Abstract
The conditions that control the migratory status of hematopoietic progenitor cells on extracellular matrix (ECM) and that decide whether a cell migrates or adheres are incompletely understood. We analyzed the migratory behavior of murine hematopoietic progenitor cells factor-dependent-cell-paterson (FDCP)-mix and purified lin−Sca1+ bone marrow cells on ECM. We found that migration on fibronectin (Fn) or laminin (Lam) becomes dependent on β1-integrins if a surface restraint force is introduced by tilting the ECM-coated culture vessels. Under these conditions, migration specifically occured on Fn and Lam, and was not detected on collagen IV-, hyaluronate-, or bovine serum albumin- coated surfaces. Migration depended on the continuous presence of hematopoietic cytokines interleukin-3 (IL-3), granulocyte colony-stimulating factor (G-CSF ), macrophage-CSF (M-CSF), granulocyte-macrophage-CSF (GM-CSF ), or stem cell factor (SCF), whereas other cytokines, such as IL-8, macrophage inflammatory protein-1α, macrophage-chemotactic and activating factor, and erythropoietin resulted in very little or no migratory response. IL-3 induced migration was synergistically enhanced by other CSFs, but was completely inhibited by addition of transforming growth factor-β1. In contrast to firm local adhesion of previously cytokine depleted progenitors that was rapidly inducible within 1 hour after exposure to cytokines, preincubation on Fn matrix for 4 to 6 hours was required before cytokines could induce migration. A sudden increase of cytokine concentration reversibly inhibited migration and induced a fully adhesive state; this effect could be prolonged by consecutive stimulation with heterologous cytokines. Whereas cytokines activated resting progenitor cells to migrate on ECM, cell migration speed was regulated by Fn concentration. These results indicate that β1-integrin–mediated progenitor cell adhesion and migration are differentially regulated by external stimuli and suggest that this regulation corresponds to different activation states of β1-integrins in hematopoietic progenitor cells.
BLOOD CELL formation involves the interaction of hematopoietic progenitor cells and stromal cells of the bone marrow (BM) microenvironment.1-3 Long-term BM cultures (LTC) have been used to study processes involved in lodgement, proliferation, and differentiation of developing blood cells, and stromal cells have been found to deposit extracellular matrix (ECM) molecules such as fibronectin (Fn), laminin (Lam), collagen, and hyaluronic acid.4-6 In addition to receptors for cytokines, candidate hematopoietic stem cells as well as developing precursor cells express matrix adhesion molecules, and a number of studies have shown that cytokines regulate not only hematopoietic cell survival, growth, and differentiation, but also adhesion of progenitor cells to BM ECM molecules such as Fn, Lam, and collagen.7-13 Specific adhesion receptors on progenitor cells have been described that mediate interaction with ECM, including the β1-integrin adhesion molecules very late antigen-4 (VLA-4), VLA-5, and VLA-6 and the receptor for hyaluronic acid, CD44, whereas other receptors including CD31/platelet and endothelial-cell adhesion molecule-1 (PECAM-1), the β2-integrin intracellular adhesion molecule-1 and also VLA-4 mediate cell to cell interactions.14-18 The majority of hematopoietic progenitor cells express VLA-4, VLA-5, and VLA-6 on the cell surface, and cytokines such as stem cell factor (SCF ), interleukin-3 (IL-3), or granulocyte-macrophage colony-stimulating factor (GM-CSF ) have been shown to regulate the avidity of the VLA-4 and VLA-5 adhesion receptors.19,20 Conversely, activation of cytokine receptors by matrix binding through adhesion receptors may play a role in the stimulation of erythropoietic colonies in methylcellulose cultures by Fn.21 The BM ECM component Fn has been found to preferentially mediate adhesion of primitive progenitor cells to stromal cells.7,8 Miyake et al18 observed suppression of lymphopoiesis in LTC in the presence of neutralizing antibodies to VLA-4. Moreover, in vivo VLA-4 application in primates led to the peripheralization of hematopoietic progenitor cells.22 Verfaillie23 showed cooperation of the β1-integrin VLA-4 and CD44 in adhesion of hematopoietic precursor cells to the heparin-binding domain of Fn. These studies indicate that adhesion molecules function in normal hematopoiesis. Yet, in contrast to adhesional mechanisms, little is currently known about the regulation of cell migration on ECM in hematopoietic progenitors.
Migration of primitive hematopoietic precursor cells plays an important physiological role eg, during embryonic development when hematopoietic stem cells move from yolk sac to fetal liver, spleen and BM, or after myeloablative chemotherapy when the BM is colonized by reinfused stem cells that mediate recovery of hematopoiesis. Recently, it was shown that embryonic stem cell derived hematopoietic cells with a functional deletion within the β1-integrin gene fail to compete against wild-type BM cells during development of chimeric mice due to a migrational defect.24 Although transendothelial homing and migration also involve selectin-mediated rolling processes as well as release of chemoattractants,25 data are lacking on more specific function and mechanisms for β1-integrin–mediated stem cell migration. The goal of this study was to characterize the capacity of primitive hematopoietic progenitors to migrate on purified BM ECM components in culture. By incubating tilted cultures we employed gravitational force as a substitute for the restraint that progenitor cells may have to overcome in vivo, when migrating eg, within a three-dimensional BM matrix. We found that ECM molecules Fn and Lam specifically confer motility of progenitor cells through the β1-integrin receptors VLA-5 and VLA-6, and that this process is regulated in a time- and concentration-dependent manner by hematopoietic cytokines. Our findings indicate that CSFs govern not only progenitor cell survival, proliferation, and differentiation, but also regulate progenitor cell migration and adhesion.
MATERIALS AND METHODS
Antibodies. Monoclonal antibodies M1/70 (anti-Mac 1), RB6-8C5a (anti-Gr-1), TER 119 (antierythroid antigen), RA3-6B2 (anti-B220), H129.19 (anti-CD4) and 53-6.7 (anti-CD8), secondary antirat IgG, and fluorescein isothiocyanate (FITC)-conjugated LY-6A/E E13-161.7 were used for preparation of lin−Sca1+ cells and were all purchased from Pharmingen (San Diego, CA). The following affinity-purified function-blocking antibodies were used in migration and adhesion assays: clone 9EG7 directed against the β1-integrin, a gift from Dr D. Vestweber (University of Münster, Germany), clone EA-1 blocking α6-integrin function, a gift from Dr B. Imhof (Institute of Immunology, Basel, Switzerland), clones 9C10 and R1-2 (function-blocking anti-α4-integrin), 5H10-27 (function-blocking anti-α5-integrin) and IgG2a control antibody, purchased from Pharmingen. Antibodies were used at a concentration of 10 μg/mL throughout.
Chemicals and cytokines. Purified fibronectin from human plasma, murine laminin, murine collagen IV, and hyaluronic acid were purchased from Sigma (Munich, Germany). Recombinant (r) murine interleukin-1α (IL-1α), IL-3, IL-6, IL-8, SCF, GM-CSF, and r human thrombopoietin (TPO) were from Genzyme (Cambridge, MA); r human granulocyte-CSF (G-CSF ) was from Roche (Basel, Switzerland); r human macrophage-CSF (M-CSF ) was from Sigma; r murine macrophage inflammatory protein-1α (MIP-1α), r human tumor necrosis factor-α (TNF-α), r human transforming growth factor-β1 (TGF-β1) and human macrophage chemotactic and activating factor (M-CAF ) were from R&D Systems (Abingdon, UK), r human erythropoietin (EPO) was from Cilag (Sulzbach, Germany). Cell culture grade bovine serum albumin (BSA) was obtained from Boehringer Mannheim (Mannheim, Germany).
Cells. Lin−Sca1+ cells were prepared from BM cells of male BDF1 mice (Charles River, Sulzfeld, Germany) at age 6 to 10 weeks according to standard methods.26 Briefly, mice were killed and femurs were flushed with Iscove's modified Dulbecco's medium (IMDM)/20% fetal calf serum (FCS). Lin− cells were enriched from BM cells by incubation with monoclonal antibodies against Mac-1, Gr-1, TER 119, B220, CD4, and CD8, subsequent labeling with magnetic beads coupled to secondary antirat IgG antibody and passage over MACS type CS columns according to the manufacturer's instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany). Enriched lin− cells (purity >88% as determined by flow cytometric analysis) were stained with FITC-conjugated LY-6A/E monoclonal antibody E13-161.7 (Pharmingen), phycoeythrin counterstained using antibodies against Mac-1, Gr-1, TER 119, B220, CD4, and CD8 and sorted for lin−Sca1+ cells on a FACSTAR PLUS (Becton Dickinson, Heidelberg, Germany). Sca1+ cells represented 3% to 5% of lin− cells; upon reanalysis the purity of lin−Sca1+ cells was >92%. One thousand lin−Sca1+ cells formed 144 ± 12 colonies upon culture in 0.9% methylcellulose supplemented with 20% FCS/1% BSA and 100 U/mL IL-3 within 10 days of incubation at 37°C 5% CO2 . For use in migration assays, 1 U/mL of IL-3, SCF, and GM-CSF were added to lin−Sca1+ cells within 2 hours after preparation; thus >85% cellular survival (determined by trypan blue dye exclusion) throughout the assay periods were ensured. Factor-dependent-cell-paterson (FDCP)-mix multipotent murine hematopoietic progenitor cells (clone A4) were obtained from C. Heyworth and E. Spooncer (Christie Hospital, Paterson Institute, Manchester, UK). For propagation of FDCP-mix cells at a multipotent undifferentiated stage, 20% horse serum (Sigma) in IMDM supplemented with 2% IL-3 conditioned medium from the X63 cell line that is stably transfected with a cDNA encoding murine IL-327 was added. During migration assays, FDCP-mix cells were kept at a basal concentration of 1 U/mL IL-3 to ensure >90% survival (as determined by trypan blue exclusion) during all experiments unless indicated otherwise. Only batches containing >95% undifferentiated multipotent FDCP-mix cells with blast or lymphocyte-like morphology were used for experiments. Multipotency of FDCP-mix cells was confirmed regularly by differentiating cells into granulocytes and macrophages, or erythrocytes and erythroblasts by addition of GM-CSF or EPO, respectively,28 and light microscopic analysis after 7 to 9 days on cytospin slides stained with May-Grünwald/Giemsa solutions (Merck, Darmstadt, Germany).
Coating of culture surfaces. Ninety-six–well plates (Becton Dickinson) were incubated with ECM proteins or BSA dissolved in coating buffer (20 mmol/L NaH2PO4 , 150 mmol/L NaCl in H2O, pH 7.4) at 4°C in the dark overnight. After removal of the coating buffer, one wash with phosphate-buffered saline (PBS) and one blocking step with PBS/0.2% BSA, cells were suspended in IMDM containing 20% FCS (PAN-Systems, Passau, Germany)/ 0.2% BSA and seeded into the respective assays.
Migration assays. Ninety-six–well plates (flat bottom) were inoculated with either 5,000 or 10,000 FDCP-mix cells per well, or 2,000 or 5,000 lin−Sca1+ cells per well in 100 μL IMDM/20% FCS/0.2% BSA. In one instance, serum-free medium (CellGro; Cellgenix, Freiburg, Germany) was also used. Plates were positioned at an 80° angle (near vertical) immediately after seeding at 37°C and 5% CO2 . After 12 to 24 hours and accumulation of all cells at the lower end of the well, cytokines were added and plates were repositioned at a 15° angle, with the cells at the lower ends of the wells. The distribution of cells within the wells was monitored through an inverted microscope, and identical views from cultures at different experimental conditions were photographed at 400× original magnification. For evaluation of the photographs, a line was drawn along the upper margin of the coherent cell mass that remained at the lower end of the well. Cells were counted above the line on identical views 24 hours after positioning the plates at 15°.
Video imaging of individual cellular movements. Cultures were monitored on a 37°C heated table on an inverted microscope (Zeiss, Stuttgart, Germany) at 400× or 1,000× original magnification, in either horizontal or inclined positions. Video imaging was performed through a Panasonic F10 digital camera and subsequent computer processing using Audio Video Shop Software (Avid Technology, Tewksbury, MA) or National Institutes of Health image software (public domain) on a Macintosh Power PC. Twenty-minute video clips were taken and analyzed for each experimental point. Experiments were recorded with the culture plates kept at a 15° inclination unless otherwise indicated and in 5% CO2 gassed plastic bags on a 37°C heated microscope table. For evaluation, the positions of individual cells at the start of the observation period were marked (15 to 25, unless indicated otherwise). Cell movements were followed using the fast forward and reverse functions, and locations of the individual cells at the beginning and end of an observation period were documented. All cells that had moved more than 25 μm from their starting position within 20 minutes were classified as migrated.
Adhesion assays. Coating with matrix substances and inoculation were performed as described for migration assays. Assays of cytokine stimulated adhesion were then conducted essentially as described elsewhere.19 Briefly, cells were serum starved for 4 hours, labeled with 50 μCi Na251CrO7 (du Pont, Nemours, Belgium) in 500 μL IMDM for 60 minutes at 37°C. Cells were washed twice in 37°C prewarmed IMDM/0.2% BSA and were inoculated at 10,000 cells/well onto prewarmed 96-well plates containing prewarmed IMDM supplemented with 0.2% BSA. Plates were centrifuged for 5 minutes at 300g and immediately afterwards rewarmed to 37°C on a heat block within 5 minutes. Plates were incubated for 45 minutes at 37°C, 5% CO2 unless otherwise indicated. The supernatant was removed, and wells were flushed three times with IMDM/0.2% BSA. Adherent cells were lysed in 1% SDS/1% NaOH in H2O and radioactivity was analyzed in a β-counter. Reference probes with defined cell numbers served to calculate cell numbers from scintillation counting values.
3H thymidine incorporation. Ninety-six–well plates were inoculated with FDCP-mix or lin−Sca1+ cells and were allowed to grow for 24 hours at 37°C, 5%CO2 . Subsequently, 0.6 μCi 3H thymidine deoxyribose (Amersham, UK) dissolved in PBS was added and the cultures were incubated for a further 24 hours before harvest onto 96-well Luma plates (Packard, Groningen, Netherlands) and liquid scintillation counting in a β-counter.
RESULTS
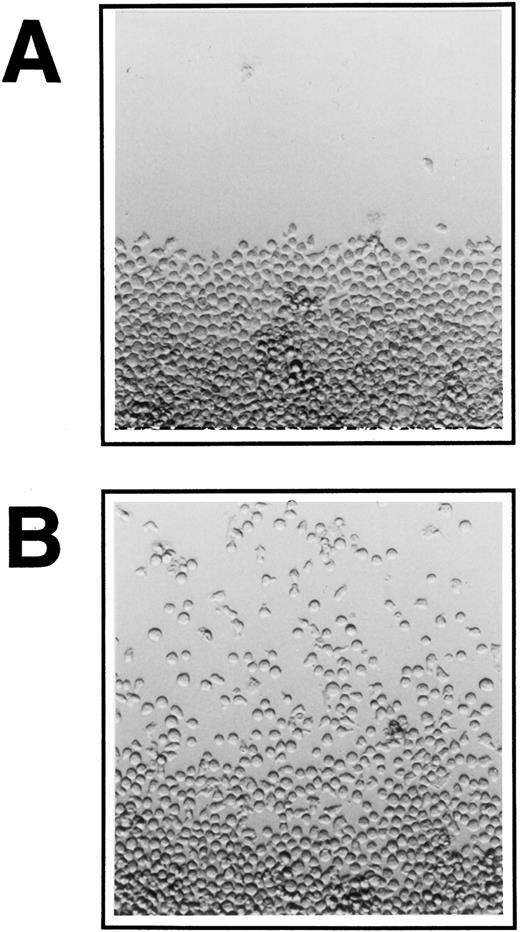
Lin−Sca1+ BM cells, a cell population highly enriched for long-term repopulating hematopoietic progenitor cells, and multipotent hematopoietic progenitor cells (FDCP-mix cells) ie, cells that are capable to differentiate into either erythroid, myeloid, or megakaryocytic cells, were analyzed for their ability to migrate on various ECM molecules. Before the start of migration experiments on ECM, the inoculated cells were allowed to accumulate at the lower end of the culture wells by positioning of the plates nearly upright at an 80° angle for 24 hours. Migration was then assayed at a 15° tilted position. Figure 1 shows FDCP-mix cells migrating on Fn-coated surface in the presence of IL-3.
Migration of FDCP-mix cells on ECM at 400× original magnification. Ten thousand cells were inoculated into 96-well plate cultures onto (A) BSA and (B) Fn-coated surface, IL-3 was added at 10 U/mL and cultures were kept at a near vertical position overnight. Cultures were then tilted at 15° towards the top end of the pictures and migration was documented photographically after 24 hours. A representative experiment is shown for each condition.
Migration of FDCP-mix cells on ECM at 400× original magnification. Ten thousand cells were inoculated into 96-well plate cultures onto (A) BSA and (B) Fn-coated surface, IL-3 was added at 10 U/mL and cultures were kept at a near vertical position overnight. Cultures were then tilted at 15° towards the top end of the pictures and migration was documented photographically after 24 hours. A representative experiment is shown for each condition.
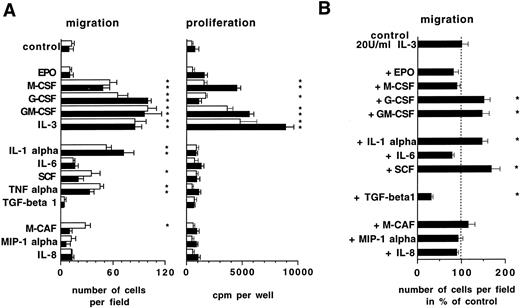
The ability of different ECM components to promote progenitor cell migration in the presence of IL-3 is shown in Table 1. Induction of migration was observed in lin−Sca1+ BM cells and FDCP-mix cells on Fn and Lam, but no significant responses were detected on Col IV, hyaluronic acid, or BSA-coated surfaces. The effect of different cytokines known to act on hematopoietic cells to elicit migration was analyzed using both lin−Sca1+ and FDCP-mix cells (Fig 2). IL-3, GM-CSF, G-CSF, M-CSF, IL-1α, SCF, and TNF-α stimulated migration on Fn, whereas of the other cytokines investigated including IL-6, EPO, and chemokines IL-8, MIP-1 α, and M-CAF, only M-CAF in lin−Sca1+ cells showed activation to a substantial degree. Cytokine induced migration was associated with an increase in cell proliferation as measured by 3H thymidine incorporation assays in the case of IL-3, GM-CSF, G-CSF, and M-CSF (Fig 2A). However, this was not the case with IL-1α, TNF-α, and SCF, which stimulated migration but did not induce a proliferative response. Therefore, cytokine-driven cell proliferation and migration are not necessarily coupled. Analysis of potential synergistic activity of cytokines to stimulate migration in FDCP-mix cells revealed that IL-3–mediated migration is further enhanced in the presence of G-CSF, GM-CSF, IL-1α, or SCF (Fig 2B). Migration was suppressed, however, in the presence of TGF-β1. These data show that cell migration on ECM is regulated in progenitor cells by hematopoietic cytokines.
Migration of Hematopoietic Progenitor Cells on Surface Coated With Various Extracellular Matrix Proteins
| Coating . | No. of Cells per Field* . | |
|---|---|---|
| . | FDCP-Mix . | lin− Sca+ BM . |
| BSA | 2 ± 1 | 7 ± 4 |
| Hyaluronic acid | 4 ± 2 | 6 ± 2 |
| Fn | 55 ± 10† | 24 ± 9† |
| Lam | 23 ± 4† | 18 ± 4† |
| Collagen IV | 4 ± 1 | 8 ± 3 |
| Coating . | No. of Cells per Field* . | |
|---|---|---|
| . | FDCP-Mix . | lin− Sca+ BM . |
| BSA | 2 ± 1 | 7 ± 4 |
| Hyaluronic acid | 4 ± 2 | 6 ± 2 |
| Fn | 55 ± 10† | 24 ± 9† |
| Lam | 23 ± 4† | 18 ± 4† |
| Collagen IV | 4 ± 1 | 8 ± 3 |
FDCP-mix A4 cells (5,000 per well) or lin− Sca+ BM cells (2,000 per well) were seeded in IMDM/FCS and 100 U/mL IL-3 into migration assays on 15°C tilted plates as described in Materials and Methods. Results are represented as means ± SD of three independent experiments.
Indicates statistically significant difference from control (Student's t-test, P < .05).
Influence of hematopoietic cytokines on progenitor cell migration and 3H thymidine incorporation. Ten thousand FDCP-mix cells (▪) or 5,000 lin−Sca1+ cells (□) were seeded into Fn-coated 96 wells and migration as well as cell proliferation were determined after 24 hours in tilted position as described in Materials and Methods. Cytokine concentrations used were: EPO, 2 U/mL; M-CSF, 50 U/mL; G-CSF, 30 ng/mL; GM-CSF, 50 ng/mL; IL-3, 100 U/mL; IL-1α, 5 ng/mL; IL-6, 10 ng/mL; SCF, 50 ng/mL; TNF-α, 10 ng/mL; TGF-β, 10 ng/mL; M-CAF, 10 ng/mL; MIP-1 α, 10 ng/mL; IL-8, 20 ng/mL. Shown are means ± SD from three independent cultures. * Indicates statistically significant difference from controls (P < .05, Student's t-test).
Influence of hematopoietic cytokines on progenitor cell migration and 3H thymidine incorporation. Ten thousand FDCP-mix cells (▪) or 5,000 lin−Sca1+ cells (□) were seeded into Fn-coated 96 wells and migration as well as cell proliferation were determined after 24 hours in tilted position as described in Materials and Methods. Cytokine concentrations used were: EPO, 2 U/mL; M-CSF, 50 U/mL; G-CSF, 30 ng/mL; GM-CSF, 50 ng/mL; IL-3, 100 U/mL; IL-1α, 5 ng/mL; IL-6, 10 ng/mL; SCF, 50 ng/mL; TNF-α, 10 ng/mL; TGF-β, 10 ng/mL; M-CAF, 10 ng/mL; MIP-1 α, 10 ng/mL; IL-8, 20 ng/mL. Shown are means ± SD from three independent cultures. * Indicates statistically significant difference from controls (P < .05, Student's t-test).
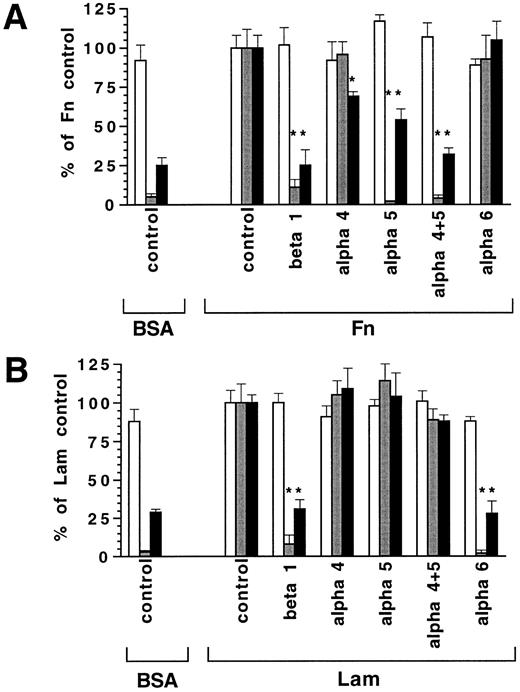
Progenitor cell migration on Fn and Lam is mediated by β1-integrins.As VLA-4 and VLA-5 are known to be expressed on a large proportion of hematopoietic progenitors and are ligands for Fn, as is VLA-6 for Lam,19 we employed antibodies that specifically interfere with the binding function of the α4-, α5-, α6-, and β1-integrin molecules to ECM. Figure 3 shows that 9EG7, an antibody blocking β1-integrin function, suppressed migration on both Fn and Lam, indicating involvement of β1-integrins in migration of hematopoietic cells. Furthermore, IL-3–induced migration on Fn could be completely abolished by 5H10-27, a neutralizing antibody to α5-integrin. In contrast, 9C10 and R1-2, two antibodies blocking α4-integrin function, did not influence Fn-mediated migration (Fig 3). Analogous results were obtained with lin−Sca1+ BM cells on Fn with 162 ± 34 (control), 182 ± 24 (anti-α4 antibodies 9C10 plus R1-2), and 61 ± 20 cells per high power field (anti-α5 antibody 5H10-27) in migration assays after 24 hours (means ± SD, n = 2). This was seen similarly in lin−Sca1+ cells when using serum-free medium, with 127 ± 17 (control), and 32 ± 6 (anti-β1 antibody) cells per field, indicating that serum was not acting as a principle source of migration stimulating activity. In contrast to the results obtained with migration on tilted plates, Fn-mediated cytokine induced adhesion in FCDP-mix cells was fully abrogated only when anti α4-integrin antibodies 9C10 and R1-2 were present in addition to the 5H10-27 anti α5-integrin antibody (Fig 3A). These data indicate a predominant role for the VLA-5 molecule in Fn-mediated migration of hematopoietic progenitors, whereas both VLA-4 and VLA-5 operate in adhesion. In analogy to the role of VLA-5 in Fn-mediated migration, migration on Lam could be completely suppressed by EA-1 antibody, which blocks α6-integrin function (Fig 3B). Therefore, VLA-5 and VLA-6 are involved in migration of lin−Sca1+ and FDCP-mix cells on Fn and Lam, respectively. Migration was blocked by anti-integrin antibodies only on tilted plates and was not detectably altered on horizontal surface, indicating that β1-integrins only became operative if a degree of restraint force was introduced by tilting of the culture plates (Fig 3).
Influence of function blocking anti-integrin antibodies on progenitor cell migration and adhesion. FDCP-mix cells (10,000 per well) were seeded on BSA, Fn, or Lam (10 μg/cm2 ) coated surfaces at 100 U/mL IL-3. Shown are the responses of migration assays on horizontal surface (□), of migration on tilted surface (), and of adhesion assays (▪) in % of the controls treated with IgG control antibody. Antibodies used were: 9C10 plus R1-2 (anti α4-integrin), 5H10-27 (anti α5-integrin), EA-1 (anti α6-integrin), 9EG7 (anti β1-integrin). Values are means ± SD from triplicate experiments. * Indicates statistically significant difference from control (P < .05, Student's t-test). Control values were: Fn/even migration, 76 ± 6%; Fn/migration on tilted surface, 51 ± 6 per field; Fn/adhesion, 58 ± 5%; Lam/even migration 66 ± 6%; Lam/migration on tilted surface, 33 ± 4 per field; Lam/adhesion, 48 ± 5%.
Influence of function blocking anti-integrin antibodies on progenitor cell migration and adhesion. FDCP-mix cells (10,000 per well) were seeded on BSA, Fn, or Lam (10 μg/cm2 ) coated surfaces at 100 U/mL IL-3. Shown are the responses of migration assays on horizontal surface (□), of migration on tilted surface (), and of adhesion assays (▪) in % of the controls treated with IgG control antibody. Antibodies used were: 9C10 plus R1-2 (anti α4-integrin), 5H10-27 (anti α5-integrin), EA-1 (anti α6-integrin), 9EG7 (anti β1-integrin). Values are means ± SD from triplicate experiments. * Indicates statistically significant difference from control (P < .05, Student's t-test). Control values were: Fn/even migration, 76 ± 6%; Fn/migration on tilted surface, 51 ± 6 per field; Fn/adhesion, 58 ± 5%; Lam/even migration 66 ± 6%; Lam/migration on tilted surface, 33 ± 4 per field; Lam/adhesion, 48 ± 5%.
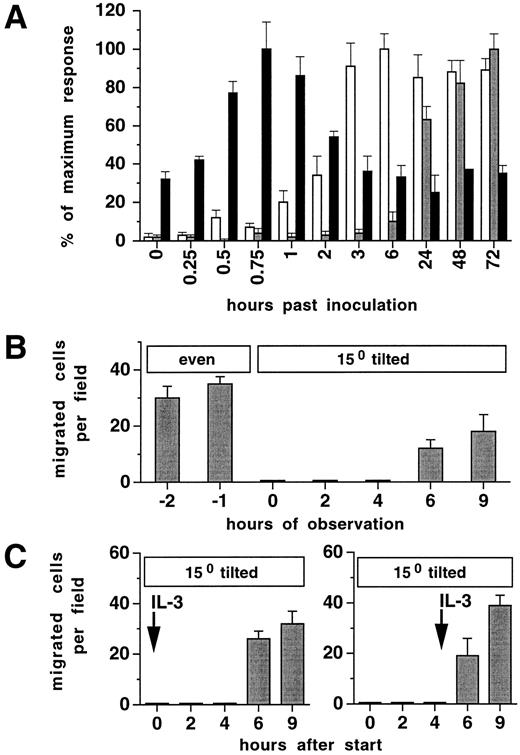
Induction of integrin-mediated migration requires previous contact with Fn.Cytokine-stimulated adhesion in FDCP-mix cells was rapidly inducible reaching maximal levels within 45 minutes and approached baseline levels within 2 to 3 hours (Fig 4A). At later time points, baseline or near baseline levels were recorded over a period of 72 hours. In contrast, migration on tilted plates was not detected before 6 hours. To determine whether contact with Fn alone, or the presence of a surface restraint driven by gravitational force was necessary to induce β1-integrin–mediated migration, additional experiments were performed. Firstly, Fn-coated cultures with cells preincubated in 100 U/mL IL-3 in a horizontal position for 24 hours were subsequently tilted to 15° under continuous video monitoring. Despite the presence of 100 U/mL IL-3, >97% of previously migrating cells accumulated on the bottom end of the well within 1 hour and were not able to further migrate (Fig 4B). This shows that although progenitor cells have been in continuous contact with Fn, binding strength between progenitors and Fn on horizontal surface was not sufficient to enable progenitors to attach to the matrix in a way strong enough to allow efficient and coordinated movement. Figure 4C shows that preincubation at a tilted position for at least 4 hours is required to induce progenitor cells to migrate. No difference was noted in progenitor cell response between cultures where 100 U/mL IL-3 was present or absent during the first 4 hours of Fn contact in a tilted position. Taken together, these results indicate that, in contrast to the rapid induction and the short-lived kinetics of cytokine-induced adhesion, induction of β1-integrin–mediated migration requires an activation period of several hours on Fn.
Induction of migration but not adhesion requires previous contact with Fn. (A) Time course of induction of horizontal migration (□), migration on tilted surface (), or adhesion (▪). Ten thousand FDCP-mix cells were assayed as described in Materials and Methods on plates precoated with Fn (10 μg/cm2 ) and in 100 U/mL IL-3. Values are means ± SD from triplicate experiments. Absolute values of the maximum responses were: Migration on horizontal surface, 85 ± 8%; migration on tilted surface, 46 ± 4 per field; adhesion, 57 ± 9%. (B and C) Previous contact on Fn and plate tilting are required to induce migration, independently of cytokine concentration in the culture medium. Ten thousand FDCP-mix cells were inoculated into 96-well plates precoated with Fn, and incubated in the presence of 100 U/mL IL-3 in a horizontal position and tilted at time point zero (B), or kept at a tilted position from the start of the experiment in the presence of 100 U/mL IL-3 from time point zero or 4 hours as indicated (C). Results in (B) and (C) are given as migrating cells analyzed by video imaging at 100× original magnification. Results represent means ± SD of triplicate determinations.
Induction of migration but not adhesion requires previous contact with Fn. (A) Time course of induction of horizontal migration (□), migration on tilted surface (), or adhesion (▪). Ten thousand FDCP-mix cells were assayed as described in Materials and Methods on plates precoated with Fn (10 μg/cm2 ) and in 100 U/mL IL-3. Values are means ± SD from triplicate experiments. Absolute values of the maximum responses were: Migration on horizontal surface, 85 ± 8%; migration on tilted surface, 46 ± 4 per field; adhesion, 57 ± 9%. (B and C) Previous contact on Fn and plate tilting are required to induce migration, independently of cytokine concentration in the culture medium. Ten thousand FDCP-mix cells were inoculated into 96-well plates precoated with Fn, and incubated in the presence of 100 U/mL IL-3 in a horizontal position and tilted at time point zero (B), or kept at a tilted position from the start of the experiment in the presence of 100 U/mL IL-3 from time point zero or 4 hours as indicated (C). Results in (B) and (C) are given as migrating cells analyzed by video imaging at 100× original magnification. Results represent means ± SD of triplicate determinations.
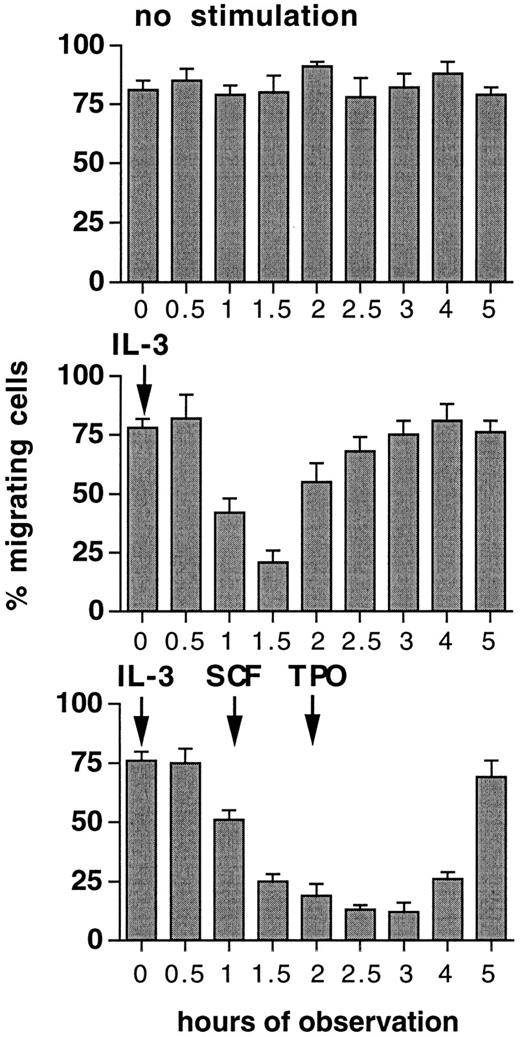
Migration is converted into adhesion by increasing cytokine concentration.To investigate if individual cells are capable to convert from a migrational state to adhesion, FDCP-mix cells were seeded on Fn-coated surface at 10 U/mL IL-3 and analyzed at a 15° angle. Cell movements were determined by time-lapse video microscopic analysis for periods of 20 minutes. Figure 5 shows that FDCP-mix cells exhibit directional cell movements. When 24 hours after induction of migration the concentration of IL-3 was subsequently increased from 10 U/mL to 100 U/mL, a complete but temporary cessation of cellular movements was induced within a relatively short period of 60 to 90 minutes (Fig 6). Whereas at time points 0 and 3 hours of this experiment, only <15% of cells were firmly adherent and resistant to removal by medium flushing as performed in adhesion assays, at time point 1.5 hours >70% of cells remained adherent. Therefore, migrating cells may reversibly convert to a full-adhesional state upon an increase in cytokine concentration. The restart of cell migration at later time points after the increase of IL-3 concentration could be counteracted by subsequent addition of SCF after a further 1 hour and TPO after 2 hours (Fig 6). This shows that sequential stimulation by heterologous cytokines may, within relatively short time periods, inhibit progenitor cell migration on ECM.
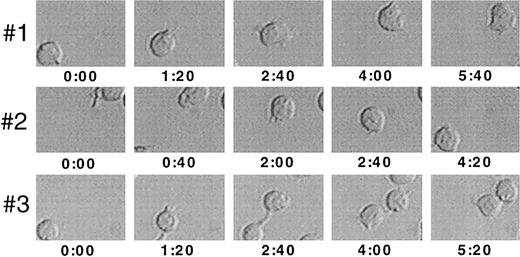
Video image analysis of migrating cells. Ten thousand FDCP-mix cells were seeded into 96-well migration assays precoated with 10 μg/cm2 Fn in the presence of 10 U/mL IL-3, and analyzed at a 15°C tilted position for a 20-minute period using time lapse video microscopy. Three representative progenitor cells, each imaged at different time points within an identical section of the observed field, are shown at 1,000× orginal magnification. Values indicated are time points in minutes:seconds after the start of the observation period.
Video image analysis of migrating cells. Ten thousand FDCP-mix cells were seeded into 96-well migration assays precoated with 10 μg/cm2 Fn in the presence of 10 U/mL IL-3, and analyzed at a 15°C tilted position for a 20-minute period using time lapse video microscopy. Three representative progenitor cells, each imaged at different time points within an identical section of the observed field, are shown at 1,000× orginal magnification. Values indicated are time points in minutes:seconds after the start of the observation period.
Inhibition of cell migration by increases in cytokine concentration or heterologous cytokine restimulation. FDCP-mix cells (10,000/well) were inoculated at 10 U/mL IL-3 into 96-well plates precoated with 10 μg/cm2 Fn and incubated for 24 hours in a 15° tilted position before observation was started. Cytokines were added at 100 U/mL as indicated during the observation period. Cell movements were analyzed by video microscopy. For each time point, 20 to 25 cells were randomly selected and cells migrated <25 μm within a 20-minute observation period were determined. Values are means ± SD from duplicate determinations.
Inhibition of cell migration by increases in cytokine concentration or heterologous cytokine restimulation. FDCP-mix cells (10,000/well) were inoculated at 10 U/mL IL-3 into 96-well plates precoated with 10 μg/cm2 Fn and incubated for 24 hours in a 15° tilted position before observation was started. Cytokines were added at 100 U/mL as indicated during the observation period. Cell movements were analyzed by video microscopy. For each time point, 20 to 25 cells were randomly selected and cells migrated <25 μm within a 20-minute observation period were determined. Values are means ± SD from duplicate determinations.
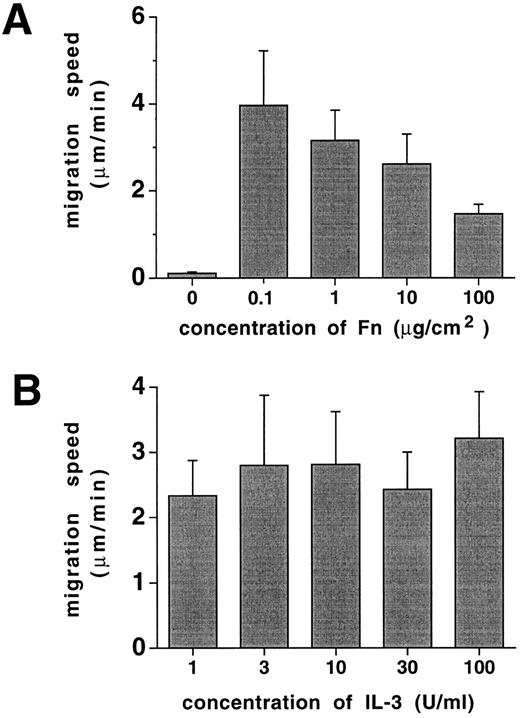
Cell migration speed on Fn is a function of Fn concentration. Whereas the proportion of progenitor cells that move on ECM is primarily regulated by cytokines, we noted that the speed of cell migration was not affected to a significant degree by cytokine concentration (Fig 7). However, migration speed depended on Fn concentration with highest cellular velocities recorded at relatively low Fn concentrations, declining with increasing Fn-coating densities (Fig 7). Therefore, the speed of progenitor cell migration is primarily a function of Fn concentration.
Progenitor cell migration speed is a function of Fn concentration. Ten thousand FDCP-mix cells were inoculated into migration assays in 96-well plates as described in Materials and Methods and assayed at a 15° tilted position. Migrational movements of individual cells were analyzed by video microscopy for each experimental point, and the average speed was calculated. (A) Cultures at 10 U/mL IL-3, with varying Fn-coating concentrations as indicated, and (B) cultures precoated with 10 μg/cm2 Fn at varying concentrations of IL-3 as indicated. Results represent means ± SD of 6 to 8 determinations.
Progenitor cell migration speed is a function of Fn concentration. Ten thousand FDCP-mix cells were inoculated into migration assays in 96-well plates as described in Materials and Methods and assayed at a 15° tilted position. Migrational movements of individual cells were analyzed by video microscopy for each experimental point, and the average speed was calculated. (A) Cultures at 10 U/mL IL-3, with varying Fn-coating concentrations as indicated, and (B) cultures precoated with 10 μg/cm2 Fn at varying concentrations of IL-3 as indicated. Results represent means ± SD of 6 to 8 determinations.
DISCUSSION
We have shown that cytokines regulate migration of hematopoietic progenitors on Fn and Lam in a dose- and time-dependent manner. VLA-5 and VLA-6 are involved in migration of hematopoietic progenitors on Fn and Lam, provided that an element of surface restraint force is involved, which was induced by tilting of the cultures. An increase in ambient cytokine concentration results in conversion of a migratory to a resident adhesive state in progenitors. These results, and the prolonged activation period required only for migration, but not for adhesion present evidence for a differential regulation of adhesive and migratory behavior in hematopoietic progenitors.
Cytokines regulate migration of hematopoietic progenitors. CSFs were major stimulators of migration on tilted surface in hematopoietic progenitors. SCF, which on its own acts as a survival factor rather than a classical CSF in hematopoiesis,29 was also a potent stimulator of hematopoietic progenitor migration. Similarly, IL-1α, which does not on its own stimulate significant hematopoietic colony formation, also proved to be a major stimulator of migration in our experiments. We did not see more than subtle increases in proliferation by IL-1α or SCF on hematopoietic progenitors under the conditions of the migration experiments. Thus, hematopoietic progenitor cell migration may be associated with, but is not strongly related to cell proliferation.
β1-integrins mediate progenitor cell adhesion and migration. VLA-4, VLA-5, and VLA-6 molecules have been found to be highly expressed in hematopoietic progenitor cell populations.19,20 The dependence of migration on the intact binding function of VLA-5 and the VLA-6 molecules as shown in experiments using function blocking antibodies is analogous to the dependence of hematopoietic progenitor adhesion on VLA-4, VLA-5, and VLA-6. This shows that integrin adhesion receptors are used by hematopoietic progenitor cells not only during permanent local adhesion, but also during migration against resistance as assessed here on tilted plates. Other than in migration assays, both VLA-4 and VLA-5 were involved in the interaction of progenitor cells with Fn in cytoadhesion assays. This is in line with previous studies who found that both VLA-4 and VLA-5 partake in adhesion to Fn in human hematopoietic progenitor cells.8-11,19,20 Also, VLA-4 but not VLA-5 was found to play a dominant role in the chemokine-induced adhesion of human peripheral blood T lymphocytes to Fn.30,31 In hematopoietic cells, a biological activity of VLA-4 is also indicated by the fact that anti-VLA-4 treatment of primates results in mobilization of hematopoietic progenitors from BM into blood.22 This effect was explained by VLA-4 mediated cell to cell adhesion, possibly involving ligands such as vascular cell adhesion molecule-1/CD106 which is present on reticular cells, sinusoidal endothelium and other hematopoietic cells, or cell-matrix adhesion.25,31 32 Our observations confirm a function for VLA-4 in progenitor cell adhesion, but suggest that VLA-5, in addition to mediating adhesion, interacts with Fn to enable migration of hematopoietic progenitors against resistance.
Cytokine-mediated adhesion and migration are differentially regulated by changes in cytokine concentration over time, and contact with ECM.In our experiments, migration was only a function of β1-integrin activation in tilted cultures, but not on horizontal surface. β1-integrin engagement also conferred the dependence of migration on cytokines. In addition, the activation of β1-integrin–mediated migration followed different kinetics than β1-integrin–mediated adhesion, and migration on tilted plates required previous contact to Fn for periods of 4 to 6 hours. This activation period indicates that migration on tilted surface, in contrast to cytokine-induced firm local adhesion or migration on an even surface, may depend on a different intracellular activation pathway, possibly including the neosynthesis or posttranslational modification of intracellular proteins. Interestingly, in fibroblasts ligand binding by β1-integrins could be induced by restraining Fn-coated latex beads.33 Also, in fibroblasts movement of β1-integrin binding beads when restrained with an optical trap intended to mimic extracellular attachment sites of different resistance, revealed that cells precisely sense a restraining force and react to this by activation of coordinated integrin movement on the cell surface.34 Our findings using ECM contact combined with plate tilting, which both together induce a shear force on all cells resting on its surface and which are both required for β1-integrin – mediated progenitor cell migration, point to a similar ability of hemtopoietic cells to sensitize traction and restraint force on Fn matrix, and to respond by activation of Fn receptor and subsequent coordinated cellular migration. Our results suggest that the induction of migration represents an “outside in”-mechanism, ie, direct activation of integrin receptor by its ligand. This is in contrast to induction of adhesion by cytokine incubation, where cytokines regulate via an “inside out” pathway the ability of β1-integrins to strongly bind to ECM.19
Our experiments also show that firm adhesion can be reversibly induced in migrating cells seeded at a certain concentration of cytokine, merely by an increase in cytokine concentration. If niches for primitive cells exist within hematopoietic marrow, local dynamic changes of CSF concentrations may therefore be a mechanism to withhold migrating primitive progenitors on site by induction of adhesion, and to release them by reversion to a migrational state. In addition, local production of TGF-β1 may be a means to withhold progenitors at certain sites within the BM stromal framework.
Taken together, we have shown that hematopoietic progenitor cells display migratory behavior on Fn or Lam matrices. CSFs stimulate this activity, which in contrast to the short-term adhesion elicited by cytokines, is a persistent process. Our experiments suggest that hematopoietic cytokines in conjunction with ECM components Fn and Lam are major mediators of progenitor cell migration. This may play a role during BM recolonization after hematopoietic transplantation, where cytokine production by marrow stroma cells and serum CSF levels are both greatly increased, and successful homing and microenvironmental localization of transplanted stem cells is likely to be a key event in hematopoietic regeneration.
ACKNOWLEDGMENT
We thank Klaus Geiger for excellent technical assistance, Dr Guido Wolff-Vorbeck for cell sorting, and Drs Clare M. Heyworth and Elaine Spooncer (Paterson Institute, Manchester, UK) for support with FDCP-mix cells. Also, we are grateful to Dr Conrad Bleul for helpful discussions, and Dr Marie Follo for critically reading the manuscript.
Supported by Deutsche Forschungsgemeinschaft (Bonn, Germany) through a grant to E.S. and through Sonderforschungsbereich 364 to R.H. and R.M.
Address reprint requests to Reinhard Henschler, MD, Department of Hematology and Oncology, University Medical Center, Hugstetter Strasse 55, 79106 Freiburg, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal