Abstract
Nonradiomimetic drugs, hydroxyurea (HU) and pipobroman (Pi), were administred to relatively young subjects with polycythemia vera (PV) in an attempt to decrease the leukemogenic risk observed in patients treated with 32P. Clinical safety, hematological efficacy, risk of carcinoma or leukemia, and frequency of progression to myelofibrosis have not yet been defined in long-term studies, and no comparative studies of HU and Pi have been conducted. Since 1980, 292 patients with PV diagnosed before the age of 65 years were randomized to receive treatment with HU (25 mg/kg/d, followed by low-dose maintenance) or Pi (1.2 mg/kg/d, followed by low-dose maintenance). Patients were followed until death or until May 1997. Drug tolerance was often poor; leg ulcers and buccal aphthous ulcers (with HU) and gastric pain and diarrhea (with Pi) sometimes required treatment change, mainly in the HU arm. Hematological stability, especially in terms of platelet count, was very often insufficient with HU (45% of cases), but the risk of thrombo-embolic event was similar in both arms. Actuarial survival was similar in the two arms and shorter than that of the reference population. The risk of leukemia was approximately 10% at the 13th year, with no significant difference between the two arms. The risk of carcinoma (when excluding the skin cancers) was similar in both groups. There was a high risk of progression to myelofibrosis in the patients treated by HU, which was significantly higher than with Pi.
THE LEUKEMOGENIC risk attributed to therapy of polycythemia vera (PV) with 32P1,2 has led, over the last 20 years, to the increased use of myelosuppressive drugs, especially hydroxyurea (HU). This alternative was clearly proposed in the recently published conclusion of the studies conducted by the Polycythemia Vera Study Group (PVSG).3 However, there has been no large-scale, long-term analysis of the efficacy, safety, and carcinogenic risk of the drug(s) proposed. The previously published series involved a limited number of cases and a relatively short follow-up, with no randomized trials comparing HU with Pipobroman (Pi), or either of these drugs with radiotherapy.
Based on the preliminary results of the PVSG, unpublished at that time, we proposed in 1980 a trial comparing HU with Pi in PV patients under the age of 65 years; older patients received myelosuppression with 32P.4 We are now able to present significant results concerning 292 cases followed for 1 to 17 years.
MATERIALS AND METHODS
Protocol. The French protocol was proposed to PV patients not previously treated by radiotherapy and/or chemotherapy. Twenty-four cases previously randomized in the phlebotomy arm of the PVSG protocols 01 or 05, and excluded from this arm,5 were allowed to enter the protocol. Randomization6 was done at a Central Office (St-Louis Hospital) once diagnosis was documented. Follow-up charts were obtained every 6 months.
The induction dosage was HU, 25 mg/kg/d and Pi, 1.25 mg/kg/d, until achievement of complete remission (ie, hematocrit lower than 50%, platelet count lower than 400.109/L). Phlebotomies were used as an emergency therapy when initial hematocrit was 55% or higher. According to previous observations, it was clear that maintenance therapy was necessary; therefore, we proposed long-term use of HU at the dose of 10 to 15 mg/kg/d, or of Pi at the dose of 0.4 to 0.7 mg/kg/d. The long-term use of aspirin was let free to the decision of the physician.
Because there was a shortage of Pi delivery in France between 1983 and 1984 and because most patients less than 65 years of age previously treated by phlebotomy in the 01 and 05 PVSG protocols had been switched without randomization to the HU arm (protocol PVSG 087), there was an excess of patients included into the HU arm and a longer follow-up in this branch. However, a separate analysis of the truly randomized cases shows that the conclusions drawn for the entire group are also valid for the truly randomized group.
This protocol was accepted by the University of Paris-VII Ethics Committee. All the patients were carefully informed about the advantages and disadvantages of the proposed treatment.8 9
Patients. Table 1 indicates that there was no significant difference between HU and Pi treatment arms in terms of clinical and hematological characteristics or risk factors.
Clinical Data at Initial Evaluation
| . | HU . | Pi . |
|---|---|---|
| . | (150 cases) . | (142 cases) . |
| Age (mean) | M = 53.2 F = 53.6 | M = 55.1 F = 53.3 |
| Sex ratio (m/f) | 0.89 | 1.20 |
| Hematological criteria (% of cases) | ||
| Increase of 2 cell lines + splenomegaly; or increase of 3 cell lines | 70 | 68 |
| Increase of red cell line + hyperleucocytosis or thrombocytosis, without splenomegaly | 25 | 24 |
| Increase of red cell line alone + abnormal value and spontaneous growth of erythropoietin BFU/E | 5 | 8 |
| Vascular risk (% of cases) | ||
| High* | 11 | 11 |
| Moderate† | 15 | 14 |
| None | 74 | 75 |
| . | HU . | Pi . |
|---|---|---|
| . | (150 cases) . | (142 cases) . |
| Age (mean) | M = 53.2 F = 53.6 | M = 55.1 F = 53.3 |
| Sex ratio (m/f) | 0.89 | 1.20 |
| Hematological criteria (% of cases) | ||
| Increase of 2 cell lines + splenomegaly; or increase of 3 cell lines | 70 | 68 |
| Increase of red cell line + hyperleucocytosis or thrombocytosis, without splenomegaly | 25 | 24 |
| Increase of red cell line alone + abnormal value and spontaneous growth of erythropoietin BFU/E | 5 | 8 |
| Vascular risk (% of cases) | ||
| High* | 11 | 11 |
| Moderate† | 15 | 14 |
| None | 74 | 75 |
High risk, severe vascular event in the antecedents.
Moderate risk, arterial hypertension, diabetes, phlebitis without pulmonary embolism.
All cases had an excess of red cell volume (more than 125% of the expected volume according to height and weight10 ); 93% had an excess of granulocyte and/or platelet count, and/or a splenomegaly, the usual criteria of PV. In 7% of the cases we accepted the diagnosis of myelo-proliferative syndrome in the presence of a pure erythrocytosis, with low serum erythropoietin value and spontaneous growth of circulating burst-forming unit–erythroid BFU-E.11 Bone marrow biopsy and karyotypic analyses were not required at the initial evaluation. Unlike patients over the age of 65 years included in a radiotherapy protocol using 32P,4 few of the cases of this study presented with major vascular risk factors (11% in the present series v 31% in the older patients). Such factors are obviously age-related.
With the exception of 6 non-French residents who returned to their country of origin and were only analyzed until their disappearance, clinical and hematological information is available up until death or until the last analysis (January to May 1997). The end points are, for each case, death or loss of follow-up, or the switch to 32P or radio-mimetic chemotherapy.
Statistical analysis. The usual methods of estimation of actuarial survival and actuarial risk of complications were used.12 However, to accurately analyze the clinical results, we took into account treatment changes or discontinuations decided by the consultant hematologist or by the physician in charge because of toxicity or insufficient efficacy (23 cases because of clinical toxicity, 23 cases because of insufficient efficiency, 6 cases decided by the physician without objective reasons, 3 cases switched to 32P because of neuro-psychiatric reasons). A protocol must accept the physician's right to personal initiative, even if it interferes with the statistical analysis of the results.13 Actuarial survival was thus calculated according to the “intention to treat,” and also to the “main treatment received.” As complications may be related to the true treatment received, these data are only presented here according to the main treatment received (“main treatment”). Because of a difference in the mean follow-up between the two arms, the risk of death or complication was calculated in reference to the expected risk (log-rank test), and the difference between the two arms was assessed by the chi-squared test. The mean life expectancy and cancer risk of the French population14 and international populations15 were used as reference.
RESULTS
Clinical tolerance of the drugs used. In contrast with 32P radiotherapy, which is nearly always perfectly well tolerated,4 continuous chemotherapy can induce clinical adverse effects ranging from merely bothersome to sometimes requiring a change of treatment. Table 2 indicates the frequency of these complications in cases followed for at least 2 years. The main toxicity of Pi was gastrointestinal and was sufficiently severe to warrant a change of treatment in 8 cases; these gastrointestinal disorders generally occurred early, during the initial phase of treatment, when relatively high doses of the drugs were used. In contrast, the toxicity of HU was essentially limited to cutaneous disorders (acne), buccal disorders (aphthous ulcers), and especially leg ulcers, which healed only when HU treatment was stopped; these complications appeared generally late (5 years or more after initiation of treatment) and they constituted a frequent reason for change of treatment. Interestingly, leg ulcers generally resolved when HU was replaced by Pi.
Clinical Side Effects of Chemotherapeutic Agents Used in PV Patients Followed for More Than 2 Years
| . | Pi (n = 108) . | HU (n = 133) . | ||
|---|---|---|---|---|
| . | . | Leading to a Change of Arm . | . | Leading to a Change of Arm . |
| No side effect | 75% of cases | 71% of cases | ||
| Gastric pain, diarrhea | 19 cases (17%) | 8 cases | 9 cases (7%) | None |
| Cystitis | None | None | 3 cases (2%) | None |
| Leg ulcer | 1 case | 1 case | 12 cases (9%) | 10 cases |
| Stomatitis | 4 cases (4%) | None | 13 cases (10%) | 4 cases |
| Dry skin, acne | 5 cases (4%) | None | 10 cases (7%) | 1 case |
| . | Pi (n = 108) . | HU (n = 133) . | ||
|---|---|---|---|---|
| . | . | Leading to a Change of Arm . | . | Leading to a Change of Arm . |
| No side effect | 75% of cases | 71% of cases | ||
| Gastric pain, diarrhea | 19 cases (17%) | 8 cases | 9 cases (7%) | None |
| Cystitis | None | None | 3 cases (2%) | None |
| Leg ulcer | 1 case | 1 case | 12 cases (9%) | 10 cases |
| Stomatitis | 4 cases (4%) | None | 13 cases (10%) | 4 cases |
| Dry skin, acne | 5 cases (4%) | None | 10 cases (7%) | 1 case |
Hematological efficacy and toxicity. Complete remission (ie, normal counts of the three myeloid lines) was obtained at the proposed doses in all but 5 cases (3 initial failures of Pi, 2 of HU). Several cases of severe thrombocytopenia (less than 50.109/L) were observed during induction treatment (2 cases with Pi, 5 cases with HU) or during the first months following introduction of maintenance treatment (5 cases with Pi, 5 cases with HU). These drug-induced thrombocytopenias or pancytopenias were observed in the oldest patients, and resolved spontaneously in a few weeks, or months.
In contrast, maintenance therapy was frequently ineffective. Table 3 indicates that long-term platelet control (lower than 400.109/L) was obtained much less often with HU than with Pi (P < .01). In these cases it may be difficult to increase the maintenance dose when the platelet count is high and hemoglobin low. Erythrocyte maintenance (hematocrit [Hct] lower than 50%) was ensured in 86% of cases, but once again with an advantage of Pi over HU (P = .11). In case of insufficient efficacy or progressive resistance, drug escalation or eventually drug switching to the other arm was used (Table 3). Treatment changes for hematological reasons were significantly more frequent in the HU arm than in the Pi arm (P = .025). Cross-resistance between the drugs was observed in half the cases. 32P was only used in the oldest cases or in patients with failure of both drugs.
Control of the Red Cell and Platelet Cell Lines on HU and Pi (in Patients Followed More Than 2 Years)
| . | . | HU (on 133 cases) . | Pi (on 108 cases) . | . | Escalation . | Further . |
|---|---|---|---|---|---|---|
| . | . | . | . | . | . | Change of . |
| . | . | . | . | . | . | Treatment . |
| Red cell line | Efficient control (Ht < 50%) | 109 cases (82%) | 97 cases (90%) | |||
| Insufficient control (Ht increasing toward 50%) | 24 cases (18%) | 11 cases (10%) | HU | 20 cases | 6 cases | |
| Pi | 8 cases | 3 cases | ||||
| Platelet line | ||||||
| (in patients with initial platelet excess) | Efficient control (<400.109/L) | 75 cases (55%) | 84 cases (78%) | |||
| Platelet count stable between 400 and 600.109/L | 43 cases (32%) | 19 cases (17%) | HU | 32 cases | 4 cases | |
| Pi | 7 cases | 1 case | ||||
| Platelet count increasing beyond 600.109/L | 15 cases (13%) | 5 cases (5%) | HU | 15 cases | 8 cases | |
| Pi | 4 cases | 1 case |
| . | . | HU (on 133 cases) . | Pi (on 108 cases) . | . | Escalation . | Further . |
|---|---|---|---|---|---|---|
| . | . | . | . | . | . | Change of . |
| . | . | . | . | . | . | Treatment . |
| Red cell line | Efficient control (Ht < 50%) | 109 cases (82%) | 97 cases (90%) | |||
| Insufficient control (Ht increasing toward 50%) | 24 cases (18%) | 11 cases (10%) | HU | 20 cases | 6 cases | |
| Pi | 8 cases | 3 cases | ||||
| Platelet line | ||||||
| (in patients with initial platelet excess) | Efficient control (<400.109/L) | 75 cases (55%) | 84 cases (78%) | |||
| Platelet count stable between 400 and 600.109/L | 43 cases (32%) | 19 cases (17%) | HU | 32 cases | 4 cases | |
| Pi | 7 cases | 1 case | ||||
| Platelet count increasing beyond 600.109/L | 15 cases (13%) | 5 cases (5%) | HU | 15 cases | 8 cases | |
| Pi | 4 cases | 1 case |
Erythrocyte macrocytosis (>100 fL, generally 115 to 130 fL) was observed in all the cases treated by HU, but not in those treated by Pi. No significant increase of fetal hemoglobin was noted in the HU arm (observed values always less than 3%).
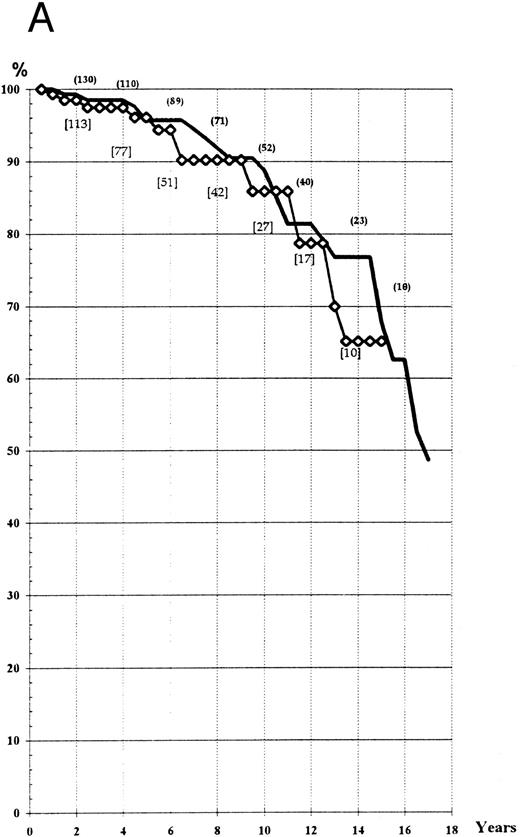
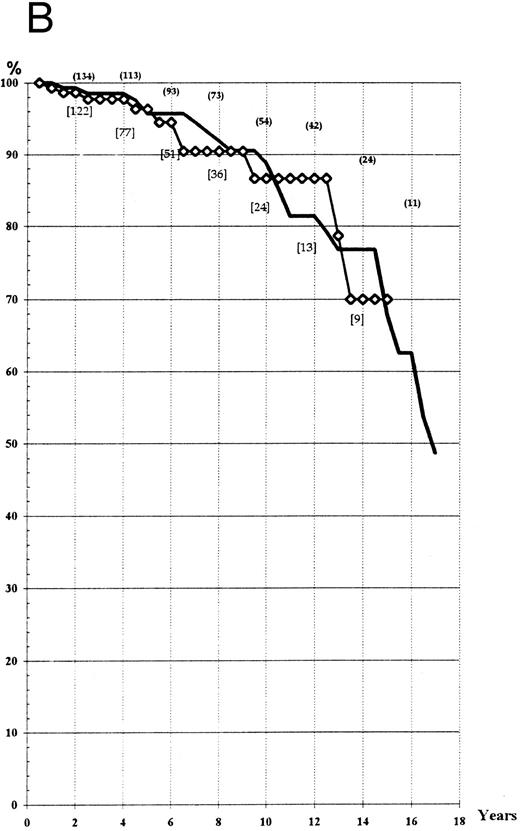
Survival. The actuarial survival in the two groups was determined according to the ‘intention to treat’ and to the ‘main treatment’ received. Life expectancy at the 14th year was about 70% in the two arms, whatever the type of analysis used (Fig 1A and B). Comparison of expected and observed deaths did not reveal any significant difference between the two groups (log-rank test, P > .3; Wilcoxon test, P > .20).
(A) Actuarial survival of the patients treated by HU or Pi (“intention to treat” analysis). (B) Actuarial survival of the patients treated by HU or Pi, taking into account any switch of therapy (“main treatment” analysis). (━), HU; (⋄), Pi.
(A) Actuarial survival of the patients treated by HU or Pi (“intention to treat” analysis). (B) Actuarial survival of the patients treated by HU or Pi, taking into account any switch of therapy (“main treatment” analysis). (━), HU; (⋄), Pi.
The mean life expectancy of a sex- and age-matched population in France is 26.5 years,14 which would lead to a 14-year survival of 83.7% (significantly higher than that observed in the PV series presented here). However, the very long-term survival still remains unknown.
Risk of vascular complications. Despite the relatively young mean age and absence of serious vascular history in 89% of the cases, 6 patients died from vascular accident and 34 patients developed a nonfatal serious vascular thrombotic event (venous [16 cases] or arterial [24 cases]).
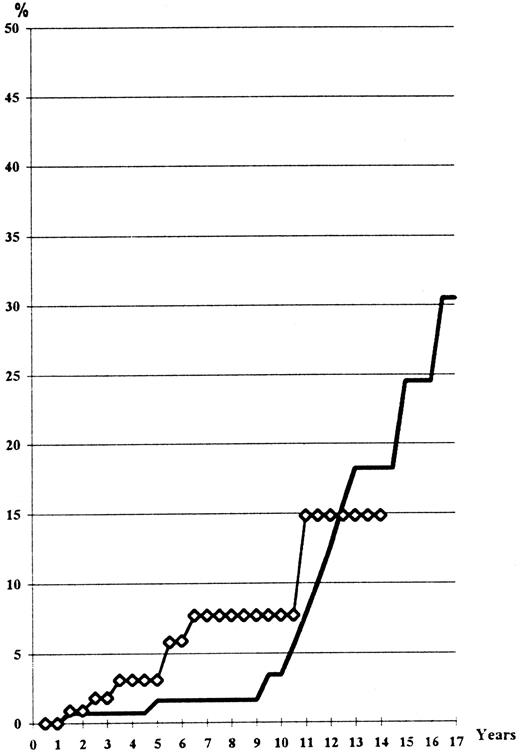
The risk is slightly higher in the HU arm in the first 8 years, but not significantly (log-rank test, P = .16); at the 14th year it is similar in the both arms (Fig 2). At the time of the vascular event, the platelet count was normal in 10 out of 14 cases in the Pi arm, but in only 13 of 26 cases in the HU arm. However, the difference is not statistically significant (chi-squared, P = .16).
Thrombo-embolic events (lethal or not) observed according to the main treatment received, including recurrent events occurring in the same patient (actuarial analysis). (━), HU; (⋄), Pi.
Thrombo-embolic events (lethal or not) observed according to the main treatment received, including recurrent events occurring in the same patient (actuarial analysis). (━), HU; (⋄), Pi.
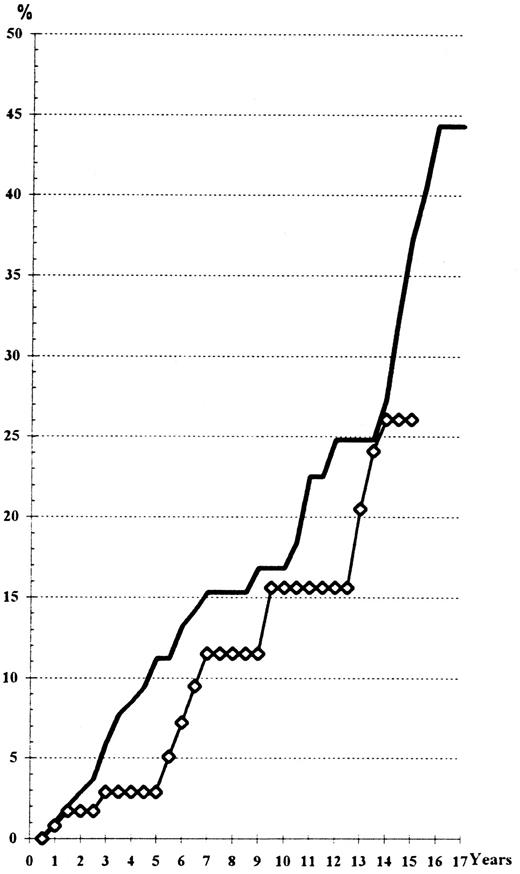
Risk of leukemia. Thirteen cases of leukemia (9 acute myelogenous leukemias [including 4 cases after myelofibrosis] and 4 myelodysplastic syndromes [refractory anemia with excess of blasts]) were observed. All of these cases died within 1 year after diagnosis, including the 4 refractory anemia with excess of blasts (RAEB) (two of them after evolution to acute myeloid leukemia [AML], another after inefficient chemotherapy, one from intercurrent femoral fracture). In comparison with the 32P-treated patients of our other trial,4 neither chronic lymphocytic leukemia nor multiple myeloma was observed, probably owing to the younger age of these chemotherapy-treated cases.
The actuarial risk of leukemia as indicated in Fig 3 is about 10% at the 13th year with no difference in the two groups (log-rank test and Wilcoxon's test, P > .30). However, a longer delay of follow-up is necessary because of the late occurrence of this complication, as shown on Fig 3. The time of occurrence of the observed cases did not differ from that observed in the 32P-treated patients.4
Actuarial risk of leukemia, according to the main treatment received. (━), HU; (⋄), Pi.
Actuarial risk of leukemia, according to the main treatment received. (━), HU; (⋄), Pi.
Risk of cancer. Sixteen patients developed a carcinoma. The actuarial cancer risk is shown in Fig 4. The incidence at the 14th year was approximately 15%, ie, a risk of 1.1% per year, which is only slightly greater than the frequency expected for this age group (0.8% per year according to French statistics).14 The type of cancer observed is shown in Table 4. The figures are too small to allow comparison with the relative frequency in France or in the world.14,15 Two cases of nonasbestos-related mesothelioma, a rare tumor, not previously reported to be associated with chemotherapy alone,16 17 should be noted because two other cases were also observed in the group of patients treated with 32P. Four cases of cutaneous cancer (basal-cell or epidermoid carcinomas) are observed in the HU arm against only one in the Pi arm.
Actuarial risk of cancer, including the skin carcinomas. (━), HU; (⋄), Pi.
Actuarial risk of evolution toward myelofibrosis (according to the main treatment received). (━), HU; (⋄), Pi.
Actuarial risk of evolution toward myelofibrosis (according to the main treatment received). (━), HU; (⋄), Pi.
Site of Cancers Observed in the Chemotherapy-Treated Patients
| . | HU . | Pi . |
|---|---|---|
| Lung | 1 | 1 |
| Pleura (mesothelioma) | 1 | 1 |
| Skin | 4 | 1 |
| Prostate | 0 | 1 |
| Breast | 0 | 1 |
| Ovary | 1 | 0 |
| Colon | 0 | 1 |
| Thyroid | 1 | 0 |
| Pancreas | 1 | 0 |
| Vagina | 1 | 0 |
| . | HU . | Pi . |
|---|---|---|
| Lung | 1 | 1 |
| Pleura (mesothelioma) | 1 | 1 |
| Skin | 4 | 1 |
| Prostate | 0 | 1 |
| Breast | 0 | 1 |
| Ovary | 1 | 0 |
| Colon | 0 | 1 |
| Thyroid | 1 | 0 |
| Pancreas | 1 | 0 |
| Vagina | 1 | 0 |
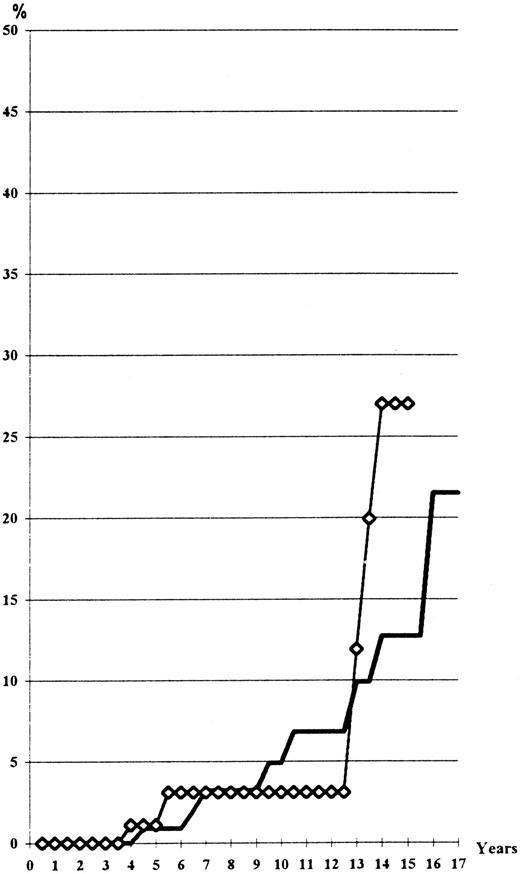
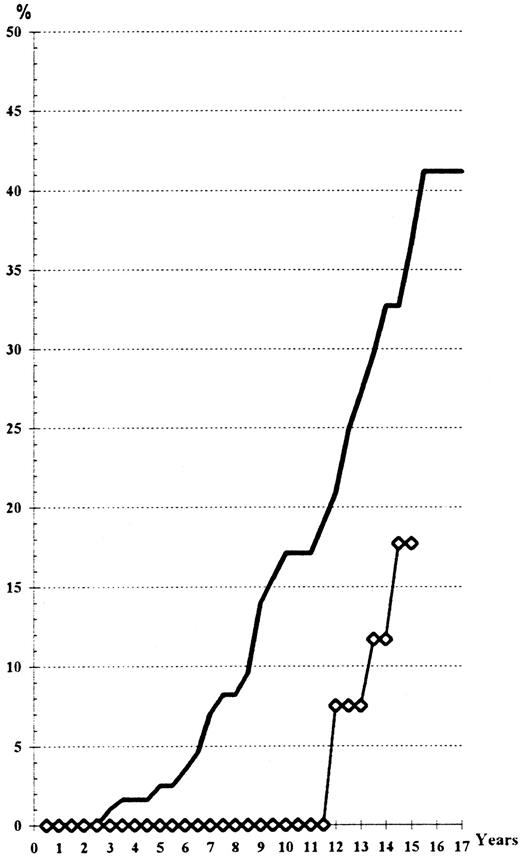
Risk of progression to a spent phase or to myelofibrosis with myeloid splenomegaly. A total of 26 cases were observed in the HU arm, with only 3 cases in the Pi group: 8 cases presented with spent phase associated with increasing splenomegaly, anemia caused by excess of plasma volume, reticulinic myelofibrosis, and 111In-transferrin splenic uptake without decreased bone marrow uptake18; 18 cases had a huge splenomegaly, collagen myelofibrosis, and low uptake of 111In-transferrin in the bone-marrow.19 The statistical difference is highly significant between the two arms (Fig 5), even when taking into account the shorter follow-up of the Pi-treated patients (log-rank test, P = .03; Wilcoxon's test, P = .01). Seven patients from among these cases have now died (4 of them from acute leukemia). The surviving cases have been followed for 1 to 9 years after the diagnosis of myelofibrosis (mean 2.9 years), so that one could expect in the next few years an adverse influence of this complication on the survival curve of patients treated with HU. The frequency (40% at the 16th year) and the early onset (as soon as the 4th year) are greater in HU-treated cases than in patients treated with 32P,4 but only slightly different from those of patients treated by phlebotomy alone.5 Six of the 23 HU-treated cases and the 3 Pi-treated cases developing myelofibrosis had been phlebotomized (protocols 01 and 05 of the PVSG) for more than 2 years before initiation of myelosuppressive treatment. Seventy-six percent of the cases who developed myelofibrosis had a permanently high platelet count (more than 400.109/L) despite maintenance treatment with a myelosuppressive agent. In contrast, only 38% of the cases that did not develop myelofibrosis had permanently excessive platelet counts (P < .03).
DISCUSSION
Because radiotherapy (32P) or radiomimetic chemotherapy (busulfan-melphalan) is generally considered to be the main cause of leukemic transformation in PV,1,2 and because long-term administration of phlebotomy is rarely accepted by the patient and favors or accelerates the development of myelofibrosis,5 we proposed in patients of 65 years or younger the use of nonradiomimetic drugs, HU or Pi.
In contrast with the small published series,20-23 we observed frequent toxicity from these drugs, which may hinder their long-term use and sometimes requires a switch of treatment. The most bothersome complications were gastrointestinal disorders with Pi and aphtous and leg ulcers with HU. These complications did occur in more than 20% of the cases, but often late, which explains why their frequency has been underestimated in the literature. On the other hand, during the induction phase, HU and Pi may cause severe thrombocytopenia in older patients. This risk requires a strict hematological monitoring during the first few weeks of treatment. Thus, chemotherapy treatment of PV patients is not as easy, innocuous, and well-tolerated as it is generally believed.
Though doses higher than those generally used for maintenance of 32P-induced remissions were used, the efficacy of maintenance therapy was often insufficient, particularly for controlling the platelet count. Despite dose escalation, the platelet count permanently exceeded 400.109/L in 22% of cases treated with Pi and in 45% of cases treated with HU. This insufficient efficacy was responsible for a significant number of treatment changes, namely in HU patients. However, dose escalation is often impossible because of a low hematocrit value. As in other series24 25 no statistically significant correlation between the vascular risk and the platelet count was observed even if a better maintenance of the platelet count in the Pi arm seems to be associated with a slightly lower risk of vascular events, at least in the first 6 to 8 years.
These young subjects have a long life expectancy. The length of this study's follow-up, at least for the Pi arm, is still not sufficient to precisely calculate the median survival,26 but in both groups mortality did exceed that expected for a matched control population. The high occurrence of late-occurring myelofibrosis in patients treated with HU raises concern about an excess of late mortality in this group of cases.
An important point concerns the risk of leukemia, approximating 10% at the 13th year. This number is not significantly different from that observed in 32P-treated patients.4 However, the number of cases followed at very long term is still too low to allow precise evaluation of the risk at long term. No significant difference was observed between the HU- and the Pi-treated cases (log-rank test) despite the fact that Pi is an alkylating and mutagenic agent in vitro.27 The suggestion that HU is not leukemogenic, which seems unlikely as it inhibits DNA repair,28,29 is not supported by our results. A similar risk of leukemia is also observed in the HU-treated thrombocythemias.30 Though no case of leukemia has been published in patients treated with HU for psoriasis or sickle-cell anemia, the follow-up of these studies is still short,31-33 and it is possible that the leukemogenic potential is observed only in preleukemic myeloproliferative syndromes, as it was also suggested for 32P.34
The slight excess of carcinomas observed in the chemotherapy-treated PV patients still requires a longer follow-up. A slight, but still not significant, excess of skin cancers occurred in the HU arm and also in the HU-maintained patients of the 32P-treated group.4 Similar data have been previously reported.35
A striking finding of the present study is the considerable relative risk of myelofibrosis observed in the patients treated with HU, a risk that is significantly higher than that observed in the Pi-treated cases. In line with the suggestion that myelofibrosis develops in response to secretion of fibrogenic cytokines, especially platelet-derived growth factor, in a persistently hyperplastic bone marrow,36-38 HU was less effective than Pi in controlling the platelet count. The bone marrow density, particularly the megakaryocytic line, was not decreased with HU.21 A significant correlation between the poor maintenance of the platelet count and the occurrence of myelofibrosis was shown in the present series.
Too little data are available in the literature on chemotherapy use in PV to allow comparison with our results. All published series with HU report a leukemia rate in the order of 8% to 10%, despite the small number of cases receiving HU alone and the short follow-up.7,21-23,38-40 These data are difficult to analyze because they were not calculated by the actuarial method and the date of occurrence of leukemia was often not specified. Two studies reported that leukemia occurred earlier than in the 32P-treated patients.41 42
Only two published reports refer to the leukemogenic risk of pipobroman. One reports the absence of leukemia in a series of 54 cases, but the median follow-up was of only 3.5 years.43 The other paper,20 with a slightly longer follow-up (median, 5 years) of 73 cases, indicates a leukemogenic risk of 9% at the 7th year. The authors of these two studies have not updated their results.
We are informed of two unpublished French series using Pi. One series by J.F. Bernard and P. Boivin includes 147 nonrandomized PV cases of all ages.44 As in our study, frequent gastrointestinal disorders are reported. All but 7 cases obtained good control of the platelet count. The median actuarial survival calculated for the patients under the age of 65 years was 19 years. Fourteen patients died from leukemia, myelodysplasia, or lymphoma between 3.5 and 18 years after starting treatment (actuarial incidence of 11.3% at the 12th year). Only 4 cases of progression to myelofibrosis were observed (between 3 and 14 years after the diagnosis). The second unpublished series, by F. Bauters and P. Fenaux, includes 37 cases under the age of 65 years who were treated for 2 to 10 years. This investigation reports good hematological efficacy, low mortality at the present time (4 deaths from vascular accident), absence of leukemia, and only one case of progression to myelofibrosis. However, these two studies cannot be combined with our results for comparative analysis of Pi versus HU because of the absence of randomization and the fact that the inclusion and follow-up criteria were not similar to those adopted in our protocol. However, these two series are in line with our findings concerning the risks and advantages of Pi.
As emphasized by Fayers,26 statistical analysis implies not only a sufficient number of cases but also a sufficient number of events (deaths, complications) to be analyzed. Despite the large number of cases included in this protocol, the number of events is probably still too small to allow these conclusions to be considered as final. However, several relevant points can be made:
The safety of the two drugs is less satisfactory than what was generally considered. Fortunately, the complications are not the same for the two drugs, allowing an effective switch.
HU ensures less effective hematological control than Pi, especially regarding platelets.
An actuarial leukemia rate of 10% at the 13th year was observed that was similar to that observed with 32P alone. Thus, the expected advantage of chemotherapy over radiotherapy has not been confirmed.
The rate of progression to myelofibrosis with HU was considerable and was much higher than that observed with myelosuppression by 32P or Pi.
ACKNOWLEDGMENT
We are indebted to Roberte Beaune, Marthe De Rosa, and Mickaele Le Bail for their secretarial assistance, and to Dr Salaun and Pr Chomienne for the linguistic review of our manuscript. Seventy-four consultants in hematology or in internal medicine departments evaluated and followed the patients included in the present protocol. We mention here only those who contributed for more than 5 cases to the present study: Drs Allard (Meaux); Belanger and Varet (Paris-Necker); Brahimi (Troyes); Brouet, Fermand, Mariette, and Marullo (Paris-Saint-Louis); Casassus (Aubervilliers); Castaigne and Gruyer (Versailles); Dupuy (Paris-Lariboisière); Gabreau (Auxerre); Gandhour (Rennes); Goguel (Boulogne); Grange (Paris-Bichat); Lejeune (Bondy); Lenoble and Echard (Montfermeil); and Rousselot and Witte (Chartres). We also acknowledge the private physicians of our patients, who followed them and informed us of clinical events. Finally, we acknowledge Professors J.F. Bernard and P. Boivin (Clichy), and Professors F. Bauters and P. Fenaux (Lille), who sent us the unpublished results of their series of cases treated by pipobroman.
Supported in part by a grant from the National Institute of Health and Medical Research (contract no. 93CN10)
Address reprint requests to Yves Najean, MD, DSc, Department of Nuclear Medicine, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, 75475 Paris Cedex 10, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal