Abstract
We conducted a prospective randomized multicenter clinical trial comparing the effects of granulocyte-macrophage colony-stimulating factor (GM-CSF ) as an adjunct to intensive chemotherapy in patients of 61 years and older with untreated newly diagnosed acute myeloid leukemia (AML). Patients were randomized to either receive daunomycin-cytosine arabinoside with GM-CSF or daunomycin-cytosine arabinoside (control arm). Based on the rationale that GM-CSF might sensitize the leukemic cells to the cytotoxicity of chemotherapy as well as enhance white blood cell regeneration, GM-CSF was given during chemotherapy as well as after chemotherapy. Patients were treated with one, and in case of a partial response, with two remission induction cycles. When a complete remission was attained they received one additional cycle of consolidation therapy. Of 318 evaluable patients with a median age of 68 years, 157 were randomized to receive GM-CSF and 161 were assigned to control therapy. The effect of GM-CSF on treatment was evaluated according to intention-to-treat. Complete remission was achieved in 56% of the patients in the GM-CSF group and 55% of the control patients (P = .98). Recovery of neutrophils was significantly faster in GM-CSF–treated patients. The median time of recovery of neutrophils towards 0.5 × 109/L was 23 days in the GM-CSF group versus 25 days in the control group (P = .0002) with the percentages of patients who recovered being 81% and 71%, respectively. With a median follow-up of 36 months, the probabilities of survival at 2 years after randomization were estimated at 22% for individuals assigned to the GM-CSF treatment as well as for control patients (P = .55). Disease-free survival at 2 years compared 15% and 19% for the two treatment groups (P = .69). The number of nights spent in the hospital, number of transfusions, and frequencies and types of hemorrhages and infections did not differ either. The cytogenetic results at diagnosis of this study in elderly AML shows that there is a relatively high numerical representation of patients with abnormal cytogenetics (55% of documented cases), who showed significantly inferior response rates and survival duration. We conclude that, except for a faster neutrophil recovery, GM-CSF during and after induction chemotherapy does not improve the clinical outcome of elderly patients with AML.
ACUTE MYELOID LEUKEMIA (AML) is seen at all ages, but more than half of the patients with AML are 60 years old or older.1,2 Intensified cytotoxic therapy and bone marrow transplantation have improved the prognosis of AML in recent years in young and middle-aged adults.3 However, these modern approaches of therapy have, as yet, appeared of only little benefit to individuals with AML older than 60 years. The reasons for a lack of improvement of treatment outcome in the elderly probably relate to the fact that aged people have limited abilities to tolerate the toxicity associated with intensive chemotherapy. In addition, aged individuals suffer from AML which is a priori more resistant to chemotherapy.4 Therefore, one may assume that any effort at improvement of the efficacy of treatment should be directed at supporting the patients' tolerances to chemotherapy or overcoming primary drug resistance in patients of greater age.
The results of a variety of studies, all conducted in patients with AML of 60 years of age or more, have indicated that these individuals have an approximately 50% probability of entering complete remission (CR).5-12 Approximately 30% of remission induction failures are caused by early death or death during the hypoplastic phase postchemotherapy, ie, mainly because of infections.4 The higher death rate would support the notion that elderly individuals are less able to tolerate the consequences of severe infections. Approximately 10% of these patients die during or within 1 week after completion of chemotherapy (early death) and 20% of the patients expire during the subsequent postchemotherapy interval (hypoplastic death). Elderly patients who experience significant toxicity after the first cycle of chemotherapy are generally withdrawn from additional efforts of treatment. Thus, the obstacles to offer adequate therapy to older patients with AML are still considerable. There has been hope that the hematopoietic growth factors might hasten hematopoietic recovery and, thus, have a role in reducing the incidence and severity of complications and mortality.
Resistance of AML to chemotherapy has been the second major cause of treatment failure in patients aged 60 years or more. The proportion of patients showing primary resistance to chemotherapy as the cause of remission induction failure is approximately 20% to 30%.4 Thus, primarily refractory leukemia is another main determinant of unfavorable outcome. The hematopoietic growth factors have also received considerable interest because of their ability in vitro to enhance the killing of leukemic blasts or leukemic colony-forming cells by cytotoxic drugs. Exposure of leukemic cells to cytarabine in the context of growth factor stimulation has shown an increase in formation of cytarabine-triphosphate (Ara-CTP), increased DNA uptake of radiolabeled cytarabine in leukemic cells, and, in addition, enhancement of cytotoxicity.13-18 These in vitro observations have set the stage for introducing hematopoietic growth factors in clinical protocols. It was argued that the simultaneous administration of hematopoietic growth factors in vivo with chemotherapy might prime the leukemic cells and render the cells more susceptible to cell killing by cytarabine in vivo.
Here the investigators present the results of a clinical randomized phase III study in which granulocyte-macrophage colony-stimulating factor (GM-CSF ) was applied concomitantly with chemotherapy with the objective of enhancing chemotherapy efficacy. GM-CSF was also given after chemotherapy to accelerate myeloid recovery and mitigate morbidity and mortality.
MATERIALS AND METHODS
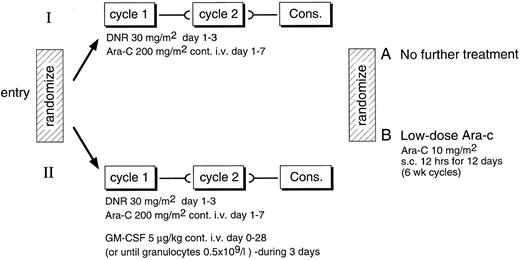
Study design. (Fig 1) Patients aged 61 years and older were entered after informed consent was given in a phase III study in which they were randomized to receive either daunomycin (30 mg/m2 intravenous bolus on days 1, 2, and 3) and cytarabine (200 mg per continuous infusion [c.i.]) on days 1 to 7 as induction therapy (control group) or the same chemotherapy for induction combined with GM-CSF. The comparison was not blinded. In case of a partial response to the induction cycle, patients were planned to receive a second identical course of treatment. Complete responders were to receive one cycle of consolidation therapy that consisted of the same therapy but with one day of daunomycin only. The choice of the daunomycin dose was 30 mg/m2, which is less than is usually used in young adults. A reduced daunomycin scheme had produced similar response rates in elderly patients.19 20 The main objective of the study was to evaluate the effects of GM-CSF on the response rate. In addition, the survival, disease-free survival (DFS), duration of postchemotherapy cytopenia, frequency of infectious complications, and number of days spent in the hospital during and after induction chemotherapy in these individuals were evaluated. After consolidation patients were assigned according to a second randomization to receive either eight cycles of 12-day cytarabine (low dose) at 6-week intervals or no maintenance chemotherapy.
Eligibility. Patients aged 61 years and more with newly diagnosed AML were eligible, including M0-M6 according to the French-American-British (FAB) classification.21 22 Patients with secondary leukemias after previous chemotherapy or previous myelodysplasia (MDS) were also included. Patients were not eligible if they had previously been treated for AML or MDS with chemotherapy or hematopoietic growth factors, if they were refractory to platelet transfusion, or if they were in poor general condition with severe hepatic, cardiac, pulmonary, or renal disease.
GM-CSF trial drug. Molgrastim (Sandoz, Basel, Switzerland) is an Escherichia coli–derived GM-CSF. It was applied at 5 μg/kg/day c.i. starting at day 1 (1 day before the start of chemotherapy) and given during and after the chemotherapy until granulocyte recovery of 0.5 × 109/L (for 3 days) but not beyond day 28. As an alternative to the intravenous route, GM-CSF could be administered once daily subcutaneously, when the patients were discharged from the hospital at the end of the hypoplastic phase. In case of white blood cell counts (WBCs) of 30 × 109/L or more at diagnosis, the start of treatment with GM-CSF was postponed until WBCs had dropped during the first days of chemotherapy to less than 20 × 109/L. If, during treatment, the WBCs would increase to 50 × 109/L or more, GM-CSF was to be interrupted until the counts had declined to 20 × 109/L. GM-CSF was discontinued in case of signs of progressive leukemia and in case of serious toxicity considered to be attributable to GM-CSF.
Criteria of response and evaluation of outcome. CR was defined by a normocellular bone marrow containing less than 5% blast cells including monocytoid cells, less than 10% blast cells and promyelocytes, and less than 50% erythroid cells; no evidence of extramedullary leukemia; and recovery of peripheral blood values to platelet counts of at least 100 × 109/L and polymorphonuclear neutrophils (PMNs) of at least 1.5 × 109/L. Partial remission (PR) was defined by bone marrow smears containing between 5.1% and 25% blasts and less than 5% circulating blast cells. Failures of response were classified as treatment resistance when there was no reduction of the leukemic cell infiltration in the marrow or a reduction that would not meet the criteria of PR or CR. Hypoplasia followed by leukemic regrowth was also classified as resistant disease. Early death was defined as death before the completion of the first cycle of induction therapy, and hypoplastic death was defined as death after the completion of induction cycle (1 or 2) before hematological recovery. Relapse was defined as recurrence of leukemia after initial CR as documented by cytological or pathological evaluation of bone marrow or blood smears or pathological diagnosis of extra medullary leukemia. Standard cytogenetic techniques, including direct preparations, incubation of cells for 24 or 48 hours, and banding techniques were used23 at diagnosis to karyotype the leukemia. Normal (NN) cytogenetics (which included the deletion of the Y chromosome), abnormal cytogenetics (AA), and a mosaicism of abnormal and normal karyotypes (AN) were recorded. The NN score was applied only when a minimum number of 20 mitoses had been evaluated; in case of an examination of less metaphases, the analysis was considered inadequate.
Abnormalities 16q(22), t(15; 17), and t(8; 21) were considered good risk abnormalities. NN karyotypes were classified as intermediate risk. Deletions of the long arm of chromosomes 5 and 7 (5q-, 7q-), or the entire chromosomes (−5, −7) and all other specific cytogenetic abnormalities were considered poor risk abnormalities.24 25 Karyotypic abnormalities involving three chromosomes or more but without any of the specific poor risk or good risk aberrations were designated as complex anomalies.
Maintenance chemotherapy. Patients, after consolidation and continuing in CR, were randomized between no further therapy (arm A) and low dose Ara-C chemotherapy (arm B). Arm B patients received cytosine arabinoside at 10 mg/m2 subcutaneously every 12 hours on days 1 through 12 at 42-day intervals for a total of 8 cycles or until relapse.
Supportive care. All patients received prophylactic platelet transfusions when platelet counts dropped below 20 × 109/L. Red blood cells were usually transfused to maintain the hematocrit above 30%. Daily fluid input and output were monitored. In case of a significantly positive fluid balance, or increase of body weight, diuretics (eg, furosemide) were applied, and in case of insufficient effect (increase of body weight of 3 kg or more) GM-CSF treatment was interrupted. Patients were put on prophylactic antibiotics and antifungal agents depending on the local recommendations of each of the participating institutions, and in case of fever, broad spectrum antibiotics were initiated. The choice of antimicrobial agents was adjusted according to sensitivity data whenever a pathogen was isolated.
Statistical methods. Data were analyzed based on the intention to treat. The relationship between the initial categorized ordered variables (WBC, age, performance status, sex, de novo/secondary AML, FAB cytological type, and cytogenetics) and the CR rate was tested with the χ2 test for linear trend. The usual χ2 test26 with the correction for continuity was used to test the relationship between the treatment randomized and the CR evaluated after induction. The treatment difference of the incidence of different types of screened toxicities observed during the induction period (d0-d28) was tested using usual χ2 test (for binary toxicities) or the χ2 test for linear trend (for graded toxicities). The 95% confidence interval (CI) of the difference was computed using the confidence interval analysis (CIA) program.27
Overall survival was calculated from the date of first randomization until date of death, whatever the reason. Patients still alive were considered as “censored” observations at the date of last follow-up. The DFS was calculated for patients who achieved CR after induction from the date of CR until the date of first relapse or death in first CR. Patients who did not relapse or died were considered as censored observations at the date of last follow-up. Actuarial curves were computed according to the Kaplan-Meier technique.26 The standard error was calculated according to the Greenwood formula.26 The difference between the curves according to the treatment groups was tested using the logrank test.26 The prognostic significance of different initial variables was tested using the logrank test (for binary variables) or the logrank test for linear trend (for ordered variables). The relative risk (RR) of having an event per time unit in one treatment group versus another, along with its 95% CI, was computed by using the odds-ratio technique.27
The aim of the trial was to detect an increase of the CR rate from 50% to 65% (alpha = 0.05, beta = 0.15) by adding the GM-CSF to the induction chemotherapy; the hope was that such an increase in the CR rate, if apparent, would lead to an increase in the survival at 2 years from 15% to 25%. Therefore, it was planned to enter 310 patients, to evaluate their remission status after subsequent treatment steps (particularly after the induction course), and follow them until death. The final analysis was planned to be performed once 256 deaths had been reported to see whether or not a significant difference in outcome would appear between both treatment groups (logrank test, alpha = 0.05, beta = 0.20).
The time to platelet and PMN recovery after the first cycle of induction was calculated from the day of start of induction course 1 until the platelet count reached level 20 or 50 × 109/L and PMN count reached 0.5 or 1 × 109/L, respectively. Only patients who recovered to reach these respective values were included in the statistical analysis. The same statistical techniques (Kaplan-Meier and logrank test) were used to estimate and to compare the proportion of patients who recovered from the start of induction cycle in the two treatment groups.
RESULTS
Response to treatment. The study was open for entry between November 1990 and October 1994, during which 326 patients have been registered. Eight patients were considered ineligible or inevaluable. Reasons of ineligibility and inevaluability were poor physical condition (n = 1), other malignant disease (n = 4), and incomplete data (n = 3). Of the 318 eligible and evaluable patients, 157 patients were randomized to chemotherapy with GM-CSF, and 161 patients were randomized to the control arm with no GM-CSF. Clinical and hematological characteristics of the patients are summarized in Table 1. The age ranged between 61 and 88 years, with a median value of 68 years in each of the two treatment groups. The proportion of patients with a poor pretreatment performance was slightly but not significantly greater in the GM-CSF group (Table 1).
Patient Characteristics
| . | Control Group (%) . | GM-CSF Group (%) . |
|---|---|---|
| Sex | ||
| Male | 52 | 56 |
| Age | ||
| 61-69 | 64 | 58 |
| 70-79 | 33 | 39 |
| ≥80 | 3 | 3 |
| WBC* | ||
| <30 | 72 | 70 |
| 30-99 | 21 | 20 |
| ≥100 | 7 | 10 |
| FAB cytology | ||
| M0 | 6 | 4 |
| M1 | 21 | 23 |
| M2 | 36 | 35 |
| M3 | 2 | 1 |
| M4 | 11 | 12 |
| M5 | 19 | 17 |
| M6 | 2 | 4 |
| Unknown | 3 | 3 |
| Cytogenetics† | ||
| Normal karyotype | 22 | 32 |
| Good risk abnormality | 3 | 2 |
| Intermediate risk abnormality | 21 | 19 |
| Poor risk abnormality | 12 | 11 |
| Failure of analysis/not done | 42 | 36 |
| Performance status‡ | ||
| Normal (0) | 32 | 31 |
| Ambulatory (1) | 49 | 43 |
| Bed ridden less than 50% of time (2) | 16 | 22 |
| Bed ridden ≥ 50% (3) | 2 | 5 |
| AML | ||
| Secondary | 22 | 22 |
| De novo | 78 | 78 |
| Total | 161 | 157 |
| . | Control Group (%) . | GM-CSF Group (%) . |
|---|---|---|
| Sex | ||
| Male | 52 | 56 |
| Age | ||
| 61-69 | 64 | 58 |
| 70-79 | 33 | 39 |
| ≥80 | 3 | 3 |
| WBC* | ||
| <30 | 72 | 70 |
| 30-99 | 21 | 20 |
| ≥100 | 7 | 10 |
| FAB cytology | ||
| M0 | 6 | 4 |
| M1 | 21 | 23 |
| M2 | 36 | 35 |
| M3 | 2 | 1 |
| M4 | 11 | 12 |
| M5 | 19 | 17 |
| M6 | 2 | 4 |
| Unknown | 3 | 3 |
| Cytogenetics† | ||
| Normal karyotype | 22 | 32 |
| Good risk abnormality | 3 | 2 |
| Intermediate risk abnormality | 21 | 19 |
| Poor risk abnormality | 12 | 11 |
| Failure of analysis/not done | 42 | 36 |
| Performance status‡ | ||
| Normal (0) | 32 | 31 |
| Ambulatory (1) | 49 | 43 |
| Bed ridden less than 50% of time (2) | 16 | 22 |
| Bed ridden ≥ 50% (3) | 2 | 5 |
| AML | ||
| Secondary | 22 | 22 |
| De novo | 78 | 78 |
| Total | 161 | 157 |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; WBC, white blood cell count; FAB, French-American-British Classification; AML, acute myeloid leukemia.
×109/L.
Cytogenetic categories as defined in Materials and Methods. Complex chromosome abnormalities were apparent in 4 patients of the control arm and 3 patients of the GM-CSF arm (ie, 8% of evaluated cases).
The comparison of the differences in performance status between the two treatment groups (performance status grade 0 v 1 v ≥2) gives a P value of .23 (χ2 test for linear trend).
CR after one or two cycles of induction chemotherapy was achieved in 55% of patients in the control group and in 56% of patients of the GM-CSF group (P = .98). Most of these individuals attained CR after the induction cycle I (Table 2). Reasons for not attaining CR were related to resistance to chemotherapy, prolonged hypoplasia, death in hypoplasia, and early death (Table 2).
Response of AML to Induction Chemotherapy
| Total . | Control Group . | GM-CSF Group . | ||
|---|---|---|---|---|
| . | 161 . | (100%) . | 157 . | (100%) . |
| Complete response | ||||
| After remission induction cycle I | 73 | (45) | 79 | (50) |
| After remission induction cycle I/II | 89 | (55) | 88 | (56) |
| No complete response | ||||
| Early death | 5 | (3) | 0 | (0) |
| Death in hypoplasia | 16 | (10) | 22 | (14) |
| Resistance to chemotherapy | 51 | (32) | 47 | (30) |
| Total . | Control Group . | GM-CSF Group . | ||
|---|---|---|---|---|
| . | 161 . | (100%) . | 157 . | (100%) . |
| Complete response | ||||
| After remission induction cycle I | 73 | (45) | 79 | (50) |
| After remission induction cycle I/II | 89 | (55) | 88 | (56) |
| No complete response | ||||
| Early death | 5 | (3) | 0 | (0) |
| Death in hypoplasia | 16 | (10) | 22 | (14) |
| Resistance to chemotherapy | 51 | (32) | 47 | (30) |
Abbreviations: AML, acute myeloid leukemia; GM-CSF, granulocyte-macrophage colony-stimulating factor.
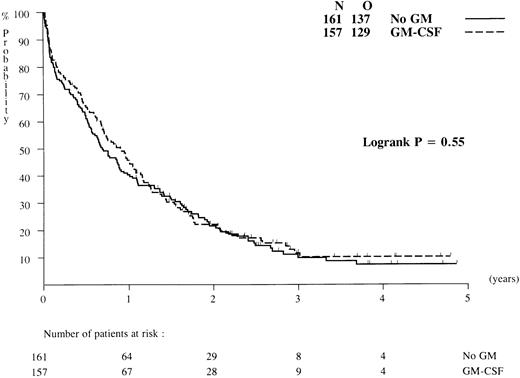
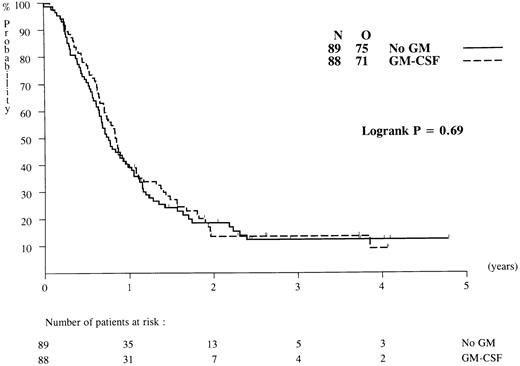
Overall survival and DFS. Patients have been followed for a median of 36 months after diagnosis. Probabilities of overall survival at 2 years after randomization were identical, ie, 22% (standard error [SE] = 3.5%) in the GM-CSF and the control groups (P = .55; Fig 2), and the RR estimate was 0.93 (95% CI = [0.73; 1.10]). Of the 177 complete responders to chemotherapy 11 patients died in first CR (5 patients in the GM-CSF group and 6 patients in the control group). All other causes of death after CR were related to recurrence of leukemia. The death rates in the two arms were similar: 67 of 89 (GM-CSF ) versus 61 of 88 (no GM-CSF ). DFS probabilities at 2 years after randomization were estimated at 14% (SE = 4.1%) in the GM-CSF group and 19% (SE = 4.3%) in the control arm (P = .69; Fig 3), and the RR estimate was 0.94 (95% CI4 = [0.68; 1.30]). If all patients randomized are considered (including ineligible and nonevaluable cases), the treatment comparison remained practically the same.
Duration of overall survival of patients randomized to chemotherapy with or without GM-CSF (results based on an intention-to-treat analysis). N, number of patients in each group; O, observed number of deaths.
Duration of overall survival of patients randomized to chemotherapy with or without GM-CSF (results based on an intention-to-treat analysis). N, number of patients in each group; O, observed number of deaths.
DFS from CR of patients randomized to chemotherapy with or without GM-CSF. N, number of patients; O, observed number of relapses/deaths.
DFS from CR of patients randomized to chemotherapy with or without GM-CSF. N, number of patients; O, observed number of relapses/deaths.
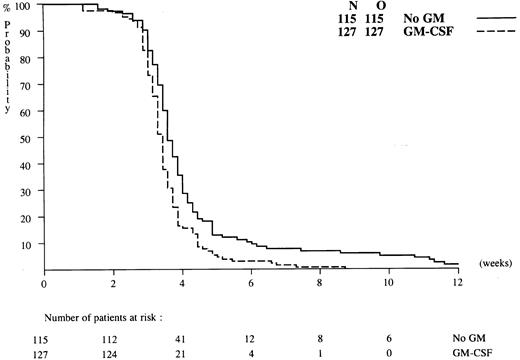
GM-CSF treatment, hematopoietic recovery, and hospitalization. The patients in the GM-CSF group received GM-CSF during an average of 18 days (median, 20; range, 0-46). GM-CSF administration started on day 0 in 69% of the patients. In 21.5% of the patients the GM-CSF administration began between 1 and 4 days after the start of the chemotherapy, in 7.4% between 4 and 6 days, and in 2.5% with a delay of more than 6 days after commencing chemotherapy. This was generally because of a leucocytosis of 30 × 109/L or more because the protocol required GM-CSF to be postponed in that case. In a majority of the patients GM-CSF therapy was stopped prematurely, mainly because of possible toxicity of GM-CSF. The reasons for discontinuation of GM-CSF treatment, except for early PMN recovery (28 cases), were related to reappearance of blasts in the blood (8 cases), doubling of circulating blast counts (4 cases), and toxicity (63 cases). Figure 4 presents the time of recovery of PMNs towards 0.5 × 109/L. Median times to neutrophil recovery of 0.5 × 109/L after induction cycle I in GM-CSF treated patients and control patients were 23 and 25 days, respectively (P = .0002). In addition, the proportion of patients who recovered to PMN of 0.5 × 109/L was greater in the GM-CSF group than in the control group: 81% (127/157) versus 71% (115/161). The median times of recovery to a platelet value of 20 × 109/L in the GM-CSF and control groups were identical, ie, 22 days. The median (range) number of days spent in the hospital during induction cycle I were 32.5 days (range, 3 to 75) in the GM-CSF group and 32.0 days (range, 11 to 98) in the controls (P = .87).
Time to neutrophil recovery towards 0.5 × 109/L for patients randomized to induction therapy with or without GM-CSF. Y-axis shows percentages of patients with PMN less than 0.5 × 109/L.
Time to neutrophil recovery towards 0.5 × 109/L for patients randomized to induction therapy with or without GM-CSF. Y-axis shows percentages of patients with PMN less than 0.5 × 109/L.
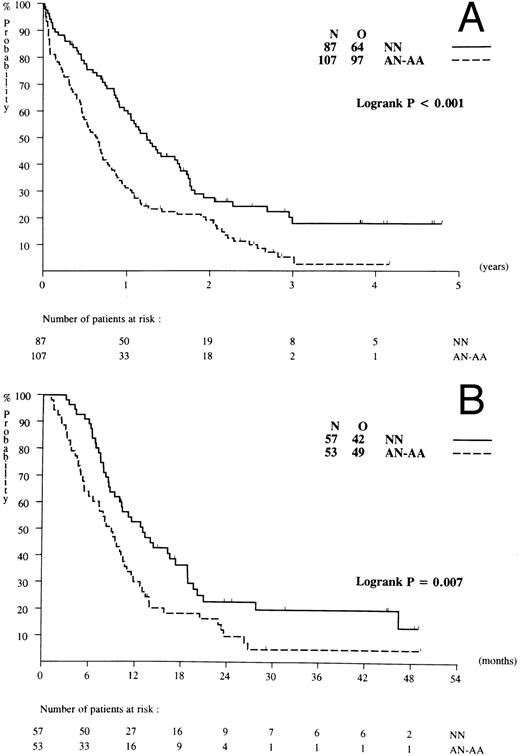
Prognostic factors for response and survival. The prognostic impact of WBC, age, sex, performance status, FAB classification, de novo AML versus secondary AML, and cytogenetics at diagnosis with regard to treatment outcome were assessed. There was no apparent relationship between age, sex, or FAB type and response or survival. Patients with a WBC of 100 × 109/L or more (n = 27), a poor performance status, and abnormal cytogenetics (AA and AN) showed a significantly reduced probability of attaining a CR (Table 3). Specific cytogenetic abnormalities of favorable risk [ie, abn(16), t(8; 21), t(15; 17)] were present in 8 patients (ie, 4% of examined cases) only. Unfavorable type abnormalities (−5, 5q-, −7, 7q-, complex, or other cytogenetic abnormalities) were shown in 36 patients (19% of examined cases) and intermediate risk type abnormalities in 63 patients (ie, 32% of examined cases). Among the subsets with cytogenetics of unfavorable and intermediate risk the CR probabilities were 36% and 54%, respectively (P = .008). Finally, of the above listed factors, abnormal cytogenetics (Fig 5A; P < .001) and poor risk cytogenetic abnormalities (P < .001), correlated with significantly shorter overall survival. Abnormal karyotype (AA and AN; Fig 5B; P < .007) and poor risk karyotypes also predicted for inferior DFS probabilities (P = .009).
Prognostic Factors of Patients With AML for Response to Remission Induction Treatment
| . | Number of Patients . | CR Rate (%) . | P Value . |
|---|---|---|---|
| WBC | |||
| <30 | 226 | 58 | .0193-150 |
| 30-99 | 65 | 57 | |
| ≥100 | 27 | 30 | |
| Age | |||
| 61-69 yrs | 194 | 56 | .833-150 |
| 70-79 yrs | 115 | 56 | |
| ≤80 yrs | 9 | 44 | |
| Performance (WHO) | |||
| 0 | 100 | 66 | |
| 1 | 146 | 54 | .0083-150 |
| 2 | 60 | 43 | |
| 3 | 12 | 50 | |
| AML | |||
| De novo | 248 | 56 | .963-151 |
| Secondary | 69 | 55 | |
| Cytogenetics3-152 | |||
| NN | 87 | 65 | .043-151 |
| AN-AA | 107 | 50 | |
| Good risk | 8 | 75 | .0083-150 |
| Intermediate risk | 63 | 54 | |
| Poor risk | 36 | 36 |
| . | Number of Patients . | CR Rate (%) . | P Value . |
|---|---|---|---|
| WBC | |||
| <30 | 226 | 58 | .0193-150 |
| 30-99 | 65 | 57 | |
| ≥100 | 27 | 30 | |
| Age | |||
| 61-69 yrs | 194 | 56 | .833-150 |
| 70-79 yrs | 115 | 56 | |
| ≤80 yrs | 9 | 44 | |
| Performance (WHO) | |||
| 0 | 100 | 66 | |
| 1 | 146 | 54 | .0083-150 |
| 2 | 60 | 43 | |
| 3 | 12 | 50 | |
| AML | |||
| De novo | 248 | 56 | .963-151 |
| Secondary | 69 | 55 | |
| Cytogenetics3-152 | |||
| NN | 87 | 65 | .043-151 |
| AN-AA | 107 | 50 | |
| Good risk | 8 | 75 | .0083-150 |
| Intermediate risk | 63 | 54 | |
| Poor risk | 36 | 36 |
Abbreviations: AML, acute myeloid leukemia; CR, complete remission; WBC, white blood cell count; WHO, World Health Organization; NN, normal; AN, abnormal and normal; AA, abnormal.
χ2 test for linear trend.
χ2 test.
Cytogenetics was not done or reported or was not successful in 124 patients (CR rate in this group was 54%). Complex chromosome abnormalities were shown in 17 patients of whom 57% attained CR. NN, AA, AN, good risk, intermediate risk, or poor risk categories as defined in Materials and Methods.
Probability of overall survival and DFS in relation to cytogenetics. (A) Survival in relation to NN (normal karyotype) and AN-AA (abnormal karyotype). (B) DFS in relation to NN and AN-AA.
Probability of overall survival and DFS in relation to cytogenetics. (A) Survival in relation to NN (normal karyotype) and AN-AA (abnormal karyotype). (B) DFS in relation to NN and AN-AA.
Fever. The median duration of fever (Fig 5) in association with induction cycle I was 10.0 days in the GM-CSF arm (range, 0 to 40) and 6.0 days in controls (range, 0 to 41; P < .001).
Supportive care. The patients in the GM-CSF group received antibiotic treatment for fever or infections during a median of 20 days (range, 0 to 40). In patients assigned to the control, arm antibiotics were administered during a median of 16 days (range, 0 to 80). Both patients in the control and GM-CSF groups received a median number of 12 platelet transfusions (range, 0 to 100). The median number of red blood cell transfusions given to either the GM-CSF– and control-treated patients did not differ significantly: 14 (range 2 to 48) versus 12 (range, 2 to 40).
Toxicity. The frequencies of hemorrhages, liver abnormalities, oral toxicity, nausea, cardiac rhythm abnormalities, neurotoxicity, bone pain, phlebitis, and infections were similar in both groups. However, the patients in the GM-CSF group experienced more diarrhea, renal abnormalities, fever, cutaneous toxicity, hypotension, fluid retention, and chills. Table 4 shows the frequencies of the latter complications during induction cycle I in each treatment group.
Relative Frequency (%) of Complications During Induction Cycle I
| . | Control Group . | GM-CSF . |
|---|---|---|
| . | . | Group . |
| Hemorrhage | ||
| Only petechiae | 21 | 26 |
| Other bleedings | 26 | 25 |
| Liver | ||
| Mild-moderate (1.25-5 × normal) | 31 | 37 |
| Severe (≥5 × normal) | 11 | 10 |
| Oral | ||
| Erythema | 17 | 14 |
| Ulcers and other toxicity | 7 | 12 |
| Nausea/vomiting | 30/35 | 32/34 |
| Diarrhea4-150 | ||
| Less than 2 days | 23 | 23 |
| More severe | 18 | 30 |
| Renal4-150 | ||
| Mild-moderate (1.26-5 × normal) | 21 | 31 |
| Severe (≥5 × normal) | 3 | 3 |
| Fever4-150 | ||
| Subfebrile (<38°C) | 12 | 11 |
| ≥38°C | 71 | 81 |
| Cutaneous4-150 | ||
| Erythema/desquamation/papules etc | 43 | 55 |
| Cardiac rhythm | 22 | 22 |
| Neurotoxicity | 11 | 12 |
| Bone pain | 6 | 8 |
| Hypotension4-151 | 13 | 26 |
| Fluid retention‡ | 56 | 71 |
| Chills‡ | 18 | 37 |
| Phlebitis4-150 | 8 | 17 |
| Infection | 77 | 78 |
| . | Control Group . | GM-CSF . |
|---|---|---|
| . | . | Group . |
| Hemorrhage | ||
| Only petechiae | 21 | 26 |
| Other bleedings | 26 | 25 |
| Liver | ||
| Mild-moderate (1.25-5 × normal) | 31 | 37 |
| Severe (≥5 × normal) | 11 | 10 |
| Oral | ||
| Erythema | 17 | 14 |
| Ulcers and other toxicity | 7 | 12 |
| Nausea/vomiting | 30/35 | 32/34 |
| Diarrhea4-150 | ||
| Less than 2 days | 23 | 23 |
| More severe | 18 | 30 |
| Renal4-150 | ||
| Mild-moderate (1.26-5 × normal) | 21 | 31 |
| Severe (≥5 × normal) | 3 | 3 |
| Fever4-150 | ||
| Subfebrile (<38°C) | 12 | 11 |
| ≥38°C | 71 | 81 |
| Cutaneous4-150 | ||
| Erythema/desquamation/papules etc | 43 | 55 |
| Cardiac rhythm | 22 | 22 |
| Neurotoxicity | 11 | 12 |
| Bone pain | 6 | 8 |
| Hypotension4-151 | 13 | 26 |
| Fluid retention‡ | 56 | 71 |
| Chills‡ | 18 | 37 |
| Phlebitis4-150 | 8 | 17 |
| Infection | 77 | 78 |
P < .05.
P < .01.
P < .001.
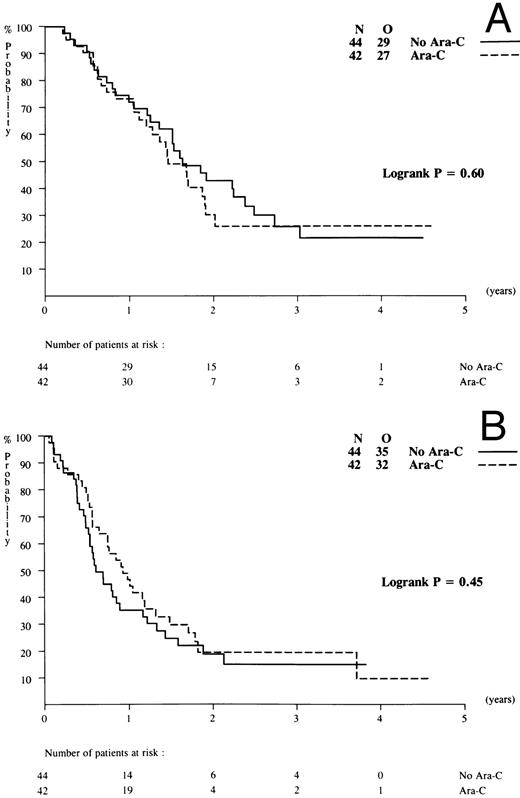
Maintenance treatment. Of 177 complete responders, a total of 88 patients were randomized, 44 patients per arm. The other patients refused to receive further therapy or showed early relapse. Two patients were ineligible, so that 44 patients on arm A (no maintenance) and 42 patients on arm B (low-dose Ara-C) were evaluated with a median follow-up of 2 years. DFS (from the time of second randomization) at 2 years was estimated at 19% (SE = 6.3%) in arm A and 20% (SE = 6.9%) for arm B. There were no differences in the frequencies or types of relapse (bone marrow, central nervous system, or extramedullary) nor in DFS and overall survival between patients on arm A or B maintenance (Fig 6).
Outcome according to maintenance treatment randomized: no cytarabine versus low-dose cytarabine. (A) Overall survival and (B) DFS.
Outcome according to maintenance treatment randomized: no cytarabine versus low-dose cytarabine. (A) Overall survival and (B) DFS.
DISCUSSION
This randomized study considered the role of GM-CSF as an adjunct to chemotherapy in elderly patients with AML. Based on in vitro data, it was hypothesized that GM-CSF might improve the response of AML to cytotoxic therapy by combining chemotherapy with GM-CSF stimulation.13-18 It also was assumed that GM-CSF might possibly reduce the duration of leucopenia after chemotherapy and thereby also reduce the frequencies of bacterial and fungal infections and decrease morbidity or mortality. The results of the study reported here show that treatment with daunomycin-cytosine arabinoside combined with GM-CSF does not result in better response rates or longer survival of patients with AML of 61 years and older. The percentages of patients who attained CR after induction treatment in the GM-CSF and the control treated patients were similar. Thus, apparently the coadministration of GM-CSF with chemotherapy does not increase chemosensitivity to any measurable extent as a consequence of AML cell “sensitization.” The use of GM-CSF postchemotherapy did not prevent the complications during the hypoplastic phase to any significant extent either. As a result, morbidity or mortality were not reduced. Why did the addition of GM-CSF to induction chemotherapy not produce any significant benefit? There may be various reasons to explain the lack of clinical efficacy of GM-CSF in the context of chemotherapy in patients with AML at higher age. The results were analyzed according to intention-to-treat. Many patients, because of possible toxicity related to GM-CSF treatment, discontinued GM-CSF treatment, so that in practice more than 40% of the patients allocated to the GM-CSF arm of the study could not receive the intended dose or schedule of GM-CSF. However, an analysis based on actual application of GM-CSF treatment (instead of intention-to-treat) would not be appropriate. It would on one hand exclude good cases with early PMN recovery (n = 28) but on the other hand exclude poor risk cases with progressive leukemia on GM-CSF treatment (n = 12). To avoid such defavoring and favoring effects, an analysis based on the actual application of GM-CSF treatment might be done excluding early discontinuation because of toxicity only. The latter comparison yielded identical CR rates for control treatment (CR = 55.3%; n = 161 cases) and actual GM-CSF treatment (CR = 55.3%; n = 94 actual GM-CSF–treated cases). Thus, the analysis according to actual treatment, instead of the intention-to-treat approach, did not show any differences either. Hence, it is unlikely that the withdrawals would have compromised any possible advantage of GM-CSF treatment. Why did the use of GM-CSF fail to improve treatment outcome? First, the 2-day reduction of duration of neutropenia may not have been significant enough to modify the death rate, especially because the neutrophil effect comes late when mucosal damage is no longer at its maximum. A second possible explanation for a lack of a measurable benefit of GM-CSF might relate to the fact that in vivo dosaging of GM-CSF for sensitizing the AML cells to the cytotoxic effects of chemotherapy was suboptimal. The toxicity profile of GM-CSF treatment would suggest that higher dosages of GM-CSF cannot realistically be applied to patients of this age category. GM-CSF is assumed to mainly enhance cytotoxicity of the chemotherapeutic agent cytarabine through cell cycle activation. Thirdly, besides the GM-CSF dose, we cannot exclude the possibility that daunomycin, also included in the chemotherapy regimen, counteracted cell cycle activation by GM-CSF and precluded a therapeutic effect of GM-CSF at this point. We did not examine, and, therefore, cannot be certain, that recruitment of the AML blasts to the active phase of the cell cycle after the administration of GM-CSF was truly achieved in vivo. A fourth explanation for the inability to extrapolate the in vitro efficacy of GM-CSF to an in vivo success might relate to the endpoints considered in vitro. The in vitro colony-forming cells might not be representative of the in vivo leukemia repopulating progenitor cells, and, therefore, the in vitro data of chemotherapy priming may not at all predict for better efficacy in vivo.28 A possible stimulation of residual leukemic cells by postchemotherapy as suggested by a recent European Organisation for the Research and Treatment of Cancer (EORTC) randomized trial in younger AML patients,29 counteracting an eventual priming effect of the prechemotherapy administration, is not probable because we did not document in the present study an overt leukemic regrowth stimulated by GM-CSF. Finally, other types of drug resistance (eg, multidrug resistance to daunomycin) rather than resistance to cytarabine might be considered as predominant causes of failure in elderly patients with AML30 so that GM-CSF priming would be less likely to modify chemotherapy responsiveness. Whatever the reason, other strategies would need to be explored to enhance the efficacy of chemotherapy with the aim of increasing the response rate (“percentage remission”) and remission duration (“quality of remission”). Clearly, GM-CSF does not offer a solution to the problem that approximately 40% of patients with AML at a higher age still do not respond to induction chemotherapy.
It is of note that patients with good risk cytogenetic features represent only a minimal fraction of patients with AML at a high age. The results of retrospective studies had previously suggested that AMLs with favorable cytogenetics are comparatively under-represented among patients of over 60 years of age.30 31 The results of the prospective analysis of this study confirm the prognostic impact of the quantitative over-representation of poor-risk types AML in elderly subjects. NN cytogenetics were scored according to a strict criterion of a minimum of 20 evaluated metaphases. As many as 55% of evaluable patients had chromosome abnormalities and only 3% presented with favorable cytogenetics. Thus, it is conceivable that the majority of these patients have an intrinsically unfavorable type of disease, so that any effort at improving outcome of this category of patients is comparatively unlikely to meet success. In fact, both CR rates and survival probabilities in patients with chromosome abnormalities or unfavorable types of cytogenetic abnormalities were significantly less as compared with those in elderly patients who have AML with normal cytogenetics.
The intervals towards neutrophil recovery of 0.5 × 109 and 1.0 × 109/L (not shown) were significantly shorter in the GM-CSF treatment arm. In contrast, times of platelet recovery to 20 × 109/L and 50 × 109/L did not differ significantly between the treatment groups. Although there was an advantage for GM-CSF–treated patients regarding more rapid neutrophil recovery after chemotherapy, this difference did not result in fewer infections, shorter hospitalization, or a reduced morbidity or mortality. Apparently the quantitative benefit with regard to neutrophil recovery comes too late or is too small to be clinically meaningful.
Moderate to severe toxicities were seen more frequently in patients treated with GM-CSF. Adverse events reported were diarrhea, renal abnormalities, fever, cutaneous abnormalities, hypotension, fluid retention, and chills. Patients on GM-CSF experienced more days of fever than did control patients. These toxicities were considerable, and most of these were based on objective measurements. Therefore, although the study was not double blinded, they were most likely to be attributed to the GM-CSF treatment. Early phase I and II studies with GM-CSF and granulocyte colony-stimulating factor (G-CSF ) given after completion of chemotherapy in patients with AML did not provide indications of inhibition of leukemia clearance, stimulation of leukemic outgrowth, or recurrence of leukemia,32-34 although in vitro data had indicated that these cytokines might activate AML cell proliferation in culture.35 This had created caution for negative effects of growth factor treatment. This study and two previous randomized studies in elderly subjects have dealt with the use of GM-CSF after chemotherapy.11,36 One other phase III study in patients of higher age has explored a potential role of G-CSF.12 In our study and three11,12,36 other prospectively controlled studies, an enhanced regeneration of neutrophils was apparent. A reduction of the length of neutropenia by 2 to 7 days was seen in G-CSF– or GM-CSF–treated patients. However, the reduction of the duration of neutropenia was not associated with a decrease of the death rate in any of these studies. In one study12 the complete response rate improved significantly as a result of growth factor treatment after chemotherapy. The latter study differed in that G-CSF was employed. Why the complete response rate was better in only two of the four studies remains unexplained at the present time. In fact, the mortality rates were not different and in only one of the studies a reduction of the percentage of resistant leukemia was reported,12 which would have contributed to a greater response probability in case of G-CSF therapy. It is of note that, in none of the previous studies was the hematopoietic growth factor applied concurrently with the chemotherapy with the objective of chemotherapy priming (as was done in our study), but only after completion of chemotherapy. The days of hospitalization of the patients or the incidence of bacterial or fungal infections were not modified in any of the studies as a result of growth factor therapy. Survival did not improve in patients on either G-CSF or GM-CSF treatment. In only one study36 was GM-CSF therapy associated with a better median survival; but the CR rate did not improve, and placebo-treated controls showed an exceptionally poor survival (ie, median of 4.8 months), which is worse than is usually seen. It may also be noted that the latter study used a glycosylated GM-CSF preparation and included patients between 55 and 70 years and, thus, considered a population of a comparatively lower age.36
Low-dose cytarabine postremission was evaluated by randomization in approximately half of the patients with a complete response but did not result in better overall survival or DFS. Because the second randomization was done in only 88 cases, the statistical power of the latter analysis was limited. In another study in elderly AML patients, the use of a similar schedule of low-dose Ara-C improved the DFS significance.37 However, it should be noted that in that particular study a less intensive dose of cytarabine (100 mg/m2 every day) was employed, which may have conditioned for an additional benefit of low-dose Ara-C as maintenance treatment. Here we employed 200 mg/m2 cytarabine during induction, which might have eliminated any possible added advantage of low-dose cytarabine.
In summary, based on the results of the study reported here, we conclude that daunomycin-cytosine arabinoside in adjunct with GM-CSF coadministered with and after chemotherapy is not an effective treatment strategy compared with daunomycin-cytosine arabinoside alone.
APPENDIX
Participating investigators and institutions: B. Löwenberg (Daniel den Hoed Cancer Center, Rotterdam, The Netherlands); E. Archimbaud (Hôpital Edouard Herriot, Lyon, France); G. Ossenkoppele (Free University Hospital, Amsterdam, The Netherlands); G.E.G. Verhoef and M.A. Boogaerts (University Hospital, Leuven, Belgium); E. Vellenga (University Hospital, Groningen, The Netherlands); P. Wijermans (Leyenburg Hospital, The Hague, The Netherlands); Z. Berneman (University Hospital, Antwerpen, Belgium); A.W. Dekker (University Hospital, Utrecht, The Netherlands); P. Sonneveld (University Hospital, Rotterdam, The Netherlands); P. Stryckmans (Institut Bordet, Brussels, Belgium); P. Muus and Th. de Witte (University Hospital, Nijmegen, The Netherlands); U. Jehn (Klinikum Groszhadern, Ludwig University, Munich, Germany); H. Schouten (University Hospital, Maastricht, The Netherlands); R. Willemze (University Hospital, Leiden, The Netherlands); R. Zittoun (Hôpital Hôtel Dieu, Paris, France); J. van der Lelie (University Hospital, Amsterdam, The Netherlands); D. Selleslag (University Hospital St. Jan, Brugge, Belgium); I. Sousa (Institute of Oncology, Coimbra, Portugal); M. Hayat (Institut Gustave Roussy, Villejuif, France); W. Feremans (University Hospital Erasme, Brussels, Belgium); G. Fillet (CHU, Liège, Belgium); M. Ribeiro (San Joan Hospital, Porto, Portugal); and S. Suciu, G. Solbu, and M. Dardenne (EORTC, Data Center, Brussels, Belgium).
Supported by Grants No. 5U 10 CA 11488-20 through 5U 10 CA 11488-26 from the National Cancer Institute (Bethesda, MD), by Sandoz (Basel, Switzerland), and by Grant No. OG91-077 from the Netherlands Health Council (Amstelveen, The Netherlands).
The content of this report is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the other supporting organizations.
Address reprint requests to B. Löwenberg, MD, PhD, Daniel den Hoed Cancer Center/University Hospital Rotterdam, PO Box 5201, 3008 AE Rotterdam, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal