Abstract
The administration of low dose interleukin-2 (IL-2) results in a selective expansion of natural killer (NK) cells in vivo, and promotes the differentiation of NK cells from hematopoietic precursor cells in vitro. We have previously shown that stem cell factor (SCF ), the ligand to the c-kit tyrosine kinase receptor, enhances IL-2–induced NK cell proliferation and differentiation in vitro. Here, we investigated the effects of SCF plus IL-2 delivered to mice in vivo. Eight-week-old C57BL/6 mice were treated with a continuous subcutaneous infusion of IL-2 (1 × 104 IU/d) plus a daily intraperitoneal dose of SCF (100 μg/kg/d), IL-2 alone, SCF alone, or vehicle alone for 8 weeks. The in vivo serum concentration of IL-2 ranged between 352 ± 12.0 pg/mL and 606 ± 9.0 pg/mL, achieving selective saturation of the high affinity IL-2 receptor, while the peak SCF serum concentration was 296 ± 13.09 ng/mL. Alone, the daily administration of SCF had no effect on the expansion of NK cells. The continuous infusion of IL-2 alone did result in a significant expansion of NK1.1+CD3− cells compared to mice treated with placebo or SCF. However, mice treated with both SCF and IL-2 showed an increase in the absolute number of NK cells that was more than twofold that seen with IL-2 alone, in the spleen (P ≤ .005), bone marrow (P ≤ .025), and blood (P < .05). NK cytotoxic activity against YAC-1 target cells was significantly higher for mice treated with SCF plus IL-2, compared to mice treated with IL-2 alone (P ≤ .0005). Interferon-γ (IFN-γ) production in cytokine-activated splenocytes was also greater for the SCF plus IL-2 group, over IL-2 treatment alone (P ≤ .01). The effect of SCF plus IL-2 on NK cell expansion was likely mediated via NK cell precursors, rather than mature NK cells. In summary, we provide the first evidence that SCF can significantly enhance expansion of functional NK cells induced by the prolonged administration of low dose IL-2 in vivo. Since the NK cell is a cytotoxic innate immune effector and a potent source of IFN-γ, this therapeutic strategy for NK cell expansion may serve to further enhance innate immune surveillance against malignant transformation and infection in the setting of cancer and/or immunodeficiency.
NATURAL KILLER (NK) cells are bone marrow (BM)-derived large granular lymphocytes that mediate non-major histocompatibility complex (MHC) restricted cytotoxicity, and are a critical component of the innate immune response to viral and obligate intracellular parasitic infection.1-4 Murine NK cells lack CD3 and can express the NK1.1 surface antigen, and are functionally characterized by their ability to spontaneously lyse tumor-derived target cells such as YAC-1.5 Murine6 and human7,8 NK cells constitutively express functional forms of the interleukin-2 receptor (IL-2R). The daily subcutaneous administration of low dose IL-2 can selectively expand NK cells in vivo in both mice9 and humans.10,11 Further, numerous studies have described the differentiation of NK cells from murine and human BM hematopoietic precursor cell (HPC) cultures with IL-2 in vitro.12-19
The proto-oncogene c-kit encodes a tyrosine kinase receptor that is expressed on normal HPCs at various stages of maturation.20-23 Stem cell factor (SCF, kit ligand, or steel factor) is a ligand for the c-kit receptor.24-26 While SCF alone has little effect on HPC differentiation,27 it acts synergistically with numerous hematopoietic growth factors, such as IL-3, IL-6, erythropoietin (EPO), granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor (GM-CSF ), to augment their effects on HPC differentiation in vitro28-30 and in vivo.31-35 A subset of human NK cells has been shown to express the c-kit receptor, and exposure to SCF in vitro promotes their survival and potentiates their proliferative response to IL-2.36,37 Highly purified human CD34+ HPCs are induced to differentiate and expand (6- to 16-fold) into CD56+ NK cells in the presence of IL-2 plus SCF, while the absence of SCF led to a dramatic reduction in NK cell expansion without affecting differentiation.19 Collectively, these data suggested that SCF may enhance IL-2–induced NK cell expansion in vivo, and the current study was performed to test this hypothesis.
MATERIALS AND METHODS
Animals. Six-week-old female C57BL/6 mice were obtained from Taconic Farms (Germantown, NY), and were housed in microisolator cages within a pathogen-free animal facility at the Roswell Park Cancer Institute. Mice were 8 weeks of age with an average weight of 23 g when treatment was initiated.
Cell lines. NK cytotoxicity assays were performed with the NK-sensitive target cell line YAC-1, grown in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS; Sigma, St Louis, MO), anti-PPLO agent, and antibiotics (GIBCO-BRL, Grand Island, NY).
Antibodies. Hamster anti-mouse CD3-fluorescein isothiocyanate (FITC), rat anti-mouse IL-2Rβ-FITC, rat anti-mouse c-kit-FITC, mouse anti-mouse NK1.1-phycoerythrin (PE) conjugated monoclonal antibodies (MoAbs), rat anti-mouse CD16/CD32 (Fc Block), and rat PE- and FITC-conjugated nonreactive mouse isotype control MoAb were purchased from Pharmingen (San Diego, CA). PE- and FITC-conjugated nonreactive mouse isotype control MoAbs were purchased from Becton Dickinson (San Jose, CA), Unlabeled nonreactive mouse Ig (IgG1 ) was obtained from Sigma, and hamster Ig (IgG1 ) was obtained from Pharmingen.
Cytokines. Recombinant-methionyl human (rh)IL-2 (specific activity 7.4 × 106 U/mg, <2.5 endotoxin units (EU) / mg protein) and polyethylene glycol (PEG)-complexed recombinant rat (rr)SCF (<1 EU / mg protein) were kindly provided by Amgen Inc (Thousand Oaks, CA). For in vitro stimulation of unfractionated splenocytes, rhIL-2 (Hoffman-LaRoche, Nutley, NJ, specific activity 1.5 × 107 U/mg) and murine rIL-12 (Genetics Institute, Cambridge, MA, specific activity 4.6 × 106 U/mg) were used. For in vivo pharmacokinetic (PK) studies rhSCF-PEG (Amgen) was used.
Drug administration. Four treatment groups of mice consisted of (1) placebo (vehicle only) (n = 5); (2) rrSCF plus vehicle (n = 6); (3) rhIL-2 plus vehicle (n = 6); (4) rhIL-2 plus rrSCF (n = 6), all for 8 weeks duration. IL-2 was administered via Alzet osmotic mini-pumps (Alza Corp, Palo Alto, CA), set to deliver 1 × 104 IU per day in a vehicle of phosphate-buffered saline (PBS; GIBCO) containing 0.1% syngeneic mouse serum (Taconic Farms). The osmotic pumps were inserted subcutaneously (s.c.) on the dorsum of the mice following the manufacturer's instructions and replaced every 10 days. Mice not treated with IL-2 were fitted with mini-pumps set to deliver an equal volume of vehicle as the IL-2–treated mice. SCF was injected intraperitoneally (i.p.) once daily at a dose of 100 μg/kg body weight in a volume of 100 μL PBS containing 0.1% syngeneic mouse serum. Mice not treated with SCF received a daily i.p. injection of vehicle only. All mice were therefore manipulated identically with regard to drug administration. PK for the administration of IL-2 via the s.c. pumps and SCF i.p. injection were performed by collecting serum at the indicated time points, and assayed by enzyme-linked immunosorbent assay (ELISA) for IL-2 (Endogen, Woburn, MA) or SCF (Amgen Inc). PK studies for SCF-PEG were performed with rhSCF-PEG so that exogenously administered SCF-PEG and endogenously produced mouse SCF could be distinguished. The ELISA used to measure rhSCF-PEG levels (Amgen) does not cross react with mouse SCF. Serum samples were not collected frequently enough to calculate area under the curve. All animal procedures were approved by the Institute Animal Care and Use Committee.
Isolation of spleen, BM, and blood cells. Mice were anesthetized with ether and killed by cervical dislocation. Blood was obtained by direct cardiac puncture. Spleens were excised, weighed, disrupted, and passed through stainless steel mesh into RPMI-1640 supplemented with 10% fetal calf serum (FCS). Splenocytes were then gently pipetted to create a single cell suspension. In some instances indicated below, splenocytes were then passed through a nylon wool column, and the nonadherent NK1.1+CD3− lymphocytes were sorted to >98% purity on a FACStar Plus flow cytometer (Becton Dickinson). BM from each mouse was obtained from two femurs by flushing the bone shaft with RPMI-1640 plus 10% FCS with a 27 gauge needle. Contaminating red blood cells (RBCs) were removed from the single cell suspensions of all tissues by incubation for 5 minutes at room temperature with sterile ammonium chloride/potassium lysis buffer. After a final resuspension in RPMI-1640 plus 10% FCS, the total number of cells from each tissue were enumerated with a hemocytometer.
Cytotoxicity assay. Unfractionated splenocytes were assessed for NK cytotoxic activity in a standard 51Cr release assay against YAC-1 tumor cells. One million YAC-1 target cells were labeled with 100 μCi of 51Cr for 1 hour, washed four times, and plated at 5 × 103 cells per well in a 96-well V-bottom plate. Splenocytes were freshly isolated from each mouse and incubated with YAC-1 target cells at an E/T ratio of 200:1 for 4 hours at 37°C in a total volume of 200 μL, in the absence of any exogenous cytokines. One hundred microliters of supernatant was then procured from each well and counted on a gamma counter to determine experimental release. Spontaneous release was determined from labeled target cells incubated in medium alone, while maximal release was obtained from labeled targets lysed in 1% NP-40. Percent specific cytotoxicity = [(experimental release − spontaneous release) / (maximal release − spontaneous release)] × 100.
Flow cytometric analysis. Approximately 1 × 106 spleen, BM, and blood cells were each incubated with nonreactive, unlabeled mouse and hamster Ig for 15 minutes on ice, and then stained with PE- and FITC-conjugated nonreactive isotype control MoAb, or anti-NK1.1-PE plus anti-CD3-FITC MoAb, for 30 minutes on ice. Cells were then washed twice with PBS and resuspended in 1 mL of 1% formalin in PBS, and forward scatter, side scatter, and fluorescence data were collected on 10,000 events using a FACScan (Becton Dickinson, Mountain View, CA). Flow cytometric data were then analyzed using the Winlist software program (Verity Software House, Topsham, ME), and results displayed in 2 parameter orthographic dot plots, with percentages indicating the fraction of total ungated events collected. Control PE- and FITC-stained cells were used to set quadrant gates, with >99% of control events designated as background and nonspecific fluorescence.
Analysis of cytokine production. 5 × 105 unfractionated splenocytes from each mouse were plated with 200 μL medium (RPMI-1640 plus 10% FCS), rhIL-2 (10 ng/mL) plus rmIL-12 (30 ng/mL), in 96-well U-bottom plates. Cell cultures were then incubated at 37°C for 72 hours, after which cell-free supernatants were harvested and assayed by ELISA (Endogen) for murine IFN-γ.
Proliferation and immunomodulation assays. Purified (>98%) NK1.1+CD3− splenocytes were plated in RPMI-1640 supplemented with 10% FCS, 5 μmol/L β-mercaptoethanol (BME) and antibiotics at a concentration of 1 × 105/well. Cells were cultured for 24 and 48 hours in one of four conditions: (1) medium alone; (2) rrSCF-PEG (100 ng/mL); (3) rhIL-2 (10 ng/mL); (4) SCF plus IL-2. Cells were then procured, preincubated with Fc Block (Pharmingen), and stained with anti-NK1.1-PE and either anti-c-kit-FITC, anti-IL-2Rβ-FITC, or isotype control-FITC MoAb. Ten thousand events were collected and analyzed as described above. For assessment of proliferation, 5 × 104 sorted NK1.1+CD3− cells were plated in triplicate in 200 μL of medium with an increasing concentration of rhIL-2 (0.001 nmol/L, 0.01 nmol/L, 0.1 nmol/L, 1 nmol/L, and 10 nmol/L) with and without 100 ng/mL rrSCF-PEG, for 72 hours at 37°C. During the final 24 hours of incubation, 3H-thymidine was added to the cultures. Cells were then procured and proliferation was assessed by measuring 3H-thymidine incorporation. A similar assay was performed for 7 days, at which time cells were counted using a hemocytometer and viability assessed by vital dye exclusion.
Statistical analysis. All comparisons between treatment groups were performed using the unpaired Student's t-test, with P < .05 designated as significant.
RESULTS
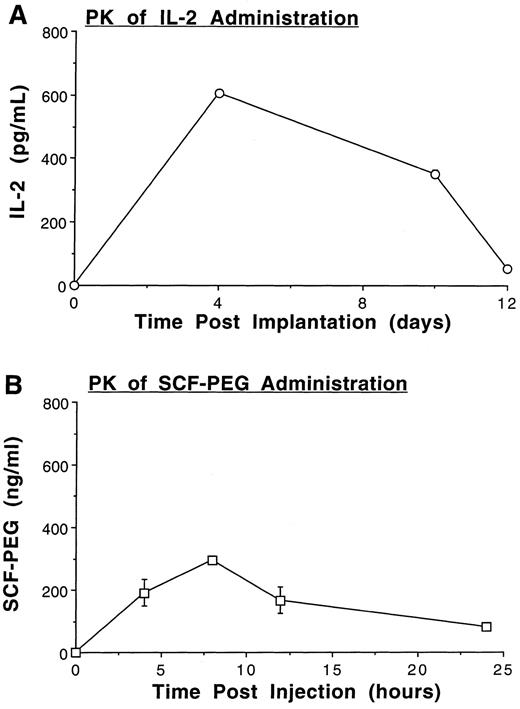
Pharmacokinetics of IL-2 and SCF administration in vivo. Eight-week-old female C57BL/6 mice were treated with a continuous s.c. infusion of IL-2 at a dose of 1 × 104 IU per day via osmotic mini-pumps, and/or SCF at a dose of 100 μg/kg body weight by once-daily i.p. injection, each for 8 weeks duration. PK of IL-2 delivered by this method resulted in serum concentrations in the range of 352 ± 12.0 pg/mL to 606 ± 9.0 pg/mL (21 to 88.5 picomolar). Thus, serum IL-2 concentrations were capable of saturating the high affinity IL-2Rαβγ, and partially saturating the intermediate affinity IL-2Rβγ (Fig 1A).38 A single i.p. injection of rSCF resulted in measurable serum concentrations over 24 hours, peaking at 296.93 ± 13.09 ng/mL (Fig 1B).
Pharmacokinetics of rhIL-2 and rhSCF-PEG treatment in mice. (A) 1 × 104 IU per day rIL-2 was delivered by constant subcutaneous infusion via Alzet osmotic pump, and measurement of serum IL-2 was performed by ELISA on days 0, 4, 10, and 12 after implantation. (B) PK of rhSCF-PEG administered as a single i.p. injection of 100 μg/kg body weight. Serum levels were measured by ELISA 0, 4, 8, 12, and 24 hours after injection. Results for each cytokine represent the mean ± SEM obtained from two mice with each measurement performed in duplicate.
Pharmacokinetics of rhIL-2 and rhSCF-PEG treatment in mice. (A) 1 × 104 IU per day rIL-2 was delivered by constant subcutaneous infusion via Alzet osmotic pump, and measurement of serum IL-2 was performed by ELISA on days 0, 4, 10, and 12 after implantation. (B) PK of rhSCF-PEG administered as a single i.p. injection of 100 μg/kg body weight. Serum levels were measured by ELISA 0, 4, 8, 12, and 24 hours after injection. Results for each cytokine represent the mean ± SEM obtained from two mice with each measurement performed in duplicate.
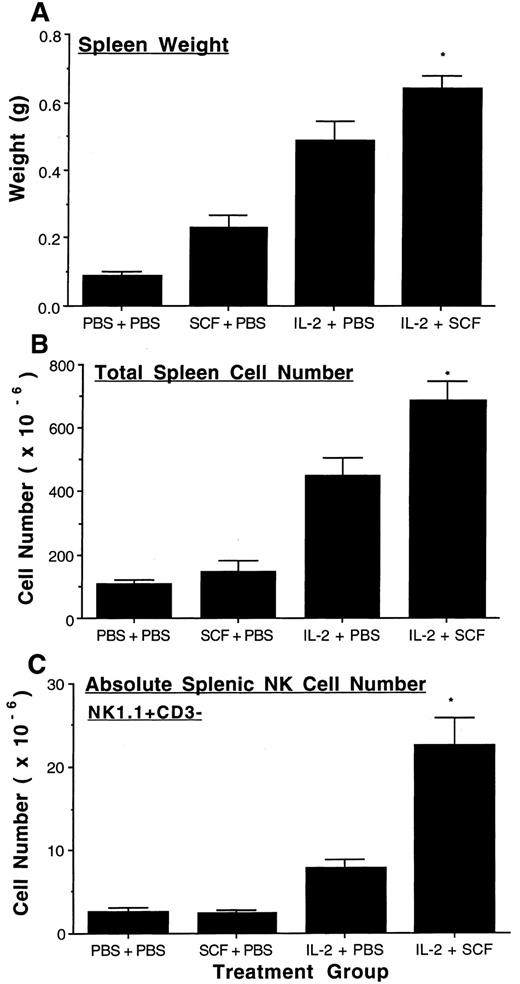
SCF enhances IL-2–mediated expansion of murine NK cells in the spleen. Administration of SCF alone or IL-2 alone resulted in a significant increase in spleen weight more than that seen with placebo (P ≤ .005 and P ≤ .0005, respectively). However, the concomitant administration of SCF and IL-2 resulted in a further significant increase in spleen weight more than that seen with either factor alone (Fig 2A, IL-2 v IL-2 plus SCF, P < .05). Total splenic cell number was significantly increased with IL-2 treatment more than either placebo or SCF alone (P ≤ .0005), and the combination of SCF plus IL-2 resulted in a further significant increase in total cell number compared to IL-2 treatment alone (P ≤ .025, Fig 2B). Flow cytometric analysis of the spleen cell populations showed that SCF did not increase the absolute number of NK1.1+CD3− NK cells more than placebo, however, IL-2 did (P ≤ .005). Moreover, the combination of SCF and IL-2 increased the absolute number of splenic NK1.1+CD3− NK cells more than twofold above IL-2 treatment alone (P ≤ .005, Fig 2C). The percentage of splenic NK1.1+CD3− NK cells in mice treated with SCF plus IL-2 was also significantly increased more than that seen with IL-2 alone (P < .005). Thus, for each splenic index the combination of SCF and IL-2 treatment resulted in a significant increase more than that measured with each factor alone.
SCF enhances IL-2 mediated expansion of splenic NK cells. Four groups of C57BL/6 mice (n = 5 to 6 per group) were treated with placebo, SCF, IL-2, or a combination of SCF plus IL-2, as described in Materials and Methods. After 8 weeks, spleens were excised and weighed (A) and the absolute number of spleen cells were determined by cell enumeration with a hemocytometer (B). 1 × 106 spleen cells were stained with NK1.1-PE and CD3-FITC MoAb and analyzed by flow cytometry for the NK1.1+CD3− NK cell population. There were 2.9 ± 0.2% NK1.1+CD3− cells in the control group, 1.8 ± 0.3% in the SCF group, 1.8 ± 0.1% in the IL-2 group, and 3.2 ± 0.2% in the SCF plus IL-2 group. The SCF plus IL-2 group had a significantly greater increase in percent NK1.1+CD3− cells compared to the IL-2 alone group (P < .005). Absolute splenic NK cell number was then calculated by multiplying the percentage NK1.1+CD3− NK cells by the total spleen cell number (C). The results represent the mean weights, total splenic cell number, and absolute splenic NK cell number of each treatment group ± SEM. Statistical significance comparing the SCF plus IL-2 group to the IL-2 alone group are indicated by the asterisk, and represent P < .05 (A), P ≤ .025 (B) and P ≤ .005 (C).
SCF enhances IL-2 mediated expansion of splenic NK cells. Four groups of C57BL/6 mice (n = 5 to 6 per group) were treated with placebo, SCF, IL-2, or a combination of SCF plus IL-2, as described in Materials and Methods. After 8 weeks, spleens were excised and weighed (A) and the absolute number of spleen cells were determined by cell enumeration with a hemocytometer (B). 1 × 106 spleen cells were stained with NK1.1-PE and CD3-FITC MoAb and analyzed by flow cytometry for the NK1.1+CD3− NK cell population. There were 2.9 ± 0.2% NK1.1+CD3− cells in the control group, 1.8 ± 0.3% in the SCF group, 1.8 ± 0.1% in the IL-2 group, and 3.2 ± 0.2% in the SCF plus IL-2 group. The SCF plus IL-2 group had a significantly greater increase in percent NK1.1+CD3− cells compared to the IL-2 alone group (P < .005). Absolute splenic NK cell number was then calculated by multiplying the percentage NK1.1+CD3− NK cells by the total spleen cell number (C). The results represent the mean weights, total splenic cell number, and absolute splenic NK cell number of each treatment group ± SEM. Statistical significance comparing the SCF plus IL-2 group to the IL-2 alone group are indicated by the asterisk, and represent P < .05 (A), P ≤ .025 (B) and P ≤ .005 (C).
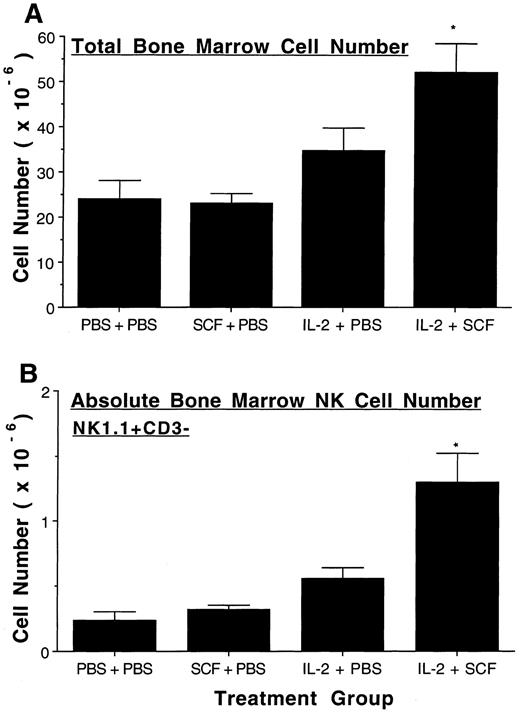
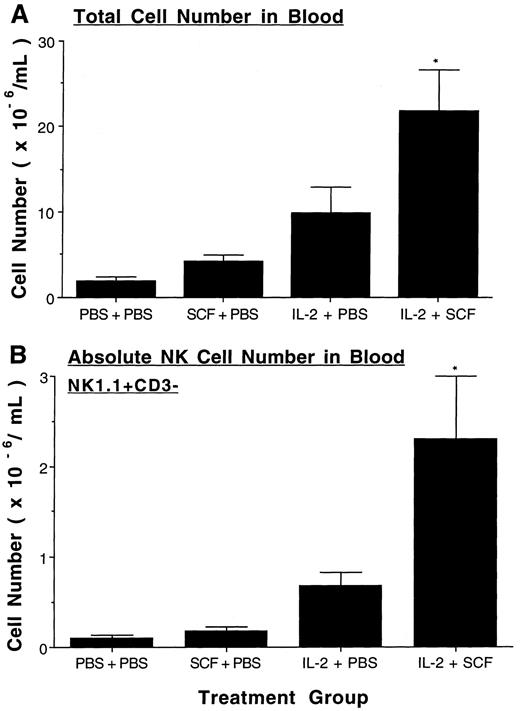
SCF enhances IL-2–mediated expansion of murine NK cells in BM and blood. Treatment with SCF alone did not alter the total number of BM cells or the absolute number of BM NK1.1+CD3− NK cells compared to placebo-treated controls. Treatment with IL-2 alone significantly increased both the total cell number (P ≤ .025) and the absolute number of NK cells (P ≤ .005) in BM over placebo and SCF alone. The total number of BM cells significantly increased even further when SCF was combined with IL-2 (P < .05, Fig 3A), and the absolute number of NK1.1+CD3− NK cells doubled with the administration of SCF and IL-2, compared to IL-2 treatment alone (P ≤ .025, Fig 3B). The percentage of BM NK1.1+CD3− NK cells in mice treated with SCF plus IL-2 was also significantly increased over that seen with IL-2 alone (P < .005). Likewise, total blood leukocyte number and absolute NK cell number did not increase with SCF alone, but were significantly increased in IL-2 treated mice over both placebo- and SCF-treated mice (P ≤ .025 and P ≤ .005, respectively, Fig 4). The combination of SCF and IL-2 treatment further increased both the total blood leukocyte number and absolute NK cell number over that seen with IL-2 treatment alone (P < .05, Fig 4). However, the percentage of blood NK1.1+CD3− NK cells in mice treated with SCF plus IL-2 was not significantly increased over that seen with IL-2 alone.
SCF enhances IL-2 mediated expansion of BM NK cells. After eight weeks of treatment, BM was procured from two femurs in each mouse and the absolute number of BM cells were determined by cell enumeration with a hemocytometer (A). 1 × 106 BM cells were then stained with NK1.1-PE and CD3-FITC and analyzed by flow cytometry for NK1.1+CD3− NK cell populations. There were 1.3 ± 0.02% NK1.1+CD3− cells in the control group, 1.4 ± 0.2% in the SCF group, 1.7 ± 0.1% in the IL-2 group, and 2.4 ± 0.2% in the SCF plus IL-2 group. The SCF plus IL-2 group had a significantly greater increase in percent NK1.1+CD3− cells compared to the IL-2 alone group (P < .005). Absolute BM NK cell number was calculated by multiplying total BM cells by the percent NK cells in the BM (B). Results represent the mean absolute number from 5 to 6 mice per treatment group ± SEM. Statistical significance comparing the SCF plus IL-2 group to the IL-2 alone group is indicated by the asterisk, and represent P < .05 (A), and P ≤ .025 (B).
SCF enhances IL-2 mediated expansion of BM NK cells. After eight weeks of treatment, BM was procured from two femurs in each mouse and the absolute number of BM cells were determined by cell enumeration with a hemocytometer (A). 1 × 106 BM cells were then stained with NK1.1-PE and CD3-FITC and analyzed by flow cytometry for NK1.1+CD3− NK cell populations. There were 1.3 ± 0.02% NK1.1+CD3− cells in the control group, 1.4 ± 0.2% in the SCF group, 1.7 ± 0.1% in the IL-2 group, and 2.4 ± 0.2% in the SCF plus IL-2 group. The SCF plus IL-2 group had a significantly greater increase in percent NK1.1+CD3− cells compared to the IL-2 alone group (P < .005). Absolute BM NK cell number was calculated by multiplying total BM cells by the percent NK cells in the BM (B). Results represent the mean absolute number from 5 to 6 mice per treatment group ± SEM. Statistical significance comparing the SCF plus IL-2 group to the IL-2 alone group is indicated by the asterisk, and represent P < .05 (A), and P ≤ .025 (B).
SCF enhances IL-2–mediated expansion of NK cells in the blood. After eight weeks of treatment absolute leukocyte number per mL in blood was obtained (A) and then multiplied by the percent NK1.1+CD3− NK cells determined by flow cytometry to yield the absolute number of NK cells per mL of blood (B). There were 4.8 ± 0.8% NK1.1+CD3− cells in the control group, 4.5 ± 0.3 in the SCF group, 10.3 ± 2.8% in the IL-2 group, and 9.8 ± 1.1% in the SCF plus IL-2 group. Results represent the mean absolute number from 5 to 6 mice in each treatment group ± SEM. Statistical significance comparing the SCF plus IL-2 group to the IL-2 alone group are indicated by the asterisk, and represent P < .05 (A and B).
SCF enhances IL-2–mediated expansion of NK cells in the blood. After eight weeks of treatment absolute leukocyte number per mL in blood was obtained (A) and then multiplied by the percent NK1.1+CD3− NK cells determined by flow cytometry to yield the absolute number of NK cells per mL of blood (B). There were 4.8 ± 0.8% NK1.1+CD3− cells in the control group, 4.5 ± 0.3 in the SCF group, 10.3 ± 2.8% in the IL-2 group, and 9.8 ± 1.1% in the SCF plus IL-2 group. Results represent the mean absolute number from 5 to 6 mice in each treatment group ± SEM. Statistical significance comparing the SCF plus IL-2 group to the IL-2 alone group are indicated by the asterisk, and represent P < .05 (A and B).
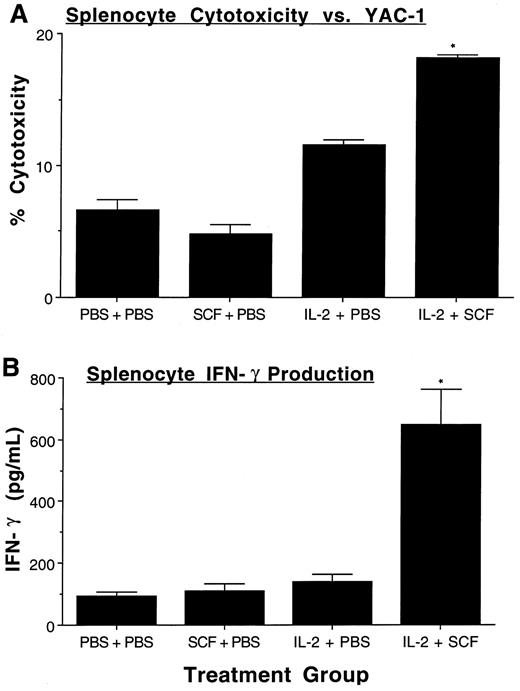
SCF augments IL-2–mediated increases in NK cell function. After observing the increase in NK1.1+CD3− NK cells, we sought to confirm those data by assessing NK cell function. When equal numbers of splenocytes were obtained from mice treated with placebo, SCF, IL-2, or SCF plus IL-2, and assayed for their ability to lyse YAC-1 target cells in the absence of any exogenous cytokines, cytotoxicity was greatest with splenocytes obtained from mice treated with SCF plus IL-2 (IL-2 v IL-2 plus SCF, P ≤ .0005, Fig 5A). In a second assay of NK cell function, equal numbers of unfractionated splenocytes from each of the four treatment groups were plated and stimulated in vitro with a combination of rhIL-2 (10 ng/mL) and rmIL-12 (30 ng/mL) for 72 hours, a known costimulus for NK IFN-γ production.39 IFN-γ production was significantly greater in splenocytes obtained from mice treated with SCF plus IL-2, compared with all other groups (P ≤ .01, Fig 5B). Collectively, these functional data support the phenotypic data showing a significant expansion of NK1.1+CD3− NK cells in mice treated with the combination of SCF plus IL-2 over that seen with placebo or either cytokine alone.
Functional assessment of NK cell expansion. Fresh single cell suspensions of unfractionated splenocytes were obtained from mice in each of the indicated treatment groups (n = 5 to 6 per group) and then assayed for NK cytotoxicity against YAC-1 targets (200:1 E/T ratio) in a standard 4 hour 51Cr release assay, without the addition of exogenous cytokines (A). Unfractionated splenocytes were also cultured (2 × 105 cells per well) in rhIL-2 plus rmIL-12 for 72 hours, after which cell-free culture supernatants were harvested and assayed for murine IFN-γ by ELISA (B). Results represent the mean percent cytotoxicity (A) or IFN-γ production (B) ± SEM. For both assays, the percentage NK1.1+CD3− cells were 2.9 ± 0.2% NK1.1+CD3− cells in the control group, 1.8 ± 0.3% in the SCF group, 1.8 ± 0.1% in the IL-2 group, and 3.2 ± 0.2% in the SCF plus IL-2 group. The SCF plus IL-2 group had a significantly greater increase in percent NK1.1+CD3− cells compared to the IL-2 alone group (P < .005). Statistical significance comparing the SCF plus IL-2 group to the IL-2 alone group are indicated by the asterisk, and represent P ≤ .0005 (A), and P ≤ .01 (B).
Functional assessment of NK cell expansion. Fresh single cell suspensions of unfractionated splenocytes were obtained from mice in each of the indicated treatment groups (n = 5 to 6 per group) and then assayed for NK cytotoxicity against YAC-1 targets (200:1 E/T ratio) in a standard 4 hour 51Cr release assay, without the addition of exogenous cytokines (A). Unfractionated splenocytes were also cultured (2 × 105 cells per well) in rhIL-2 plus rmIL-12 for 72 hours, after which cell-free culture supernatants were harvested and assayed for murine IFN-γ by ELISA (B). Results represent the mean percent cytotoxicity (A) or IFN-γ production (B) ± SEM. For both assays, the percentage NK1.1+CD3− cells were 2.9 ± 0.2% NK1.1+CD3− cells in the control group, 1.8 ± 0.3% in the SCF group, 1.8 ± 0.1% in the IL-2 group, and 3.2 ± 0.2% in the SCF plus IL-2 group. The SCF plus IL-2 group had a significantly greater increase in percent NK1.1+CD3− cells compared to the IL-2 alone group (P < .005). Statistical significance comparing the SCF plus IL-2 group to the IL-2 alone group are indicated by the asterisk, and represent P ≤ .0005 (A), and P ≤ .01 (B).
In vitro effects of SCF and/or IL-2 on mature NK1.1+CD3− splenocytes. To better understand the mechanism of in vivo NK cell expansion, we assessed mature NK cell expression of c-kit and IL-2Rβ, NK cell survival, and NK cell proliferation in the presence of SCF, IL-2, or SCF plus IL-2. On fresh NK1.1+CD3− splenocytes, 13.2 ± 0.27% coexpressed c-kit, and 89.04 ± 0.4% coexpressed IL-2Rβ. Cells cultured for 24 and 48 hours under conditions described above did not increase c-kit or IL-2Rβ expression on NK1.1+ cells (data not shown). Further, neither proliferation or survival of NK1.1+ cells were enhanced when cultured for 3 days or 7 days, respectively, in concentrations of IL-2 that were comparable to those obtained in vivo (ie, 0.01 to 0.1 nmol/L). The addition of rrSCF to these cultures had no effect on any of these parameters (data not shown). Hence, the effects of SCF and IL-2 on mouse NK cells in vivo are not likely to be predominantly mediated by expansion at the level of the mature NK1.1 cell compartment.
DISCUSSION
This report provides the first in vivo evidence that SCF can augment IL-2–induced expansion of murine NK cells. Significant expansion in absolute NK cell number over that seen with IL-2 alone was observed after 8 weeks of treatment with SCF plus IL-2 in all three hematopoietic compartments examined (ie, the spleen, BM, and blood). Therapy with SCF alone resulted in a modest increase in spleen weight, but did not result in any significant increase in total cell number or NK cell number in any tissue examined, compared to placebo-treated mice. Therefore, SCF itself does not appear to induce NK cell differentiation or expansion of NK cells in this system, but rather serves to enhance NK cell differentiation and expansion induced by IL-2.
To further characterize these measured increases in NK cell number, we assessed NK cell effector function in unfractionated splenocytes obtained from mice treated with placebo, SCF, IL-2, or the combination of SCF and IL-2. Cytotoxicity against YAC-1 target cells and early IFN-γ production following cytokine costimulation are both properties of murine NK cells.4 39 These analyses showed significantly greater NK effector function in unfractionated splenocytes obtained from mice treated with SCF plus IL-2 versus mice treated with IL-2 alone. These data are consistent with the significant increase in the number and percentage of NK1.1+CD3− spleen cells observed in mice treated with the combination of SCF plus IL-2 over those treated with IL-2 alone.
SCF combines with a number of cytokine receptor super family ligands to enhance the production of erythrocytes and leukocytes.27-30,35 SCF's synergy with EPO was recently shown to result from an activated c-kit–induced tyrosine phosphorylation of the EPO receptor's cytoplasmic domain not used by JAK2.40 SCF's synergy with GM-CSF in the human MO7e cell line occurs through increases in the cyclin dependent kinase inhibitor (cki) p21cip-1 cellular protein, and decreases in the cki p27kip-1.41 This alteration in the cki ratio was found to be correlated with increased cyclin dependent kinase 2 (cdk2) kinase activity, phosphorylation of the retinoblastoma (Rb) gene product, and ultimately enhanced proliferation. The mechanism by which activation of the c-kit tyrosine kinase receptor may enhance the signal induced by IL-2 binding to the IL-2R in NK cells or HPCs is currently unknown. The IL-2Rβ and IL-2Rγ are signal transducing subunits and members of the cytokine receptor super family. Whether mechanisms described above may be operative in the synergy observed between c-kit and IL-2Rβγ is not known.
The point of action of exogenously administered IL-2 and SCF in the NK cell differentiation schema is not known, but several possible scenarios exist. Recent studies performed on mice with 17β-estradiol-induced BM ablation showed that short-term (3 day) in vitro culture with IL-2 was able to substitute for the BM microenvironment, allowing maturation of functional NK1.1+CD3− NK cells.42 Mice with 17β-estradiol-induced BM ablation have functionally immature (nonlytic) NK cells in the spleen, and the authors showed that activation of the IL-2R supports maturation into lytic NK cell effectors. This is consistent with our observations that IL-2 alone expands functional NK cells in mice. Additionally, Aiba et al43 have recently examined the cytokine requirements for maturation of murine NK cell progenitors from the fetal thymus into NK cell colonies in a cell-free culture system. These studies revealed that SCF was indispensable for formation of immature NK cell colonies, and both IL-2 and IL-15 increase the frequency of these colonies. Based on these cytokine requirements, one possible point of action of exogenously administered SCF and IL-2 is at the point of lineage commitment to NK cell development.
Our previous in vitro work has explored IL-2- and SCF-induced NK cell production from purified human CD34+ HPCs in humans. Twenty-one day culture in low dose IL-2 alone resulted in CD56+ NK cell differentiation with little NK cell expansion, while culture in SCF alone resulted in a modest expansion (5- to 13-fold) with no NK cell differentiation. When human HPCs were cultured in both IL-2 and SCF, we observed NK cell differentiation with considerably greater NK cell expansion (6- to 16-fold) compared to that seen with IL-2 alone.19 Identical results were obtained in vitro with CD34+ HPCs, SCF and IL-15, a cytokine that also uses the β and γ components of the IL-2R for binding and signal transduction.30 Collectively these in vitro human data also suggest that IL-2 and SCF can synergize for NK cell production at the level of an NK precursor cell. We have shown that at concentrations obtained in vivo, combinations of SCF and IL-2 have essentially no effect on survival or proliferation of mature mouse NK1.1+ cells, further supporting the notion that this effect is mediated via differentiation and expansion of HPCs. Indeed, other investigators have recently shown that c-kit+Sca-2+NK1.1− mouse HPCs can differentiate and expand into NK1.1+ NK cells in the presence of SCF, Flt3 ligand, IL-6, IL-7, and IL-15, the latter of which signals through components of the IL-2R (V. Kumar, personal communication, May 1997).
Low dose IL-2 has been administered by daily s.c. injection to patients with cancer and immunodeficiency for 8 to 12 weeks. The therapy produces low (ie, picomolar) concentrations of IL-2 in vivo, and consistently results in selective expansion of NK cells.10 11 The in vivo data presented in this report would suggest that the combination of SCF plus IL-2 might provide a greater and more consistent expansion of these innate immune effector cells than low dose IL-2 alone. Given their cytolytic properties and potent IFN-γ production, such NK cell expansion may be beneficial in patients with cancer and/or immunodeficiency.
ACKNOWLEDGMENT
We thank Dr Keith Langley at Amgen, Inc for performing the ELISA quantitation of serum rhSCF-PEG levels, and Vinay Kumar for critical review of the manuscript.
Supported by National Institutes of Health (Bethesda, MD) Grants CA68326, CA65670, and CA68458. T.A.F. is the recipient of the Howard Hughes Medical Institute (Chevy Chase, MD) Research Fellowship for Medical Students. W.E.C. is the recipient of the American Society of Clinical Oncology (Alexandria, VA) Young Investigator Award.
Address reprint requests to Michael A. Caligiuri, MD, The Ohio State University, Arthur G. James Cancer Center, 300 W 10th Ave, Columbus, OH 43210.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal