tubes containing EDTA. Complete blood counts were determined using a Baker-9000 automated cell counter (Biochem ImmunoSystems, Plano, TX). Bone marrow was harvested by flushing both femoral bones with 3 mL of α-minimum essential medium (α-MEM) containing 2% fetal bovine serum (FBS). Manual leukocyte differentials were performed on Wright-stained blood smears or cytospin preparations of bone marrow mononuclear cells.
Flow cytometry.Bone marrow mononuclear cell preparations were incubated with the indicated antibody at 4°C for 30 minutes in phosphate-buffered saline (PBS) containing 0.1% sodium azide and 1% fetal calf serum to block nonspecific binding. The following panel of lineage-restricted antibodies were used. Myeloid: fluorescien isothiocyanate (FITC)-conjugated rat antimouse Gr-1 (RB6-8C5, IgG2b , Pharmingen, San Diego, CA); erythroid: phycoerythrin (PE)-conjugated rat antimouse Ter-119 (M1/70, IgG2b , Pharmingen); B lymphoid: FITC-conjugated rat antimouse B220 (M1/70, IgG2b , Pharmingen); and T lymphoid: FITC-conjugated rat antimouse CD3 (M1/70, IgG2b , Pharmingen). PE-conjugated rat IgG2b (R35-38, Pharmingen) and FITC-conjugated rat IgG2b (R35-38, Pharmingen) were used as isotype controls. All cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Hematopoietic progenitor cell assays.Bone marrow cells were enumerated using a hemacytometer. A total of 2.5 × 104 bone marrow mononuclear cells were plated in 1 mL of methylcellulose media (MethoCult M3230; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with the indicated cytokines and placed at 37°C in a humidified chamber with 5% CO2 for 7 to 8 days. Recombinant murine cytokines were used at the following concentrations: GM-CSF (10 ng/mL, R&D Systems), IL-3 (10 ng/mL, R&D Systems), and IL-6 (500 ng/mL, R&D Systems) To examine cell morphology, entire methylcellulose cultures were harvested and washed extensively to remove the methylcellulose. Leukocyte differentials were performed on Wright-stained cytospins of the hematopoietic cells. Colonies were classified by examination of stained whole-plate preparations of bone marrow cells cultured in semisolid agar for 7 to 8 days with either IL-3 or GM-CSF as previously described.12
Colony-forming unit-spleen (CFU-S) assay.Day 12 CFU-S numbers were determined as described.13 Bone marrow mononuclear cells (5 × 104) were injected into lethally irradiated (900 cGy, single dose) wild-type recipient mice. Mice were killed after 12 days, their spleens harvested, and macroscopic colonies counted after overnight fixation in Tellesniczky's solution. No colonies were observed in saline injected controls (data not shown).
IL-6 administration to mice.Recombinant murine IL-6 (R & D Systems) was administered by daily subcutaneous injection at a dose of 20 μg/kg/d for 5 days. Peripheral blood was obtained before the first IL-6 dose and 4 to 6 hours after the final IL-6 dose. Peripheral blood leukocyte levels were analyzed as described above. A total of four wild-type and four G-CSFR −/− mice were studied. No leukocyte responses were detected in control (saline injected) mice (data not shown).
Statistical analysis.Data are presented as mean ± standard deviation (SD). Statistical significance was assessed by Student's t-test unless otherwise stated in the text.
RESULTS
Generation of IL-6 × G-CSFR doubly deficient mice.In the original description of G-CSFR–deficient mice, we analyzed the phenotype of the homozygous null mutation on a outbred NIH Swiss Black × 129 Sv genetic background.3 In the current study, the G-CSFR null mutation on a C57BL/6 × 129 Sv genetic background was used to minimize the effect of strain differences on phenotype, as the IL-6 null mutation was also on a C57BL/6 × 129 Sv genetic background.11 A comparison of the phenotype of G-CSFR (−/−) mice on the two genetic backgrounds showed only minor differences. The G-CSFR–deficient mice were intercrossed with IL-6–deficient mice, and the resulting doubly heterozygous animals were intercrossed to obtain viable doubly-deficient homozygous mice (Fig 1). The expected number of IL-6–deficient and G-CSFR–deficient mice were obtained. However, significantly fewer IL-6 × G-CSFR doubly-deficient animals were obtained (four of 174 offspring [v 10.9 expected]; P = .014 according to a binomial distribution). Breeding pairs for wild type, IL-6–deficient, G-CSFR–deficient, and doubly-deficient mice were established, and their progeny were used in all subsequent studies. The average litter size of IL-6 × G-CSFR–deficient intercrosses was significantly smaller than wild-type (average litter size ± SD: 7.7 ± 1.7 [wild-type] v 4.3 ± 3.5 [IL-6 × G-CSFR–deficient], P < .01). No significant decrease in litter size was observed with singly IL-6–deficient or G-CSFR–deficient mice. Despite the decrease in live birth rate, doubly-deficient mice appeared healthy and had normal growth and development. Except for a single IL-6 × G-CSFR–deficient mouse that developed a pyogenic infection at the site of a retro-orbital blood draw, no other mice developed clinically apparent infections.
Serum IL-6 levels are not elevated in G-CSFR–deficient mice. Resting levels of serum IL-6 were measured using an ELISA to determine whether the neutropenia present in G-CSFR–deficient mice induced the production of IL-6. As expected, no immunoreactive IL-6 was detected in either IL-6–deficient mice (n = 8) or G-CSFR × IL-6–deficient mice (n = 8). Low levels of IL-6 were detected in the sera of three of eight G-CSFR–deficient mice (range, 16 to 30 pg/mL). Similar results were obtained with wild-type mice (three of eight mice had detectable IL-6 levels; range, 16 to 40 pg/mL). These data indicate that the impaired granulopoiesis present in G-CSFR–deficient mice does not result in a constitutive increase in IL-6 production.
Loss of IL-6 significantly impairs the residual granulopoiesis in G-CSFR–deficient mice.Peripheral blood counts were examined at 4 to 5 weeks and 15 weeks of age in mice of each genotype (Table 1). No significant difference in resting levels of total white blood cells, red blood cells, or platelets was observed. In addition, none of the mice had a significant alteration in the levels of circulating eosinophils or lymphocytes, although a mild lymphocytosis was often observed in G-CSFR–deficient and doubly deficient mice. As we previously have shown, G-CSFR–deficient mice are neutropenic with levels of circulating neutrophils approximately 25% that of wild-type mice. In agreement with a previous report, the loss of IL-6, by itself, had no effect on resting levels of circulating neutrophils.14 However, the loss of IL-6 in the G-CSFR–deficient mice resulted in a further significant decrease in circulating neutrophil levels to approximately 10% that of wild-type mice. Interestingly, the negative effect of the loss of IL-6 on the residual granulopoiesis in G-CSFR–deficient was less striking in 15-week-old mice.
Peripheral Blood Counts
| Age (wk) . | Peripheral Blood Parameter . | Genotype . | |||
|---|---|---|---|---|---|
| . | . | Wild-Type . | IL-6 (−/−) . | G-CSFR (−/−) . | IL-6 × GR (−/−) . |
| 4-5 | WBC (×10−9/L) | 6.51 ± 1.42 | 6.06 ± 2.28 | 6.86 ± 2.45 | 7.03 ± 2.07 |
| RBC (×10−12/L) | 7.76 ± 0.64 | 7.98 ± 0.74 | 8.45 ± 1.24 | 7.33 ± 1.06 | |
| Platelet (×10−12/L) | 0.85 ± 0.28 | 0.77 ± 0.16 | 0.98 ± 0.19 | 0.96 ± 0.25 | |
| PMN (%) | 10.05 ± 3.03 | 12.60 ± 3.30 | 2.53 ± 1.27* | 0.84 ± 0.34*† | |
| PMN (×10−9/L) | 0.64 ± 0.17 | 0.73 ± 0.26 | 0.17 ± 0.09* | 0.06 ± 0.03*† | |
| Eosinophil (×10−9/L) | 0.17 ± 0.10 | 0.10 ± 0.06 | 0.08 ± 0.09 | 0.09 ± 0.06 | |
| Lymph (×10−9/L) | 5.37 ± 1.35 | 5.00 ± 1.98 | 6.30 ± 2.32 | 6.65 ± 1.99 | |
| 15 | WBC (×10−9/L) | 11.14 ± 2.84 | 8.32 ± 2.06 | 8.67 ± 3.24 | 7.00 ± 3.54 |
| RBC (×10−12/L) | 9.39 ± 0.56 | 9.15 ± 0.50 | 10.11 ± 0.77 | 9.53 ± 0.90 | |
| Platelet (×10−12/L) | 1.36 ± 0.20 | 1.21 ± 0.12 | 1.22 ± 0.25 | 1.05 ± 0.28 | |
| PMN (%) | 13.71 ± 4.66 | 13.89 ± 3.99 | 2.62 ± 1.06* | 2.07 ± 0.89* | |
| PMN (×10−9/L) | 1.48 ± 0.58 | 1.19 ± 0.59 | 0.23 ± 0.14* | 0.14 ± 0.09* | |
| Eosinophil (×10−9/L) | 0.26 ± 0.22 | 0.19 ± 0.10 | 0.15 ± 0.09 | 0.14 ± 0.08 | |
| Lymph (×10−9/L) | 8.72 ± 2.26 | 6.58 ± 1.47 | 7.79 ± 2.86 | 6.34 ± 3.32 | |
| Age (wk) . | Peripheral Blood Parameter . | Genotype . | |||
|---|---|---|---|---|---|
| . | . | Wild-Type . | IL-6 (−/−) . | G-CSFR (−/−) . | IL-6 × GR (−/−) . |
| 4-5 | WBC (×10−9/L) | 6.51 ± 1.42 | 6.06 ± 2.28 | 6.86 ± 2.45 | 7.03 ± 2.07 |
| RBC (×10−12/L) | 7.76 ± 0.64 | 7.98 ± 0.74 | 8.45 ± 1.24 | 7.33 ± 1.06 | |
| Platelet (×10−12/L) | 0.85 ± 0.28 | 0.77 ± 0.16 | 0.98 ± 0.19 | 0.96 ± 0.25 | |
| PMN (%) | 10.05 ± 3.03 | 12.60 ± 3.30 | 2.53 ± 1.27* | 0.84 ± 0.34*† | |
| PMN (×10−9/L) | 0.64 ± 0.17 | 0.73 ± 0.26 | 0.17 ± 0.09* | 0.06 ± 0.03*† | |
| Eosinophil (×10−9/L) | 0.17 ± 0.10 | 0.10 ± 0.06 | 0.08 ± 0.09 | 0.09 ± 0.06 | |
| Lymph (×10−9/L) | 5.37 ± 1.35 | 5.00 ± 1.98 | 6.30 ± 2.32 | 6.65 ± 1.99 | |
| 15 | WBC (×10−9/L) | 11.14 ± 2.84 | 8.32 ± 2.06 | 8.67 ± 3.24 | 7.00 ± 3.54 |
| RBC (×10−12/L) | 9.39 ± 0.56 | 9.15 ± 0.50 | 10.11 ± 0.77 | 9.53 ± 0.90 | |
| Platelet (×10−12/L) | 1.36 ± 0.20 | 1.21 ± 0.12 | 1.22 ± 0.25 | 1.05 ± 0.28 | |
| PMN (%) | 13.71 ± 4.66 | 13.89 ± 3.99 | 2.62 ± 1.06* | 2.07 ± 0.89* | |
| PMN (×10−9/L) | 1.48 ± 0.58 | 1.19 ± 0.59 | 0.23 ± 0.14* | 0.14 ± 0.09* | |
| Eosinophil (×10−9/L) | 0.26 ± 0.22 | 0.19 ± 0.10 | 0.15 ± 0.09 | 0.14 ± 0.08 | |
| Lymph (×10−9/L) | 8.72 ± 2.26 | 6.58 ± 1.47 | 7.79 ± 2.86 | 6.34 ± 3.32 | |
Manual 300-count leukocyte differentials were performed on blood smears from sex-matched mice. A total of 8 and 6 mice of each genotype were analyzed at 4 to 5 and 15 weeks, respectively. Data represent the mean ± SD.
P < .001 compared with wild-type mice.
P < 0.01 compared with G-CSFR (−/−) mice.
To further characterize the defect in granulopoiesis, we next examined hematopoiesis in the bone marrow. No significant difference in bone marrow cellularity was detected (Table 2). As reported previously, the loss of the G-CSFR resulted in a significant reduction in cells of the myeloid lineage.3 A similar, but more severe, loss of myeloid cells was observed in the bone marrow of IL-6 × G-CSFR–deficient mice. Although there was a mild shift to less mature cells within the myeloid series, no absolute block to myeloid maturation was noted. Interestingly, the loss of myeloid cells in the bone marrow of G-CSFR–deficient and doubly-deficient mice was associated with a proportional increase in lymphoid cells. To characterize this lymphocytosis further, we analyzed a subset of the bone marrow samples by flow cytometry for the expression of a panel of lineage restricted antigens (see Materials and Methods). A significant increase in B lymphocytes was detected in the bone marrow of G-CSFR–deficient and doubly-deficient mice (data not shown); this result is consistent with an earlier report indicating that G-CSF plays an inhibitory role in B lymphopoiesis.15 The flow cytometry results were otherwise consistent with the manual leukocyte differentials.
Bone Marrow Analysis
| Nucleated Cell Types . | Wild-type . | IL-6 (−/−) . | G-CSFR (−/−) . | IL-6 × GR (−/−) . |
|---|---|---|---|---|
| . | (% ± SD) . | (% ± SD) . | (% ± SD) . | (% ± SD) . |
| Total nucleated cells (×10−6) (per femur) | 22.6 ± 4.0 | 20.8 ± 2.7 | 25.1 ± 8.4 | 21.3 ± 3.7 |
| Myeloblasts | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 |
| Promyelocytes | 1.4 ± 0.6 | 1.6 ± 0.5 | 0.8 ± 0.2 | 1.0 ± 0.5 |
| Myelocytes | 3.2 ± 1.0 | 3.8 ± 1.5 | 2.2 ± 0.9 | 2.2 ± 0.9 |
| Metamyelocytes neutrophil | 17.2 ± 4.7 | 16.7 ± 3.7 | 10.2 ± 4.7* | 9.0 ± 3.2† |
| Band and segmented neutrophil | 26.4 ± 8.0 | 24.2 ± 6.4 | 9.2 ± 2.6‡ | 5.5 ± 1.2‡ρ |
| Eosinophil lineage | 3.7 ± 2.5 | 3.2 ± 2.0 | 2.1 ± 1.2 | 5.2 ± 3.9 |
| Lymphocyte lineage | 23.1 ± 7.8 | 23.8 ± 2.5 | 44.8 ± 5.1‡ | 40.4 ± 5.8† |
| Normoblasts | 24.7 ± 8.0 | 26.3 ± 10.1 | 30.2 ± 6.6 | 36.9 ± 7.7* |
| Myeloid/erythroid ratio | 2.4 ± 1.8 | 2.2 ± 1.4 | 0.8 ± 0.3 | 0.6 ± 0.2* |
| Nucleated Cell Types . | Wild-type . | IL-6 (−/−) . | G-CSFR (−/−) . | IL-6 × GR (−/−) . |
|---|---|---|---|---|
| . | (% ± SD) . | (% ± SD) . | (% ± SD) . | (% ± SD) . |
| Total nucleated cells (×10−6) (per femur) | 22.6 ± 4.0 | 20.8 ± 2.7 | 25.1 ± 8.4 | 21.3 ± 3.7 |
| Myeloblasts | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 |
| Promyelocytes | 1.4 ± 0.6 | 1.6 ± 0.5 | 0.8 ± 0.2 | 1.0 ± 0.5 |
| Myelocytes | 3.2 ± 1.0 | 3.8 ± 1.5 | 2.2 ± 0.9 | 2.2 ± 0.9 |
| Metamyelocytes neutrophil | 17.2 ± 4.7 | 16.7 ± 3.7 | 10.2 ± 4.7* | 9.0 ± 3.2† |
| Band and segmented neutrophil | 26.4 ± 8.0 | 24.2 ± 6.4 | 9.2 ± 2.6‡ | 5.5 ± 1.2‡ρ |
| Eosinophil lineage | 3.7 ± 2.5 | 3.2 ± 2.0 | 2.1 ± 1.2 | 5.2 ± 3.9 |
| Lymphocyte lineage | 23.1 ± 7.8 | 23.8 ± 2.5 | 44.8 ± 5.1‡ | 40.4 ± 5.8† |
| Normoblasts | 24.7 ± 8.0 | 26.3 ± 10.1 | 30.2 ± 6.6 | 36.9 ± 7.7* |
| Myeloid/erythroid ratio | 2.4 ± 1.8 | 2.2 ± 1.4 | 0.8 ± 0.3 | 0.6 ± 0.2* |
Cell counts and 500-count manual leukocyte-differentials were performed on mononuclear cells recovered from the femurs of 6 to 7 sex-matched mice of each genotype. Data represent the mean ± SD.
P < .05 compared with wild-type mice.
P < .01 compared with wild-type mice.
P < .001 compared with wild-type mice.
ρ P < .01 compared with G-CSFR (−/−) mice.
Collectively, these data show that the loss of IL-6 significantly impairs the residual granulopoiesis present in G-CSFR–deficient mice. However, the absence of a block in myeloid maturation and the presence of mature morphologically normal neutrophils in IL-6 × G-CSFR–deficient mice indicates that neither IL-6 nor G-CSFR are absolutely required for granulopoiesis.
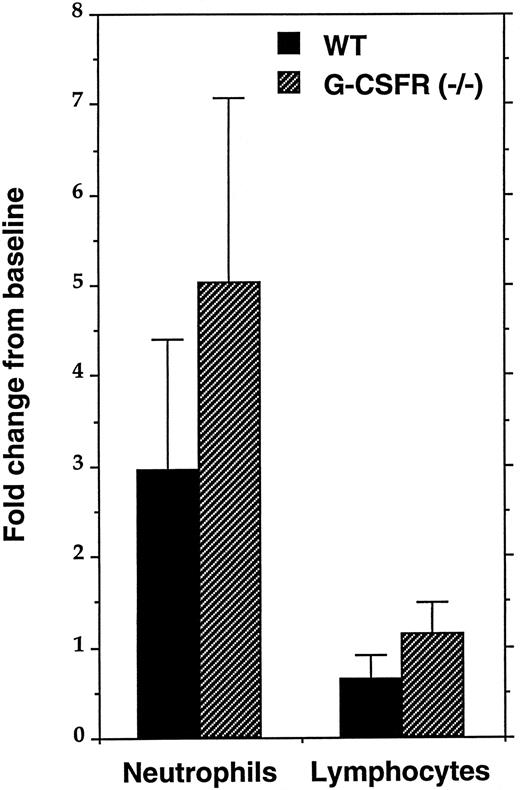
IL-6 stimulates granulopoiesis in vivo in G-CSFR–deficient mice.IL-6 has been implicated in the stress granulopoiesis response to certain infections; specifically, IL-6–deficient mice have a blunted neutrophilic response to infection with either Listeria monocytogenes7 or Candida albicans.8 However, it is not clear whether IL-6 directly stimulates granulopoiesis or instead stimulates the production of another cytokine such as G-CSF. To address this issue, we measured the in vivo neutrophil response to IL-6 in G-CSFR–deficient mice. Consistent with a previous report, in wild-type mice, IL-6 stimulates an approximately threefold increase in the level of circulating neutrophils and a modest decrease in the level of circulating lymphocytes (Fig 2).6 A fivefold increase in the number of circulating neutrophils was detected in G-CSFR–deficient mice after IL-6 administration; no change in the level of circulating lymphocytes was detected. These data show that IL-6 can stimulate granulopoiesis in vivo in a G-CSFR independent fashion.
In vivo response to IL-6. Four sex- and age-matched mice of each genotype were injected subcutaneously with murine rIL-6 (20 μg/kg) for 5 days. Complete blood counts and 300-count manual leukocyte differentials were performed before the first injection and 4 hours after the last injection of IL-6. The fold change from baseline in the absolute level of circulating neutrophils and lymphocytes is shown. Data represent the mean ± SD.
In vivo response to IL-6. Four sex- and age-matched mice of each genotype were injected subcutaneously with murine rIL-6 (20 μg/kg) for 5 days. Complete blood counts and 300-count manual leukocyte differentials were performed before the first injection and 4 hours after the last injection of IL-6. The fold change from baseline in the absolute level of circulating neutrophils and lymphocytes is shown. Data represent the mean ± SD.
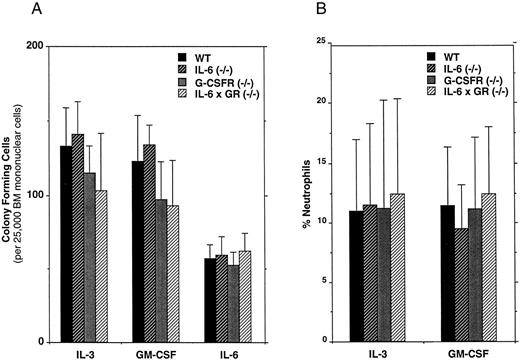
The number of committed myeloid progenitors is near normal in IL-6 × G-CSFR–deficient mice.Cytokines have been postulated to play an important role in the lineage commitment of multipotential progenitors.16 We previously have shown that the loss of G-CSFR signals results in a modest, but significant, reduction in the number of myeloid progenitors present in the bone marrow of G-CSFR–deficient mice.3 Because the loss of IL-6 in the G-CSFR–deficient mice further impaired granulopoiesis, we also examined the number of myeloid progenitors in IL-6 × G-CSFR–deficient mice (Fig 3A). In methylcellulose cultures, IL-3 and GM-CSF predominantly stimulate myeloid progenitors (CFU-GM, CFU-G, and CFU-M), while IL-6 predominantly stimulates the committed granulocytic progenitor, CFU-G. In contrast to a previous report,14 we detected no decrease in the absolute number of myeloid progenitors in the bone marrow of IL-6–deficient mice. However, in cultures of IL-6 × G-CSFR–deficient bone marrow cells, we did detect a modest and reproducible (but nonsignificant) reduction in the number of hematopoietic colonies that formed in response to IL-3 or GM-CSF. Interestingly, IL-6 stimulated the formation of a similar number of colonies in cultures of bone marrow cells isolated from mice of each genotype (Fig 3A), indicating that the loss of both IL-6 and G-CSFR had no effect on the generation of CFU-G. These data show that the number of myeloid progenitors present in the bone marrow of IL-6 × G-CSFR–deficient marrow is near normal.
Hematopoietic progenitor assays. (A) Bone marrow mononuclear cells were plated in methylcellulose-containing media supplemented with the indicated cytokine. Hematopoietic colonies containing greater than 30 cells were scored after 7 to 8 days (these colonies include CFU-GM, CFU-M, and CFU-G). Six sex- and age-matched mice of each genotype were analyzed. Data represent the mean ± SD. No significant differences in progenitor frequency were detected among the different genotypes. (B) Neutrophil production in hematopoietic colonies. The percentage of neutrophils was determined by manual 300-count leukocyte differentials on Wright-stained cytospins of cells recovered from entire methylcellulose cultures. Cultures were stimulated with the indicated cytokine for 8 days. Data represent the mean ± SD.
Hematopoietic progenitor assays. (A) Bone marrow mononuclear cells were plated in methylcellulose-containing media supplemented with the indicated cytokine. Hematopoietic colonies containing greater than 30 cells were scored after 7 to 8 days (these colonies include CFU-GM, CFU-M, and CFU-G). Six sex- and age-matched mice of each genotype were analyzed. Data represent the mean ± SD. No significant differences in progenitor frequency were detected among the different genotypes. (B) Neutrophil production in hematopoietic colonies. The percentage of neutrophils was determined by manual 300-count leukocyte differentials on Wright-stained cytospins of cells recovered from entire methylcellulose cultures. Cultures were stimulated with the indicated cytokine for 8 days. Data represent the mean ± SD.
The in vitro production of neutrophils from IL-6 × G-CSFR–deficient progenitors stimulated with IL-3 or GM-CSF is normal.The presence of normal-appearing neutrophils in IL-6 × G-CSFR–deficient mice suggested that other hematopoietic cytokines may be stimulating the residual granulopoiesis. To explore this possibility, we examined the ability of myeloid progenitors from IL-6 × G-CSFR–deficient mice to produce mature neutrophils in vitro in response to IL-3 or GM-CSF. The methylcellulose cultures described above were harvested in total and leukocyte differentials performed (Fig 3B). As expected, both IL-3 and GM-CSF stimulated the production of a small number of mature appearing neutrophils from wild-type progenitors. Surprisingly, a similar number of mature neutrophils was observed in cultures with IL-6 × G-CSFR–deficient progenitors. To determine whether the neutrophils produced in cultures of IL-6 × G-CSFR–deficient bone marrow cells arose exclusively from a specific type of myeloid progenitor, the number of CFU-GM and CFU-G colonies present in these cultures was quantitated. Bone marrow cells from mice of each genotype were stimulated with IL-3 or GM-CSF in soft agar cultures and the resulting colonies stained in situ. Interestingly, the relative number of CFU-GM and CFU-G detected in these assays was equivalent regardless of the genotype (data not shown). Collectively, these data show that the ability of IL-6 × G-CSFR–deficient progenitors to produce neutrophils in vitro in response to IL-3 or GM-CSF is normal.
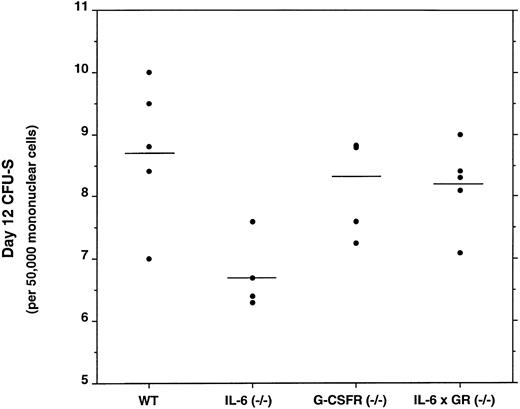
The number of day 12 CFU-S is decreased in the bone marrow of IL-6–deficient mice, but normal in G-CSFR and IL-6 × G-CSFR–deficient mice.Several recent reports have suggested that the both IL-6 and G-CSFR may play roles in the regulation of primitive multipotential hematopoietic progenitors.17-19 To determine whether the loss of G-CSFR signals would have a similar effect, we measured the number of day 12 CFU-S in the bone marrow of mice of each genotype (Fig 4). As reported previously, IL-6–deficient mice have a modest, but statistically significant, reduction in the number of day 12 CFU-S to approximately 70% that of wild-type (P < .05).14 In contrast, no decrease in the absolute number of day 12 CFU-S was detected in the bone marrow of G-CSFR–deficient or, surprisingly, IL-6 × G-CSFR–deficient mice.
Frequency of day 12 CFU-S in the bone marrow. Five sex- and age-matched donors of each genotype were analyzed. Each data point represents the mean number of day 12 CFU-S detected in a minimum of five recipient animals injected with bone marrow mononuclear cells from a single donor. The horizontal lines represent the mean of this data.
Frequency of day 12 CFU-S in the bone marrow. Five sex- and age-matched donors of each genotype were analyzed. Each data point represents the mean number of day 12 CFU-S detected in a minimum of five recipient animals injected with bone marrow mononuclear cells from a single donor. The horizontal lines represent the mean of this data.
DISCUSSION
Hematopoietic cytokines clearly play an important role in the regulation of granulopoiesis. The relative importance of individual cytokines to granulopoiesis and their mechanisms of action is less clear. In this study, we have examined the contribution of the G-CSFR and IL-6 to granulopoiesis using mice carrying homozygous null mutations for both the G-CSFR gene and the IL-6 (cytokine) gene. We show that the loss of IL-6 significantly worsens the chronic neutropenia present in G-CSFR–deficient mice. Further, we show that IL-6 can stimulate granulopoiesis in vivo in the absence of G-CSFR signals. Together, these data support a role for IL-6 as a major independent regulator of granulopoiesis. However, we also provide evidence that neither G-CSFR or IL-6 signals are required for the commitment of multipotential progenitors to the myeloid lineage. Likewise, we show that G-CSFR and IL-6 signals are not required for the terminal differentiation of progenitor cells to mature neutrophils.
Significantly fewer (40% of predicted based on a Mendelian pattern of inheritance) IL-6 × G-CSFR–deficient mice were obtained from double heterozygous intercrosses. Further, the litter sizes of IL-6 × G-CSFR–deficient mice intercrosses were reduced to approximately 50% of wild-type. Because no neonatal deaths were observed, these data suggest that a combined deficiency of IL-6 and G-CSFR leads to excessive intrauterine loss, whereas loss of IL-6 or G-CSFR alone does not. The mechanism(s) for this intrauterine loss is currently unknown, however, maternal factors are unlikely given the decrease in live birth rate of IL-6 × G-CSFR–deficient mice to double heterozygous mothers. Because both IL-6 and G-CSFR are expressed in fetal trophoblasts cells20,21; it is possible that IL-6 × G-CSFR–deficient embryos have a partial defect in placentation. Studies are planned to determine the time and cause of intrauterine death.
Several previous studies have implicated IL-6 as an important regulator of granulopoiesis. Indeed, we previously have shown that IL-6, like G-CSF, predominantly stimulates the formation of granulocytic colonies in methylcellulose cultures of bone marrow mononuclear cells.3 In agreement with a previous study, we detected no alteration in the basal level of circulating neutrophils in IL-6–deficient mice.14 However, the additional loss of IL-6 resulted in a significant worsening of the neutropenia seen in young adult G-CSFR–deficient mice. These results are in contrast to the results observed with GM-CSF × G-CSF–deficient mice; in this case, the loss of GM-CSF had no apparent effect on granulopoiesis in adult G-CSF–deficient mice.5 Interestingly, the neutropenia present in IL-6 × G-CSFR–deficient mice partially corrected with advancing age suggests that other cytokines may be compensating for the loss of IL-6 in older mice. Alternatively, it is possible that subclinical infections may have played a role in stimulating neutrophil production in older IL-6 × G-CSFR–deficient mice.
Neutrophil counts can increase dramatically in response to certain infections. Elevated levels of serum IL-6 are often detected in patients with sepsis.22 The observation that the neutrophil response to infection with Listeria monocytogenes7 or Candida albicans8 is defective in IL-6–deficient mice suggests that IL-6 may be a key regulator of stress granulopoiesis. However, the observation that the serum level of G-CSF also is often elevated during infections raises the possibility that the ability of IL-6 to stimulate granulopoiesis is indirect and mediated by G-CSF.22 In this study, we observed a significant increase in the level of circulating neutrophils in G-CSFR–deficient mice after the administration of IL-6 showing the ability of IL-6 to stimulate granulopoiesis in the absence of G-CSFR signals. Collectively, these data suggest that IL-6 is a major independent regulator of granulopoiesis in vivo and may play a direct role in stimulating neutrophil production during certain infections.
McKinstry et al23 recently reported that primitive (Rhlo lin−Ly6A/E+c-kit+) murine hematopoietic progenitors express the G-CSFR. This observation is consistent with reports showing a proliferative effect of G-CSF on purified primitive progenitors in vitro.18 19 In the present study, no defect in the production of primitive day 12 CFU-S progenitors in G-CSFR–deficient mice was detected. Although hematopoietic stem cell function has not been tested directly, it seems unlikely that the G-CSFR plays a major role in the regulation of primitive hematopoietic progenitors in vivo. A modest reduction in the absolute number of day 12 CFU-S was observed in the bone marrow of IL-6–deficient mice, a reduction that was not seen in IL-6 × G-CSFR–deficient mice. This difference, although intriguing, is small and of questionable biologic significance.
The role of cytokines in the lineage commitment and terminal differentiation of multipotential progenitors is controversial.16 With respect to the G-CSFR, several studies have shown that the addition of G-CSF to cultures of certain multipotential hematopoietic cell lines results in their granulocytic differentiation.24-27 This observation has lead to the hypothesis that G-CSFR signals play an active (instructive) role in directing lineage-commitment and granulocytic differentiation. Our results do not support this hypothesis. The number of committed myeloid progenitors in IL-6 × G-CSFR–deficient mice is near normal. Further, the presence of normal appearing neutrophils in IL-6 × G-CSFR–deficient mice indicates that neither IL-6 nor G-CSFR are absolutely required for granulocytic differentiation. This conclusion is supported by the observation that IL-6 × G-CSFR–deficient progenitors are able to produce significant numbers of neutrophils in vitro in response to either IL-3 or GM-CSF. Collectively, these data show that the commitment of multipotential progenitors to the myeloid lineage and their terminal differentiation into mature neutrophils does not require either IL-6 or G-CSFR signals.
Our results are most consistent with a model in which the major role of cytokines in granulopoiesis is to provide growth and survival signals to granulocyte-lineage committed progenitors. In this model, cytokines are not required either for lineage commitment or terminal differentiation. Because it is likely that progenitor cells coexpress multiple receptors for hematopoietic cytokines,28 this model predicts that each of these cytokines should be able to stimulate granulopoiesis with the magnitude of their effect dependent on the strength of the proliferative signal delivered. Thus the “knock out” of one (or more) hematopoietic cytokines would not be predicted to completely abrogate granulopoiesis. A key feature of granulopoiesis is its ability to increase production rapidly in response to stress. In this model, cytokines produced in response to stress act directly on a preformed pool of committed progenitors allowing for the rapid amplification of granulopoiesis. In addition to their effect on proliferation and survival, certain cytokines may generate specific signals that modulate neutrophil effector functions.29 Studies are in progress to examine the effect of specific G-CSFR mutations on neutrophil function.
ACKNOWLEDGMENT
We thank Nancy Link for her expert technical assistance. We thank Timothy Graubert and Peter Westerveld for their critical review of this manuscript.
Supported by the James S. McDonnell Foundation (St Louis, MO) and by the Monsanto-Searle/Washington University Biomedical Research Program (St Louis, MO).
Address reprint requests to Daniel C. Link, MD, Washington University Medical School, Division of Bone Marrow Transplantation and Stem Cell Biology, Box 8007, 660 South Euclid Ave, St Louis, MO 63110-1093.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal