Abstract
All-trans retinoic acid (tRA) is a potent differentiation agent that is effective therapy for acute promyelocytic leukemia (APL). However, 5% to 25% of patients develop retinoic acid syndrome, a potentially life-threatening complication in which the pathogenesis relates to adhesive alterations of APL cells. Therefore, we investigated the relationship between tRA-induced differentiation and the adhesive properties of APL cells. After confirming differentiation-related morphological changes of NB-4 cells in response to tRA, we showed that homotypic aggregation of NB-4 cells grown in tRA for 72 hours is dose-dependent with a median effective dose of approximately 50 nmol/L. Maximal aggregation occurred at mean and peak therapeutic serum concentrations (100 and 1,000 nmol/L, respectively). Aggregation also increased with the length of tRA exposure over 168 hours. Aggregation was inhibited by neutralizing antibodies against LFA-1 and ICAM-2. Notably, antibodies directed against VLA-4, other β2 integrins (Mac-1 and p150), or other potential LFA-1 counterstructures that were expressed on the cell surface (ICAM-1 and ICAM-3) did not block aggregation. Aggregation occurred with similar kinetics regardless of the presence of phorbol ester or the “activating” monoclonal antibody (MoAb) KIM 185, suggesting that the avidity of LFA-1 is not modulated on NB-4 cells in a manner similar to other leukocytes. Consistent with the prompt clinical effectiveness of methyl prednisolone sodium succinate (MPSS) in retinoic acid syndrome, MPSS rapidly inhibited homotypic aggregation in a dose-dependent manner. Thus, tRA alters the adhesive properties of APL cells by inducing the expression of high-avidity β2 integrins, aggregation is inhibited by LFA-1 and ICAM-2 MoAb, and tRA effects are rapidly reversible by MPSS. Taken together, our findings provide a clinically relevant system for study of LFA-1/ICAM-2 interaction and suggest a mechanism in part for retinoic acid syndrome and the effectiveness of MPSS in ameliorating retinoic acid syndrome.
ACUTE PROMYELOCYTIC leukemia (APL) differs from other types of acute myeloid leukemia (AML) by unique morphological, immunophenotypic, and chromosomal [t(15; 17)] findings.1,2 The translocation between a retinoic acid receptor gene (RAR-α) on chromosome 17 and the promyelocytic leukemia gene locus (pml ) on chromosome 15 ultimately gives rise to PML/RAR-α fusion protein.1,3-5 The chimeric protein apparently contributes to leukemogenesis by inhibiting differentiation or by promoting cell survival through inhibiting apoptosis.6,7 Fortuitously, APL cells are responsive to all-trans retinoic acid (tRA), a potent regulator of growth and differentiation that causes differentiation of APL cells into mature granulocytes both in vivo and in vitro.8-11 Although the molecular mechanisms involved in tRA-dependent differentiation are not completely understood, it has been widely confirmed that tRA induces complete remission in more than 80% of APL patients by inducing differentiation of the leukemic cells.1,8,10-19 However, tRA therapy has two major clinical limitations: (1) retinoic acid syndrome is potentially lethal and causes progressive hypoxemia and multiorgan failure in 5% to 25% of APL patients,8,9,11,20,21 and (2) retinoid resistance develops in almost all patients.8,12-15,17,21 22 To identify molecular targets for pharmaceutical prevention of these side effects, it is of great importance to understand the cellular and molecular alterations induced by tRA in cases of APL.
The cellular and molecular mechanisms of retinoic acid syndrome are poorly understood, and the proposed mechanisms involve ill-defined changes in the adhesive qualities and cytokine secretion of leukemic cells during tRA-induced differentiation.1,8,20 The clinical syndrome is characterized by fever, episodic hypotension, and effusions,20 suggesting a role for cytokines and other soluble mediators. Organ infiltration by leukemic cells was quite prominent in two patients in whom postmortem studies were performed,20 indicating that tRA induces leukemic cells to acquire functions of mature leukocytes including leukemic cell-endothelial cell (heterotypic) adhesion followed by vascular extravasation. Migration of these cells into tissues such as lung and kidney may explain the respiratory distress and renal impairment seen clinically. Adhesion of leukemic cells to each other (homotypic adhesion) induced by tRA could also lead to respiratory and organ compromise by promoting formation of leukoaggregates and leukostasis, a finding present in cases of AML with hyperleukocytosis23 such as may be seen in APL after tRA therapy.11,21 Pertinent to our study, steroid therapy has been shown to reduce the fatality and occurrence of retinoic acid syndrome.16 18-20
Although changes in the adhesive properties and surface expression of adhesion molecules on APL cells may contribute to retinoic acid syndrome, these changes on APL cells in response to tRA are not well characterized. Homotypic and heterotypic adherence of leukocytes is the result of a multistep adhesion cascade.24-29 The leukocyte or β2 integrins (lymphocyte function associated antigen-1 [LFA-1, CD11a/CD18], Mac-1 [CR3, CD11b/CD18], p150, 95 [CD11c/CD18], and alpha d)30 and their cell surface counterstructures (intercellular adhesion molecules [ICAM]-1, -2, and -3) participate in both homotypic and heterotypic leukocyte adhesion.25,29 In addition, a member of the β1 integrins, very late antigen-4 (VLA-4, CD49d/CD29), has also been shown to be involved in heterotypic interaction of leukocytes with endothelium through interaction with vascular cell adhesion molecule-1 (VCAM-1).25,27,28 In normal leukocytes participation of integrins in cell adhesion requires activation, and many stimuli have been shown to increase the avidity of integrins on leukocytes for their counterstructures, including chemotactic stimuli and pharmacological agents.31 The biological importance of the β2 integrins is shown in leukocyte adhesion deficiency-1 (LAD-1) patients who have a genetic lack of β2 integrins.32 As a result, these patients have numerous in vitro leukocyte adhesion abnormalities, recurrent bacterial infections, and delayed wound healing.
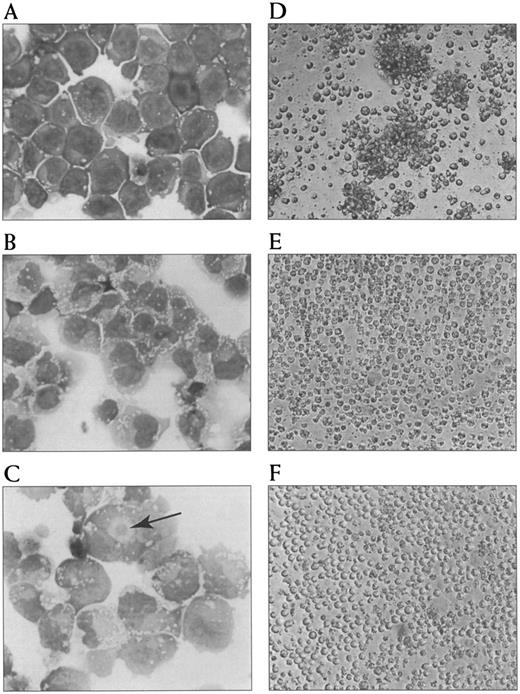
Photomicrographs of morphological changes and aggregation in response to tRA and its inhibition by LFA-1 α and ICAM-2 MoAb. Morphological features of NB-4 cells untreated (A), treated with 100 nmol/L tRA for 24 hours (B), and treated with 100 nmol/L tRA for 72 hours (C). An arrow indicates an eosinophilic perinuclear hof. Aggregation of NB-4 cells treated with tRA for 72 hours (D) and its inhibition by LFA-1 α (E) and ICAM-2 (F ) MoAb are shown.
Photomicrographs of morphological changes and aggregation in response to tRA and its inhibition by LFA-1 α and ICAM-2 MoAb. Morphological features of NB-4 cells untreated (A), treated with 100 nmol/L tRA for 24 hours (B), and treated with 100 nmol/L tRA for 72 hours (C). An arrow indicates an eosinophilic perinuclear hof. Aggregation of NB-4 cells treated with tRA for 72 hours (D) and its inhibition by LFA-1 α (E) and ICAM-2 (F ) MoAb are shown.
To investigate the in vitro biological response of APL cells to retinoids, we benefit from NB-4,33 a cell line derived from a patient with APL. The NB-4 cell line is the only true promyelocytic leukemia cell line with the characteristic chromosomal translocation t(15; 17)(p21; q23) and immunophenotype (HLA-DR negativity), although HL60 and PL1 have been inaccurately reported to also be promyelocytic cell lines.7,33,34 tRA-induced differentiation of NB-4 cells has been shown to be associated with induction of transcription, immunophenotypic changes, and morphological alteration.34 Notably, Mac-1 and p150 have been shown to be expressed at higher levels after tRA treatment.33 34
We have investigated tRA-induced adhesive alterations on NB-4 cells as a first step toward understanding the relationship between tRA-induced differentiation and the adhesive properties of leukemic cells in APL. We first confirm the morphological changes of NB-4 cells in response to therapeutic concentrations of tRA. We then show that tRA induces homotypic aggregation of APL cells through induction of LFA-1 expression and that aggregation is mediated by LFA-1 and ICAM-2. tRA effects were rapidly reversible by methylprednisolone. These findings suggest a mechanism in part for retinoic acid syndrome and for the effectiveness of corticosteroids in ameliorating retinoic acid syndrome.
MATERIALS AND METHODS
Chemicals and reagents.tRA and ethylene diaminetetraacetate (EDTA) were obtained from Sigma Chemical (St Louis, MO). tRA was dissolved in 100% ethanol at 3 mg/mL and stored at −20°C in the dark until use or disposed of after 2 weeks. Phorbol 12-myristate 13-acetate (PMA; Sigma) was prepared in 1 mg/mL stocks in ethanol and used at a final concentration on 50 ng/mL. Methylprednisolone sodium succinate (MPSS; Solu-Medrol, Upjohn, Kalamazoo, MI) was prepared as a 40-mg/mL solution and used immediately. The monoclonal antibodies (MoAb) directed against LFA-1 α (TS 1/22) and shared β2 subunit (IB4) were purified from hybridoma lines (ATCC, Rockville, MD) as previously described in detail.35 Blocking antibodies directed against ICAM-1 (R6.5) were a gift of Dr R. Rothlein (Boehringer Ingelheim, Ridgefield, CT). Neutralizing MoAb directed against VLA-4 (HP 2/1) and ICAM-2 (BT-1) were purchased from Immunotech (Westbrook, ME). Neutralizing MoAb directed against Mac-1 α (D12) and p150 α (SHCL3) subunits were supplied by Collaborative Bioresearch (Bedford, MA). Fluorescein isothiocyanate (FITC)–labeled β1 subunit (K20) and purified ICAM-3 (HPZ/19) MoAb were purchased from Dako (Carpinteria, CA) and Immunotech (Westbrook, ME), respectively. F(ab′)2 fragments of IB4 and R6.5 were made as previously described.36 MoAb directed against an activation epitope of LFA-1, KIM 185 was a gift from Dr Bruce Edwards (Lovelace Research Institute, Albuquerque, NM).
Cell culture.The human promyelocytic cell line NB-433 (a gift from C. Willman, University of New Mexico, Albuquerque, NM) was grown in serum-free AIM-V media (GIBCO-BRL, Gaithersburg, MD) that consists of DMEM, Ham Nutrient Mixture F12, HEPES buffer, human serum albumin, human transferrin, and recombinant human insulin supplemented with L-glutamine (2 mmol/L), 0.1 units/mL of penicillin G sodium, and 0.1 μg/mL streptomycin.37 Cultures were maintained at 3 to 5 × 105 cells/mL to ensure high viability. Cells were incubated at 37°C in an air/5%-CO2 atmosphere. tRA was added directly to cultures at the indicated final concentrations. Viability was determined using trypan blue exclusion techniques.
Morphology.Morphology was assessed by light microscopy of Wright's-stained cytocentrifuge preparations. Briefly, cells (150 μL at a concentration of 75,000 cells/mL) were pelleted onto glass slides in a cyto-centrifuge (Shandon, Pittsburg, PA) at 500 rpm for 5 minutes, and allowed to air-dry. The slides with the cyto-centrifuge preparation were Wright's stained on a Sakura automated stainer (Minneapolis, MN).
Immunophenotyping and fluorescence-activated cell sorter (FACS) analysis.NB-4 cells were untreated or treated with tRA (100 nmol/L or 1,000 nmol/L), phorbol 12-myristate 13-acetate (PMA; 50 ng/mL for 4 hours), KIM 185 (10 μg/mL for 4 hours), or a combination of these reagents. Cells were washed three times with cold phosphate buffered saline, and 1 × 106 cells were stained by direct or indirect immunofluorescence as previously described in detail.38 39 All samples were evaluated in parallel. As the negative control for indirect immunofluorescence, a nonspecific mouse immunoglobulin G (IgG; MOPC, Bionetics, Kensington, MD) was substituted in the first step. FITC-conjugated goat anti-mouse IgG (Becton Dickinson, Bedford, MA) was the indirect reagent and was used alone as the negative control for direct immunoflorescence. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Five thousand events were acquired. The specific mean linear fluorescence intensity was determined by subtraction of control fluorescence using WINLIST software (Verity, Topsham, ME). Results were expressed as fold increase over samples untreated with tRA using the following calculation: specific mean linear fluorescence of the treated sample ÷ specific mean linear fluorescence of untreated sample. Each experiment was repeated three times, and the mean and SD were determined. Untreated NB-4 cells were analyzed each day to determine the mean fluorescence of the untreated sample as well as to verify that a shift in expression was not occurring during normal cell culture.
Aggregation assay.A homotypic aggregation assay was performed as previously described by Keizer et al40 and modified by Zapata et al.41 Briefly, NB-4 cells were washed two times with serum-free AIM-V media and resuspended at a concentration of 1 × 106 cells/mL. In a final volume of 150 μL, 100 μL of cells with potentially blocking concentrations of MoAb (10 μg TS1/22 and IB4; 20 μg all other MoAb) and as indicated PMA, MPSS, or EDTA were seeded in 96-well flat-bottomed microtiter plates (VWR Scientific, Albuquerque, NM). Cells with MoAb were allowed to settle at 37°C in humidified air with 5% CO2 . Cells were visualized and counted by inverted phase microscopy. Within each well the number of aggregates as well as the total number of free (single) cells were counted. Percent aggregation was determined by the following equation:
where each condition was performed in duplicate, and the SD was determined. Each experiment was performed a minimum of three times. Photomicrographs were taken with a Olympus OM-1 camera (Lake Success, NY) coupled to the microscope.
RESULTS
tRA induces morphological changes in NB-4 cells.NB-4 cells were grown in two concentrations of tRA (100 nmol/L and 1,000 nmol/L) corresponding to mean and peak therapeutic serum values, respectively.42 Cell cultures were observed by inverted phase microscopy, and then Wright's-stained cytocentrifuge preparations were prepared and examined each day by light microscopy (Fig 1). Before treatment with tRA, a uniform population of myeloblasts was seen, and spontaneous morphological differentiation or cell clumping did not occur during the 7-day culture period. Upon exposure to tRA, increasing numbers of cells were seen to spread and clump spontaneously in culture each of the 7 days. Qualitatively, there was not an apparent difference between the two doses of tRA. By Wright's-stain examination, cells exposed to tRA did develop more abundant cytoplasm and eosinophilic perinuclear hofs (a morphological change in the Golgi apparatus). The nuclei also became increasingly more irregular, and many nuclei become bilobed or rarely multilobed, all of which are features suggestive of myeloid differentiation. Virtually all cells had some identifiable morphological change by day 3; however, further changes particularly in nuclear contours were seen on days 4 through 7. Differences in the rate or type of differentiation at the different doses of tRA was not apparent by Wright's stain.
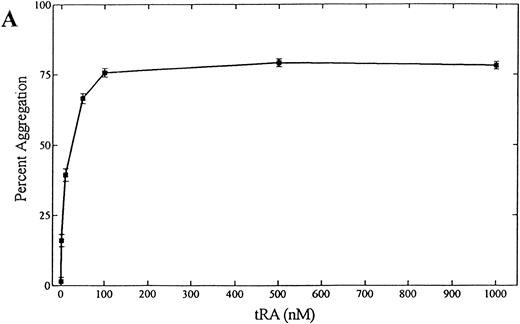
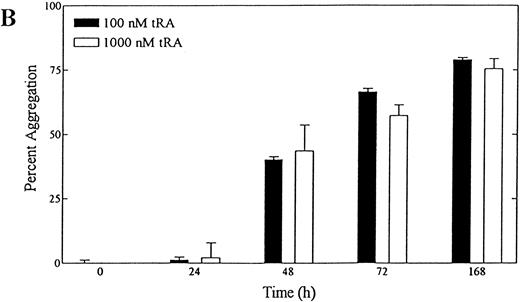
tRA stimulates homotypic adhesion of NB-4 cells.To quantitate tRA-stimulated aggregation, NB-4 cells were grown in serum-free media for 72 hours, and their ability to aggregate was measured in a static homotypic adhesion assay (Figs 1D and 2A). Homotypic aggregation was dose-dependent between 1 and 100 nmol/L with a median effective dose of approximately 50 nmol/L. Maximal aggregation was observed at concentrations of tRA that represents mean (100 nmol/L) and peak (1,000 nmol/L) serum levels obtained during therapy.42 Aggregation also increased with the length of tRA exposure using clinically relevant concentrations over 168 hours (Fig 2B). To verify that the clumping was not caused by cell death, viability of cells directly obtained from each well was measured using trypan blue and light microscopy at the end of each assay. Cells exposed for 24, 48, and 72 hours had similar levels of viability (>90%). In contrast, cells exposed for 168 hours had much lower levels of viability (25% to 48%, range in three experiments), raising the possibility that some of the cell clumping related to cell death. In addition, similar magnitude and time course of aggregation was seen in cells grown in serum (data not shown). Because NB-4 cells treated with 100 nmol/L tRA for 72 hours showed near maximal aggregation and maintained high viability, this condition was chosen for further study.
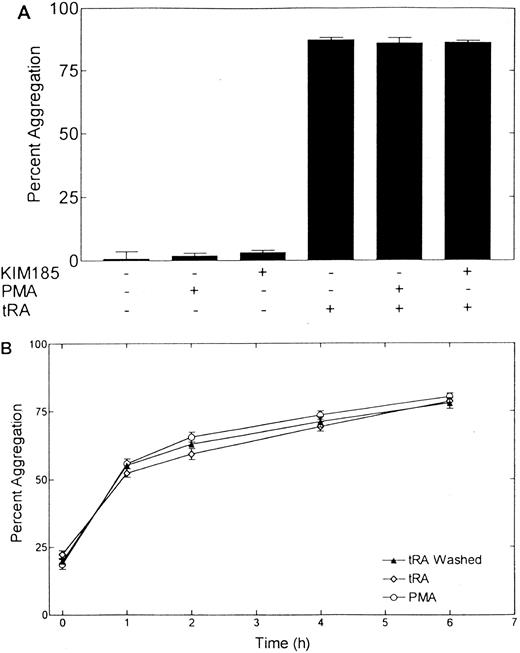
(A) Dose dependence of tRA-induced NB-4 aggregation. NB-4 cells were exposed to increasing concentrations (0 to 1,000 nmol/L) of tRA for 72 hours. (B) Time dependence of tRA-stimulated NB-4 aggregation. NB-4 cells were exposed to two concentrations of tRA (100 nmol/L and 1,000 nmol/L) for 24, 48, 72, and 168 hours. Homotypic aggregation was measured as described in Materials and Methods. The graph (A) and histogram (B) depict the percent aggregation ± SD. Data represent results from three separate experiments in duplicate.
(A) Dose dependence of tRA-induced NB-4 aggregation. NB-4 cells were exposed to increasing concentrations (0 to 1,000 nmol/L) of tRA for 72 hours. (B) Time dependence of tRA-stimulated NB-4 aggregation. NB-4 cells were exposed to two concentrations of tRA (100 nmol/L and 1,000 nmol/L) for 24, 48, 72, and 168 hours. Homotypic aggregation was measured as described in Materials and Methods. The graph (A) and histogram (B) depict the percent aggregation ± SD. Data represent results from three separate experiments in duplicate.
Anti–LFA-1 and ICAM-2 MoAb as well as EDTA inhibit tRA-induced homotypic adhesion of NB-4 cells.After exposure to tRA for 72 hours, NB-4 cells were evaluated for the ability to adhere homotypically in the presence of MoAb directed at molecules known to be involved in cell-cell adhesion. MoAb concentrations were used that were known to inhibit adhesion in other cell-cell adhesion assays.
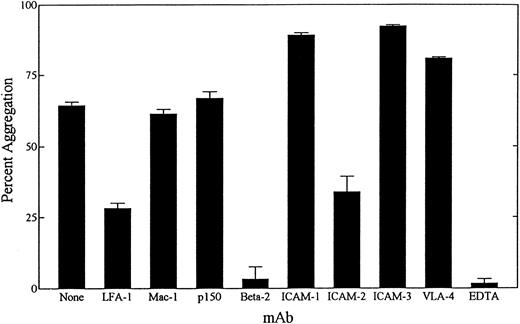
Anti–LFA-1 α chain and β chain MoAb-inhibited homotypic adhesion of tRA stimulated NB-4 cells (Fig 1E, Fig 3). In contrast, anti–Mac-1, anti-p150, or anti–VLA-4 did not inhibit aggregation. Inhibitory MoAb directed against the counterstructures of LFA-1 (ICAM-1, -2, and -3) were also evaluated for their ability to inhibit homotypic aggregation of NB-4 cells. Strikingly, MoAb directed against ICAM-2 but not ICAM-1 or ICAM-3 were able to inhibit aggregation (Fig 1F, Fig 3). To verify that potential aggregation effects caused by Fc portion of MoAb were not occurring, F(ab′)2 fragments directed against the β chain and ICAM-1 MoAb were used. In addition, the MoAb used did not induce spontaneous aggregation of NB-4 cells (data not shown).
Inhibition of NB-4 cell aggregation by MoAb and 10 mmol/L EDTA. NB-4 cells were exposed to 100 nmol/L tRA for 72 hours. Homotypic aggregation was measured as described in Materials and Methods. Blocking concentrations of the indicated MoAbs were added at the initiation of the assay, and the percent aggregation ± SD was determined at 6 hours. Data represent results from three separate experiments performed in duplicate.
Inhibition of NB-4 cell aggregation by MoAb and 10 mmol/L EDTA. NB-4 cells were exposed to 100 nmol/L tRA for 72 hours. Homotypic aggregation was measured as described in Materials and Methods. Blocking concentrations of the indicated MoAbs were added at the initiation of the assay, and the percent aggregation ± SD was determined at 6 hours. Data represent results from three separate experiments performed in duplicate.
tRA-induced aggregation of NB-4 cells was tested for divalent cation dependence. Because LFA-1–dependent homotypic aggregation requires divalent cations, particularly Mg2+, Ca2+, and Mn2+.29 43 Serum-free AIM-V media contains magnesium and calcium (concentrations are proprietary to GIBCO), allowing for NB-4 aggregation that was completely inhibited in 10 mmol/L EDTA (Fig 3).
Aggregation of NB-4 cells is not enhanced by phorbol ester or “activating” MoAb directed against LFA-1.PMA, a potentially potent enhancer or inducer of aggregation by increasing the avidity of LFA-1 for its counterstructures, did not induce aggregation of NB-4 cells (Fig 4A). PMA (50 ng/mL) also did not alter the magnitude of aggregation of NB-4 cells treated with 100 nmol/L tRA for 72 hours (Fig 4A). In addition to PMA, we also evaluated the activation state of LFA-1 using the MoAb KIM 185 (Fig 4A).44 This MoAb binds directly to LFA-1 and increases the avidity of LFA-1 for its counterstructures. KIM 185 did not induce the aggregation of NB-4 cells or alter the aggregation of NB-4 cells exposed to tRA.
Aggregation of NB-4 cells in the presence of PMA or activating MoAb KIM 185. (A) The effect of PMA (50 ng/mL) and KIM 185 (10 μg/mL) on the aggregation of NB-4 cells and NB-4 cells exposed to 100 nmol/L tRA for 72 hours is shown. Aggregation was measured at 6 hours as described in Materials and Methods The histogram depicts the percent aggregation ± SD. The combination of modulators added to the assay are indicated on the x-axis. Data represent results from three separate experiments performed in duplicate. (B) Kinetics of NB-4 cell adhesion after exposure to 100 nmol/L tRA for 72 hours. Cells were washed and then allowed to aggregate in the presence of tRA (tRA), absence of tRA (tRA washed), or tRA and 50 ng/mL PMA (PMA). The percent aggregation at different time points was determined. Data represents results from three separate experiments performed in duplicate.
Aggregation of NB-4 cells in the presence of PMA or activating MoAb KIM 185. (A) The effect of PMA (50 ng/mL) and KIM 185 (10 μg/mL) on the aggregation of NB-4 cells and NB-4 cells exposed to 100 nmol/L tRA for 72 hours is shown. Aggregation was measured at 6 hours as described in Materials and Methods The histogram depicts the percent aggregation ± SD. The combination of modulators added to the assay are indicated on the x-axis. Data represent results from three separate experiments performed in duplicate. (B) Kinetics of NB-4 cell adhesion after exposure to 100 nmol/L tRA for 72 hours. Cells were washed and then allowed to aggregate in the presence of tRA (tRA), absence of tRA (tRA washed), or tRA and 50 ng/mL PMA (PMA). The percent aggregation at different time points was determined. Data represents results from three separate experiments performed in duplicate.
We also examined the kinetics of NB-4 cell aggregation after 72 hours of exposure to 100 nmol/L tRA (Fig 4B). Significant cell aggregation was observed at 1 hour (54%) after beginning the assay and was maximal at 6 hours (73%). Additional aggregation was not observed at 24 hours (data not shown). Some aggregation (approximately 15%), consisting predominantly of 2 to 5 cell aggregates, was observed at the initiation of the assay. Vigorous pipeting or increased cell washing did not decrease this background aggregation, suggesting that these small aggregates are rapidly formed (within minutes) at this concentration before our ability to manually count the completely disaggregated state. Aggregation occurred with similar kinetics in cells treated with tRA for 72 hours, washed to remove the tRA, and then allowed to aggregate over 6 hours in the absence of tRA (Fig 4B). Finally, the presence of PMA (50 ng/mL) also did not alter the kinetics of aggregation.
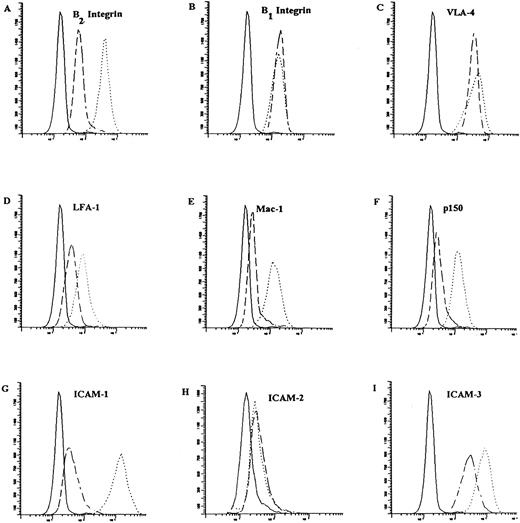
Regulated expression of leukocyte integrins and their counterstructures on tRA-treated cells.Aggregation in the presence of tRA raises the possibility that the drug may be altering the expression state of the integrin receptors. Accordingly, the level of expression of the leukocyte integrins (LFA-1, Mac-1, and p150), their counterstructures (ICAM-1, -2, -3), and VLA-4 were determined by flow cytometric analysis at 100- and 1,000-nmol/L concentrations of tRA after 24 and 72 hours of exposure (Fig 5). After 72 hours, tRA induces a twofold to sevenfold increase in the expression of the leukocyte integrins, LFA-1, Mac-1, and p150. In contrast, the expression of the β1 integrins as measured by CD29 and, in particular, the VLA-4 α subunit remained nearly constant. The counterstructures for the β2 integrins were expressed on NB-4 before treatment with tRA, but after exposure the level of expression of ICAM-1 and ICAM-3 increased 30- and 3-fold, respectively. An intermediate level of induced expression was seen at 24 hours for LFA-1, Mac-1, p150, ICAM-1, and ICAM-3. Interestingly, ICAM-2 is constitutively expressed and the level of expression is not altered at 24 or 72 hours. A statistically significant difference in level of expression or time course of expression among any of the tested adhesion molecules was not observed on cells treated with 100 or 1,000 nmol/L tRA in three experiments analyzed in parallel (data not shown).
Surface expression of adhesion molecules on NB-4 cells in response to treatment with 100 nmol/L tRA for 72 hours. The surface expression of integrins (except β1 integrin) and their counterstructure were determined as described in Materials and Methods using indirect immunofluorescence. β1 integrin (CD29) expression was determined by direct immunofluorescence. As a negative control for indirect immunoflroescence, a nonspecific IgG was substituted in the first step and FITC conjugated goat anti-mouse IgG was used as the indirect reagent. FITC conjugated goat anti-mouse IgG alone was used as the negative control for direct staining. Representative histograms are presented that show surface expression on NB-4 cells (- - - -) and NB-4 cells exposed to 100 nmol/L tRA for 72 hours (⋅⋅⋅⋅⋅⋅) as compared with a negative control ( — ). The surface expression of (A) LFA-1, (B) Mac-1, (C) p150, (D) β2 subunit, (E) β1 subunit, (F ) VLA-4, (G) ICAM-1, (H) ICAM-2, and (I) ICAM-3 are shown. The fold increase in expression of these surface receptors is described in the text. Fold increase in text represents three separate experiments with all samples run in parallel.
Surface expression of adhesion molecules on NB-4 cells in response to treatment with 100 nmol/L tRA for 72 hours. The surface expression of integrins (except β1 integrin) and their counterstructure were determined as described in Materials and Methods using indirect immunofluorescence. β1 integrin (CD29) expression was determined by direct immunofluorescence. As a negative control for indirect immunoflroescence, a nonspecific IgG was substituted in the first step and FITC conjugated goat anti-mouse IgG was used as the indirect reagent. FITC conjugated goat anti-mouse IgG alone was used as the negative control for direct staining. Representative histograms are presented that show surface expression on NB-4 cells (- - - -) and NB-4 cells exposed to 100 nmol/L tRA for 72 hours (⋅⋅⋅⋅⋅⋅) as compared with a negative control ( — ). The surface expression of (A) LFA-1, (B) Mac-1, (C) p150, (D) β2 subunit, (E) β1 subunit, (F ) VLA-4, (G) ICAM-1, (H) ICAM-2, and (I) ICAM-3 are shown. The fold increase in expression of these surface receptors is described in the text. Fold increase in text represents three separate experiments with all samples run in parallel.
The effect of PMA and KIM 185 on the expression of LFA-1 was also examined (data not shown). PMA or KIM 185 did not alter the expression levels of LFA-1 on NB-4 cells or NB-4 cells treated with 100 nm tRA for 72 hours.
Homotypic adhesion of tRA exposed cells is inhibited by methylprednisolone.MPSS inhibits neutrophil aggregation (Larson, Lynam, and Sklar, unpublished observation, May 1996) and is effective in the treatment of neutrophil adhesion-dependent diseases (such as adult respiratory distress syndrome) and retinoic acid syndrome.16 18-20 Therefore, we evaluated its effect on tRA-induced homotypic aggregation of NB-4 cells (Fig 6). MPSS at concentrations of 0.5 and 1.0 mg/mL was effective at inhibiting homotypic aggregation 60% and 95%, respectively. Inhibition of aggregation was seen 1 hour after MPSS. Maximal inhibition of aggregation was present by 6 hours. Notably, at 6 hours similar high viability among cells treated and untreated with MPSS was observed (>90%).
Effect of MPSS on NB-4 aggregation. The dose dependence of MPSS on tRA-induced NB-4 cell aggregation is shown. NB-4 cells were exposed to 100 nmol/L tRA for 72 hours. Homotypic aggregation is measured as described in Materials and Methods. Data represent three experiments done in duplicate in which the percent aggregation ± SD is determined.
Effect of MPSS on NB-4 aggregation. The dose dependence of MPSS on tRA-induced NB-4 cell aggregation is shown. NB-4 cells were exposed to 100 nmol/L tRA for 72 hours. Homotypic aggregation is measured as described in Materials and Methods. Data represent three experiments done in duplicate in which the percent aggregation ± SD is determined.
DISCUSSION
Adhesive alterations of APL cells in response to tRA are clinically important and have not been well studied. In the present study, we investigated the adhesive characteristics of the APL cell line NB-4 as a first step toward understanding the relationship between tRA-induced differentiation and the adhesive properties of APL cells. The homotypic aggregation model system that we used measures aggregation, determines specific molecules involved in adhesive alterations, and evaluates pharmacological agents that may affect aggregation. We found that exposure of NB-4 cells to tRA promotes LFA-1/ICAM-2–dependent homotypic aggregation. Aggregation is not dependent on the continual presence of tRA, is not enhanced by phorbol ester or an activating LFA-1 MoAb, but is rapidly inhibited by MPSS. In addition, these findings provide evidence that the LFA-1/ICAM-2 pathway is differentially regulated since aggregation is not inhibited by a limited panel of MoAbs directed to ICAM-1 and -3, although these counterstructures are present and induced 30- and 3-fold by tRA, respectively. Finally, these findings support a mechanism of retinoic acid syndrome in which cellular adhesion plays an integral role.
Homotypic aggregation was progressively induced over 168 hours of exposure to therapeutic doses of tRA. Aggregation is attributable in large part to LFA-1 and its counterreceptor ICAM-2, and is near maximal after 72 hours of tRA exposure. Expression of the β2 integrins (LFA-1, Mac-1, and p150), ICAM-3, and ICAM-1 is induced 2- to 30-fold over 72 hours of tRA exposure, suggesting that RA, a known regulator of transcription,34 leads to increased surface expression at least in part by increased transcription of these molecules. On the other hand, tRA does not appear to have this effect on ICAM-2, VLA-4, or β1 integrin as surface expression of these molecules remains constant. The lack of induction of ICAM-2 expression is consistent with observations of ICAM-2 expression on other cell types.45-47 Alpha d was not studied because a MoAb is not yet available to us.30
tRA-induced aggregation of NB-4 cells represents a novel cell adhesion model for the study of LFA-1–dependent aggregation. Aggregation was not enhanced by PMA or the activating MoAb KIM 185. We and others have observed that PMA or KIM 185 promotes enhanced aggregation on a variety of cell types through conversion of LFA-1 from a low to high avidity state.43-45,48,49 PMA induces a signaling pathway that converts LFA-1 to a high avidity state, and KIM 185 binds directly to LFA-1 converting LFA-1 to a high avidity conformation. Both agents induce aggregation after conversion of LFA-1 to a high avidity state. However, the avidity of surface LFA-1 molecules on tRA-treated NB-4 cells is not regulated in a manner similar to other leukocytes. Furthermore, the alteration in cellular processes leading to NB-4 cell aggregation are different from tRA-induced aggregation of another myeloid leukemia cell line, HL-60.50 Treatment of HL-60 cells with tRA will not induce homotypic aggregation of HL-60 cells, although a high avidity state of LFA-1 is induced. Transfection of a constitutively active Ras gene, however, will allow for tRA induced aggregation that is LFA-1/ICAM-1–dependent, although ICAM-2 is present on the cell surface.51 In contrast, our results show that tRA-induced NB-4 cell aggregation is associated with LFA-1/ICAM-2 interaction and the avidity is not regulated.
LFA-1 on tRA-treated NB-4 cells appears to preferentially interact with ICAM-2 rather than ICAM-1 or -3, although these latter counterstructures are prevalent and induced 30- and 3-fold, respectively, on the cell surface. Two mechanisms that may act in isolation or in concert may explain this observation: (1) LFA-1 is present in a conformational state that allows for preferential interaction with ICAM-2 relative to other counterstructures or (2) ICAM-1 and -3 are topographically present in inaccessible locations, making ICAM-2 the preferred ligand to LFA-1. The former proposition is supported by studies showing that different stimuli and conformations of LFA-1 promote differential adhesion to ICAM-1, -2, or -3.45,49,50 The latter proposal is affirmed by the topographic restriction of ICAM-2 on natural killer cell–sensitive targets,52 a finding that indicates surface location of integrin counterstructure may play a role in the ability to bind LFA-1 molecules. Finally, although ICAM-2 plays a predominant role in tRA-induced NB-4 aggregation, a role for ICAM-1 or -3 cannot be excluded since this assay or MoAb panel may lack sensitivity to detect their relative contribution.
Our observations further support the contention made by others that cellular adherence pathways are relevant to the pathogenesis of retinoic acid syndrome.8,20 Retinoic acid syndrome occurs days to weeks after initiating tRA therapy.8,9,11,20 Consistent with this observation, induction of integrin expression and cellular aggregation of NB-4 cells occurs progressively over several days in response to therapeutic levels of tRA. In addition, MPSS is effective in resolving and decreasing fatality from retinoic acid syndrome,16,18-20 and tRA-induced homotypic aggregation was inhibited by MPSS in a dose-dependent manner. Notably, the concentration of MPSS found to be inhibitory to NB-4 aggregation is similar to that which inhibits integrin-dependent homotypic adherence of neutrophils in vitro (Larson, Lynam, and Sklar, unpublished, May 1996). Furthermore, these in vitro findings are consistent with the clinical observation that relatively high bolus doses of MPSS (30 mg/kg) are effective in treating retinoic acid syndrome, adult respiratory distress syndrome, arthritis, and ischemic repair.53-58 The peak and mean serum concentration of MPSS and its bioavailability in patients treated for these diseases has not been reported. However, a 1,800 to 2,400 mg bolus (60 to 80 kg individual) of MPSS could theoretically lead to a transient serum level of approximately 0.5 mg/mL over very brief periods, which may be periods that may be sufficient for MPSS effects. Leukoaggregates in vessels were not detected in the two cases of retinoic acid syndrome that were studied posthumously by previous investigators.20 However, one patient was treated with steroids and the other with chemotherapy just before death, raising the possibility that the rapid action of these therapies disrupted the leukoaggregates, but tissue infiltration was still significant enough to cause death. The mechanism by which MPSS inhibits aggregation is likely complex, because MPSS has been shown to coincidentally inhibit several cellular processes that are necessary for integrin-dependent aggregation including cytoskeletal organization, vesicular trafficking, membrane fluidity, and nonspecific competitive binding to surface receptors.59-62 We have shown an in vitro effect of MPSS solely to draw a correlation with a clinical observation.
We have shown that tRA induces APL cells to aggregate homotypically through an LFA-1/ICAM-2–mediated pathway that is rapidly inhibited by MPSS. Although our study has focused on measuring homotypic aggregation, it has implications for leukemic cell-endothelial cell (heterotypic) interactions as well. The expression of the β2 integrins, LFA-1, Mac-1, and p150, and their counterstructures ICAM-1 and -3 are dramatically increased on APL cells by tRA at doses that represent peak or average serum values attained in patients. Because these molecules may participate in a variety of heterotypic interactions, it is likely that interactions of APL cells with endothelium are also altered by tRA therapy. Taken together, our findings and future studies have important implications in understanding adhesion biology as well as the pathogenesis of tRA-induced complications of APL therapy.
ACKNOWLEDGMENT
We thank Sheryl Curtin for her assistance in the preparation of this manuscript.
Supported in part by grants from the National Institutes of Health (IDeA PAR-96-012 and GM 37696) and American Cancer Society (ACS-IRG-192 and ACS-CB-162).
Address reprint requests to Richard S. Larson, MD, PhD, UNM Cancer Center Room B88-A, 900 Camino de Salud, NE, Albuquerque, NM 87131-5636.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal