Abstract
Erythrocyte dehydration is an important feature of sickle cell disease, leading to increased sickle hemoglobin polymerization and decreased red blood cell survival. Substantial in vivo dehydration appears to occur in reticulocytes or in an even younger subset of reticulocytes that are positive for transferrin receptor. Previous studies have suggested both sickling-dependent and sickling-independent components of dehydration for these cells. Two types of investigations are reported here. The first series of experiments explored the possibility that fetal hemoglobin (HbF ) content influences the in vivo dehydration of very young, transferrin receptor-positive (T+) cells. These studies confirmed that in most patients the T+ cells in the densest fraction lacked HbF (T+F−). However, T+F− and T+F+ cells appeared to have the same tendency to become moderately dense. The second type of investigation examined moderately dense T+ cells with normalized K+ content and determined the effect of HbF content on KCl cotransport-mediated dehydration in oxygenated incubations. Under these conditions, both T+F− and T+F+ cells had an equal tendency to become more dense by this pathway. Taken together, these studies indicate that at least some young sickle cells become moderately dense due to higher KCl cotransport activity independent of HbF content (and by inference, independent of sickling). However, to become very dense, it appears that further dehydration through a sickling-mediated pathway is required. We suggest that the dehydration of young sickle cells occurs in two steps, with the first dominated by KCl cotransport and the second having an important sickling-dependent component.
NORMAL RETICULOCYTES are approximately 25% larger than red blood cells (RBCs) and have a correspondingly low cellular hemoglobin concentration.1 In sickle cell disease, there is a variable degree of dehydration of young RBCs, manifested by their presence in the more dense fractions after separation on a density gradient. In previous studies,2 we have separated SS RBCs into three density fractions. Fraction I (1.073 to 1.083 g/mL) contains transferrin receptor-positive cells (T+; a less mature subset of reticulocytes) that have a normal reticulocyte density. Fraction II (1.083 to 1.094 g/mL) contains about one-half of the total number of T+ cells, which have mean corpuscular hemoglobin concentration (MCHC) values similar to mature AA RBCs, but are nevertheless abnormally dense for reticulocytes and are referred to here as moderately dense. Fraction III (>1.094 g/mL) contains hyperdense T+ cells. There is no clear understanding of the genesis and fate of these young dense cells. They may have a relatively stable density after quickly reaching a volume set point, perhaps before release from the bone marrow, and would thus mature into RBCs with about the same level of hydration. Alternatively, they may be undergoing continual loss of K+ and water and would soon be nonviable.
It has been shown that immature hyperdense sickle cells, either reticulocytes3 or the younger T+ cells,4 have little or no fetal hemoglobin (HbF ). In view of the antisickling effect of HbF, these findings provide indirect evidence for a contribution from sickling-dependent pathways to severe dehydration of very young sickle cells. However, there also appears to be a component of dehydration in young sickle cells that does not depend on sickling. A study from our laboratory indicated that fraction II or fraction III T+ cells, after normalization of hydration and K+ content with nystatin, had a greater tendency to dehydrate during short oxygenated incubations than did fraction I T+ cells normalized to the same conditions.2 Chloride replacement and inhibitor studies indicated that this density increase was mediated by KCl cotransport, suggesting that those T+ cells that had become dense in vivo had a higher activity of this pathway than did age-matched cells that had remained light. There was no deoxygenation in these experiments, so these density changes were not directly dependent on polymerization or sickling and provided evidence for a sickling-independent component of T+ cell dehydration. It is possible that the presence of HbF could have a sickling-independent effect on KCl cotransport by diluting the amount of sickle hemoglobin (HbS) within the cell. This is plausible because HbS and other similar β chain mutant hemoglobins have been shown to increase KCl cotransport activity even in the heterozygous state.5 Furthermore, the presence of HbF could modify such factors as membrane oxidation and phosphorylation state and thereby change KCl cotransport activity. Evidence against a direct effect of HbF on KCl cotransport activity was provided in a study by Fabry et al6 in which cells having a KCl cotransport-mediated high volume regulatory decrease (H-VRD) in hypotonic medium were separated from cells having a low volume regulatory decrease (L-VRD). It was found that HbF-containing reticulocytes (F retics) were enriched in the H-VRD cells to the same extent as those without HbF, indicating that the presence of HbF had little or no effect on KCl cotransport activity in young sickle cells.
The experiments presented here tested the following two hypotheses: (1) that the presence of HbF within a cell protects that cell from becoming moderately dehydrated while very young; and (2) that the presence of HbF alters the KCl cotransport activity of very young sickle cells with normalized K+ content under isotonic conditions. In the first set of experiments, a two-color flow cytometric assay was used to determine the density distribution of T+ SS reticulocytes and to compare cells with HbF (T+F+) and without (T+F−). These studies indicated that moderate in vivo dehydration was independent of HbF, whereas more severe dehydration, in most patients, was dependent on HbF. In the second type of experiment, the hydration and K content of moderately dense cells were normalized with nystatin, and a KCl cotransport-mediated density increase induced in T+ cells by oxygenated incubation at pH 7.2. In these experiments, the T+F− and T+F+ cells exhibited an equal tendency to become more dense, indicating that KCl cotransport activity was unrelated to HbF content and providing additional evidence that moderate density increase in very young cells is not dependent on HbF.
MATERIALS AND METHODS
Blood samples were obtained in heparin anticoagulant from patients with homozygous sickle cell disease under a protocol approved by the University of Cincinnati Institutional Review Board. Experiments were started either on the day of collection or after overnight storage in plasma at 4°C. In all experiments, RBCs were separated into three primary fractions as previously described2 using a discontinuous arabinogalactan gradient with density ranges of 1.073 to 1.083 (fraction I), 1.083 to 1.094 (II), and greater than 1.094 (III) g/mL. The percentage of total RBCs present in each fraction was determined.
Experiments measuring T+F− and T+F+ density distributions. For each density fraction, 10 μL of RBCs was washed three times with phosphate-buffered saline (PBS) and mixed with 167 μL of a 10:1 dilution of mouse monoclonal antihuman transferrin receptor; (Dako M074, diluted in PBS/1% bovine serum albumin [BSA]; Dako, Carpenteria, CA). Equivalent mixtures were also prepared with nonspecific mouse IgG1 (isotypic controls). After 30 minutes of incubation at room temperature, cells were washed three times with PBS/1% BSA, 335 μL of biotinylated rabbit antimouse (Dako E0464, 200:1) was added, and the mixture was incubated for 30 minutes at room temperature. After three washes with PBS/1% BSA, the cells were brought to 35 μL and an equal volume of phycoerythrin-Cy5-avidin (Southern Biotechnology 7200-13; Southern Biotechnology, Birmingham, AL) was added, followed by 30 minutes of incubation at room temperature. After washing, the cells were brought to 100 μL, and a 5-μL aliquot was diluted to 1 mL with PBS for flow cytometry to determine the percentage of T+ cells before permeabilization. The remaining cells were fixed with 4% formaldehyde and permeabilized at −15°C by sequential exposure to 50/50 acetone/water, acetone, and 50/50 acetone/water, as described.7 After permeabilization, the cells were resuspended in an equal volume of PBS/3% BSA, and 4 μL of this suspension was added to 8 μL of anti-HbF–fluorescein isothiocyanate (FITC) (kindly provided by Dr Thomas Campbell, Isolab, Inc, Akron, OH) that had been diluted to 0.625 mg/mL. The cells were incubated for 30 minutes at room temperature, washed once with PBS, and resuspended in PBS for flow cytometry (Coulter XL-MCL; Coulter, Hialeah, FL). Permeabilized RBCs were gated on forward and side light scatter, and FITC fluorescence was selected based on the nadir between the HbF− and HbF+ cells. For each density fraction, the percentages of cells falling in the quadrants corresponding to T+F− and T+F+ cells in the isotypic control were subtracted from the percentages in the sample incubated with anti-TfR to give true positives. The number of cells of each type in each fraction, based on an arbitrary total of 10,000 RBCs, was calculated from these data and the percentage of total RBCs in each fraction.
In vitro density shift experiments. Fraction II cells (1.083 to 1.094 g/mL) were used for these experiments on the day after primary density separation. RBCs were treated with nystatin as previously described2 to normalize K+ content and to obtain a more homogeneous density distribution. Cells were then washed twice in ice-cold HBIS 7.2 (135 mmol/L NaCl, 20 mmol/L HEPES, 5 mmol/L KCl, 1 mmol/L Na2HPO4 , 1 mmol/L CaCl2 , 1 mmol/L MgCl2 , 10 mmol/L glucose, and 290 to 300 mOsm/kg, pH 7.2, at 37°C), and the hemoglobin and hematocrit levels were measured for calculation of MCHC. One aliquot of cells was kept on ice and is called postnystatin. A second aliquot was incubated in HBIS 7.2 for 20 minutes at 37°C. A third aliquot was incubated in HBIN 7.2, which is identical to HBIS 7.2 except that Cl− is replaced with NO−3. After incubation, all samples were washed in ice-cold HBIS 7.4, and the following samples were loaded onto secondary density gradients: prenystatin, postnystatin, HBIS 7.2 incubated, and HBIN 7.2 incubated. Secondary gradients were discontinuous and consisted of the following arabinogalactan densities and volumes in 1.4 × 8.9 cm polyallomer tubes: 1.086 (3.3 mL), 1.094 (3.3 mL), 1.103 (3.3 mL), and greater than 1.15 (0.6 mL). Four secondary RBC density fractions were isolated by centrifugation (45 minutes at 20,000 rpm, Ti41 rotor, 22°C). The number of RBCs in each secondary fraction was quantitated, each fraction was stained with anti-TfR–FITC, and the percentage of T+ cells in each secondary fraction was quantitated by flow cytometry, allowing the calculation of the absolute number of T+ cells in each fraction and the percentage of the total number of T+ cells in each fraction.2 Of the four sets of secondary fractions, two were selected for fixation and permeabilization. One set was the HBIS 7.2 incubation. The other was either the HBIN 7.2 incubation or the unincubated postnystatin set. Secondary fraction 4 always contained very few cells and was not included. Staining for TfR and isotypic control, fixation, permeabilization, staining for HbF, and flow cytometry were as described above. A density score (DS) for T+F+ and T+F− cells analogous to that previously described,2 but for three fractions rather than for four, was calculated as DS = (P1 × 1) + (P2 × 2) + (P3 × 3), where Pn is the percentage of the total number of appropriate cells in fraction n. The difference in this density score between control cells and cells incubated in HBIS 7.2 is a measure of the density shift during the incubation.
RESULTS
Experiments measuring T+F− and T+F+ density distributions. Figure 1 shows typical flow cytometric output for the two-color TfR/HbF assay as applied to the three density fractions. The F cell region was defined by the nadir between negative and positive cells, whereas TfR positivity was determined with the aid of the isotype control. Comparison of the percentage of T+ cells before and after permeabilization with acetone indicated that some TfR was lost during this procedure. The losses were larger in the denser fractions, most likely because the average TfR antigen density on the dense T+ cells was lower. Compared with intact cells, the percentage of T+ cells after acetone treatment was an average of 6% lower for fraction I, 33% lower for fraction II, and 58% lower for fraction III. There was no correlation between the loss of TfR and the number of F cells in a sample, indicating that the loss was not selective for F or non-F cells. However, the high loss in fraction III makes conclusions concerning this fraction less definitive.
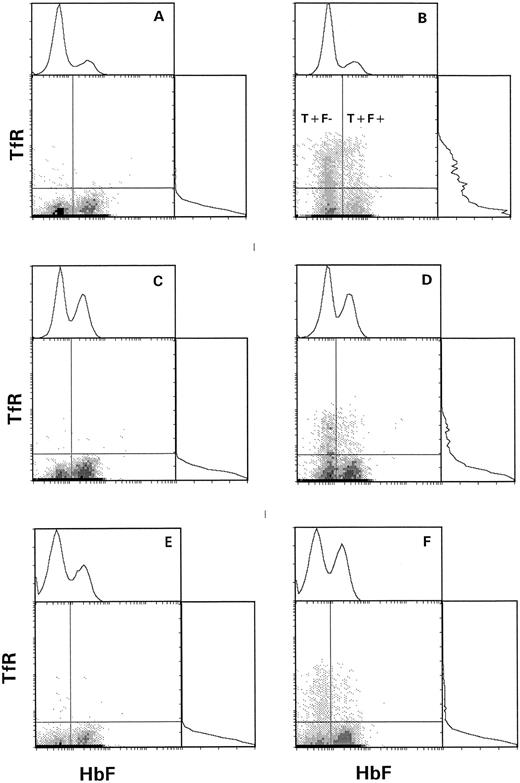
Two-parameter analysis of HbF and TfR in density-fractionated sickle cells. F cell analysis is on the x-axis, with positive and negative regions determined by the nadir between the two peaks. The positive region for transferrin receptor was determined with the aid of matched nonspecific IgG (idiotype control). The regions containing T+F− and T+F+ cells are indicated. (A) Fraction I, idiotype control. (B) Fraction I, anti-TfR. (C) Fraction II, idiotype control. (D) Fraction II, anti-TfR. (E) Fraction III, idiotype control. (F ) Fraction III, anti-TfR.
Two-parameter analysis of HbF and TfR in density-fractionated sickle cells. F cell analysis is on the x-axis, with positive and negative regions determined by the nadir between the two peaks. The positive region for transferrin receptor was determined with the aid of matched nonspecific IgG (idiotype control). The regions containing T+F− and T+F+ cells are indicated. (A) Fraction I, idiotype control. (B) Fraction I, anti-TfR. (C) Fraction II, idiotype control. (D) Fraction II, anti-TfR. (E) Fraction III, idiotype control. (F ) Fraction III, anti-TfR.
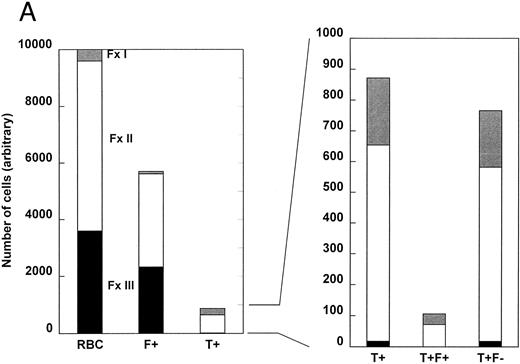
Figure 2 shows two examples of the density distribution of sickle RBCs, F+ cells, T+ cells, T+F+ cells, and T+F− cells. The number of cells of each type in each of the three density fractions is based on an arbitrary total of 10,000 cells. On the left, the distribution of all RBCs is shown. In Fig 2A, approximately 4% (400/10,000) of the RBCs were in fraction I, 60% in fraction II, and 36% in the densest fraction III. This patient had a high number of F+ cells (57%), which is depicted in the second bar. Only 1% of the F+ cells were in fraction I, with 58% in fraction II and 41% in fraction III. About 8.7% of the RBCs in Fig 2A were T+, and almost all of these were in fractions I and II. The right panel of Fig 2 shows the distribution of T+ cells on an expanded scale, in which the small number of T+ cells in fraction III can be seen. For this patient, most of the T+ cells are in fraction II. The two bars on the far right of Fig 2 depict the distribution of very young cells that contain HbF (T+F+) or do not contain HbF (T+F−). For the patient represented by Fig 2A, none of the hyperdense T+ cells contains HbF.
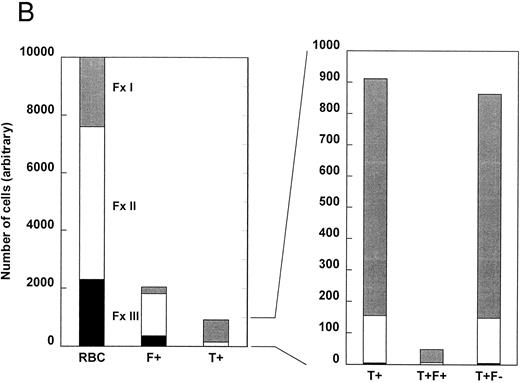
The density distribution of all RBCs, F+ cells, T+ cells, T+F+ cells, and T+F− cells. Sickle cells were separated by density and, after permeabilization, each fraction was analyzed for HbF and TfR by two-parameter flow cytometry. An arbitrary total of 10,000 cells was selected for analysis, and the number of each subtype (T+F+ and T+F−) in each density fraction was calculated from the percentage of total cells in each fraction and the percentage of cells in each fraction that belonged to that subtype. Subtype percentages were corrected by subtracting the corresponding percentage in the HbF/idiotype control. The data for total F+ cells was calculated using an average of the idiotype control and specific antibody values. (A) Cells from a patient with a higher number of F+ cells. (B) Cells from a patient with a lower number of F+ cells.
The density distribution of all RBCs, F+ cells, T+ cells, T+F+ cells, and T+F− cells. Sickle cells were separated by density and, after permeabilization, each fraction was analyzed for HbF and TfR by two-parameter flow cytometry. An arbitrary total of 10,000 cells was selected for analysis, and the number of each subtype (T+F+ and T+F−) in each density fraction was calculated from the percentage of total cells in each fraction and the percentage of cells in each fraction that belonged to that subtype. Subtype percentages were corrected by subtracting the corresponding percentage in the HbF/idiotype control. The data for total F+ cells was calculated using an average of the idiotype control and specific antibody values. (A) Cells from a patient with a higher number of F+ cells. (B) Cells from a patient with a lower number of F+ cells.
Figure 2B represents a patient having a lower number of F+ cells (20% of total RBCs), a higher percentage of fraction I cells, and about the same percentage of T+ cells (9.1%). In this patient, most of the T+ cells (83%) were in fraction I. In the right panel of Fig 2B, the relative distribution of T+ cells between fractions I and II was similar for T+F+ and T+F− cells. Again, the only T+ cells in fraction III were T+F−.
Table 1 shows the density distributions of the various cell types for 7 patients with sickle cell disease and 3 high reticulocyte controls. As expected, a larger fraction of the sickle T+ cells were in the denser fractions when compared with the controls. There is considerable interpatient variability, but a number of trends are apparent from the average values. About 7.5% of the sickle RBCs were in the lightest fraction I, with the remainder equally divided between fraction II and III. On average, about 5% of the sickle cells were T+, of which 36% were in fraction I and thus were normally hydrated for young reticulocytes. These data, showing most of the sickle T+ cells in the denser fractions, are consistent with our previously published density distributions for these cells.2
The Number of Cells (Based on a Total of 10,000 Cells) of Each Type in Each Density Fraction
| Experiment No. . | RBCs . | F+ . | T+ (after permeabilization) . | T+F+ . | T+F− . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | I . | II . | III . | Total . | I . | II . | III . | Total . | I . | II . | III . | Total . | I . | II . | III . | Total . | I . | II . | III . | Total . |
| 1 | 400 | 6,000 | 3,600 | 10,000 | 85 | 3,288 | 2,326 | 5,699 | 218 | 636 | 18 | 872 | 34 | 72 | 0 | 106 | 184 | 564 | 18 | 766 |
| 2 | 2,400 | 5,300 | 2,300 | 10,000 | 228 | 1,454 | 359 | 2,041 | 756 | 150 | 5 | 911 | 41 | 7 | 0 | 48 | 715 | 143 | 5 | 863 |
| 3 | 700 | 1,500 | 7,800 | 10,000 | 189 | 407 | 2,156 | 2,752 | 53 | 137 | 152 | 342 | 5 | 14 | 19 | 38 | 48 | 123 | 133 | 304 |
| 4 | 400 | 3,000 | 6,600 | 10,000 | 155 | 1,050 | 1,842 | 3,047 | 64 | 375 | 198 | 637 | 5 | 63 | 20 | 88 | 59 | 312 | 178 | 549 |
| 5 | 750 | 4,800 | 4,450 | 10,000 | 333 | 3,034 | 2,416 | 5,783 | 124 | 149 | 18 | 291 | 41 | 58 | 0 | 99 | 83 | 91 | 18 | 192 |
| 6 | 140 | 6,430 | 3,420 | 10,000 | 31 | 2,793 | 1485 | 4,309 | 6 | 118 | 16 | 140 | 1 | 28 | 4 | 33 | 5 | 90 | 12 | 107 |
| 7 | 480 | 4,760 | 4,760 | 10,000 | 48 | 493 | 615 | 1,156 | 22 | 165 | 42 | 229 | 0 | 17 | 1 | 18 | 22 | 148 | 41 | 211 |
| Average | 753 | 4,541 | 4,705 | 10,000 | 153 | 1,788 | 1,600 | 3,541 | 178 | 247 | 64 | 489 | 18 | 37 | 6 | 61 | 159 | 210 | 58 | 427 |
| C1* | 3,840 | 5,361 | 798 | 10,000 | 342 | 64 | 7 | 413 | ||||||||||||
| C2† | 8,000 | 1,600 | 400 | 10,000 | 128 | 10 | 5 | 143 | ||||||||||||
| C3‡ | 1,440 | 6,060 | 2,500 | 10,000 | 190 | 120 | 20 | 330 | ||||||||||||
| Average | 4,427 | 4,340 | 1,233 | 10,000 | 220 | 65 | 11 | 295 | ||||||||||||
| Experiment No. . | RBCs . | F+ . | T+ (after permeabilization) . | T+F+ . | T+F− . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | I . | II . | III . | Total . | I . | II . | III . | Total . | I . | II . | III . | Total . | I . | II . | III . | Total . | I . | II . | III . | Total . |
| 1 | 400 | 6,000 | 3,600 | 10,000 | 85 | 3,288 | 2,326 | 5,699 | 218 | 636 | 18 | 872 | 34 | 72 | 0 | 106 | 184 | 564 | 18 | 766 |
| 2 | 2,400 | 5,300 | 2,300 | 10,000 | 228 | 1,454 | 359 | 2,041 | 756 | 150 | 5 | 911 | 41 | 7 | 0 | 48 | 715 | 143 | 5 | 863 |
| 3 | 700 | 1,500 | 7,800 | 10,000 | 189 | 407 | 2,156 | 2,752 | 53 | 137 | 152 | 342 | 5 | 14 | 19 | 38 | 48 | 123 | 133 | 304 |
| 4 | 400 | 3,000 | 6,600 | 10,000 | 155 | 1,050 | 1,842 | 3,047 | 64 | 375 | 198 | 637 | 5 | 63 | 20 | 88 | 59 | 312 | 178 | 549 |
| 5 | 750 | 4,800 | 4,450 | 10,000 | 333 | 3,034 | 2,416 | 5,783 | 124 | 149 | 18 | 291 | 41 | 58 | 0 | 99 | 83 | 91 | 18 | 192 |
| 6 | 140 | 6,430 | 3,420 | 10,000 | 31 | 2,793 | 1485 | 4,309 | 6 | 118 | 16 | 140 | 1 | 28 | 4 | 33 | 5 | 90 | 12 | 107 |
| 7 | 480 | 4,760 | 4,760 | 10,000 | 48 | 493 | 615 | 1,156 | 22 | 165 | 42 | 229 | 0 | 17 | 1 | 18 | 22 | 148 | 41 | 211 |
| Average | 753 | 4,541 | 4,705 | 10,000 | 153 | 1,788 | 1,600 | 3,541 | 178 | 247 | 64 | 489 | 18 | 37 | 6 | 61 | 159 | 210 | 58 | 427 |
| C1* | 3,840 | 5,361 | 798 | 10,000 | 342 | 64 | 7 | 413 | ||||||||||||
| C2† | 8,000 | 1,600 | 400 | 10,000 | 128 | 10 | 5 | 143 | ||||||||||||
| C3‡ | 1,440 | 6,060 | 2,500 | 10,000 | 190 | 120 | 20 | 330 | ||||||||||||
| Average | 4,427 | 4,340 | 1,233 | 10,000 | 220 | 65 | 11 | 295 | ||||||||||||
Nine-week-old premature (28 weeks) infant, hematocrit level of 22%.
Ten-month-old infant with biliary atresia without hepatic failure, status post-Kasai procedure, anemia presumed secondary to documented gastrointestinal bleeding, hematocrit level of 27%.
Seven-week-old premature (30 weeks) infant, hematocrit level of 23%.
On average, F+ cells are 35.4% of the total RBCs in these patients. However, only 12.5% of the T+ cells contain HbF. This most likely reflects the survival advantage of F+ cells in the circulation, perhaps while still very young, and is consistent with the differences found between F cells and F reticulocytes using slide-based methods8 and the difference in F content between all cells and isolated T+ cells4 as measured by high-performance liquid chromatography. The T+ cells are the best measure available of the number of F cells in a cohort of RBCs leaving the marrow. However, even these cells may have undergone a prior selection process, either before or after entering the circulation. On the other hand, the T− cells represent a wide range of ages, from 1 or 2 days up to the maximum survival of cells in the circulation. Therefore, the enrichment ratio of 3:1 most likely underestimates total enrichment when the entire span from hemoglobin synthesis to older cells is considered. It appears that the differential survival of F and non-F cells is an important, perhaps controlling, determinant of blood HbF level and must be accounted for when studying the effect of genetic factors (eg, haplotype) or treatment on blood HbF level. In 5 of the 7 patients, there were few if any T+F+ cells in the densest fraction III. The 2 patients who appeared to have T+F+ cells in this fraction also had a very high number of total RBCs in this fraction (66% and 78%). The lack of fraction III T+F+ cells in most patients is consistent with our previous study using immunomagnetically separated T+ cells and with data from other investigators.3 4
The tendency of a particular type of young cell to become moderately dehydrated may be estimated by calculating the ratio of the number of cells of that type in fraction I to the number in fraction II. These ratios for T+F+ and T+F− cells, shown in Table 2, are not different (P = .65 by paired t-test). Thus, moderate dehydration in vivo appears to be independent of HbF content for very young cells.
The Ratio of T+F+ and T+F− Cells in Light (Fraction I) and Moderately Dense (Fraction II) Fractions
| Patient No. . | T+F+ . | T+F− . | ||||
|---|---|---|---|---|---|---|
| . | Fraction I . | Fraction II . | Ratio . | Fraction I . | Fraction II . | Ratio . |
| 1 | 34 | 72 | 0.47 | 184 | 564 | 0.33 |
| 2 | 41 | 7 | 5.8 | 715 | 143 | 5.0 |
| 3 | 5 | 14 | 0.36 | 48 | 123 | 0.39 |
| 4 | 5 | 63 | 0.079 | 59 | 312 | 0.19 |
| 5 | 41 | 58 | 0.71 | 83 | 91 | 0.91 |
| 6 | 1 | 28 | 0.036 | 5 | 90 | 0.055 |
| 7 | 0 | 17 | 0 | 22 | 148 | 0.15 |
| Patient No. . | T+F+ . | T+F− . | ||||
|---|---|---|---|---|---|---|
| . | Fraction I . | Fraction II . | Ratio . | Fraction I . | Fraction II . | Ratio . |
| 1 | 34 | 72 | 0.47 | 184 | 564 | 0.33 |
| 2 | 41 | 7 | 5.8 | 715 | 143 | 5.0 |
| 3 | 5 | 14 | 0.36 | 48 | 123 | 0.39 |
| 4 | 5 | 63 | 0.079 | 59 | 312 | 0.19 |
| 5 | 41 | 58 | 0.71 | 83 | 91 | 0.91 |
| 6 | 1 | 28 | 0.036 | 5 | 90 | 0.055 |
| 7 | 0 | 17 | 0 | 22 | 148 | 0.15 |
Paired t-test of ratios: P = .65.
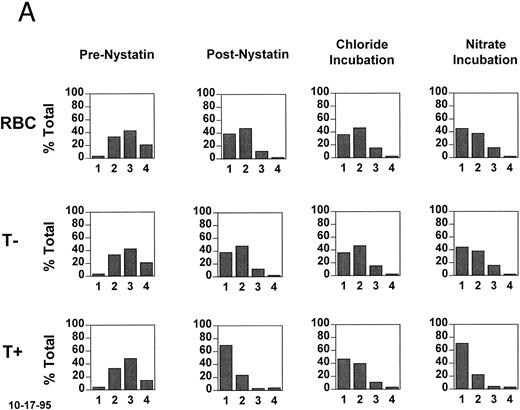
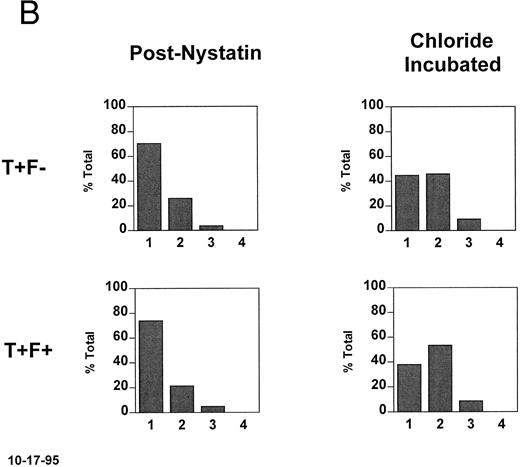
In vitro density shift experiments. To determine whether HbF has an effect on the activity of the KCl cotransporter in SS reticulocytes, cells from fraction II (57% to 67% of total RBCs, 4 experiments with 3 patients) were rehydrated and the K was normalized with nystatin.2 The normalized cells were incubated for 20 minutes in the oxygenated state at pH 7.2, conditions known to activate the KCl cotransporter.2 Figure 3A shows the density distributions of all RBCs as well as the T− and T+ subpopulations. Initially (prenystatin) T+ and T− cells have the same density distribution, because all of the cells come from primary fraction II. After nystatin treatment, the rehydrated cells have lower densities, with the T+ subpopulation having more cells in the lowest density fraction. After incubation in chloride medium at pH 7.2, there was no shift in the density of T− cells, whereas the T+ cells became more dense. This density shift is not seen in nitrate medium, indicating that it is chloride dependent.
Density changes that occur in T+ sickle cells during short oxygenated incubations and their dependence on HbF. Fraction II cells were treated with nystatin to normalize their K+ content and hydration and incubated for 20 minutes at pH 7.2 in the presence and absence of chloride. A secondary density fractionation was performed and the T+ cells were identified. (A) The secondary density distributions of all RBCs, T− cells, and T+ cells before nystatin treatment, after nystatin treatment, and after incubation of the normalized cells in the presence or absence of chloride. The expected chloride-dependent density shift in T+ cells takes place. (B) For the postnystatin and chloride-incubated samples, cells were also analyzed for HbF, and the density distributions of T+F− and T+F+ cells were determined. Both subtypes had the same tendency to become more dense when incubated under these conditions.
Density changes that occur in T+ sickle cells during short oxygenated incubations and their dependence on HbF. Fraction II cells were treated with nystatin to normalize their K+ content and hydration and incubated for 20 minutes at pH 7.2 in the presence and absence of chloride. A secondary density fractionation was performed and the T+ cells were identified. (A) The secondary density distributions of all RBCs, T− cells, and T+ cells before nystatin treatment, after nystatin treatment, and after incubation of the normalized cells in the presence or absence of chloride. The expected chloride-dependent density shift in T+ cells takes place. (B) For the postnystatin and chloride-incubated samples, cells were also analyzed for HbF, and the density distributions of T+F− and T+F+ cells were determined. Both subtypes had the same tendency to become more dense when incubated under these conditions.
In Fig 3B, the T+ cells from each secondary density fraction were analyzed on the basis of their HbF content (T+F− and T+F+). The postnystatin density distributions of T+F− and T+F+ cells were the same, and during chloride incubation the density shifts were similar. Density scores were calculated as described in Materials and Methods and are given in Table 3 for four experiments. There is no consistent difference in the density shift of T+F− and T+F+ cells, indicating that HbF content does not affect KCl cotransport activity in these age-matched (T+) SS reticulocytes.
The Change in DS of T+F+ and T+F− Cells During Incubation in Chloride Medium
| Experiment No. . | T+F+ DS . | T+F− DS . | ||||
|---|---|---|---|---|---|---|
| . | Cont . | Cl Inc . | Δ . | Cont . | Cl Inc . | Δ . |
| 13-150 | 178 | 248 | 70 | 171 | 246 | 75 |
| 2 | 131 | 170 | 39 | 133 | 164 | 31 |
| 3 | 131 | 177 | 46 | 126 | 168 | 42 |
| 4 | 119 | 153 | 34 | 118 | 141 | 23 |
| Experiment No. . | T+F+ DS . | T+F− DS . | ||||
|---|---|---|---|---|---|---|
| . | Cont . | Cl Inc . | Δ . | Cont . | Cl Inc . | Δ . |
| 13-150 | 178 | 248 | 70 | 171 | 246 | 75 |
| 2 | 131 | 170 | 39 | 133 | 164 | 31 |
| 3 | 131 | 177 | 46 | 126 | 168 | 42 |
| 4 | 119 | 153 | 34 | 118 | 141 | 23 |
Paired t-test, Δ DS for T+F+v Δ DS for T+F−P = .29.
For experiment no. 1, the control was incubated in a buffer that contained NO−3 rather than Cl−. For the remaining experiments, the control was the unincubated postnystatin sample.
DISCUSSION
The current studies were performed to clarify the role of HbF in the dehydration of young sickle cells. Specifically, our aim was to determine (1) whether HbF influenced the in vivo formation of the moderately dense T+ cells in fraction II and (2) whether the KCl cotransport activity of nystatin-normalized T+ cells from fraction II was dependent on the presence of HbF.
In the first set of experiments, the number of T+F+ and T+F− cells in each density fraction was determined, and the ratio of cells in fraction I to cells in fraction II was the same for each cell type. Therefore, the presence or absence of HbF appears to play little or no role in the moderate in vivo dehydration of the cell. However, in most patients, only T+F− cells were present in the higher density fraction III. These data, together with results from previous studies,3,4 9 indicate that the presence of HbF is an important factor for the in vivo formation of more severely dehydrated young cells.
Another, less likely interpretation is that cells lacking HbF preferentially become moderately dense, but then either progress to higher density or are removed from the circulation such that there appears to be no selection of T+F− cells in fraction II compared with fraction I. For this to occur, the entrance of T+F− into fraction II from fraction I minus the removal of T+F− from fraction II would have to be equivalent to the entrance of T+F+ into fraction II. It seems unlikely that these rates would match in each patient.
To clarify the role of HbF in KCl cotransport activation, the second set of studies was performed. The fraction II cell population was isolated, normalized with nystatin treatment, and incubated at pH 7.2 to approximate the low end of the physiologic pH range. Previous studies2 have shown measurable density shifts in this fraction during short incubations even at pH 7.4, but the lower pH value was used to further activate KCl cotransport and to induce larger density shifts. As expected, this fraction exhibited a Cl-dependent density shift of T+ cells during a short, oxygenated incubation. When T+F+ and T+F− cells were quantitated in each secondary fraction, it was apparent that, under these conditions, the young cells that contain HbF had the same tendency to become more dense like the age-matched cells that lacked HbF. Therefore, it appears that the presence of HbF does not directly influence KCl cotransport-mediated dehydration.
Bookchin et al10-13 have proposed a mechanism for young sickle cell dehydration in which initial K loss from reticulocytes is due to sickling-mediated Ca entry and activation of the K(Ca) channel, leading to partial dehydration and decreased intracellular pH through Donnan effects. Lower pH activates KCl cotransport, creating a vicious cycle of dehydration, acidification, and KCl cotransport activation that may persist after cytoplasmic Ca returns to normal. In this context, the effect of HbF to prevent reticulocyte dehydration would be inhibition of the initial sickling-dependent Ca influx. Because in this proposed mechanism KCl cotransport activity is dependent upon sickling, young cells that contain HbF should remain hydrated. However, the studies presented here suggest that T+F+ cells have the same tendency in vivo as T+F− cells to become moderately dense and thus do not support a mechanism in which initial dehydration is HbF dependent. These data are consistent with a model for sickle cell dehydration in which initial K loss is mediated by KCl cotransport. Cells that have a high activity of this transporter would tend to undergo rapid dehydration and to enter the more dense fractions while still T+. Cells with lower KCl cotransport activity remain in the light fraction while T+.
The presence of an initial KCl cotransport-mediated dehydration is consistent with the properties of this cotransporter and with observations in other hemogobinopathies. KCl cotransport is thought to mediate the normal decrease in volume that occurs in erythroid cells. There is evidence that it is under the control of cellular factors such as phosphorylation state14 and intracellular protein concentration.15 If indeed this pathway mediates the volume reduction of normal erythroid cells, then its spontaneous activity would be expected in young sickle cells. Although HbS and other mutant hemoglobins may have an effect on its activity/set point, it is unlikely that they would induce a completely uncontrolled and terminal level of cell dehydration by this mechanism. In homozygous CC disease, there are many dehydrated cells in the circulation that cannot be a consequence of sickling-dependent mechanisms. The pathway most likely to be responsible is KCl cotransport, which is known to be highly active in these cells.16 Therefore, KCl cotransport appears to provide a consistent first step of dehydration for sickling and nonsickling cell types.
Once an S-containing young cell becomes partially dehydrated, it is much more likely to sickle in the circulation. The magnitude of this effect may be inferred from the measurements by d'Onofrio et al1 of RBC and reticulocyte indices in normal subjects and from the dependence of HbS polymerization on concentration. The cell hemoglobin concentration mean (CHCM) of normal RBCs was 31.6 ± 1.02 (1 SD) and the CHCM of normal reticulocytes was 26.3 ± 1.50. Sickle reticulocytes with a normal CHCM of about 26 (and there are many such sickle reticulocytes in fraction I) will be much less likely to sickle than the partially dehydrated reticulocytes in fraction II with the CHCM of a normal RBC (∼32). The delay time of sickling for light reticulocytes would be about 100 times longer than that of reticulocytes in fraction II.17,18 Therefore, the second stage of dehydration, in which young cells progress from moderatly dense to hyperdense, leading to early removal from the circulation, is more likely than the first stage to be sickling-dependent and therefore HbF-dependent. This is consistent with the finding that hyperdense (>1.092 or >1.094 in our studies) T+ cells have little or no HbF.4 Partially dehydrated young cells that do not contain HbF would have a high likelihood of sickling and activating sickling-dependent pathways for further dehydration. One pathway with the high capacity required for fast dehydration is the Ca2+-dependent K+ channel [K(Ca)], which may be activated by sickling-induced Ca2+ influx. Because this pathway is not normally activated, but depends on a pathologic increase of cytoplasmic calcium concentration for activation, it is more likely to be uncontrolled and lead to severe dehydration. Ionophore-mediated increased intracellular calcium can indeed cause fast, extreme RBC dehydration.19
In a recently published study, the K(Ca) inhibitor clotrimazole was administered to patients with sickle cell disease.20 There was a decrease in the density of circulating RBCs, but little or no difference in the hydration of reticulocytes during clotrimazole treatment. These findings support a model in which sickling-independent mechanisms predominate in reticulocyte dehydration while sickling-dependent pathways mediate cation loss in more mature cells.
In summary, we propose a model of sickle cell dehydration in which the activity of the KCl cotransporter is the primary determinant of initial dehydration of the SS reticulocyte. This model shares a number of features with that proposed by Schwartz et al21 in that KCl cotransport leads to the formation of intermediate density cells and K(Ca) is primarily responsible for generation of very dense cells. Cotransporter activity may be determined by a variety of factors, including stochastic variations in transport or regulatory protein expression among differentiating erythroblasts, but is independent of HbF levels. Moderately dehydrated reticulocytes (and mature RBCs) would be more susceptible to sickling-dependent mechanisms, including sickling-induced activation of K(Ca) channels, and this process would be mitigated by the presence of HbF. There is evidence that at least some reticulocytes sickle more readily than more mature cells,10,22 and in vitro experiments indicate that T+ reticulocytes are more sensitive to deoxygenation-induced density changes than mature cells.23 In addition, the adhesion receptors on immature cells24-27 may make them more subject to sequestration and extended periods of deoxygenation. According to our model of sickle cell dehydration, all cells that contain HbF and the cells that lack HbF but have lower levels of KCl cotransport would have a greater chance of surviving past the reticulocyte stage. Once past this stage, the cells appear to be more resistant to density changes during in vitro continuous or cyclic deoxygenation22 23 and from this point slower dehydration mechanisms may dominate.
Supported by National Institutes of Health Grant No. RO1HL51174.
Address reprint requests to Robert S. Franco, PhD, Research Professor, Hematology/Oncology Division, Mail Location 508, University of Cincinnati College of Medicine, 231 Goodman St, Cincinnati, OH 54267-0508.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal