Abstract
We investigated the representation of T cells in patients who had been treated for Hodgkin's disease (HD). We found a marked depletion in both CD4 and CD8 naive T-cell counts that persists up to 30 years after completion of treatment. In contrast, CD4 and CD8 memory T-cell subsets recovered to normal or above normal levels by 5 years posttreatment. Thus, the previously-reported long-term deficit in total CD4 T-cell counts after treatment for HD is due to specific depletion of naive T cells. Similarly, total CD8 T-cell counts return to normal by 5 years only because CD8 memory T cells expand to higher than normal levels. These findings suggest that the treatment (mediastinal irradiation) results in a longterm dysregulation of T-cell subset homeostasis. The profound depletion of naive T cells may explain the altered T-cell function in treated patients, including the poor response to immunization after treatment for HD. Further, in some individuals, we identified expansions of unusual subsets expressing low levels of CD8. Eight-color fluorescence-activated cell sorting analyses showed that these cells largely express CD8αα homodimers and CD57, consistent with the phenotype of potentially extrathymically derived T cells. In addition, these cells, both CD4+ and CD4−, are probably cytotoxic lymphocytes, as they express high levels of intracellular perforin. In adults treated for HD, an increased activity of extrathymic T-cell differentiation may partially compensate for the loss of thymic-derived T cells.
PREVIOUS STUDIES have shown that radiation therapy decreases the absolute CD4 T-cell counts in patients treated for Hodgkin's disease (HD) and that these cells appear to regenerate slowly after treatment.1 In addition, long-term persistence of in vitro functional abnormalities, eg, decreased proliferation in response to ConA or phytohemagglutinin (PHA) stimulation, has also been reported in these patients.2,3 Furthermore, the antibody response to pneumococcal vaccine was profoundly impaired in patients who had received intensive treatment for HD.4 However, there is no satisfactory explanation for these long-term functional defects, in view of the eventual recovery of the CD4 and the CD8 T-cell counts to normal or near-normal levels.
Recent flow cytometric studies have further subdivided CD4 and CD8 T cells into several phenotypically and functionally distinct subsets.5-9 In particular, the combination of CD45RA and CD62L, together with CD4 or CD8, distinguishes naive and memory T-cell subsets. Naive T cells are cells that have been released from the thymus into the peripheral circulation, but have not yet encountered antigen. In vitro studies have shown that naive and memory subsets have distinct functional capacities. For example, memory subsets do not proliferate as well as naive subsets in response to generic mitogenic stimuli; however, the memory subsets produce a wider variety and greater amounts of many cytokines.5,10 11
The radiation used to treat HD often is directed at the mediastinum, including the thymus, and therefore is likely to impair the ability of the thymus to generate naive T cells. In turn, this may lead to a specific loss of these cells in the periphery and underlie the functional defects observed in peripheral blood mononuclear cells of patients treated for HD. We studied the effect of mediastinal radiation on T-cell subsets in patients treated many years previously for HD, and now report that such treatment results in a long-term depletion of naive T cells with normal or increased numbers of memory T cells. In addition, we find an increase in “unusual” T-cell subsets, normally rare in healthy individuals, that may herald an increase in extrathymic differentiation processes that compensate for the loss of naive T-cell generation.
The resultant T-cell compartment in individuals treated for HD is in many ways similar to that found in human immunodeficiency virus (HIV)-infected adults. During the progression of HIV disease, HIV-infected adults show a decrease in the representation of naive T cells12 and a compensatory increase in “unusual” T-cell subsets such as those we describe here (De Rosa et al, in preparation). Thus, for HIV-infected adults, as well as for the individuals treated for HD studied in this report, there is a profound change in the homeostatic regulation of T-cell subsets; we suggest that this dysregulation may be due in part to irreversible thymic destruction that accompanies both pathologic states.13
MATERIALS AND METHODS
Study Subjects
The study population comprised a group of 75 patients with HD referred to Stanford University Medical Center between 1965 and 1996. Nineteen patients were studied before treatment and a separate group of 56 patients were studied at a single time point after treatment (ranging between 4 months and 32 years after completion of treatment). Patients were staged according to the Ann Arbor classification system,14 and the clinical characteristics of the study participants are summarized in Table 1. Patients who were studied after the completion of therapy were without evidence of disease when tested. In addition, we studied 11 patients treated for non-Hodgkin's Lymphoma (NHL); nine of these 11 individuals were more than 50 years old at the time of sampling.
Clinical Data of Healthy Individuals and Patients With HD
| . | . | . | Stage of Disease . | Age (yr) at Analysis . | Age (yr) at Diagnosis . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . |
| . | . | Sex . | I . | II . | III . | IV . | Median . | Median . |
| Category . | No. . | M:F . | No. Subjects (Symptoms A/B) . | (range) . | (range) . | |||
| . | . | . | . | . | . | . | . | . |
| Healthy controls | 34 | 17:17 | 40.5 | |||||
| (24.8-59.8) | ||||||||
| HD | 19 | 13:6 | 4 | 11 | 1 | 3 | 31.1 | |
| Pretreatment | (4/0) | (11/0) | (1/0) | (3/0) | (17.2-58.9) | |||
| Posttreatment | 56 | 21:35 | 8 | 33 | 9 | 6 | 40.0 | 32.0 |
| (7/1) | (30/3) | (6/3) | (5/1) | (17.3-72.2) | (14.8-50.8) | |||
| . | . | . | Stage of Disease . | Age (yr) at Analysis . | Age (yr) at Diagnosis . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . |
| . | . | Sex . | I . | II . | III . | IV . | Median . | Median . |
| Category . | No. . | M:F . | No. Subjects (Symptoms A/B) . | (range) . | (range) . | |||
| . | . | . | . | . | . | . | . | . |
| Healthy controls | 34 | 17:17 | 40.5 | |||||
| (24.8-59.8) | ||||||||
| HD | 19 | 13:6 | 4 | 11 | 1 | 3 | 31.1 | |
| Pretreatment | (4/0) | (11/0) | (1/0) | (3/0) | (17.2-58.9) | |||
| Posttreatment | 56 | 21:35 | 8 | 33 | 9 | 6 | 40.0 | 32.0 |
| (7/1) | (30/3) | (6/3) | (5/1) | (17.3-72.2) | (14.8-50.8) | |||
All 56 posttreatment patients received radiation therapy including thymic irradiation. Thirty-two of them also had chemotherapy in addition to radiotherapy (15, Stanford V: nitrogen mustard, adriamycin, vincristine, vinblastine, bleomycin, prednisone, ancillary drugs; 6, MOPP; mechlorethamine, vincristine, procarbazine, prednisone; 4, VBM: vinblastine, bleomycin, methotrexate; 3, PAVe: procarbazine, melphalan, vinblastine; 2, ABVD: doxorubicin, bleomycin, vinblastine, dacarbazine; 2, MOPP/ABVD).
All of the 56 patients treated for HD received mediastinal irradiation (range, 2,550 to 4,400 cGy), and 35 of these also received more extensive radiation above and below the diaphragm (range, 2,550 to 5,060 cGy total). In addition to the radiotherapy, 32 of the patients treated for HD also received various combinations of chemotherapy typical for lymphoid malignancy (Table 1). Six of 11 NHL patients received chemotherapy in addition to irradiation (range, 3,000 to 4,500 cGy). Thirty-four healthy individuals, roughly similar in sex and age distribution to the patients with HD, were also studied (Table 1).
Fluorescence-Activated Cell Sorting (FACS) Analysis
From each patient or healthy control, blood was drawn by venipuncture for FACS analysis and for a complete blood count (CBC; performed by an accredited commercial laboratory). For three-color analysis, all fluorochrome-conjugated monoclonals were obtained from PharMingen (San Diego, CA). Ficoll-Paque was obtained from Pharmacia AB (Uppsala, Sweden). Biotin, flavin-deficient RPMI-1640 (hereafter, RPMI) was obtained from Irvine Scientific (Santa Ana, CA). Other chemicals were obtained from Sigma Chemical Co (St Louis, MO).
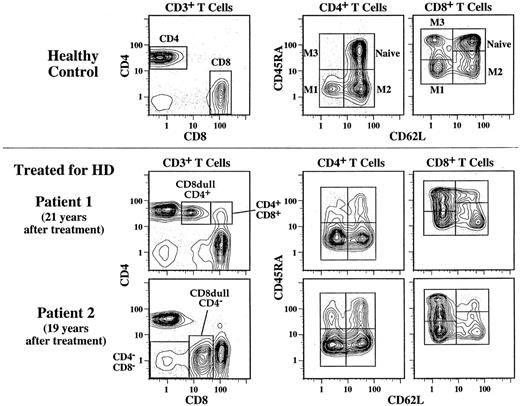
Peripheral blood mononuclear cells (PBMC) were prepared from 7 mL of heparinized blood by Ficoll-Paque density centrifugation. For analyses of CD4 and CD8 T-cell subsets in this report, we used the following antibody combinations: (1) fluorescein-conjugated (FITC) CD3, phycoerythin (PE) CD8, and CyChrome (Cy5-PE) CD4; (2) FITC CD62L, PE CD45RA, and Cy5-PE CD4; (3) FITC CD62L, PE CD45RA, and Cy5-PE CD8. The gates used to identify these subsets are shown in Fig 1. Naive cells are identified as coexpressing high levels of CD62L and CD45RA.
CD4 and CD8 T-cell subset frequencies determined by three-color flow cytometry. To determine the CD4 and CD8 T-cell frequencies, the staining combination of CD3, CD4, and CD8 was used. The 5% probability plots shown on the left have first been gated on lymphocytes as determined by forward and side scatter, and then they have been gated on CD3 to identify T cells. In addition to CD4 and CD8 single positive T cells, CD4−CD8−, and CD4+CD8+ T cells can also be distinguished. In a few individuals treated for HD, we can identify two unusual T-cell subsets that express dull levels of CD8 and are either CD4+ or CD4−. The naive and memory CD4 and CD8 T-cell frequencies were determined by the staining combinations of CD62L, CD45RA, and either CD4 or CD8. Shown on the right are contour plots first scatter gated on lymphocytes and then gated on either CD4+ or CD8bright cells. Naive cells and three memory subsets (M1, M2, and M3) can be distinguished by this method. Shown are examples for a healthy control and two patients who were treated for HD more than 15 years before analysis. The naive T-cell depletion noted in these two individuals is typical of that seen in the posttreatment HD subjects.
CD4 and CD8 T-cell subset frequencies determined by three-color flow cytometry. To determine the CD4 and CD8 T-cell frequencies, the staining combination of CD3, CD4, and CD8 was used. The 5% probability plots shown on the left have first been gated on lymphocytes as determined by forward and side scatter, and then they have been gated on CD3 to identify T cells. In addition to CD4 and CD8 single positive T cells, CD4−CD8−, and CD4+CD8+ T cells can also be distinguished. In a few individuals treated for HD, we can identify two unusual T-cell subsets that express dull levels of CD8 and are either CD4+ or CD4−. The naive and memory CD4 and CD8 T-cell frequencies were determined by the staining combinations of CD62L, CD45RA, and either CD4 or CD8. Shown on the right are contour plots first scatter gated on lymphocytes and then gated on either CD4+ or CD8bright cells. Naive cells and three memory subsets (M1, M2, and M3) can be distinguished by this method. Shown are examples for a healthy control and two patients who were treated for HD more than 15 years before analysis. The naive T-cell depletion noted in these two individuals is typical of that seen in the posttreatment HD subjects.
B-cell frequencies were determined by either of these two combinations: (1) FITC CD20, PE CD5, Cy5-PE CD3 (ie, CD20+ CD3−), or (2) FITC CD62L, PE CD5, Cy5-PE CD19 (ie, CD19+). Natural killer (NK) cell frequencies were determined by either of these two combinations: (1) FITC CD7, PE CD16, Cy5-PE CD5, (ie, CD16+ CD5−) or (2) FITC CD16, PE CD56, Cy5-PE CD3 (ie, CD16+ or CD56+ and CD3−).
After staining, cells were washed three times in RPMI and then resuspended in 0.5% paraformaldehyde in RPMI. Three-color flow cytometric analysis was performed on a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA) interfaced to a VAX 6300 computer (Digital Computer, Maynard, MA). For each cell, data for forward scatter, side scatter, and the fluorescences of FITC (515-545 nm bandpass filter), PE (577-592 nm), and Cy5-PE (650-690 nm) were collected. For each stain, data from 30,000 to 50,000 cells were collected and analyzed by FACS-Desk software. For subset frequencies, a lymphocyte scatter gate was used. In addition, gates uniquely identifying CD4 or CD8 T cells and their subsets (as shown in Fig 1) were applied.
For further analyses of special subsets, we used the following 7- or 8-color antibody combinations: (1) FITC CD4, PE TcR γδ, Cy5-PE CD8, texas red (TR) CD3, allophycocyanin (APC) CD57, Cy7-APC CD45RA and cascade blue (CasBlu) CD8β; (2) FITC CD62L, PE CD11a, Cy5-PE CD56, TR CD4, APC CD57, Cy7-APC CD45RA, CasBlu CD3 and Cy7-PE CD8; (3) FITC TcR Vδ1, PE TcR Vδ2, Cy5-PE CD16, TR CD3, APC CD4, Cy7-APC CD57, CasBlu CD5, and Cy7-PE CD8. The FITC Vδ1 antibody was obtained from T Cell Diagnostics (Woburn, MA). The antibodies to CD8β and CD56 were kindly provided by E. Reinherz (Dana Farber Cancer Institute) and C. Prussin (NIAID, NIH), respectively. All other antibodies were provided by PharMingen (San Diego, CA). Eight-color FACS analysis is described in detail elsewhere.15 Data analysis was performed using FlowJo (TreeStar, Inc, San Carlos).
To stain for intracellular perforin, we used the δG9 antibody kindly supplied by Dr E. Podack (University of Miami),16 which we conjugated to FITC. After staining for surface antigens, cells were washed in ice-cold phosphate-buffered saline (PBS) and resuspended at 2 × 106/mL in ice-cold PBS. An equal volume of 4% formaldehyde is added and mixed; cells are incubated for 20 minutes. The cells are centrifuged and washed twice with ice-cold PBS with 1% bovine serum albumin (BSA). Cells are resuspended in 150 μL of 0.5% Saponin (in PBS/BSA) and incubated for 10 minutes. Cells are centrifuged and resuspended in 25 μL of the Saponin buffer containing the appropriate amount of δG9. After 30 minutes, cells are washed twice with 150 μL of the Saponin buffer, twice with PBS/BSA, and finally resuspended in PBS/BSA for FACS analysis.
Absolute numbers of the subsets reported here were calculated by multiplying their representation among lymphocytes by the absolute lymphocyte count determined from the CBC. For instance, the number of CD4 T cells per microliter of whole blood was calculated by multiplying the fraction of lymphocytes that were CD3+ and CD4+ (from FACS gating) by the absolute lymphocyte count per microliter of blood.
Statistical Analysis
For analyses and display of statistical comparisons, we used JMP for the Apple Macintosh (SAS Institute, Cary, NC). Comparisons of distributions were performed by the nonparametric two-sample Wilcoxon rank test.
RESULTS
Changes in Lymphocyte Subsets in Patients With HD Before Treatment
As shown in Fig 2 and 3, the overall CD4 T-cell count (P = .05), the naive CD4 T-cell count (P = .002), the naive CD8 T-cell count (P = .0005), the B-cell count (P = .0005), and the NK cell count (P = .02) were significantly decreased in the 19 HD patients before treatment when compared with the 34 healthy controls. There was no significant difference between these two groups for the total CD8 T-cell count or the memory CD4 and CD8 T-cell counts. When the CD4 and CD8 memory subsets are further subdivided based on the expression of CD62L (as explained below and shown in Fig 1), there were also no significant differences between the comparable memory subsets in the control group and the group of patients with HD before treatment.
CD4 and CD8 T-cell subset counts before and after treatment for HD. Shown are the medians and interquartile ranges (error bars) of the absolute counts for the total, memory, and naive CD4 and CD8 subsets in the following groups: healthy controls (n = 34), HD patients before treatment (n = 19), subjects who had been treated for HD within 2.5 years (n = 23), 2.5 to 5 years (n = 10), and more the 5 years (n = 23) before analysis. Naive and memory subsets were determined based on expression of CD62L and CD45RA as shown in Fig 1. Subjects were treated for HD with either radiation alone or combined radiation and chemotherapy. Significant differences between the healthy controls and any of the other groups are shown along the top; significant differences between the HD patients before treatment and the posttreatment HD subjects are shown below this. Note that the control group and posttreatment HD groups were appoximately matched for age; however, five of the treated HD subjects were older than the oldest control individual (60 years). Because older age may have an effect on the representation of T-cell subsets, we also analyzed the data excluding these five older HD patients. The significance of the decrease in the naive CD4 and CD8 T-cell counts, as well as the decrease in the total CD4 T-cell count, was not changed.
CD4 and CD8 T-cell subset counts before and after treatment for HD. Shown are the medians and interquartile ranges (error bars) of the absolute counts for the total, memory, and naive CD4 and CD8 subsets in the following groups: healthy controls (n = 34), HD patients before treatment (n = 19), subjects who had been treated for HD within 2.5 years (n = 23), 2.5 to 5 years (n = 10), and more the 5 years (n = 23) before analysis. Naive and memory subsets were determined based on expression of CD62L and CD45RA as shown in Fig 1. Subjects were treated for HD with either radiation alone or combined radiation and chemotherapy. Significant differences between the healthy controls and any of the other groups are shown along the top; significant differences between the HD patients before treatment and the posttreatment HD subjects are shown below this. Note that the control group and posttreatment HD groups were appoximately matched for age; however, five of the treated HD subjects were older than the oldest control individual (60 years). Because older age may have an effect on the representation of T-cell subsets, we also analyzed the data excluding these five older HD patients. The significance of the decrease in the naive CD4 and CD8 T-cell counts, as well as the decrease in the total CD4 T-cell count, was not changed.
WBC, neutrophil, B-cell, and NK cell counts before and after treatment for HD. Shown are the medians and interquartile ranges (error bars) of the absolute counts for the white blood cells, neutrophils, B cells, and NK cells in the same groups as shown in Fig 2. Significant differences between the healthy controls and any of the other groups are shown along the top; significant differences between the HD patients before treatment and the posttreatment HD subjects are shown below this.
WBC, neutrophil, B-cell, and NK cell counts before and after treatment for HD. Shown are the medians and interquartile ranges (error bars) of the absolute counts for the white blood cells, neutrophils, B cells, and NK cells in the same groups as shown in Fig 2. Significant differences between the healthy controls and any of the other groups are shown along the top; significant differences between the HD patients before treatment and the posttreatment HD subjects are shown below this.
Lymphocyte Subset Changes in HD After Treatment
Short-term effect of treatment. Within 2.5 years of treatment completion, many of the subsets we analyzed were significantly decreased compared with controls (the total CD4 T-cell count and all CD4 subsets, the total CD8 T-cell count and the naive CD8 T-cell count). Only the neutrophil, B cell, NK cell, and CD8 memory T-cell counts had recovered to normal or above normal levels within 2.5 years after treatment (Figs 2 and 3).
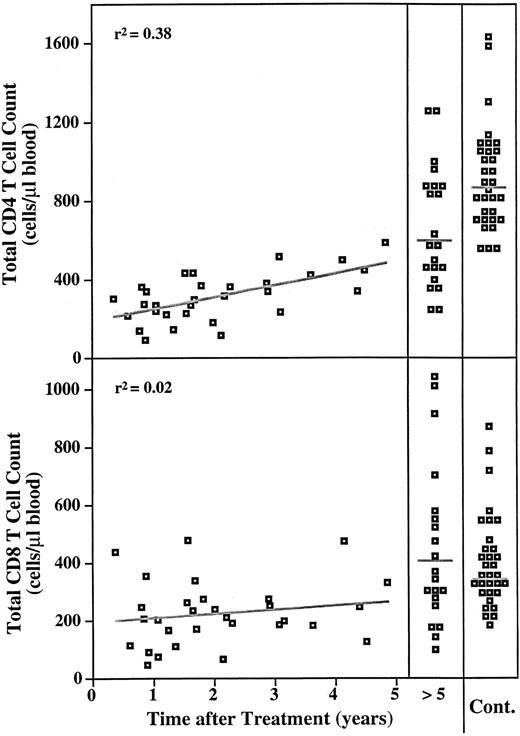
Figure 4 shows that during the first 5 years posttreatment, there is a gradual recovery of the total CD4 T-cell count (r2 = .38), although the counts remain below normal. The total CD8 T-cell count does not show this gradual pattern of recovery, likely due to the early expansion of CD8 memory T cells (see below).
Time course of total CD4 and CD8 T-cell counts after treatment for HD. The total CD4 and CD8 T-cell counts are plotted versus years after completion of treatment for the group of subjects who had been treated for HD up to 5 years before analysis (n = 33). Counts for subjects who were analyzed more than 5 years after completion of treatment are grouped together (n = 23). The distribution of CD4 and CD8 counts for 34 healthy individuals is shown on the right for comparison. The standard least squares fit is shown. Gray lines on the distributions for the groups on the right indicate the medians.
Time course of total CD4 and CD8 T-cell counts after treatment for HD. The total CD4 and CD8 T-cell counts are plotted versus years after completion of treatment for the group of subjects who had been treated for HD up to 5 years before analysis (n = 33). Counts for subjects who were analyzed more than 5 years after completion of treatment are grouped together (n = 23). The distribution of CD4 and CD8 counts for 34 healthy individuals is shown on the right for comparison. The standard least squares fit is shown. Gray lines on the distributions for the groups on the right indicate the medians.
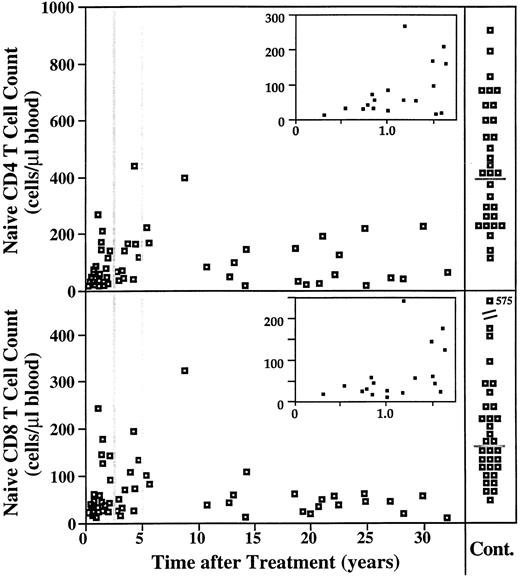
Long-term effect of treatment. Figures 2 and 3 show that by 2.5 to 5 years after completion of treatment, most subsets recovered to normal levels. In the group analyzed more than 5 years posttreatment, the only subsets that remained decreased compared with controls were the total CD4 T-cell count and the naive CD4 and naive CD8 T-cell counts (Figs 2 and 3). Figure 5 shows the striking and long-term depletion in the naive T-cell counts even up to 30 years posttreatment. Immediately after treatment, there is a recovery phase lasting approximately 2 years, during which the naive T-cell compartment may show slight expansion. However, for naive T cells, the equilibrium level achieved and maintained for decades after that is much below normal for both absolute cell counts and representation (ie, percent of T cells).
Time course of naive CD4 and CD8 T-cell counts after treatment for HD. The naive CD4 and CD8 T-cell counts are plotted versus years after completion of treatment for all subjects in this study who had been treated for HD (n = 56). Counts for 34 healthy individuals are shown on the right for comparison (gray lines indicate the medians for the control group). The inset for each graph shows a magnification of the acute recovery phase, lasting about 1.5 years.
Time course of naive CD4 and CD8 T-cell counts after treatment for HD. The naive CD4 and CD8 T-cell counts are plotted versus years after completion of treatment for all subjects in this study who had been treated for HD (n = 56). Counts for 34 healthy individuals are shown on the right for comparison (gray lines indicate the medians for the control group). The inset for each graph shows a magnification of the acute recovery phase, lasting about 1.5 years.
In contrast to the naive T-cell counts, the memory CD4 T-cell count returns to normal levels by 5 years posttreatment. CD4 memory T cells can be further subdivided based on expression of CD62L, as shown in Fig 1. Within 2.5 years of treatment completion, both the CD62L−(labeled as M1 in Fig 1) and the CD62L+ (M2) CD4 memory subsets are below normal levels. For the group of subjects analyzed 2.5 to 5 years after treatment completion, the CD62L−(M1) subset has returned to normal levels, but the CD62L+ subset remains significantly decreased compared with control levels (P = .01). By 5 years posttreatment, both these CD4 memory subsets are not different compared with controls.
The CD8 memory T cells have recovered to normal levels even by 2.5 years. In fact, it is an expansion of CD8 memory cells above normal that accounts for the recovery of the total CD8 T-cell count to normal levels after treatment. CD8 memory cells can also be divided into CD62L+ (labeled as M2 in Fig 1) and CD62L− (M1 and M3 in Fig 1) subsets. It is the CD62L−memory subsets that recover early and then expand above normal levels after 2.5 years. Within 2.5 years, the CD62L+ (M2) subset is the only CD8 memory subset significantly below normal levels (P = .001). Comparing all patients who had completed treatment more than 2.5 years before analysis, the CD8 M2 subset is not significantly different from controls, and both of the CD62L− memory subsets (M1 and M3) are significantly above normal levels (P = .003 and P = .0003, respectively).
There was no significant difference in the long-term naive T-cell depletion resulting from treatment with radiation alone or combined treatment with radiation and chemotherapy. For those subjects analyzed more than 2.5 years after completion of treatment, the CD4 and CD8 naive T-cell counts were not significantly different between the group that received radiation alone (n = 18) and the group that received combined radiation/chemotherapy (n = 13, P > .2). We do not have enough data to compare the effects of different types of chemotherapy (each combined with radiation), as there are too few people in each category. However, naive cells appear decreased for all types of chemotherapy included in this study (listed in Table 1). The stage of disease did not affect the resulting naive T-cell depletion because there was no significant difference in naive T-cell counts when the posttreatment patients were grouped by stage of disease (data not shown).
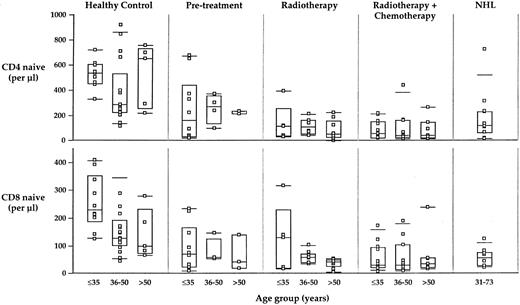
The age at the time of treatment probably affects the rate of recovery of cell counts17; longitudinal analysis must be completed to confirm this hypothesis. However, we found no effect of age on the number of naive CD4 or CD8 counts after the acute recovery phase (Fig 6). Thus, the basic insult to the homeostatic regulation of T-cell subset counts appears to be quantitatively equivalent at any (adult) age of treatment.
Relationship of naive CD4 and CD8 counts to age. There is a slight trend to lower numbers of naive CD4 and CD8 cells in older healthy controls (but it is not statistically significant). There is no statistically significant effect of age on the absolute numbers of naive CD4 or CD8 cells in any of the HD-treated populations. While the rate of increase in cell counts during the acute recovery phase may be age-dependent, the equilibrium naive cell counts do not show an age dependence. The far right panel shows naive CD4 and CD8 counts for 11 individuals treated with radiotherapy or radio- plus chemotherapy for NHL; these individuals have a similar long-term loss of naive T-cell counts, suggesting that the treatment, identical to that used for the HL patients, is itself an important contributor to the long-term lack of naive T-cell regeneration.
Relationship of naive CD4 and CD8 counts to age. There is a slight trend to lower numbers of naive CD4 and CD8 cells in older healthy controls (but it is not statistically significant). There is no statistically significant effect of age on the absolute numbers of naive CD4 or CD8 cells in any of the HD-treated populations. While the rate of increase in cell counts during the acute recovery phase may be age-dependent, the equilibrium naive cell counts do not show an age dependence. The far right panel shows naive CD4 and CD8 counts for 11 individuals treated with radiotherapy or radio- plus chemotherapy for NHL; these individuals have a similar long-term loss of naive T-cell counts, suggesting that the treatment, identical to that used for the HL patients, is itself an important contributor to the long-term lack of naive T-cell regeneration.
Unusual T-cell subsets appear in patients after treatment for HD. In a few individuals in the group treated for HD, we were able to identify expansions in two unusual T-cell subsets that expressed dull levels of CD8 and were either CD4+ or CD4− (examples shown in Fig 1). For the CD8dull CD4− T-cell subset, 9 (17%) posttreatment HD patients had absolute counts above the highest count found in controls (61/μL, Fig 7). For the CD8dull CD4+ T-cell subset, 10 (19%) posttreatment HD patients had counts above normal (>19/μL Fig 7). Five of these individuals had elevated levels for both of these subsets. The individuals who had elevations in these subsets were not different from the other posttreatment HD subjects in terms of age, stage of disease, type of treatment, or time since completion of treatment.
Unusual CD8dull T-cell subset counts before and after treatment for HD. The gates for these CD8dull subsets (either CD4+ or CD4−) are shown in Fig 1. Although the majority of the posttreatment HD patients had counts within the normal range, several individuals had counts above the highest levels found in controls. Five of these individuals had elevated levels of both of these subsets.
Unusual CD8dull T-cell subset counts before and after treatment for HD. The gates for these CD8dull subsets (either CD4+ or CD4−) are shown in Fig 1. Although the majority of the posttreatment HD patients had counts within the normal range, several individuals had counts above the highest levels found in controls. Five of these individuals had elevated levels of both of these subsets.
Phenotype of the CD8dull T Cells Expanded in Patients Treated for HD
To further investigate the phenotype of these subsets, we recalled five individuals who had elevated counts in either or both of these subsets (four subjects had high CD8dull CD4+ T-cell counts and three subjects had high CD8dull CD4− T-cell counts, of which two individuals had high counts for both subsets). Unique identification of these cells requires three-color immunophenotyping; thus, further characterization of their phenotype requires the ability to measure several additional antigens simultaneously. For these analyses, we used the eight-color flow cytometric methods we recently developed (an example is shown in Fig 8).
The phenotype of the CD8dull subsets determined by eight-color flow cytometry. Shown is one subject who was treated for HD 22 years before analysis and has elevated levels of both the CD8dullCD4+ subset (top panels) and the CD8dullCD4−subset (middle panels). The CD8bright subset from this subject is shown in the lower panels for comparison. The panel on the left has been gated first on lymphocytes using forward and side scatter and then on T cells using CD3. The gates for the CD8dull and CD8bright subsets are shown in this panel. Surface marker expression measured from three different eight-color antibody combinations are shown (see Materials and Methods). The percentages of cells within the indicated subset that express the marker are shown. Bivariate data is shown as 5% probability contour plots with outlying events shown as dots.
The phenotype of the CD8dull subsets determined by eight-color flow cytometry. Shown is one subject who was treated for HD 22 years before analysis and has elevated levels of both the CD8dullCD4+ subset (top panels) and the CD8dullCD4−subset (middle panels). The CD8bright subset from this subject is shown in the lower panels for comparison. The panel on the left has been gated first on lymphocytes using forward and side scatter and then on T cells using CD3. The gates for the CD8dull and CD8bright subsets are shown in this panel. Surface marker expression measured from three different eight-color antibody combinations are shown (see Materials and Methods). The percentages of cells within the indicated subset that express the marker are shown. Bivariate data is shown as 5% probability contour plots with outlying events shown as dots.
Lineage markers. Because CD8dull cells often express CD8αα homodimers, as opposed to CD8bright cells, which express primarily CD8αβ heterodimers, we measured the expression of both CD8α and CD8β on these subsets. We found that virtually all of the CD8dull CD4+ cells and the majority of the CD8dull CD4− cells, did not express CD8β, but did express CD8α (Fig 9 and Table 2). Thus, the CD8 molecules on these cells consist primarily of CD8αα homodimers, a phenotype consistent with T cells that have been educated extrathymically.18
Expression of the CD8αα homodimer, CD57, and perforin on CD8dull subsets. (Left panels) Shown are the percentages of cells in either the CD8dullCD4+, CD8dullCD4−, or the CD8bright subsets that express CD8αα homodimers or CD57. Subjects had been treated for HD 3 to 25 years before analysis. Four had elevated levels (<19/μL) of the CD8dullCD4+ cells; three had elevated levels (<61/μL) of the CD8dullCD4−cells; two of these subjects had elevated levels of both subsets. The gray box indicates the median and interquartile range for the expression of CD57 on CD8bright cells in a group of 19 healthy controls. The gray bars below and above this box are the 10th and 90th percentiles of the distribution. (Right panels). T cells from two healthy individuals who have enough CD4+CD8dull (top plots) or CD4−CD8dull (bottom) were stained for intracellular perforin in addition to the immunophenotyping stains. The CD8dull populations exhibit a high degree of perforin, including the CD57− cells within the CD4−CD8dull population. These data suggest that the CD8dull cells may have cytotoxic function in the body. The perforin expressing (CD57+) CD4+ T cells in the CD8− fraction were entirely negative for CD8, ie, they are not contaminating cells from the CD8dull fraction and represent yet another phenotypically distinct subset of cells.
Expression of the CD8αα homodimer, CD57, and perforin on CD8dull subsets. (Left panels) Shown are the percentages of cells in either the CD8dullCD4+, CD8dullCD4−, or the CD8bright subsets that express CD8αα homodimers or CD57. Subjects had been treated for HD 3 to 25 years before analysis. Four had elevated levels (<19/μL) of the CD8dullCD4+ cells; three had elevated levels (<61/μL) of the CD8dullCD4−cells; two of these subjects had elevated levels of both subsets. The gray box indicates the median and interquartile range for the expression of CD57 on CD8bright cells in a group of 19 healthy controls. The gray bars below and above this box are the 10th and 90th percentiles of the distribution. (Right panels). T cells from two healthy individuals who have enough CD4+CD8dull (top plots) or CD4−CD8dull (bottom) were stained for intracellular perforin in addition to the immunophenotyping stains. The CD8dull populations exhibit a high degree of perforin, including the CD57− cells within the CD4−CD8dull population. These data suggest that the CD8dull cells may have cytotoxic function in the body. The perforin expressing (CD57+) CD4+ T cells in the CD8− fraction were entirely negative for CD8, ie, they are not contaminating cells from the CD8dull fraction and represent yet another phenotypically distinct subset of cells.
Expression of Differentiation, Activation, and NK Markers on CD4, CD8, and CD8dull Subsets
| Subset* . | Cells/μL† . | Frequency of Subset With Phenotype‡ . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | CD45RA+ . | CD45RA− . | CD45RA− . | CD45RA+ . | CD57+ . | CD8αα+ . | CD16+ . | CD56+ . | TcRγδ+ . |
| . | . | CD62L+ . | CD62L− . | CD62L+ . | CD62L− . | . | . | . | . | . |
| CD4+CD8− | 630 | 5.9 | 57 | 34 | 2.9 | 18 | NA | 0.6 | 6.5 | 0.5 |
| (240-1100) | (0.1-14) | (42-86) | (14-47) | (0.4-7.4) | (11-33) | (0.1-1.1) | (1.7-18) | (0.1-1.1) | ||
| CD8+CD4− | 620 | 7.5 | 48 | 14 | 30 | 52 | 5.7 | 3.3 | 24 | 1.5 |
| (270-830) | (1.5-17) | (22-77) | (11-17) | (11-57) | (38-78) | (0.8-16) | (1.7-6.1) | (11-36) | (0.7-2.5) | |
| CD8dullCD4+ | 55 | 1.5 | 60 | 7.2 | 27 | 79 | 93 | 2.2 | 41 | 1.3 |
| (27-68) | (0.2-2.4) | (16-92) | (5.3-10) | (0.6-76) | (59-92) | (83-99) | (0.6-3.1) | (14-74) | (0.8-2.0) | |
| CD8dullCD4− | 103 | 4.3 | 40 | 5.8 | 49 | 65 | 59 | 8.2 | 36 | 27 |
| (90-114) | (2.1-6.5) | (13-66) | (5.6-5.9) | (23-75) | (49-77) | (50-71) | (3.7-17) | (18-56) | (5.0-52) | |
| Subset* . | Cells/μL† . | Frequency of Subset With Phenotype‡ . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | CD45RA+ . | CD45RA− . | CD45RA− . | CD45RA+ . | CD57+ . | CD8αα+ . | CD16+ . | CD56+ . | TcRγδ+ . |
| . | . | CD62L+ . | CD62L− . | CD62L+ . | CD62L− . | . | . | . | . | . |
| CD4+CD8− | 630 | 5.9 | 57 | 34 | 2.9 | 18 | NA | 0.6 | 6.5 | 0.5 |
| (240-1100) | (0.1-14) | (42-86) | (14-47) | (0.4-7.4) | (11-33) | (0.1-1.1) | (1.7-18) | (0.1-1.1) | ||
| CD8+CD4− | 620 | 7.5 | 48 | 14 | 30 | 52 | 5.7 | 3.3 | 24 | 1.5 |
| (270-830) | (1.5-17) | (22-77) | (11-17) | (11-57) | (38-78) | (0.8-16) | (1.7-6.1) | (11-36) | (0.7-2.5) | |
| CD8dullCD4+ | 55 | 1.5 | 60 | 7.2 | 27 | 79 | 93 | 2.2 | 41 | 1.3 |
| (27-68) | (0.2-2.4) | (16-92) | (5.3-10) | (0.6-76) | (59-92) | (83-99) | (0.6-3.1) | (14-74) | (0.8-2.0) | |
| CD8dullCD4− | 103 | 4.3 | 40 | 5.8 | 49 | 65 | 59 | 8.2 | 36 | 27 |
| (90-114) | (2.1-6.5) | (13-66) | (5.6-5.9) | (23-75) | (49-77) | (50-71) | (3.7-17) | (18-56) | (5.0-52) | |
Abbreviation: NA, not applicable.
Subsets are defined on the expression of CD3, CD4, and CD8, as shown in Fig 1 and 7.
Absolute count of each subset, expressed as cells per microliter of whole blood. The average and range are given for 3 to 5 individuals.
Frequency of each subset expressing the marker or combination of markers shown (as shown by the example in Fig 7). The average and range are given for 2 to 5 individuals.
T cells expressing γδ T-cell receptors are very rare in the CD8dull CD4+ subset, as they are in the CD4+ or CD8+ subsets, but comprise a significant fraction of the CD8dull CD4− cells (Table 2). Among these γδ T cells in this subset, one subject had a Vδ2/Vδ1 ratio below 1 (ie, a predominance of Vδ1 cells, shown in Fig 8), whereas the other two subjects had a more typical ratio above 1. In healthy adults, a vast majority of γδ T cells express Vδ2 (but in HIV-infected adults, a majority are Vδ1).
Differentiation markers. We also measured the representation amongst subsets defined by CD45RA and CD62L in the CD8dull subsets. Because the differentation of CD8dull T cells, which may be educated extrathymically, have not been defined phenotypically, it is premature to assign “naive” and “memory” to distinct subsets. Nonetheless, the CD8dull T cells are divided by the expression of CD45RA and CD62L in a manner which is similar to that for the thymically educated T cells (Fig 8). We find that very few cells in either CD8dull subset coexpressed CD45RA and CD62L (Table 2), and only a minority express CD62L at all (which is similar to the CD8bright memory cells that are expanded after treatment). In fact, the majority of CD8dull T cells express CD45RA and are CD62L−.
Functional markers. We also measured the expression of CD57, as this is a marker known to identify a subset of cells that expands after bone marrow transplantation19 and in HIV disease.20 A large percentage of the CD8dull cells (both CD4+ and CD4−) express CD57 (Fig 9 and Table 2). There is also a higher percentage of CD57+ cells among CD8bright cells in patients treated for HD compared with controls (see Fig 9). Although more treated HD patients must be tested to determine if this difference is statistically significant, the trend is consistent with that seen for other states in which T-cell development is highly altered. We also measured the expression of CD16 and CD56, which are expressed on a minority of CD3+ T cells, often in association with CD57. The expression of these NK markers is highly variable between subjects (Table 2); this variation may be due to an underlying variation in subsets of the CD8dull T cells that we have not yet defined.
In addition, we measured the intracellular perforin expression in T cells from healthy individuals who had a relatively high representation of the CD8dull populations (Fig 9). The CD8dull T cells, in general, had uniformly high levels of perforin expression, suggesting that these may have cytotoxic function in vivo. In addition, a very high fraction of these cells express γ-interferon (IFN) after stimulation (data not shown), consistent with the interpretation of these cells as being differentiated effector populations. However, it is possible that the CD8dull populations in the patients treated for HD are more heterogeneous and have both perforin-expressin and perforin-negative T cells.
DISCUSSION
Altered T-Cell Homeostasis After Treatment for HD
It is well known that the absolute numbers of leukocytes in the blood is under strict homeostatic control. This regulation mechanism extends to fine T-cell subsets: in healthy adults, an average of 50% of T cells (either CD4 or CD8) are CD45RA+CD62L+, or naive. There is little variation in the representation of this subset, or even among the major memory subsets, within an individual over an extended period of time (M. Roederer, data not shown). However, in certain pathological states, there is a profound change in the representation of these functionally and phenotypically distinct subsets.
For example, in HIV disease, there is a progressive loss of naive T cells, of both CD4 and CD8 lineages.12 In addition, there is an expansion of “unusual” T-cell subsets similar to some of those described here (De Rosa et al, in preparation). The basic mechanism underlying these changes is not determined; however, it is possible that destruction of the thymus12 21 can account for many of these changes. To explore this hypothesis, we examined adults who had been treated for HD by (at least) mediastinal irradiation, which may be expected to irreversibly destroy the thymus.
Interestingly, we found that in individuals who had not been treated for HD already display a loss of naive T cells compared with healthy controls. This loss becomes much more significant after treatment and is maintained for at least three decades. In contrast, all of the memory T-cell subsets were unaffected before treatment and recovered to normal or above normal levels within a few years after treatment. Mackall et al17 showed that in young adults there was a much slower recovery of the naive compartment following chemotherapy than the memory; here we show that (at least for treatment of HD), there is a permanent alteration in the homeostatic control of fine T-cell subsets at equilibrium. In addition, we could find no effect of age (our subjects were all older than 17 years) on the degree to which this control mechanism has been altered.
The total number of CD4 or CD8 cells returns almost to normal in these subjects (after 5 years). This suggests that the homeostatic mechanisms controlling overall T-cell number is relatively intact. Thus, to explain the selective lack of naive T cells, a mechanism that selectively or at least preferentially affects these subsets must be invoked.
In the most general sense, we observe a basic insult to the mechanism of fine T-cell subset homeostasis. Because the thymus is the main source of naive T cells,22,23 we suggest that an explanation accounting for these findings is irreversible damage to this organ due either to the disease or to the treatment (the damage is not necessarily complete, as there is an acute recovery phase during which some naive T-cell increase occurs). Indeed, there is evidence of thymic destruction in HD before therapy (R. Hoppe, unpublished data), which may explain the observed pretreatment depletion of naive T cells. While the functionality of the thymus in adults is controversial, there is considerable evidence that it is still populated by lymphocytes,24-28 even in centenarians,28 retains terminal deoxytransferase activity,26 and continues to repopulate the T-cell compartment well past puberty.29
Certainly, there are other mechanisms that may account for the selective inability of naive T cells to regenerate. For example, some investigators have suggested that T-cell populations might be largely maintained (but not replenished) by mechanisms independent of thymus,30 ie, peripheral expansion of mature T cells without differentiation. Our data would suggest that such peripheral expansion would be primarily restricted to the memory populations, thus causing a selective expansion of memory T cells, while naive T-cell numbers remain relatively low.
Implications for the Functionality of the Immune System
Naive subsets proliferate more than memory subsets in response to generic mitogenic stimuli5,10,11; in addition, naive T cells have a significantly different cytokine profile than do memory T cells. Thus, a change in the representation of naive T cells will change the functionality of a bulk population of lymphocytes, even if there is no change in the functionality of any given cells within that bulk population. Thus, the significantly lower representation of naive T cells in lymphocytes of patients treated for HD shown here is certainly a major contributor to the long-term impairment of bulk T-cell proliferation in response to ConA or PHA observed in such patients2,3 and that there may not necessarily be any dysfunctionality at the cellular level. Further, although the depletion of naive CD4 and CD8 T cells is most dramatic posttreatment, there is a decrease in these subsets before treatment (Fig 3). Thus, our data can also explain the decreased proliferative response to PHA found in patients with HD before treatment.31 Indeed, to determine whether or not there is any functional defect at the cellular level, analysis of purely-sorted, functionally homogeneous subsets is required.
The selective loss of T-cell subsets may also explain the reports of impaired antibody response to pneumococcal vaccine in patients who have received recent intensive treatment for H.4 Prophylactic immunization is often necessary in HD because patients with HD frequently are splenectomized to identify splenic involvement. Splenectomized patients are at increased risk from infections caused by encapsulated bacteria, particularly Streptococcus pneumoniae, Haemophilus influenzae Type b, and Neisseria meningitidis.32-35 Because of the fulminant nature of these infections, prophylactic measures, particularly immunization, are essential in splenectomized patients.
A diverse pool of naive T cells is necessary to produce an immune response to a new antigen. In addition, the CD4 memory subset that expresses CD62L (ie, CD62L+CD45RA−or “M2”) has been shown to induce immunoglobulin production in neonatal naive B cells.36 The loss of both of these subsets in subjects who had been treated for HD may explain the poor response to immunization in this group. On the other hand, patients with HD who have not yet received treatment have an essentially normal humoral immune response to vaccination.37 Consistent with this observation, we found, compared with control subjects no pretreatment difference in the CD4 M2 memory subset and only a modest decline in the naive subset before treatment.
Expansion of Unusual T Cells: Compensation?
In a few individuals in the group treated for HD, we were able to identify a significant expansion of two unusual T-cell subsets that expressed dull levels of CD8 and were either CD4+ or CD4−. Because CD8dull cells have previously been shown to express the CD8αα homodimer, we investigated the phenotype of these subsets using eight-color flow cytometric analysis that included both anti-CD8α and anti-CD8β antibodies. We confirmed that both of these subsets expressed predominantly the CD8αα homodimer. Expression of this homodimeric form of CD8 has been associated with extrathymic T-cell development, particularly in the fetal intestine.18 CD4+ iIEL have been observed both in mice and humans, but their development remains a mystery.38-40 Using allogeneic transfer studies,39,40 it was shown that some of these cells could arise from mature T cells (which were likely, but not definitively, of postthymic origin). On the other hand, CD4+ intestinal intra-epithelial lymphocytes (iIEL) were found in a jejunal location with high levels of recombinase activity.38
Another line of evidence that these cells are derived from extrathymic development is the expression of CD57 by both of the CD8dull subsets. The anti-CD57 monoclonal antibody recognizes a sulfated carbohydrate determinant41 expressed on various glycoproteins of different cell lines42; in both healthy controls and the treated populations studied here, virtually all T cells expressing CD57 lack expression of CD28 (data not shown). Persistently elevated numbers of CD57+CD8+ T cells have been associated with a number of clinical disorders including HIV infection,20 persistent cytomegalovirus (CMV) infection,43 as well as in long-time survivors of bone marrow transplants.19 After bone marrow transplantation, these CD57+ cells are among the earliest cells to appear in the peripheral blood. Over time, these cells are replaced by CD57−CD28+ T cells that are more likely to be thymic-derived. CD57+ cells (both CD4+ and CD8+) are also expanded in HIV disease, where viral-induced destruction of the thymus occurs.
Together, these observations suggest that expansion of CD8dull subsets in subjects treated for HD may be a reflection of increased extrathymic T-cell development, perhaps as a consequence of impaired thymic function resulting from the mediastinal irradiation. This increased extrathymic development may be stimulated by the relative paucity of T cells after treatment for HD. While these cells may be cytotoxic in nature, it remains unknown as to the relative immunocompetence of these cells: what sorts of immune responses can they generate and how protective are they for the individual?
ACKNOWLEDGMENT
We thank Dr Mark Prichard for his contribution to the genesis of this project. We are grateful to the Dr David Parks, Adam Treister, Martin Bigos, Richard Stovel, Tom Nozaki, and Wayne Moore of the Stanford Shared FACS Facility for expert advice and technical assistance, especially with the eight-color FACS technology, to members of the Herzenberg laboratory for critical comments and advice, and to Dr Michael McCune for discussions and ideas about thymic function in adults.
N.W. and S.D. contributed equally to this work.
Supported in part by Grant Nos. CA42509, AI31770, AI07290, and LM04836 from the National Institutes of Health, Bethesda, MD.
Address reprint requests to Mario Roederer, PhD, Beckman B011, Department of Genetics, Stanford University, Stanford, CA 94305.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal