Abstract
Granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood progenitor cell (PBPC) collections are increasingly emerging as the graft of choice in many centers for autologous transplantation, and with increasing frequency for allogeneic transplantation. However, the role of myeloid cytokines in lymphoid function, lymphoid progenitors, and immune-mediated antitumor responses is not known. We studied PBPC collections from normal donors mobilized with G-CSF (10 μg/kg). CD56+/CD3− natural killer (NK) cells sorted from PBPC products exhibited a diminished ability to kill tumor targets, were less responsive in acquiring increased cytolysis with interleukin-2 (IL-2), and proliferated less than NK cells from normal unprimed peripheral blood. This abnormality was not explained by a change in phenotype of NK cells normally circulating in the blood after G-CSF administration. We could not demonstrate any direct suppressive effect on normal unprimed NK cell proliferation or cytotoxicity by culture with pharmacologic concentrations of G-CSF. We next evaluated the effects of G-CSF on CD34+ NK cell progenitors. CD34+/CD2+, CD34+/CD7+, and CD34+/CD10+ progenitors were markedly diminished in G-CSF–mobilized PBPC products. CD34+ cells cultured in limiting dilution assays showed a sixfold decrease in NK cell progenitors when derived from G-CSF–mobilized CD34+ PBPCs compared with CD34+ cells derived from unprimed marrow. The finding of decreased NK cell function, inhibited proliferation, and diminished cloning frequency after treatment with G-CSF could be mimicked in vitro by culture of primitive marrow progenitors (CD34+, lineage-negative, HLA-DR−) on stromal layers in the presence of exogenous G-CSF. The findings presented here show that G-CSF administration to normal donors decreases NK cell function and the relative frequency of NK cell progenitors within the CD34+ progenitor population. Overcoming this diminished lymphoid capacity may be important to facilitate early posttransplant immunotherapy. Our in vitro model will be used in future studies to determine the mechanism of the G-CSF–induced suppression of NK cell progenitors, which may occur early in the differentiation process.
PRIMITIVE PROGENITORS have been known to circulate in the peripheral blood of animals and man.1-3 Commercially available cytokines (granulocyte colony-stimulating factor [G-CSF] and granulocyte-macrophage CSF [GM-CSF]) have dramatically changed the clinical practice of marrow transplantation where the use of peripheral blood progenitor cell (PBPC) grafts are routine for autologous transplantation. More recently, increased use of PBPCs as a graft source for allogeneic transplantation is being reported. Accelerated neutrophil engraftment after transplantation and the ease of donor procurement are among the major known advantages of this progenitor source for transplantation. However, there are many questions that remain to be answered as to whether growth factor–mobilized PBPC grafts may function differently from unprimed marrow.
There is growing interest in the lymphocyte and lymphoid progenitor capacity of different stem cell sources. Recent studies suggest that the graft-versus-host disease (GVHD) incidence is similar for allogeneic mobilized PBPC transplants compared with standard unprimed marrow.4,5 This was unexpected since there are about 10-fold more lymphocytes infused with mobilized PBPC grafts, suggesting that GVHD reactivity is not dose-dependent after a threshold number of lymphocytes are infused or, alternatively, that the lymphocytes in growth factor–mobilized PBPC grafts function differently. The other potential importance of immune function in the graft is its ability to support a graft-versus-leukemia (GVL) effect. Depletion of lymphocytes alters GVL reactivity,6 and although the mechanisms are not well understood, cytotoxic T cells,7 natural killer (NK) cells, 8 or LAK cells may be involved.9 The lymphoid capacity of the graft may also be important in the autologous transplant setting for its ability to support early posttransplant immunotherapy.10-13
In the laboratory, primitive progenitors from unprimed normal marrow can give rise to phenotypic and functional NK cells when cultured in the presence of interleukin-2 (IL-2) and in direct contact with primary allogeneic stromal layers.14,15 A functional limiting dilution assay can be used to quantify the number of clonogenic progenitors within these populations. We have found that several phenotypically distinct progenitors can give rise to NK cells. For example, CD34+/CD7+bright cells give rise to NK cells with a cloning frequency of up to 17%, suggesting that these cells may represent an intermediately differentiated but already lymphoid committed progenitor population. CD34+/CD10+ and CD34+/CD2+ cells have also been described as lymphoid committed progenitors that can give rise to NK cells.16 17 In this study, we evaluate PBPC products for the ability to support NK cell functions and for the NK cell progenitor differentiating capacity.
MATERIALS AND METHODS
Mobilized PBPCs.Normal healthy donors were selected using the standard criteria of the American Association of Blood Banks for blood donors.18 All samples were obtained using guidelines approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota. PBPC donors received a daily dose of 10 μg/kg/d human recombinant G-CSF (Neupogen; Amgen Inc, Thousand Oaks, CA) subcutaneously for 5 days. G-CSF was given as a single morning dose. PBPC collections were performed between 2 and 24 hours after the last dose of G-CSF with a Fenwal CS-3000 PLUS (Baxter Healthcare Corp, Round Lake, IL) as previously described.19
Normal unprimed blood or bone marrow.Peripheral blood or bone marrow was obtained from normal donors after provision of informed consent using guidelines approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota. Bone marrow mononuclear cells (BMMNCs) or peripheral blood mononuclear cells (PBMNCs) were obtained by Ficoll-Hypaque (specific gravity, 1.077; Sigma Diagnostics, St Louis, MO) density gradient centrifugation.
Purification of NK cells.NK cells were isolated from PBMNCs or PBPCs using the MACS selection system as described by the manufacturer (Miltenyi Biotec, Auburn, CA). The depleted PBMNCs from normal donors were labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 or anti-CD5 monoclonal antibody (MoAb) and phycoerythrin (PE)-conjugated anti-CD56 MoAb (250 ng/106 cells; Becton Dickinson, Bedford, MA) and sorted on a FACS Star-Plus flow cytometer (Becton Dickinson) equipped with a Consort 32 computer (Hewlitt Packard 340; Hewlitt Packard, Palo Alto, CA) into a CD56+/CD3− or CD56+/CD5− population. CD56+bright and CD56+dim subpopulations were determined using established criteria for CD56+bright cells.20-22
Purification of primitive progenitors.BMMNCs or PBPCs were enriched for CD34+ cells using an avidin-biotin column as recommended by the manufacturer (Cellpro, Bothel, WA). Resultant cells were stained with PE-conjugated anti-CD34 antibody (HPCA-2; Becton Dickinson) for isolation of CD34+ cells or CD34-biotin (Cellpro) for multicolor sorting as previously described.15 FITC-conjugated antibodies against CD2, CD3, CD4, CD5, CD7, CD8, CD10, and CD19 were used for the lineage (Lin) cocktail (Becton Dickinson), PE-conjugated anti-HLA-DR (DR) was used, and streptavidin SA670 (GIBCO BRL, Grand Island, NY) was used as the third fluorescent color.
NK cell cultures.PBMNCs or sorted NK cells were placed in a 2:1 (vol/vol) Dulbecco's modified Eagle's medium (DMEM)/Ham's F12–based NK cell medium supplemented with 10% heat-inactivated human AB serum (North American Biologicals, Miami, FL) as previously described.22 Fresh unstimulated or overnight (14 to 18 hours) IL-2–activated cells (1,000 U/mL recombinant IL-2; Amgen, Thousand Oaks, CA) were incubated in 5% CO2 at 37°C before testing for cytotoxicity.23 Long-term IL-2 culture of NK cells (4 weeks) was performed by plating purified NK cells (1 × 105) in 1 mL medium in 24-well plates either in tissue culture wells alone or in direct contact with irradiated M2-10B4 (a murine fibroblast stromal cell line provided by Dr Connie Eaves, PhD, Vancouver, Canada) as previously described.24 At day 7, culture volumes were doubled to 2.0 mL, and then half-medium changes were performed every 5 to 7 days. Short-term (2 to 4 days) thymidine incorporation assays24 were used to test effects of G-CSF on sorted NK cells alone or in contact with CD14+ monocytes isolated by MACS selection (Miltenyi Biotec). Ten thousand CD56+/CD3− cells were plated in 1,000 U/mL IL-2 (with or without G-CSF as indicated) for 48 or 96 hours and then pulsed with thymidine for 16 hours to determine proliferation.
Culture of NK cell progenitors.CD34+ NK cell progenitors were plated on pre-established allogeneic irradiated (2,500 cGy) stroma as previously described.14,15 Ten thousand CD34+/Lin−/DR− cells from unprimed marrow or G-CSF–mobilized PBPCs were cultured in NK cell media with 1,000 U/mL IL-2 for 5 weeks. CD34+ progenitors were plated in limiting dilution assay (LDA) in 96-well plates (22 replicates at four dilutions: 1,200 to 2,400, 400 to 800, 133 to 266, and 45 to 90 cells per well) with established marrow stroma for 5 weeks. Wells were determined as positive by addition of 5,00051Cr-labeled K562 cells (American Type Culture Collection [ATCC], Rockville, MD) resulting in a specific lysis greater than 3 standard deviations above spontaneous lysis. The frequency of NK cell progenitors in each population was calculated as the reciprocal of the concentration of cells that resulted in 37% negative wells using Poisson statistics25 and the weighted-mean method.26 G-CSF was added to some cultures only once at the time of initial plating or at each weekly half-media change as indicated.
Maintenance of long-term bone marrow cultures.Cultures were maintained in a humidified atmosphere at 37°C and 5% CO2 . At day 7, the culture volume was doubled with fresh NK cell media. Subsequently, at weekly intervals, the cultures were fed by carefully removing half of the supernatant to avoid removing nonadherent cells and replacing it with fresh NK cell media. For cells cultured in a Transwell insert, 50% of the media from the lower well was changed weekly. At week 5, the wells were washed thoroughly to harvest the cells, and the cells were analyzed for expansion, cytotoxicity, and phenotypic characteristics. All cell populations were counted in a hemocytometer to determine fold expansion: fold expansion is the number of cells present at week 5 divided by the number of sorted cells inoculated on day 0.
Phenotype and cytotoxicity.Cell surface antigens were determined by direct staining of cells with mouse MoAbs. FITC- or PE-coupled antibodies (Becton Dickinson) were directed at CD2, CD3, CD7, CD8, CD16, and CD56. FITC- and PE-coupled isotype-matched Igs were used as controls. All analyses were performed with the FACS Star Plus flow cytometer equipped with the Consort 32 computer. Cytotoxicity assays were performed in triplicate using K562 (ATCC) and Raji (ATCC) cell lines in a 4-hour 51Cr-release assay. To control for target variability, frozen targets (expanded from the same batch) were thawed every 4 weeks, and in addition, some experiments were performed against the same batch of targets.
Statistics.Results for experimental points obtained from multiple experiments are reported as the mean ± 1 SEM. Significance levels were determined by two-sided Student t test analysis.
RESULTS
Effects of in vivo G-CSF on mature NK cells.PBPC collections were obtained from normal donors after 5 days of mobilization with G-CSF (10 μg/kg). The peripheral white blood cell count, CD34 content, and myeloid progenitor content of the PBPC collections have been reported elsewhere.19 Lymphocyte function was first assessed by testing unstimulated cytotoxicity against K562 targets, and was diminished in G-CSF PBPC products compared with unprimed PBMNCs. IL-2 stimulation (1,000 U/mL for 16 hours) increased PBMNC killing of Raji targets, but had little effect on G-CSF PBPCs. As expected, there was a twofold to fourfold decrease in the percent of lymphoid cells in the G-CSF –mobilized PBPC product compared with unprimed PBMNCs, which can be explained by expansion of the myeloid pool.
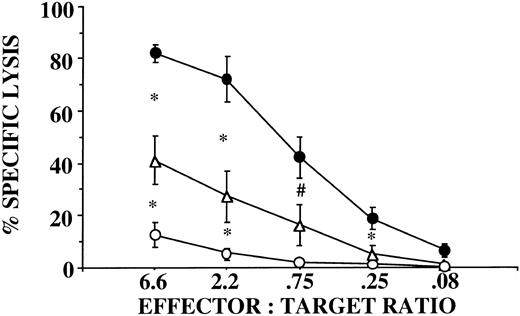
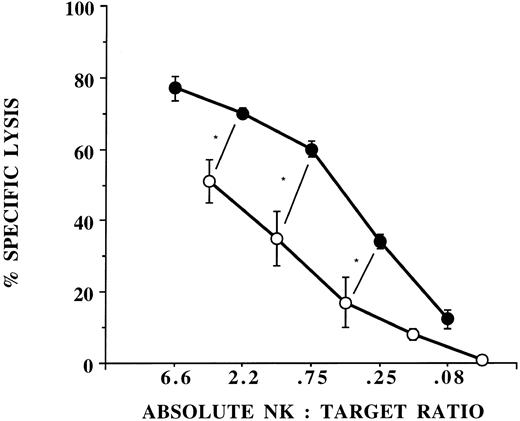
Several possible mechanisms may account for the decreased killing by G-CSF–mobilized PBPCs: (1) a dilution of effectors by myeloid cells; (2) a decreased function due to contact with CD14+ monocyte suppressor cells27; and (3) a consequence of the lymphapheresis procedure itself (collection, anticoagulation, etc.). The first two possibilities were evaluated by purification of CD56+/CD3− NK cells from PBPCs using flow cytometry. Sorted NK cells from PBPC collections exhibited significantly less lytic function against K562 compared with NK cells purified from unprimed PBMNCs, suggesting a functional abnormality of NK cells induced by G-CSF mobilization that persists in the absence of interactions with other cell types (Fig 1). To exclude the possibility that the decreased NK cell function was explained by the lymphapheresis procedure, we isolated NK cells directly from the peripheral blood of donors after 5 days of G-CSF that still exhibited significantly less killing of K562 targets compared with NK cells purified from unprimed blood (Fig 1). The finding that NK cells from the G-CSF product exhibited diminished killing compared with NK cells from G-CSF blood suggests that the lymphapheresis process itself contributes to a certain extent but does not entirely account for the decreased NK function induced by G-CSF.
CD56+/CD3− NK cells function abnormally in donors receiving G-CSF. NK cells were selected by flow cytometry from unprimed blood (•, n = 4), from G-CSF–mobilized PBPC products (○, n = 6), and from peripheral blood of donors receiving G-CSF (▵, n = 5). There was a statistically significant difference in unstimulated cytotoxicity against K562 targets between NK cells derived from unprimed blood and NK cells derived from G-CSF–mobilized blood (*P ≤ .05 and ;dyP = .07) or from the PBPC product collected by lymphapheresis (P ≤ .001 at all E:T ratios). There was also a statistically significant difference at the 2 highest E:T ratios (*P ≤ .05) between NK cells derived from G-CSF–mobilized blood and G-CSF PBPC products.
CD56+/CD3− NK cells function abnormally in donors receiving G-CSF. NK cells were selected by flow cytometry from unprimed blood (•, n = 4), from G-CSF–mobilized PBPC products (○, n = 6), and from peripheral blood of donors receiving G-CSF (▵, n = 5). There was a statistically significant difference in unstimulated cytotoxicity against K562 targets between NK cells derived from unprimed blood and NK cells derived from G-CSF–mobilized blood (*P ≤ .05 and ;dyP = .07) or from the PBPC product collected by lymphapheresis (P ≤ .001 at all E:T ratios). There was also a statistically significant difference at the 2 highest E:T ratios (*P ≤ .05) between NK cells derived from G-CSF–mobilized blood and G-CSF PBPC products.
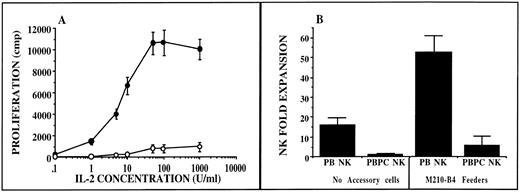
CD56+/CD3− NK cells exhibit diminished IL-2–induced proliferation in donors receiving G-CSF. Sorted NK cells derived from unprimed blood (•, n = 6) and from G-CSF–mobilized PBPC products (○, n = 5) were incubated with IL-2 and tested for short-term (day 4) proliferation as measured by thymidine incorporation (A). There was a statistically significant decrease in proliferation from NK cells derived from PBPCs v NK cells derived from unprimed blood. NK cells were then plated in IL-2–containing media alone (no accessory cells) or in direct contact with M210-B4 feeders (B). Long-term proliferation was assessed by actual cell counts after 28 days in culture. NK cell fold expansion was determined by the number of harvested cells after culture divided by the number of CD56+/CD3− NK cells initially inoculated into culture. NK cells derived from G-CSF–mobilized PBPC collections (PBPC NK, n = 6) gave rise to significantly fewer NK cells (P ≤ .001) than when cultures were initiated with NK cells from unprimed blood (PB NK, n = 10) irrespective of whether accessory cells were used in culture.
CD56+/CD3− NK cells exhibit diminished IL-2–induced proliferation in donors receiving G-CSF. Sorted NK cells derived from unprimed blood (•, n = 6) and from G-CSF–mobilized PBPC products (○, n = 5) were incubated with IL-2 and tested for short-term (day 4) proliferation as measured by thymidine incorporation (A). There was a statistically significant decrease in proliferation from NK cells derived from PBPCs v NK cells derived from unprimed blood. NK cells were then plated in IL-2–containing media alone (no accessory cells) or in direct contact with M210-B4 feeders (B). Long-term proliferation was assessed by actual cell counts after 28 days in culture. NK cell fold expansion was determined by the number of harvested cells after culture divided by the number of CD56+/CD3− NK cells initially inoculated into culture. NK cells derived from G-CSF–mobilized PBPC collections (PBPC NK, n = 6) gave rise to significantly fewer NK cells (P ≤ .001) than when cultures were initiated with NK cells from unprimed blood (PB NK, n = 10) irrespective of whether accessory cells were used in culture.
We next assessed whether the abnormal function of NK cells from G-CSF–mobilized PBPC collections was specific to cytotoxicity alone. Normal CD56+/CD3− NK cells from unprimed PBMNCs exhibit an IL-2 dose–dependent proliferation, as measured in a 4-day thymidine incorporation assay. In contrast, NK cells purified from G-CSF–mobilized PBPCs did not proliferate in response to a broad range of IL-2 concentrations (Fig 2A). We next evaluated whether NK cells from G-CSF–mobilized PBPC collections could proliferate and acquire lytic activity after longer exposures of IL-2. NK cells interact with multiple accessory cell types in addition to IL-2 for maximal proliferation. A murine stromal cell line (M210-B4) can provide both a soluble and contact-mediated factor that maximally stimulates NK cell proliferation.24 Therefore, NK cells from G-CSF–mobilized PBPC collections were plated with or without M210-B4 accessory cells for 4 weeks and assessed for proliferation. Culture of NK cells from G-CSF–mobilized PBPC collections resulted in significantly less 4-week proliferation compared with normal unprimed blood NK cells under both conditions (Fig 2B). However, in contrast to short-term IL-2 culture, NK cells from G-CSF collections that proliferated after 4 weeks acquired normal cytolytic function against K562 targets (n = 6, data not shown).
The phenotype of NK cells was evaluated to determine if an NK cell subset was altered by G-CSF administration or the lymphapheresis procedure. There was no difference in the percent of CD56+ NK cells expressing CD2, CD7, CD8, or CD16 antigens compared with NK cells from unprimed blood (Table 1). The proportion of CD56+bright NK cells in G-CSF–mobilized PBPC collections was similar to that observed from normal donors. However, the only phenotypic abnormality that could be identified in G-CSF–mobilized PBPC collections is that both CD56+bright and CD56+dim NK cells showed a significantly lower relative mean channel fluorescence compared with normal NK cells, suggesting that CD56 surface density may be lower on NK cells after G-CSF administration (Table 1).
Phenotype of NK Cells From G-CSF–Mobilized PBPCs Versus Unprimed PBMNCs
| Phenotype . | G-CSF PBPC NK Cells . | Unprimed PBMNC NK Cells . | P . |
|---|---|---|---|
| CD56+/CD2+ | 72 ± 4.6 (7) | 58 ± 5.8 (6) | NS |
| CD56+/CD7+ | 94 ± 1.9 (7) | 98 ± 0.9 (6) | NS |
| CD56+/CD8+ | 32 ± 6.2 (7) | 32 ± 6.3 (6) | NS |
| CD56+/CD16+ | 84 ± 2.5 (7) | 86 ± 4.1 (6) | NS |
| CD56+ bright | 6.3 ± 1.44 (9) | 5.7 ± 0.8 (20) | NS |
| CD56+ bright MCF | 146 ± 16 (9) | 234 ± 15.5 (20) | .002 |
| All CD56+ MCF | 44 ± 5.0 (9) | 63 ± 4.6 (20) | .018 |
| Phenotype . | G-CSF PBPC NK Cells . | Unprimed PBMNC NK Cells . | P . |
|---|---|---|---|
| CD56+/CD2+ | 72 ± 4.6 (7) | 58 ± 5.8 (6) | NS |
| CD56+/CD7+ | 94 ± 1.9 (7) | 98 ± 0.9 (6) | NS |
| CD56+/CD8+ | 32 ± 6.2 (7) | 32 ± 6.3 (6) | NS |
| CD56+/CD16+ | 84 ± 2.5 (7) | 86 ± 4.1 (6) | NS |
| CD56+ bright | 6.3 ± 1.44 (9) | 5.7 ± 0.8 (20) | NS |
| CD56+ bright MCF | 146 ± 16 (9) | 234 ± 15.5 (20) | .002 |
| All CD56+ MCF | 44 ± 5.0 (9) | 63 ± 4.6 (20) | .018 |
Data are presented as the percent of NK cells positive for each phenotype. The number in parentheses is the number of experiments.
Abbreviations: MCF, mean channel fluorescence; NS, not significant.
The mechanism of G-CSF–induced NK cell dysfunction may be direct on NK cells themselves or indirect by interaction with other cell types. To distinguish these possibilities, experiments were designed to compare IL-2–induced NK cell proliferation from normal unprimed blood with or without addition of exogenous G-CSF. There was no difference in early NK cell proliferation (day 4 thymidine incorporation) in the presence of 5 ng/mL G-CSF on sorted CD56+/CD3− NK cells plated alone (108% ± 15% of control without G-CSF, n = 6) or in direct contact with CD14+ monocytes (107% ± 9% of control without G-CSF, n = 6). Similarly, there was no difference in late proliferation (day 28 cell counts) in the presence of G-CSF when sorted CD56+/CD3− NK cells were plated alone (91% ± 17% of control without G-CSF, n = 4) or in the presence of PBMNCs separated from NK cells by a Transwell membrane (97% ± 19% of control without G-CSF, n = 4) as previously described.23 Since endogenous peak serum concentrations of G-CSF can be in the range of 26 to 85 ng/mL after G-CSF injections of 10 μg/kg,28 we further explored the direct effects of G-CSF on NK cells by evaluating proliferation and cytotoxicity using a G-CSF concentration up to 100 ng/mL. Although there is a small effect (P = .075) on proliferation at the highest G-CSF concentration, there is no effect on resting or IL-2–stimulated cytotoxicity over a wide range of effector to target (E:T) ratios (Table 2; cytotoxicity data only shown at E:T ratio 2.2). These data suggest that the marked decrease in NK cell proliferation and cytotoxicity induced by in vivo G-CSF is not mediated by a direct effect of G-CSF on NK cells themselves.
Direct Effect of G-CSF on NK Proliferation and Cytotoxicity
| G-CSF Concentration (ng/mL) . | Proliferation . | No IL-2 Resting Cytotoxicity . | IL-2–Stimulated Cytotoxicity . |
|---|---|---|---|
| . | (% of control)* . | (% specific lysis)† . | (% specific lysis)† . |
| 0 | 100 | 54 ± 2.4 | 60 ± 2.7 |
| 5 | 105 ± 2.9 | ND | ND |
| 10 | 96 ± 6.3 | 54 ± 1.1 | 75 ± 4.6 |
| 50 | 93 ± 3.2 | 56 ± 2.0 | 71 ± 2.1 |
| 100 | 79 ± 6.0‡ | 54 ± 2.5 | 70 ± 1.2 |
| G-CSF Concentration (ng/mL) . | Proliferation . | No IL-2 Resting Cytotoxicity . | IL-2–Stimulated Cytotoxicity . |
|---|---|---|---|
| . | (% of control)* . | (% specific lysis)† . | (% specific lysis)† . |
| 0 | 100 | 54 ± 2.4 | 60 ± 2.7 |
| 5 | 105 ± 2.9 | ND | ND |
| 10 | 96 ± 6.3 | 54 ± 1.1 | 75 ± 4.6 |
| 50 | 93 ± 3.2 | 56 ± 2.0 | 71 ± 2.1 |
| 100 | 79 ± 6.0‡ | 54 ± 2.5 | 70 ± 1.2 |
n = 3 donors in triplicate for all determinations.
Abbreviation: ND, not determined.
Determined after 48 hours of stimulation before thymidine pulse (G-CSF added at time 0).
Cytotoxicity presented at E:T ratio 2.2:1 against K562 after a 16-hour incubation before testing.
P = .075.
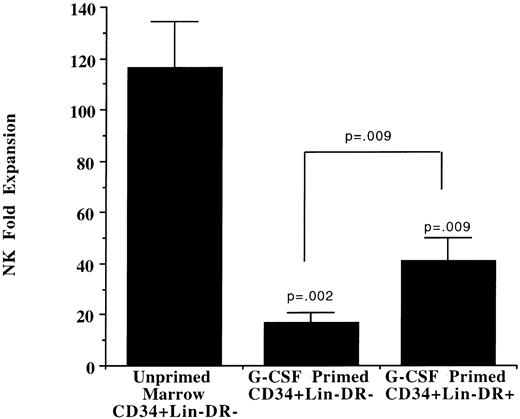
Effects of in vivo G-CSF on NK cell progenitors.Human CD34+/Lin−/DR− primitive progenitors can give rise to NK cells after culture with IL-2 in a stromal-based long-term culture system.15 Primitive progenitors from G-CSF–mobilized PBPC collections were tested for the ability to differentiate into functional NK cells. In addition to CD34+/Lin−/DR− cells, CD34+/Lin−/DR+ cells were evaluated based on the observation that the primitive progenitor phenotype may change with G-CSF administration.19 Both populations derived from G-CSF–mobilized blood gave rise to significantly fewer NK cells compared with CD34+/Lin−/DR− cells obtained from unprimed marrow (Fig 3). The NK cell progeny from these progenitors showed phenotypic characteristics similar to those described for marrow progenitors (data not shown).14 NK cell progeny from G-CSF–mobilized CD34+/Lin−/DR− or CD34+/Lin−/DR+ cells exhibited similar cytotoxicity compared with NK cells derived from unprimed marrow progenitors after the 5-week culture (data not shown).
Primitive progenitors from G-CSF–mobilized PBPC collections give rise to fewer NK cell progeny. Ten thousand primitive progenitors from unprimed marrow (CD34+/Lin−/DR−) or from G-CSF–mobilized PBPC products (CD34+/Lin−/DR− and CD34+/Lin−/DR+) were plated on pre-established allogeneic irradiated stroma for long-term NK cell culture. NK cell fold expansion was reported as the number of total cells harvested after 5 weeks. All cultured populations contained < 90% CD56+/CD3− NK cells. CD34+/Lin−/DR− cells from unprimed marrow gave rise to more NK cells than either population selected from G-CSF–mobilized PBPCs.
Primitive progenitors from G-CSF–mobilized PBPC collections give rise to fewer NK cell progeny. Ten thousand primitive progenitors from unprimed marrow (CD34+/Lin−/DR−) or from G-CSF–mobilized PBPC products (CD34+/Lin−/DR− and CD34+/Lin−/DR+) were plated on pre-established allogeneic irradiated stroma for long-term NK cell culture. NK cell fold expansion was reported as the number of total cells harvested after 5 weeks. All cultured populations contained < 90% CD56+/CD3− NK cells. CD34+/Lin−/DR− cells from unprimed marrow gave rise to more NK cells than either population selected from G-CSF–mobilized PBPCs.
Lymphoid committed progenitors are routinely found in unprimed marrow from normal donors, where 9.4% ± 1.3% of CD34+ cells stain positive with a cocktail of lymphoid antibodies (CD2, CD3, CD4, CD5, CD7, CD8, CD10, and CD19; n = 15). Since populations enriched for NK cell progenitors can be found among CD34+ cells already showing signs of lymphoid antigen expression by the presence of CD7, CD2, and CD10,15-17 we focused on these antigens in G-CSF–mobilized blood. In contrast to unprimed marrow, CD34+/CD7+, CD34+/CD2+, and CD34+/CD10+ cells are significantly depleted from G-CSF–mobilized PBPC collections (Table 3). NK cell progenitor frequency was then measured by sorting CD34+ cells irrespective of other antigens and culturing the cells in a functional LDA. G-CSF–mobilized CD34+ cells from blood resulted in an approximately sixfold lower frequency of clonogenic NK cell progenitors versus unprimed marrow CD34+ cells (0.07% ± 0.02% v 0.41% ± 0.06%, n = 5, P = .001), establishing a correlation between progenitor phenotype and the ability to give rise to clonogenic NK cell progeny.
G-CSF–Mobilized PBPCs Contain Fewer Committed Lymphoid Progenitors Than Bone Marrow
| Source of CD34+ Cells . | % CD34+ Expressing CD7+ . | % CD34+ Expressing CD2+ . | % CD34+ Expressing CD10+ . |
|---|---|---|---|
| Unprimed bone marrow | 3.0 ± 0.3 (22) | 4.8 ± 0.4 (22) | 6.4 ± 1.2 (5) |
| G-CSF–mobilized PBPCs | 0.41 ± 0.11 (8) | 0.09 ± 0.03 (8) | 1.2 ± 0.3 (6) |
| Comparison (BM v PBPCs) | P ≤ .001 | P ≤ .001 | P = .001 |
| Source of CD34+ Cells . | % CD34+ Expressing CD7+ . | % CD34+ Expressing CD2+ . | % CD34+ Expressing CD10+ . |
|---|---|---|---|
| Unprimed bone marrow | 3.0 ± 0.3 (22) | 4.8 ± 0.4 (22) | 6.4 ± 1.2 (5) |
| G-CSF–mobilized PBPCs | 0.41 ± 0.11 (8) | 0.09 ± 0.03 (8) | 1.2 ± 0.3 (6) |
| Comparison (BM v PBPCs) | P ≤ .001 | P ≤ .001 | P = .001 |
CD34+ cells were enriched from each cell source by an avidin-biotin column before phenotyping. The number in parentheses is the number of experiments.
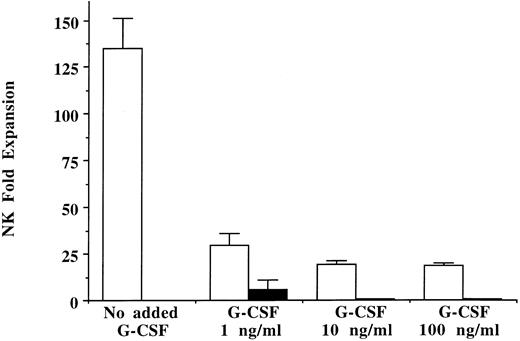
Effects of in vitro G-CSF on NK cell progenitors.Since exogenous G-CSF had no direct effect on mature NK cell proliferation, progenitors earlier in NK cell development were evaluated. The long-term NK cell culture assay, which may more fully represent the spectrum of interactions encountered in vivo, was used to test the effect of G-CSF on NK cell development. Unprimed marrow CD34+/Lin−/DR− cells were plated on irradiated allogeneic stroma with and without the addition of G-CSF (1, 10, and 100 ng/mL) only once at the time of initial plating or weekly with each half-media change (n = 3). NK cells expanded 135-fold ± 16-fold from the starting number of CD34+/Lin−/DR− cells in long-term culture in the absence of G-CSF. In contrast, adding G-CSF only once at the time of initial plating resulted in significant suppression of CD56+/CD3− NK cell progeny at all G-CSF concentrations tested (Fig 4). Weekly addition of G-CSF at the two higher concentrations (10 and 100 ng/mL) almost completely inhibited NK cell development from marrow CD34+/Lin−/DR− cells. Similarly, addition of G-CSF to long-term NK cell cultures inoculated with CD34+ cells derived from G-CSF–mobilized PBPC collections resulted in significantly less NK cell generation than in controls cultured without exogenous G-CSF (n = 4, data not shown).
In vitro addition of G-CSF to long-term NK cell cultures leads to decreased NK cell proliferation. Ten thousand CD34+/Lin−/DR− cells from unprimed marrow were inoculated into long-term NK cell culture with G-CSF (1, 10, or 100 ng/mL) added only once at culture initiation (□) or added with each weekly half-media change (▪). A large number of NK cell progeny (NK cell fold expansion determined by actual CD56+/CD3− NK cells emerging from culture) were derived from progenitors cultured in the absence of G-CSF after the 5-week culture. In contrast, exogenous addition of G-CSF significantly decreased NK cell expansion under all G-CSF conditions and concentrations (n = 3).
In vitro addition of G-CSF to long-term NK cell cultures leads to decreased NK cell proliferation. Ten thousand CD34+/Lin−/DR− cells from unprimed marrow were inoculated into long-term NK cell culture with G-CSF (1, 10, or 100 ng/mL) added only once at culture initiation (□) or added with each weekly half-media change (▪). A large number of NK cell progeny (NK cell fold expansion determined by actual CD56+/CD3− NK cells emerging from culture) were derived from progenitors cultured in the absence of G-CSF after the 5-week culture. In contrast, exogenous addition of G-CSF significantly decreased NK cell expansion under all G-CSF conditions and concentrations (n = 3).
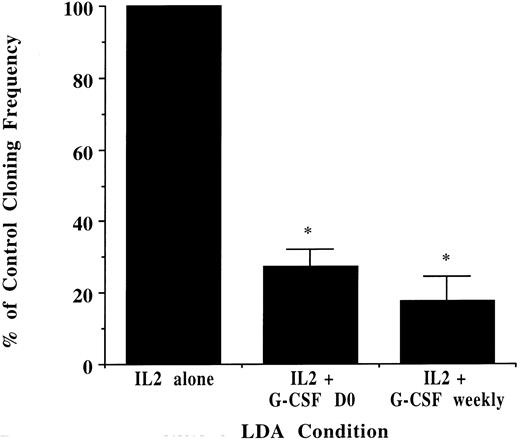
NK cells generated from unprimed marrow CD34+/Lin−/DR− cells cultured with and without G-CSF in long-term NK cell cultures were then tested for lytic function. NK cells from cultures grown without G-CSF were 95% ± 1.0% CD56+/CD3− and developed potent lytic activity against K562 targets. In contrast, NK cells from cultures with as little as 1 ng/mL G-CSF were 64% ± 3.5% CD56+/CD3− and exhibited significantly decreased killing activity even after correction for the one third fewer phenotypic NK cells (Fig 5). Coexpression of CD2, CD7, CD8, and CD16 on NK cells derived from long-term culture was not significantly different in the presence or absence of G-CSF (data not shown). Lastly, CD34+/Lin−/DR− progenitors from unprimed marrow were plated in limiting dilution with media containing IL-2 alone, IL-2 with 10 ng/mL G-CSF added on day 0 only, and IL-2 and G-CSF added weekly at each half-media change. Addition of G-CSF significantly decreased the growth of functional clonogenic progenitors as compared with assays plated in the absence of G-CSF (Fig 6).
NK cell progeny of primitive progenitors cultured with G-CSF function abnormally. CD34+/Lin−/DR− cells from unprimed marrow were cultured in long-term NK cell culture with (○) or without (•) 1 ng/mL G-CSF added only once at culture initiation. Week 5 progeny of these cultures were tested in cytotoxicity assays against K562 targets (n = 3). The E:T ratio was offset to represent the absolute NK cell number present in each population (95% NK cells without G-CSF v 64% with G-CSF), and statistics are reported using the higher absolute NK cell E:T ratio for G-CSF–derived progeny compared with the lower E:T ratio for control NK cell progeny grown without G-CSF. Differentiated NK cells grown in the absence of G-CSF exhibited potent lytic function against K562 targets, which was significantly diminished when G-CSF was added at culture initiation (*P ≤ .02).
NK cell progeny of primitive progenitors cultured with G-CSF function abnormally. CD34+/Lin−/DR− cells from unprimed marrow were cultured in long-term NK cell culture with (○) or without (•) 1 ng/mL G-CSF added only once at culture initiation. Week 5 progeny of these cultures were tested in cytotoxicity assays against K562 targets (n = 3). The E:T ratio was offset to represent the absolute NK cell number present in each population (95% NK cells without G-CSF v 64% with G-CSF), and statistics are reported using the higher absolute NK cell E:T ratio for G-CSF–derived progeny compared with the lower E:T ratio for control NK cell progeny grown without G-CSF. Differentiated NK cells grown in the absence of G-CSF exhibited potent lytic function against K562 targets, which was significantly diminished when G-CSF was added at culture initiation (*P ≤ .02).
NK cell progenitor cloning frequency decreases with exogenously added G-CSF. CD34+/Lin−/DR− cells from unprimed marrow were plated in 96-well plates in limiting dilution. The absolute cloning frequency of primitive progenitors plated without G-CSF was 0.22% ± .07%. With progenitors derived from the same donor, CD34+/Lin−/DR− cells were analyzed in LDA with G-CSF (10 ng/mL) added only once at culture initiation or at each weekly half-media change. G-CSF significantly decreased the cloning frequency of the starting population compared with that determined in the absence of G-CSF (n = 3 for all conditions, *P ≤ .001).
NK cell progenitor cloning frequency decreases with exogenously added G-CSF. CD34+/Lin−/DR− cells from unprimed marrow were plated in 96-well plates in limiting dilution. The absolute cloning frequency of primitive progenitors plated without G-CSF was 0.22% ± .07%. With progenitors derived from the same donor, CD34+/Lin−/DR− cells were analyzed in LDA with G-CSF (10 ng/mL) added only once at culture initiation or at each weekly half-media change. G-CSF significantly decreased the cloning frequency of the starting population compared with that determined in the absence of G-CSF (n = 3 for all conditions, *P ≤ .001).
DISCUSSION
The ability of G-CSF to mobilize progenitors has been demonstrated and is well established in clinical transplantation.19,29 Although much is known about myeloid function between stem cell sources, the lymphoid capacity between stem cell sources and its physiologic relevance is less well explored. In this study, we compared NK cells and NK cell progenitors from the two most commonly used stem cell sources: G-CSF–mobilized PBPC collections and unprimed marrow. CD56+/CD3− NK cells from G-CSF–mobilized products exhibit diminished cytotoxicity and proliferation. The finding that NK cells isolated from the blood of patients receiving G-CSF instead of from the PBPC product itself also exhibit diminished NK cell function suggests that the inhibition is not due to the collection procedure alone. However, the cytotoxicity of NK cells from PBPC products was slightly less than for NK cells isolated from fresh blood in donors receiving G-CSF, which may indicate that a component of the lymphapheresis itself contributes, at least in part, to the loss of NK cell activity. This is in agreement with a recent report in which the PHA response of lymphocytes from GM-CSF–mobilized PBPC products was less than the response found in ficolled blood before the collection procedure.30
The mechanism of the observed NK cell suppression by G-CSF is not clear. Involvement of monocyte suppressor cells, which have been shown to mediate T-cell suppression in GM-CSF–mobilized products,27 was not found in our study, since experiments were performed without interaction with other cell types. We could not demonstrate a direct effect of pharmacologic concentrations of G-CSF on sorted NK cells. These studies suggest that NK cells themselves are not directly inhibited by G-CSF and that other cell types or a different activation than that explored in these in vitro experiments occurs in vivo. Alternatively, suppressor cell interactions may already be mediated in vivo, and the suppressive effect is not reversible in the 24 to 48 hours in which the cells were isolated and assays performed.
Mobilization of CD34+ progenitors by G-CSF had a significant impact on the number of phenotypic and functional (determined by LDA) lymphoid committed progenitors as compared with unprimed marrow. In these studies, we cannot exclude the possibility that progenitor capacity is determined by location (blood v marrow) rather than independently by G-CSF. However, culture of marrow CD34+/Lin−/DR− progenitors with G-CSF on allogeneic irradiated stroma decreased the NK cell outgrowth, decreased the cytotoxicity of NK cell progeny, and decreased the cloning frequency of starting progenitors to differentiate along the NK cell lineage. This suggests that G-CSF itself indirectly influences NK cells and their progenitors even when G-CSF is added only once at the time of initial plating. In these long-term culture experiments, it is possible that the qualitative decrease in cytotoxicity observed with NK cell progeny obtained after supplementation with G-CSF is mediated by interaction with other cells (CD56−) present in the cultured population.
The functional NK cell abnormalities reported here are similar to the functional abnormalities of NK cells from patients with chronic myelogenous leukemia (CML). In CML, we have shown that absolute circulating NK cells decrease as CML progresses from early to advanced disease, and that the NK cells exhibit proliferation abnormalities compared with NK cells derived from normal donors.31 The proliferation abnormalities are similar to those shown here. Both situations lead to proliferation and egress of myeloid progenitors into the blood: G-CSF by benign alteration of progenitor adhesion32 and CML by abnormal adhesion as a result of the bcr/abl gene rearrangement.33 34 These observations suggest that there may be a mechanism common to the benign (G-CSF) and malignant (CML) effects on the myeloid pool that account for the abnormal NK cell function observed in both settings.
Several studies support the notion that myeloid growth factors used to mobilize stem cells may also affect the lymphoid compartment.35-38 Possible mechanisms include the observation that in vitro stimulation of granulocytes with G-CSF resulted in a contact-mediated suppression of NK cell function.39 In a nontransplant setting, giving GM-CSF to patients with aplastic anemia and myelodysplastic syndrome inhibited NK cell number and function and marrow NK cell progenitor clonogenic frequency.40 This effect could be demonstrated in vitro with GM-CSF but not with G-CSF.41 The differences between these studies and the data presented here may be due to our more primitive CD34+/Lin−/DR− starting progenitor population or, alternatively, the requirement for marrow stroma in our system.
Although the physiologic importance of our findings is not known, we hypothesize that G-CSF indirectly induces NK cell dysfunction and decreased NK cell progenitor capacity in PBPC collections, which may play a role in engraftment and subsequent immunologic reconstitution after transplantation. This may occur via secondary cytokines or via contact-mediated factors. The finding that similar results can be induced when primitive progenitors differentiate in long-term culture suggests that this model may be useful to determine whether G-CSF mediates its effects through interaction with a stromal component versus a component of the progenitor pool itself and/or its progeny. Future studies comparing progenitors (with and without G-CSF) from blood versus marrow will be used to assess the role of progenitor location as a function of their adhesion characteristics in determining lymphoid progenitor capacity.
ACKNOWLEDGMENT
The authors thank Brad Anderson for helping with the flow cytometry, Jennifer Tessmer-Tuck for excellent technical assistance, and David Stroncek and the University of Minnesota Blood Bank staff for facilitating PBPC collections.
Supported in part by National Institutes of Health Grants No. R29-HL-55417 and PO1-CA-65493, the Paul Christiansen Foundation, the University of Minnesota Bone Marrow Transplant Research Fund, and the Children's Cancer Research Fund.
Address reprint requests to Jeffrey S. Miller, MD, Division of Hematology, Box 480, University of Minnesota Cancer Center, Harvard Street at East River Road, Minneapolis, MN 55455.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal