To the Editor:
The Duffy antigen receptor for chemokines (DARC) is a seven-transmembrane domain glycoprotein of 35-45 kD that functions as receptor for the malaria parasites Plasmodium vivax and Plasmodium knowlesi and binds with high-affinity selected members of both the C-X-C and C-C classes of chemokines. DARC is expressed in human erythrocytes, on endothelial cell lining postcapillary venules throughout the body, and in vascular endothelial and epithelial cells in some nonerythroid organs.1 Furthermore, recent findings showed that a functional DARC polypeptide is also expressed in the central nervous system, exclusively by Purkinje neurons of the cerebellum.2 Interestingly, two mRNA species of 1.35 kb and 7.5 kb were detected by Northern blot analysis of whole brain extracts, whereas only the 1.35-kb transcript is present in red blood cells and in the endothelial cells of all other tissues investigated.3
These results prompted us to investigate whether (1) the expression of the two DARC mRNAs is differently regulated in various regions of brain and (2) the DARC protein detected in cerebellum is identical to the erythroid/endothelial DARC, or whether the 7.5-kb brain specific transcript should encode for a novel tissue-specific DARC polypeptide isoform.
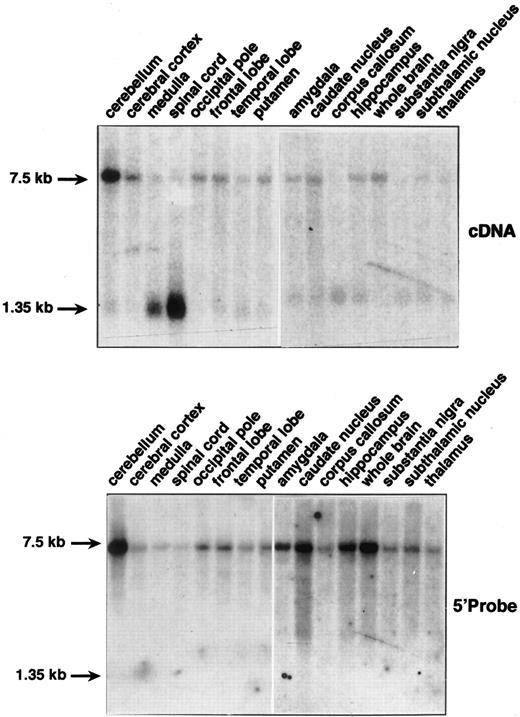
Northern blots containing mRNAs from different sections of brain were hybridized with a cDNA probe corresponding to the entire coding region of the erythroid/endothelial DARC mRNA. In all samples, both 1.35 kb and 7.5 kb mRNA species were detected, although with different intensities (Fig 1, top). Particularly, the 7.5-kb transcript was highly expressed and the 1.35-kb transcript almost undetectable in cerebellum, whereas the opposite pattern of expression was observed in spinal cord. In all other sections of brain, both transcripts were poorly expressed. This mRNA expression pattern fit well with the immunohistochemical and biochemical analysis of DARC expression in brain,2 and indicated that the promiscuous chemokine receptor of Purkinje neurons in the cerebellum is encoded by the 7.5-kb DARC transcripts.
Northern blot analysis of DARC mRNA expression in human brain tissues. Northern blots containing 2 μg of poly(A+) RNAs from multiple sections of brain (Clontech) were probed with the full-length DARC cDNA (top) and with the 5′ DARC probes (bottom) (see text). Hybridization was performed in QuickHyb solution (Stratagene, La Jolla, CA) at 68°C for 1 hour and washed under stringent conditions according to the manufacturer's instructions. Exposure was 24 hours except for the 5′ probe on amygdala to thalamus samples where exposure was 72 hours. Actin cDNA probe was used to ascertain that an equal amount of mRNA was loaded for each sample (not shown).
Northern blot analysis of DARC mRNA expression in human brain tissues. Northern blots containing 2 μg of poly(A+) RNAs from multiple sections of brain (Clontech) were probed with the full-length DARC cDNA (top) and with the 5′ DARC probes (bottom) (see text). Hybridization was performed in QuickHyb solution (Stratagene, La Jolla, CA) at 68°C for 1 hour and washed under stringent conditions according to the manufacturer's instructions. Exposure was 24 hours except for the 5′ probe on amygdala to thalamus samples where exposure was 72 hours. Actin cDNA probe was used to ascertain that an equal amount of mRNA was loaded for each sample (not shown).
As a first approach to investigate the relationship between the 1.35-kb and 7.5-kb DARC transcripts, the same Northern blots were rehybridized with PCR probes specific of the genomic regions located in 5′ of the cap site or in 3′ of the polyadenylation site identified for the 1.35-kb erythroid mRNA.3 4 No hybridization signal was detected with a 3′ probe corresponding to the 300 nucleotides downstream the poly A additional site (Genebank, accession no. X85785), suggesting that the 7.5-kb mRNA did not differ, even in part, from the 1.35-kb transcript by the use of a different polyadenylation site.
1.7 kb of the DARC 5′ flanking region was isolated by genome walking using a promoter finder kit from Clontech (Palo Alto, CA) (sequences available in Genebank, accession no. XX). Two polymerase chain reaction (PCR) fragments corresponding to nt −75 to −568 and to nt −993 to −1638 (+1 taken as the erythroid initiation site)4 were used as probes. Both probes gave the same hybridization pattern and detected only the 7.5-kb DARC mRNA in all brain tissues with the same relative intensities than those observed with the cDNA probe (Fig 1, bottom). These analyses strongly suggestesd that the additional sequences present in the 7.5-kb mRNA as compared with the 1.35-kb mRNA are located in the 5′ region.
Analysis of DARC clones isolated from a cerebellum cDNA library in λgt10 (Clontech) supported this hypothesis. Eight clones were characterized, among which 2 hybridized with both the cDNA and the 5′ DARC probes, 5 with the cDNA probe only, and 2 with the 5′ probe only. Inserts (1 kb to 2 kb in length) contained overlapping sequences extending from the polyA tracts to position −1,520 upstream the erythroid cap site. Four main observations were made from these sequence analysis: (1) the cerebellum specific transcript did not use a specific polyadenylation site because in three clones, polyA tracks were found at the same position than in the 1.35-kb erythroid/endothelial mRNA; (2) the translated sequence of the erythroid isoform is conserved in the cerebellum transcript, without insertions or deletions; (3) the translated sequence of the cerebellum transcript did not extend in 5′ of the ATG initiator codon used by the major erythroid isoform, because a non-sense codon was found 5 triplets upstream; and (4) there was no additional intron in the 5′ region of the DARC gene, at least until position −1,520, because the identified 5′ untranslated sequence of the cerebellum transcript was colinear with the genomic sequence until this position. In conclusion, we have shown that the expression of the two DARC mRNA species previously detected in the brain are differently regulated in various regions of the central nervous system. The 7.5-kb transcript encoding the functional DARC in cerebellum should contain a long 5′ untranslated region of about 4 kb. Therefore, this DARC mRNA is expressed under the control of a specific promoter different from those regulating the expression of the 1.35-kb mRNA expressed in erythroid4 and endothelial cells.5 Existence of this cerebellum-specific promoter implies that the mutation in the erythroid promoter that abolishes DARC expression in Duffy-negative red blood cells4 should not affect normal expression in brain Purkinje cells of these individuals, as demonstrated in other nonerythroid Duffy-negative tissues.6,7 Interestingly, the expression of the murine homologue of DARC is also most likely under the control of a tissue-specific promoter in brain, since Northern blot analysis revealed an abundant mRNA species of 7.5 kb in this tissue, whereas a 1.35-kb transcript was detected in mouse spleen, lung, and squeletal muscle (our unpublished data). Finally, the main conclusion arising from the present study is that, although encoded by a 7.5-kb mRNA isoform, the DARC polypeptide expressed in cerebelum is identical to the isoforms encoded by the 1.35-kb mRNA species in erythroid cells, kidney, and fetal liver3,4,5 8 as well as to those cloned from heart, fetal brain, and skeletal muscle during the course of this study (data not shown).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal