Abstract
To examine the mechanisms of CD4 reconstitution in an adult population, lymphocyte repopulation was assessed following dose-intense chemotherapy in 25 breast cancer patients, ages 33 to 69 years. Chemotherapy resulted in a greater than 60% reduction in total CD4 T cells and, in particular, a greater than 90% loss of the CD45RA+ CD4 cells. CD4 recovery was protracted, achieving less than 50% of pretreatment levels after 12 to 14 months. Two facets of the CD4 recovery were notable. First, generation of CD45RA+ CD4 cells played only a minor role in the first year, suggesting that thymic production was not the main route of CD4 regeneration. Indeed, recovery of CD45RA+ CD4 cell levels remained limited in half of the patients even after 2 years. Second, expansion of the mature peripheral CD4 cells (CD45RO+) remaining after chemotherapy was the main source of early CD4 repopulation, peaking at 3 to 6 months postchemotherapy. This expansion was limited in duration, however, and was followed by a secondary decline, such that the total CD45RO+ CD4 levels at 9 to 12 months were lower than at 6 months. When stimulated by mitogens, an increased susceptibility to apoptosis was observed in postchemotherapy CD4 cells as compared with those from normal donors. The elevated expression of markers such as HLA-DR during chemotherapy and for several months postchemotherapy is consistent with the presence of an activated T-cell population. CD4 apoptotic frequency correlated with the frequency of HLA-DR expression on T cells. Thus, CD4 recovery is constrained in adults by a limited thymic regenerative capacity and by an increased susceptibility to apoptosis within the expanding peripheral CD4 population.
A MAJOR COMPONENT of immune deficiency following transplantation or dose intensive chemotherapy is the depletion of CD4 cells. Regeneration of adequate T-cell numbers and repertoire diversity are key elements in the recovery of immune competence. In the absence of such recovery, susceptibility to opportunistic infections is increased.1 2 Furthermore, a lack of functional T cells may limit the feasibility of T-cell–based immune therapies during the period of minimal residual disease following chemotherapy.
In young children, CD4 populations recover rapidly from chemotherapy or transplantation by thymic-dependent production of naive CD4 cells, as distinguished by expression of the CD45RA isoform.3-5 This recovery is correlated with an enlargement of the thymus detectable by computed tomographic (CT) scanning or radionuclide imaging.3 Because the thymus progressively declines in function with age, the extent to which the thymus can contribute to adult T-cell recovery is unknown; in previous work “thymic rebound” failed to occur in some young adults and CD4 recovery was delayed. In transplantation studies in thymectomized and nude mice, expansion of mature peripheral CD4 cells was observed to provide an alternative route of CD4 reconstitution in the absence of the thymic route.6 This recovery was not as extensive as that observed in mice with intact thymuses, however, suggesting a limited expansion capacity.
To characterize CD4 recovery in an adult population, lymphocyte regeneration was studied in patients undergoing a dose intensive chemotherapy regimen for breast cancer. Lymphocyte populations were examined by flow cytometry before treatment, at the end of each of the two chemotherapy phases, and at 3-month intervals postchemotherapy. Regeneration of CD4 cells by thymic maturation and by expansion of existing mature CD4 cells was assessed by CD45RA and CD45RO expression on CD4 cells. Although peripheral expansion provided an early augmentation in CD45RO+ CD4 numbers, CD4 recovery was restricted both by inadequate thymic maturation and by the subsequent loss of the CD45RO cells. Because of this evidence of a secondary decline in CD4 populations, the contribution of apoptosis to restricting the recovery of CD4 populations was investigated.
MATERIALS AND METHODS
Patients. Between June 1993 and December 1994, 53 breast cancer patients (stage III to IV) entered dose intense chemotherapy (5-fluorouracil, leucovorin, adriamycin, cytoxan [FLAC]/Taxol) with cytokine support. The stage II and III patients had received no prior chemotherapy; the stage IV patients could have received prior adjuvant chemotherapy, but were previously untreated with chemotherapy for metastatic disease. All had a normal peripheral blood cell count. Chemotherapy consisted of five cycles, each lasting 3 weeks, of FLAC therapy: (5-fluorouracil (300 mg/m2 intravenous [IV] daily days 1 to 3), leucovorin calcium, 500 mg/m2 daily days 1 to 3), doxorubicin (17 mg/m2 IV daily days 1 to 3), and cyclophosphamide (500 mg/m2 IV days 1 to 3). Patients were randomized to receive either granulocyte-macrophage colony-stimulating factor (GM-CSF ) (sargramostim, Leukine [Immunex, Seattle, WA], 250 μg/m2 subcutaneously daily) or PIXY321 (375 μg/m2 subcutaneously twice a day) from days 4 through 19. Patients were required to have recovered their blood counts to an absolute neutrophil count (ANC) >1,500/μL and platelets >90,000/μL to be treated with the next cycle of chemotherapy. Patients then received five cycles, each lasting 21 days, of paclitaxel (140 mg/m2 by continuous IV infusion [CIVI] over 96 hours). Peripheral blood lymphocyte populations were assessed by flow cytometry and functional assays before the start of treatment, post-FLAC, postpaclitaxel, and during routine follow-up visits at 1, 3, 6, 9, 12, and 15 months following chemotherapy. As of June 1996, 25 patients had completed chemotherapy and 6 to 24 months of follow-up study. Data from patients who relapsed or who underwent further treatment were included only up to the follow-up visit before detection of relapse. These patients formed the basis for the lymphocyte population analysis.
In the apoptosis assays, some peripheral blood (PBL) samples were tested from patients who had been treated with a second chemotherapy protocol involving three successive 2-week cycles of adriamycin-cyclophosphamide (AC) therapy (consisting of cytoxan 2,000 mg/m2 and doxorubicin 60 mg/m2 IV on day 1 followed by 5 μg/kg/d granulocyte colony-stimulating factor (G-CSF ) on days 2 to 13), followed by three cycles of taxol therapy (paclitaxel at 140 mg/m2 by IV infusion 96 hours days 1 to 4, G-CSF days 6 to 13 5 μg/k/d).
Flow cytometric analyses. Peripheral blood, collected in heparin, was stained at 4°C with a panel of antibodies, lysed using fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson Immunocytometry Systems, Mountain View, CA), washed with FACS buffer (Hanks' Balanced Salt Solution [HBSS] without phenol red [GIBCO, Grand Island, NY] supplemented with 0.1% sodium azide and 0.2% bovine serum albumin) and analyzed on a FACSORT (BDIS, Mountain View, CA) equipped with a 480 nm argon laser. Flow cytometer data acquisition and analysis was performed initially using Lysis II software (BDIS) on an HP 340 workstation and subsequently Cellquest software (BDIS) on a Macintosh Quadra 650 computer (Apple, Cupertino, CA). Total leukocyte populations were analyzed using CD45FITC and CD14PE. In subsequent tubes only a lymphocyte population was acquired. The following antibodies were used: anti-CD4, CD8, CD20, HLA-DR, and CD56, (Caltag, San Francisco, CA); anti-CD19, CD25, and CD45RO (DAKO, Carpinteria, CA); anti-CD3, CD16, and CD45RA (Pharmingen, San Diego, CA); and CD38 and anti-CD45/CD14 (BDIS.) B cells were defined as CD19+CD20+, natural killer (NK) cells as CD3−CD16+/CD56++, CD8 cells as CD3+CD8+ and CD4 cells as CD3+CD4+. Proportions of CD45RA and CD45RO within the CD4 population were determined by staining cells with all three antibodies, acquiring the lymphocyte population, and gating during analysis on CD4+ cells only. The CD45RA+ population consisted of CD4 T cells expressing high levels of CD45RA and low to no expression of CD45RO. Intermediate CD45RAlowCD45ROlow cells were not found to be a significant population among CD4 cells (unlike CD8 cells). The numbers of CD45RO+ CD4 cells was therefore calculated by subtraction of the CD45RA+ population from the total CD4 population. Determination of total cells/μL expressing a particular phenotype was calculated by multiplying the white blood cells (WBC) (as determined by Coulter Counter, Hialeah, FL) by the frequency of that population in the lymphocyte acquisition gate and the fraction of total WBC included in the acquisition gate.
Statistical analysis. To illustrate the overall recovery of lymphocyte populations, each individual's lymphocyte subpopulations (CD4, CD8, B, or NK cells) at each time point were converted into a percentage of that individual's pretreatment populations and the means and standard errors of all patients were graphed. To test whether the populations at a given time point were different from the pretreatment levels, the log transform of the individual percentage data was compared with log 100 by the one sample sign test. Comparisons of paired individual CD45RO and CD45RA levels at the nadir, the early recovery peak, and the post-6–month decline level were performed using the Wilcoxon signed rank test. Comparisons of apoptotic frequencies in cultured CD4 cells from normal donors and postchemotherapy patients were compared by unpaired t-test. Correlations between age and CD4 or CD45RA+CD4 cell recovery and between CD4 apoptotic frequency and DR expression on T cells were tested by Spearman correlation coefficient. All statistical analyses were performed using the program Statview (Abacus Concepts, Inc, Berkeley, CA) for the Macintosh.
Induction and assay of apoptosis. Peripheral blood mononuclear cells from normal donors and from postchemotherapy patients (at 3 to 21 months of follow-up) were separated by ficoll-sodium diatrizoate gradient (Lymphocyte Separation Medium, Organon Teknika Corp, Durham, NC), washed two times in Dulbecco's Phosphate-Buffered Saline (DPBS) (GIBCO/BRL, Gaithersburg, MD) and resuspended at 3 × 106 cells/mL in RPMI 1640 (GIBCO) supplemented with penicillin/streptomycin, nonessential amino acids, sodium pyruvate and glutamine, and 5% normal AB serum (Sigma, St Louis, MO). Cells were cultured at 3 × 106 cells/well in 24-well plates (Costar, Cambridge, MA) or at 1 × 105 cells/well (in quadruplicate wells) in 96-well round bottom plates (Costar) in either medium alone, phytohemagglutinin (PHA) (0.75% final, GIBCO), or pokeweed mitogen (PWM) (10 μg/mL final, Sigma) and staphylococcal enterotoxin B (SEB) (1 μg/mL final; Sigma). Cells were harvested after 18 to 48 hours, washed, stained for 10 minutes at 37°C in 2 μg/mL Hoeschst stain (Sigma), fixed in 2% paraformaldehyde and viewed under a fluorescence microscope (Zeiss, Thornwood, NY) for changes in nuclear morphology consistent with apoptosis. To identify the frequency of apoptosis specifically in the CD4 population, harvested cells were stained with anti–CD4-FITC or CD4-PE (Caltag), washed, and resuspended in FACS buffer containing 20 μg/mL 7amino actinomycin D (7AAD, Calbiochem, San Diego, CA), as previously described.7 8 After 30 minutes at 4°C in 7AAD, cells were either analyzed immediately or were fixed in 2.0% paraformaldehyde, washed, and resuspended in 20 μg/mL actinomycin D (Sigma) in FACS buffer and analyzed within 24 hours.
RESULTS
Dose-intensive FLAC chemotherapy resulted in severe depletion of lymphocyte populations. Lymphocyte populations were assessed by flow cytometry in 25 women, ranging in age from 33 to 69 years, during the course of intensive chemotherapy for treatment of breast cancer and during a 6- to 24-month follow-up period. Although hematopoietic growth factors were used during chemotherapy, patients did not receive autologous stem cell infusions or immunomodulatory cytokines. Hence, the recovery of lymphocyte populations was dependent on lymphocytes and lymphocyte precursors remaining at the end of chemotherapy.
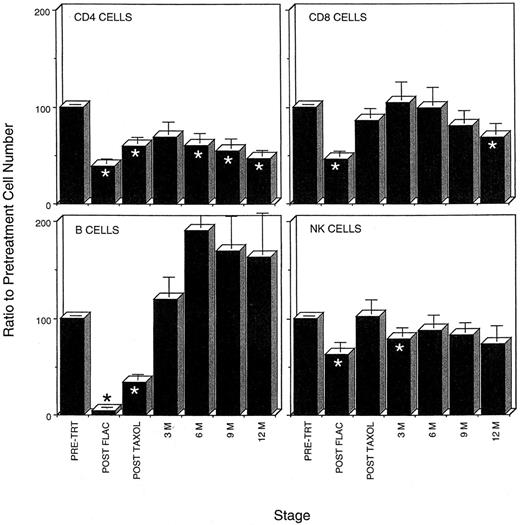
Five cycles of FLAC chemotherapy severely depleted all lymphocyte populations (Fig 1). The average lymphocyte population fell from 2,084 ± 161 cells/μL to only 721 ± 113 cells/μL. Peripheral blood B-cell populations were the most severely affected. Most peripheral blood B cells were lost within the first two cycles of chemotherapy. At the end of five cycles, B-cell populations had been reduced 96%, to fewer than 6 B cells/μL. Similarly FLAC chemotherapy resulted in a greater than 60% reduction in CD4 T cells in the peripheral blood and, in particular, a 95% loss in the number of CD45RA+ naive CD4 T cells (Table 1, Fig 1). Although not as severely affected, CD8 and NK cell populations were reduced to 46% and 62% of their pretreatment numbers (Fig 1).
Time course of loss and recovery of lymphocyte populations during chemotherapy and 1 year of follow-up in the FLAC/taxol study, showing that CD4 recovery postchemotherapy lags behind that of other lymphocyte populations. The average percentage of recovery of lymphocyte populations as compared with pretreatment levels in each patient was determined. When patient data for more than one assay was available within the 3-month time periods (see Fig 4), only that assay closest to the 3-month interval was used in this calculation. (*) Time points at which percentage recovery of lymphocyte populations differs significantly from pretreatment levels.
Time course of loss and recovery of lymphocyte populations during chemotherapy and 1 year of follow-up in the FLAC/taxol study, showing that CD4 recovery postchemotherapy lags behind that of other lymphocyte populations. The average percentage of recovery of lymphocyte populations as compared with pretreatment levels in each patient was determined. When patient data for more than one assay was available within the 3-month time periods (see Fig 4), only that assay closest to the 3-month interval was used in this calculation. (*) Time points at which percentage recovery of lymphocyte populations differs significantly from pretreatment levels.
Depletion and Recovery of CD4 Populations
| Patient No. . | Age . | Stage . | Prior Treatment . | CD4+ Count . | CD4+ CD45RA+ Count . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Before Therapy . | Nadir . | After 6 Mo . | After 12 Mo . | Before Therapy . | Nadir . | After 6 Mo . | After 12 Mo . |
| 9445 | 33 | II | 0 | 649 | 259 | 165 | * | 227 | 37 | 20 | * |
| 9446 | 33 | II | 0 | 583 | 240 | 292 | 281 | 157 | 6 | 18 | 44 |
| 9439 | 39 | II | 0 | 671 | 324 | 110 | 217 | 310 | 97 | 55 | 66 |
| 9305 | 39 | IV | 0 | 851 | 407 | 383 | 212 | ||||
| 9455 | 39 | IIIB | 0 | 750 | 331 | 346† | 187 | 22 | 36† | ||
| 9430 | 40 | IV | CA | 281 | 199 | 456 | * | 76 | 1 | 10 | * |
| 9448 | 40 | II | 0 | 1,616 | 237 | 346 | 748 | 693 | 9 | 27 | 187 |
| 9327 | 41 | IV | CMF | 336 | 187 | 536 | * | 49 | 2 | 81 | * |
| 9335 | 41 | IIB | 0 | 1,254 | 442 | 553 | 345 | 27 | 99 | ||
| 9452 | 43 | IIIB | 0 | 1,044 | 127 | 355 | * | 206 | 6 | 98 | * |
| 9407 | 46 | IV | 0 | 596 | 222 | 226 | 103 | 7 | 27 | ||
| 9338 | 46 | IV | 0 | 962 | 474 | 417 | 530 | 191 | 10 | 30 | |
| 9411 | 47 | III | 0 | 599 | 167 | 556 | 379 | 106 | 18 | 111 | 110 |
| 9349 | 48 | II | 0 | 755 | 271 | 531 | 243 | 40 | 7 | 26 | 17 |
| 9449 | 48 | III | 0 | 970 | 284 | 391 | 219 | 441 | 16 | 51 | 51 |
| 9441 | 49 | II | 0 | 958 | 205 | 256 | 437 | 28 | 43 | ||
| 9447 | 49 | IV | CMF | 538 | 421 | 517 | 379 | 83 | 28 | 47 | 18 |
| 9344 | 51 | III | 0 | 767 | 202 | 636 | 213 | 10 | 99 | ||
| 9306 | 51 | III | 0 | 1,034 | 270 | 294 | 421 | 225 | 4 | 31 | 79 |
| 9307 | 53 | III | 0 | 1,105 | 288 | 770 | * | 262 | 41 | 131 | * |
| 9436 | 54 | IIB | 0 | 1,180 | 68 | 168 | 186‡ | 229 | 5 | 13 | 20‡ |
| 9422 | 55 | II | 0 | 939 | 380 | 505 | 384‡ | 95 | 25 | 49 | 30‡ |
| 9322 | 60 | IV | TAM | 587 | 192 | 412 | * | 193 | 6 | 48 | * |
| 9409 | 68 | IV | CMF | 920 | 321 | 757 | 529‡ | 112 | 7 | 29 | 77 |
| 9424 | 69 | IIIB | 0 | 796 | 244 | 387 | 372 | 184 | 95 | 108 | |
| Patient No. . | Age . | Stage . | Prior Treatment . | CD4+ Count . | CD4+ CD45RA+ Count . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Before Therapy . | Nadir . | After 6 Mo . | After 12 Mo . | Before Therapy . | Nadir . | After 6 Mo . | After 12 Mo . |
| 9445 | 33 | II | 0 | 649 | 259 | 165 | * | 227 | 37 | 20 | * |
| 9446 | 33 | II | 0 | 583 | 240 | 292 | 281 | 157 | 6 | 18 | 44 |
| 9439 | 39 | II | 0 | 671 | 324 | 110 | 217 | 310 | 97 | 55 | 66 |
| 9305 | 39 | IV | 0 | 851 | 407 | 383 | 212 | ||||
| 9455 | 39 | IIIB | 0 | 750 | 331 | 346† | 187 | 22 | 36† | ||
| 9430 | 40 | IV | CA | 281 | 199 | 456 | * | 76 | 1 | 10 | * |
| 9448 | 40 | II | 0 | 1,616 | 237 | 346 | 748 | 693 | 9 | 27 | 187 |
| 9327 | 41 | IV | CMF | 336 | 187 | 536 | * | 49 | 2 | 81 | * |
| 9335 | 41 | IIB | 0 | 1,254 | 442 | 553 | 345 | 27 | 99 | ||
| 9452 | 43 | IIIB | 0 | 1,044 | 127 | 355 | * | 206 | 6 | 98 | * |
| 9407 | 46 | IV | 0 | 596 | 222 | 226 | 103 | 7 | 27 | ||
| 9338 | 46 | IV | 0 | 962 | 474 | 417 | 530 | 191 | 10 | 30 | |
| 9411 | 47 | III | 0 | 599 | 167 | 556 | 379 | 106 | 18 | 111 | 110 |
| 9349 | 48 | II | 0 | 755 | 271 | 531 | 243 | 40 | 7 | 26 | 17 |
| 9449 | 48 | III | 0 | 970 | 284 | 391 | 219 | 441 | 16 | 51 | 51 |
| 9441 | 49 | II | 0 | 958 | 205 | 256 | 437 | 28 | 43 | ||
| 9447 | 49 | IV | CMF | 538 | 421 | 517 | 379 | 83 | 28 | 47 | 18 |
| 9344 | 51 | III | 0 | 767 | 202 | 636 | 213 | 10 | 99 | ||
| 9306 | 51 | III | 0 | 1,034 | 270 | 294 | 421 | 225 | 4 | 31 | 79 |
| 9307 | 53 | III | 0 | 1,105 | 288 | 770 | * | 262 | 41 | 131 | * |
| 9436 | 54 | IIB | 0 | 1,180 | 68 | 168 | 186‡ | 229 | 5 | 13 | 20‡ |
| 9422 | 55 | II | 0 | 939 | 380 | 505 | 384‡ | 95 | 25 | 49 | 30‡ |
| 9322 | 60 | IV | TAM | 587 | 192 | 412 | * | 193 | 6 | 48 | * |
| 9409 | 68 | IV | CMF | 920 | 321 | 757 | 529‡ | 112 | 7 | 29 | 77 |
| 9424 | 69 | IIIB | 0 | 796 | 244 | 387 | 372 | 184 | 95 | 108 | |
Deceased, off study, or lost to follow-up.
15 months postchemotherapy.
9 months postchemotherapy.
The paclitaxel chemotherapy regimen used in this trial was much less toxic to lymphocytes than FLAC therapy. Although lymphocyte numbers were reduced during the course of each cycle of paclitaxel therapy, cumulatively the lymphocyte populations recovered from the nadir reached during FLAC therapy. B-cell levels increased 10-fold to an average of 66 B cells/μL during therapy with paclitaxel. CD8 and NK levels increased to 86% and 100% of pretreatment levels. The recovery of CD4 populations was less extensive, but nonetheless, CD4 populations increased to 59% of pretreatment levels, to an average of 391 CD4 cells/μL.
CD4 recovery was protracted in adults compared with other lymphocyte populations. During the course of the first year postchemotherapy, all lymphocyte populations except CD4 cells recovered to pretreatment levels. B-cell populations recovered to pretreatment levels within the first 3 months (Fig 1). During the remainder of the first year, B-cell numbers in the peripheral blood were higher than pretreatment levels (Fig 1). Similarly, CD8 and NK populations recovered to levels not significantly different from pretreatment numbers during most of the first year. CD4 populations, in contrast, remained low in most patients. After an early recovery to approximately 69% of pretreatment levels (448 ± 62 cells/μL), CD4 numbers declined, falling to only 45% of the pretreatment levels (358 ± 48 cells/μL) in the peripheral blood after 1 year (Fig 1 and 2). CD4 levels remained below 400 in six of 14 patients even at the end of 2 years (Fig 3A).
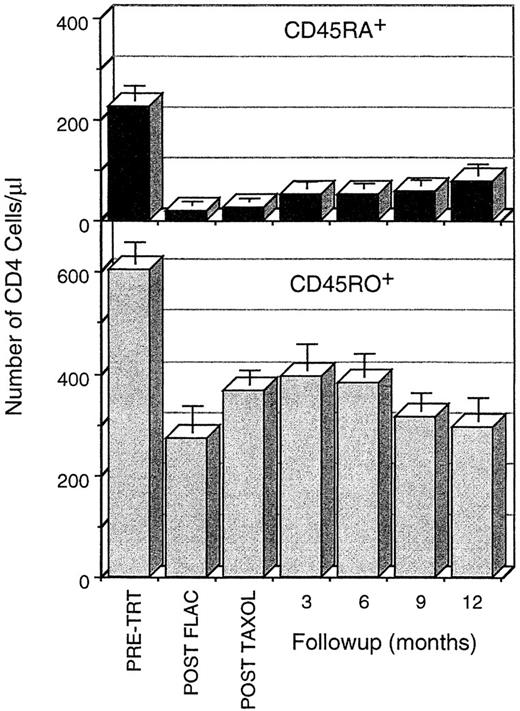
Time course of loss and recovery of CD4 cells, subdivided into the CD45RA+CD45RO− and the CD45RA−CD45RO+ subpopulations, during chemotherapy and 1 year of follow-up. CD45RA+ CD4 cells are severely depleted by FLAC chemotherapy and recover slowly during the first year postchemotherapy. CD45RO+ CD4 cells are not depleted as severely, recover rapidly in the first 3 months postchemotherapy, and then decline secondarily. Data are calculated as the mean ± SE of the CD4 cell numbers/μL; these data were calculated based on the WBC, the percentage of CD4 cells in the lymphocyte population (FACS acquistion gate), and the percentage of lymphocytes in the leukocyte populaton. When an individual was assayed more than once in the 3-month follow-up periods, only that assay closest to the 3-month interval was used in the calculation. All time points during chemotherapy and follow-up are significantly different from pretreatment. Comparison between time points is difficult because data from different patients were included at different time points.
Time course of loss and recovery of CD4 cells, subdivided into the CD45RA+CD45RO− and the CD45RA−CD45RO+ subpopulations, during chemotherapy and 1 year of follow-up. CD45RA+ CD4 cells are severely depleted by FLAC chemotherapy and recover slowly during the first year postchemotherapy. CD45RO+ CD4 cells are not depleted as severely, recover rapidly in the first 3 months postchemotherapy, and then decline secondarily. Data are calculated as the mean ± SE of the CD4 cell numbers/μL; these data were calculated based on the WBC, the percentage of CD4 cells in the lymphocyte population (FACS acquistion gate), and the percentage of lymphocytes in the leukocyte populaton. When an individual was assayed more than once in the 3-month follow-up periods, only that assay closest to the 3-month interval was used in the calculation. All time points during chemotherapy and follow-up are significantly different from pretreatment. Comparison between time points is difficult because data from different patients were included at different time points.
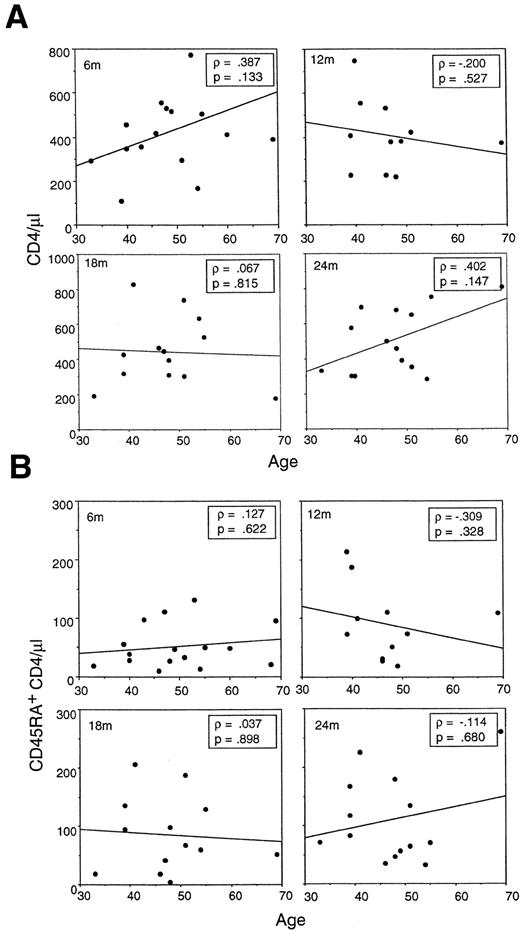
Recovery of either (A) total CD4 or (B) CD45RA+ CD4 cells does not correlate with patient age at 6, 9, or 12 months postchemotherapy. Within an adult age range from 33 to 69 years, the overall pattern of slow steady recovery of CD45RA+ CD4 cells within the first year postchemotherapy is consistent.
Recovery of either (A) total CD4 or (B) CD45RA+ CD4 cells does not correlate with patient age at 6, 9, or 12 months postchemotherapy. Within an adult age range from 33 to 69 years, the overall pattern of slow steady recovery of CD45RA+ CD4 cells within the first year postchemotherapy is consistent.
The generation of new CD4 cells through the thymus, as indicated by expression of CD45RA, contributed little to CD4 repopulation in the first year postchemotherapy. The CD4+ T-cell population consists of two subpopulations as defined by the mutually exclusive expression of low and high molecular weight isoforms of the leukocyte common antigen CD45, (CD45RO and CD45RA, respectively). CD4 cells emerging from the thymus express the CD45RA+ CD45RO− phenotype, whereas activated or memory CD4 cells express the CD45RA−CD45RO+ phenotype.9-13 Naive CD4 cells, which have not been activated following release from the thymus, however, may continue to express CD45RA; hence in normal donors, the maturation of new CD4 cells cannot be readily distinguished from existing naive CD4 cells. During chemotherapy, however, 95% of naive CD45RA+ CD45RO− CD4 cells were lost. Therefore, increases in the number of CD45RA+ CD4 cells postchemotherapy could be used to approximate new generation of CD4 cells through the thymic maturation pathway.
During the first year postchemotherapy, the proportion of CD45RA+ cells in the CD4 population increased gradually (Fig 2). At pretreatment, CD45RA+ cells constituted on average 26% of the total CD4 cells in the patient population; only after 18 months had patients returned to the pretreatment frequency of CD45RA+ cells. Similarly, the number of CD45RA+CD45RO− CD4 cells increased steadily, but very gradually, from the nadir following FLAC treatment (Figs 2 and 4; Table 1). No rapid increase in CD45RA+ CD4 cells was observed, as has been found in young children following chemotherapy or transplantation.1-3 After 1 year, the number of CD45RA+ CD4 cells remained less than one third of pretreatment levels (76 ± 20 cells/μL compared with 225 ± 30 cells/μL). Thus, generation of new CD45RA+ CD4 cells supplied a steadily increasing, but numerically small, component of CD4 cells in the first year after chemotherapy.
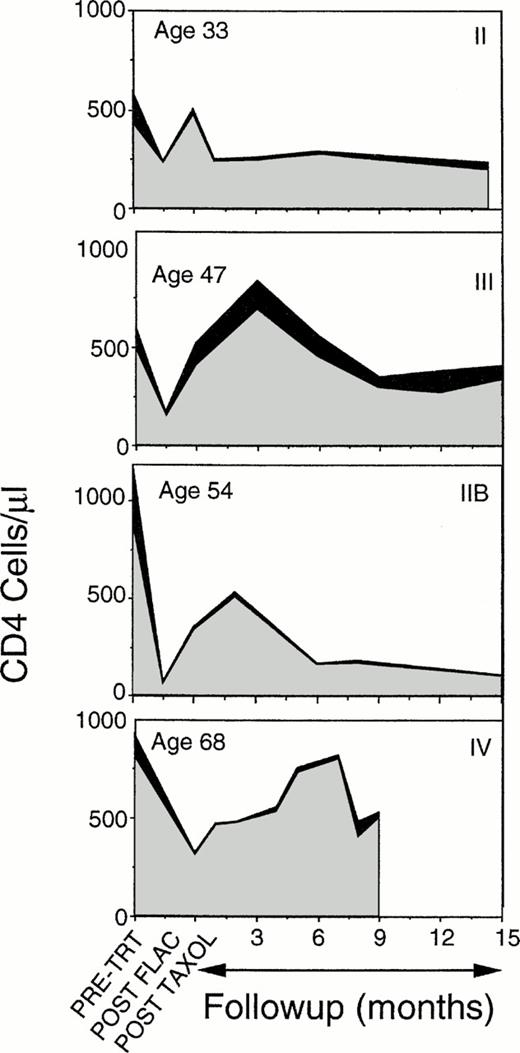
Time course of loss and recovery of CD4 cells, subdivided into the CD45RA+CD45RO− (▪) and the CD45RA−CD45RO+ () subpopulations, during chemotherapy and 15 months of follow-up in four representative patients. CD4 levels are severely reduced during chemotherapy, increase rapidly from the chemotherapy nadir to an early peak, but subsequently decline. Most of the cells involved in both the initial rise and the late drop off are CD45RO+ CD4 cells. Inflection points in the curves represent the times of assays. Patient age is listed in the upper left corner, breast cancer stage at start of treatment (II to IV) is indicated in the upper right.
Time course of loss and recovery of CD4 cells, subdivided into the CD45RA+CD45RO− (▪) and the CD45RA−CD45RO+ () subpopulations, during chemotherapy and 15 months of follow-up in four representative patients. CD4 levels are severely reduced during chemotherapy, increase rapidly from the chemotherapy nadir to an early peak, but subsequently decline. Most of the cells involved in both the initial rise and the late drop off are CD45RO+ CD4 cells. Inflection points in the curves represent the times of assays. Patient age is listed in the upper left corner, breast cancer stage at start of treatment (II to IV) is indicated in the upper right.
In a population ranging in age from 33 to 69 years, no correlation was found between age and recovery of total CD4 cells or CD45RA+ CD4 cells. The recovery of total CD4 cells or CD45RA+ CD4 cells postchemotherapy or transplantation has previously been found to be correlated with the age of the host.1-3 These studies, however, contained a significant number of pediatric or adolescent cases. Because this study included patients ranging in age from 33 to 69 years at the start of chemotherapy, the effect of age on CD4 recovery in a strictly adult population was examined. At 6 months postchemotherapy, the same time point used in the earlier pediatric studies, no significant correlation was observed between age and total CD4 recovery or recovery of CD45RA+ CD4 cells (Fig 3A and B). Because of the concern that the 6-month time point might reflect residual CD45RA+ cell survival postchemotherapy rather than new maturation, CD4 recovery was assessed for as long as 2 years after chemotherapy (Fig 3A and B). The CD45RA+ population gradually increased in essentially all patients, but age was not a determinant in CD45RA+ CD4 levels even after 2 years. Overall, recovery of total CD4 cells (or of CD45RA+ CD4 cells) was quite variable in the adult population studied, with some achieving normal numbers within 1 year and others still at low CD4 levels (<400/μL) after 2 years.
CD45RO+ peripheral CD4 cell expanded rapidly following chemotherapy, but this expansion was transient, with a secondary fall in CD4 numbers at 3 to 6 months postchemotherapy. Although the changes in CD45RA+ populations were incremental, the overall CD4 population, evaluated either as a percentage of individual pretreatment levels or as absolute counts/μL (Figs 1 and 2), was observed to follow a more complex pattern. CD4 levels initially increased concurrent with the expansion of other lymphocyte populations, but declined in the latter half of the first year. Both this early increase in total CD4 levels and the later decline were primarily due to changes in CD45RO+ CD4 cell levels (Figs 2 and 4). CD45RO+ CD4 cell numbers initially increased, recovering to 81% of pretreatment levels by 3 to 6 months postchemotherapy. This increase was short-lived, however, as CD45RO+CD4 numbers subsequently declined to less than 50% of pretreatment levels by 12 months. But a concern arises in using summary data, in that not all patients were assessed at each time point and the early peak in CD4 recovery occurred at different times in different patients (Fig 4). Hence, depending on which patients were tested at a particular time point, variation in individual levels of CD4 cells could alter the average. Examination of the individual patient data, however, shows a repeated pattern in which there is an early peak of CD4 cells, due primarily to expansion of CD45RO+ populations and a subsequent decline (Fig 4). This same pattern was observed independent of patient age or breast cancer disease stage at the start of therapy.
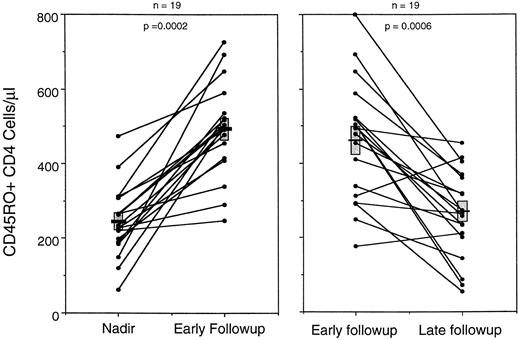
To test this model, a paired comparison was made in individual patients between the number of CD45RO+ CD4 cells at the chemotherapy nadir and at the early peak in CD4 expansion (Fig 5A). A consistent pattern was observed in which CD45RO+ CD4 cells doubled from an average of 241 cell/μL at the nadir to 491 cells/μL at the peak CD4 point in the early follow-up period. CD45RA+ CD4 populations at that early peak constituted only 55 cells/μL; this is a significant (P = .023) change from the nadir, but it is quantitatively much less than the CD45RO+ contribution to total CD4 regeneration.
Comparison of the changes in CD45RO+ CD4 cell levels for 19 patients between the chemotherapy nadir and the peak level of CD4 cells in the early months of follow-up, and between that peak and the lowest level of CD4 cells in the later months of follow-up. Data show that the levels of CD45RO+ cells increased from the nadir to the early peak, but then decreased during the latter half of the first year of chemotherapy follow-up. Data from 16 patients were included in both graphs and from six in only one graph because of missing time points. Note that the bar (▪) represents the mean and () SE of the two populations.
Comparison of the changes in CD45RO+ CD4 cell levels for 19 patients between the chemotherapy nadir and the peak level of CD4 cells in the early months of follow-up, and between that peak and the lowest level of CD4 cells in the later months of follow-up. Data show that the levels of CD45RO+ cells increased from the nadir to the early peak, but then decreased during the latter half of the first year of chemotherapy follow-up. Data from 16 patients were included in both graphs and from six in only one graph because of missing time points. Note that the bar (▪) represents the mean and () SE of the two populations.
When this early peak was then compared with the lowest point in CD4 cells during the later follow-up period (between 6 months and 1 year), the level of CD45RO+ cells consistently declined. CD45RO+ CD4 numbers fell to 260 ± 27 cells/μL, little difference from the original chemotherapy nadir levels (Fig 5B). Comparison of CD45RA+ CD4 subpopulations at the same time points in each patient showed no significant change (P = .60). Thus, the CD45RO+ CD4 cells remaining after chemotherapy undergo a rapid early expansion, but then decline.
Lymphocytes are susceptible to apoptosis for a prolonged period postchemotherapy. The observation of a pattern of rapid increase and a delayed decrease in the CD45RO+ CD4 T-cell population raised the possibility that apoptosis might be involved in the decline. Apoptosis has been shown to play an important role in the regulation of activated lymphocyte populations. When chronically activated cells are stimulated in vitro, many are induced to undergo programmed cell death.14 Recent work in human immunodeficiency virus (HIV)-infected individuals has shown that susceptibility to apoptosis is correlated not with disease state or viral load, but rather, with the activation state of T cells, specifically CD45RO and HLA-DR expression.15,16 The chronically stimulated T cells from HIV patients require no priming step, as do normal lymphocytes, but undergo apoptosis on initial stimulation.14
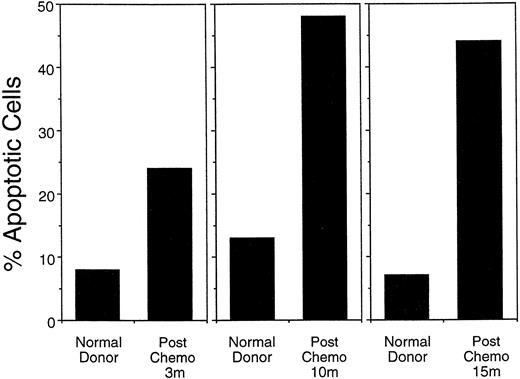
To test whether susceptibility to programmed cell death is increased in postchemotherapy lymphocytes, mononuclear cells from normal controls and postchemotherapy patients were cultured for 18 to 48 hours in PHA, stained with Hoechst stain, and assessed for apoptosis by nuclear morphology. In patients ranging from 3 months to 15 months postchemotherapy, the frequency of apoptotic nuclei was twofold to threefold higher than in normal control donors (Fig 6).
Percentage of apoptotic nuclei among peripheral blood mononuclear cells stimulated for 48 hours with PHA, stained with Hoechst stain, and assessed for nuclear morphology under a fluorescent microscope. Postchemotherapy donor's (PC) cells consistently had a higher level of apoptotic nuclei after stimulation than did normal (N) control cells. Data from the 9- and 15-month time points represent patients in the FLAC/paclitaxel protocol (#9447 and 9306, respectively). The 3-month follow-up assay was done on a patient in a similar chemotherapy protocol (ACT).
Percentage of apoptotic nuclei among peripheral blood mononuclear cells stimulated for 48 hours with PHA, stained with Hoechst stain, and assessed for nuclear morphology under a fluorescent microscope. Postchemotherapy donor's (PC) cells consistently had a higher level of apoptotic nuclei after stimulation than did normal (N) control cells. Data from the 9- and 15-month time points represent patients in the FLAC/paclitaxel protocol (#9447 and 9306, respectively). The 3-month follow-up assay was done on a patient in a similar chemotherapy protocol (ACT).
Because CD4 populations were the limiting population postchemotherapy, apoptotic cell death was then assessed by flow cytometry using 7AAD uptake to discriminate living from apoptotic CD4 populations. This recently developed technique7 8 permits the assessment of apoptosis concurrent with identification of lymphocyte subpopulations by surface markers. Furthermore, this technique permits larger numbers of cells to be assessed than can be done by examination of nuclear morphology; live/apoptotic data was typically collected on 5,000 CD4 cells. The technique was validated by sorting CD4 cell populations identified as living or apoptotic by 7AAD uptake, staining with Hoechst stain, and examining nuclear morphology. This analysis corroborated the flow cytometric data.
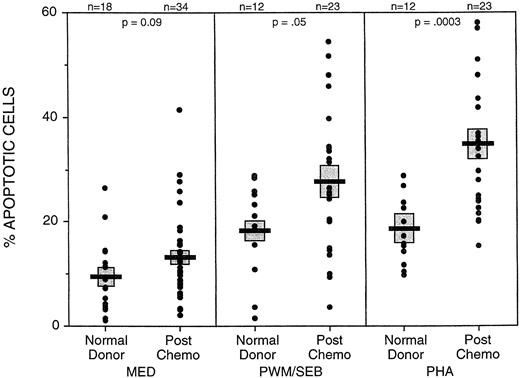
CD4 cells from postchemotherapy patients were found to undergo apoptosis at an elevated frequency when peripheral blood mononuclear cells were cultured with PHA (34.6% ± 2.8%, mean ± SE) or PWM/SEB (27.6% ± 2.9%), as compared with CD4 cells from normal controls (18.1% ± 1.8% and 18.5% ± 2.6%) (Fig 7). Patients were tested at periods from 3 to 21 months postchemotherapy. Although CD4 cells from some of the patients at later time points had apoptotic frequencies similar to those from normal donors, others continued to have elevated apoptotic frequencies. This evidence is consistent with a protracted period of increased susceptibility to apoptosis following chemotherapy.
Higher frequencies of apoptotic CD4 cells were found in unstimulated, PWM/SEB, or PHA stimulated culture from postchemotherapy patients (PC) than in normal control cultures. Cultures of PMN were cultured for 18 to 48 hours with medium alone or mitogens. Apoptosis was determined by flow cytometry using 7AAD uptake in CD4+ cells. Data include assays on patients ranging from 3 to 21 months postchemotherapy from the FLAC/paclitaxel study and the ACT study. Bar (▪) represents the mean and () SE of the assayed populations. Number of assays (n) and P value of the comparison between normal donors and postchemotherapy donors are indicated at the top.
Higher frequencies of apoptotic CD4 cells were found in unstimulated, PWM/SEB, or PHA stimulated culture from postchemotherapy patients (PC) than in normal control cultures. Cultures of PMN were cultured for 18 to 48 hours with medium alone or mitogens. Apoptosis was determined by flow cytometry using 7AAD uptake in CD4+ cells. Data include assays on patients ranging from 3 to 21 months postchemotherapy from the FLAC/paclitaxel study and the ACT study. Bar (▪) represents the mean and () SE of the assayed populations. Number of assays (n) and P value of the comparison between normal donors and postchemotherapy donors are indicated at the top.
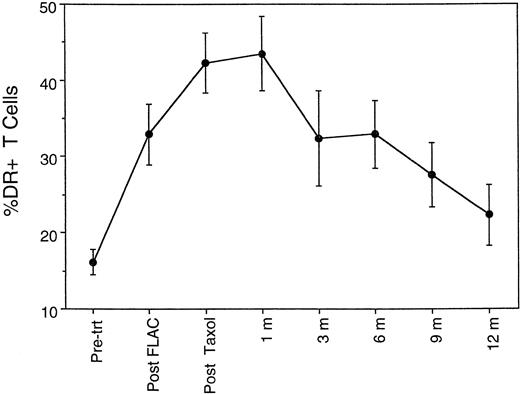
Given the apparent increased susceptibility to activation-induced apoptosis, the question arises as to whether the postchemotherapy T cells are in a chronically activated state, as has been described for HIV+ individuals.14 As noted above, the CD45RA+CD45RO− CD4 cells largely disappeared during chemotherapy, either dead or converted into CD45RA− CD45RO+ cells. The FLAC and taxol chemotherapy regimen also resulted in elevated expression of CD25, CD38, and in particular HLA-DR on CD3+ T cells (Fig 8). Both the percentage of T cells expressing these surface markers and the level of expression per cell (mean fluorescent intensity) were increased. Because of the elevated expression of these markers, the term activated is used in preference to memory in regard to the CD45RO population postchemotherapy; the overall phenotype is consistent with a broadly based activation of T-cell populations, rather than a conversion into resting memory cells. Furthermore, the elevated expression of activation markers is not a transient event. The percentage of T cells expressing HLA-DR did not return to pretreatment levels until after 9 months (Fig 8), that is, after the period of flux of CD45RO+ CD4 cell levels.
Time course of percentage of CD3+ T cells expressing HLA-DR antigen, a marker of activated T cells, during chemotherapy and 1 year of follow-up.
Time course of percentage of CD3+ T cells expressing HLA-DR antigen, a marker of activated T cells, during chemotherapy and 1 year of follow-up.
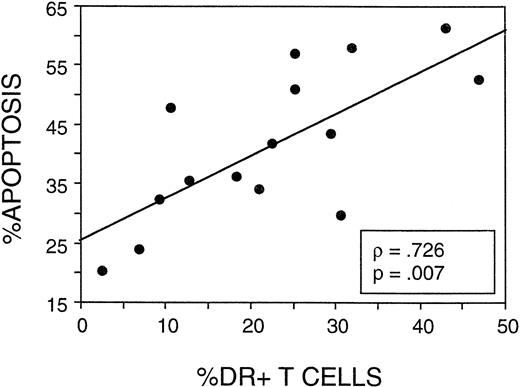
The final consideration was whether the frequency of apoptosis following stimulation in CD4 cells correlated with expression of activation markers. In 15 patients tested for apoptosis and lymphocyte activation markers, a strong correlation was observed (P = .007) between the percentage of T cells expressing HLA-DR antigen and the percentage of CD4 cells undergoing apoptosis (Fig 9). Expression of HLA-DR was only assessed on the CD3+ T cells (not on the CD4+ T-cell subset) during the longitudinal study of FLAC/taxol patients, but ongoing studies of postchemotherapy patients have shown a strong correlation between the percentage of CD4 cells expressing HLA-DR and that of CD3+ cells (data not shown). Furthermore the CD4:CD8 ratio was not reduced below an average of 1.5 during the follow-up period in this study. Hence, the elevated DR was not disproportionately attributable to CD8 cells. Thus, the T-cell population was apparently activated during chemotherapy or during the early expansion phase. This activated population declined subsequently, concurrent with a period of decline of the activated/memory CD45RO+ CD4 population. CD4 cells from postchemotherapy patients showed an increased susceptibility to apoptosis on stimulation that correlated with the overall expression of HLA-DR on T cells.
The frequency of apoptosis in PHA-stimulated CD4 cells correlated with the expression of HLA-DR on T cells. In 15 of the postchemotherapy patients assayed in Fig 7, data on the percentage of total CD3+ cells in the peripheral blood expressing HLA-DR were compared with the frequency of CD4 cells determined to undergo apoptosis after culture of PMN with PHA.
The frequency of apoptosis in PHA-stimulated CD4 cells correlated with the expression of HLA-DR on T cells. In 15 of the postchemotherapy patients assayed in Fig 7, data on the percentage of total CD3+ cells in the peripheral blood expressing HLA-DR were compared with the frequency of CD4 cells determined to undergo apoptosis after culture of PMN with PHA.
DISCUSSION
As chemotherapy regimens have become more intensive, the depletion of lymphocytes and the associated immune deficiency have become more severe.16 In this study, we have shown that CD4 recovery is delayed for more than 1 year following dose-intensive chemotherapy in an adult population. This delay was produced by defects in both thymic-dependent and thymic-independent pathways of CD4 regeneration. First, the classic route of thymic maturation of new naive CD45RA+ CD4 cells from pre-T cells provided a steadily increasing, but very limited, number of new T cells in the first year. Second, expansion of peripheral CD45RO+ CD4 cells predominated in early recovery, but this expansion was limited in size and duration.
Generation of naive CD45RA+ CD4 cells by thymic maturation apparently contributed little to CD4 recovery in the first year. Several concerns should be addressed regarding the use of the CD45RA frequency to approximate new thymic-dependent CD4 generation. Peripheral expansion of residual or newly matured CD45RA+ CD4 cells without conversion to the activated/memory phenotype or conversion of dividing cells back to the naive form could of course increase the level of CD45RA+ cells without new generation by the thymus.17,18 Both murine and human data, however, suggest that the main route of CD45RA+ CD4 cell generation is thymic maturation. Transplants into thymectomized mice do not result in expansion of residual or transferred CD4 cells expressing the high molecular weight isoforms of CD45 (CD45RB in mice); peripherally expanded cells convert to the low molecular weight CD45 isoform.6 Furthermore, expansion of CD45RA+ CD4 cells in pediatric patients postchemotherapy correlated with thymic rebound, an expansion of the thymus to a size greater than that observed prechemotherapy.3 Young adult patients lacking this thymic rebound showed minimal CD45RA recovery for several months. Thus, nonthymic dependent pathways make at most a minor contribution to the generation of CD45RA CD4 cells. An alternative concern is that continued activation of CD4 cells after the end of chemotherapy could siphon newly generated naive CD4 cells into the activated/memory phenotype pool, lowering the CD45RA frequency. Elevated percentages of T cells expressed HLA-DR for several months after chemotherapy (Fig 8), but increased expression of CD69, an early activation marker, was not observed (data not shown). Furthermore, in studies of young patients, CD45RA cells accumulated to more than 60% of the total CD4 cells within 6 months,3 hence, conversion to CD45RO+CD45RA− is not automatic in CD4 cells in the postchemotherapy period. Thus, the CD45RA+ CD4 cell level postchemotherapy provides a useful approximation of CD4 regeneration along the thymic-dependent pathway.
The current study identified no correlation between age and CD4 recovery. Rather, newly matured naive CD4 cells were found to play a minor role in CD4 reconstitution throughout the adult age range in the first year. Other studies, which have found an inverse correlation between age and total CD4 recovery or CD45RA+ CD4 recovery, have included a significant pediatric or adolescent component.3-5 Examined strictly in an adult population, age differences may not be a major factor in the level of thymopoiesis. The diminished thymic function in adults may be due to a combination of factors. The adult thymus is smaller than the prepubertal thymus and does not generally expand (show “thymic rebound”) in man in the early postchemotherapy period.3 Furthermore, aged thymuses produce fewer CD4 cells than young thymuses following lethal irradiation and transplantation with young bone marrow (Mackall, in preparation). Indeed, the aged thymus replaces depleted CD4 cells at a lower rate than the young thymus, even when the the method of depletion is not toxic to the thymus, as in aged versus young mice treated with anti-CD4.19 Finally, the thymus is disorganized by chemotherapy drugs and does not recover function immediately even in young mice.20 Hence, chemotherapy may have exacerbated the deficits of aged thymuses.
A key observation of this study was that expansion of mature peripheral T cells was the main contributor to the early rise in CD4 cell levels postchemotherapy. The CD45RO+ CD4 cells expanded significantly even during the rounds of paclitaxel therapy and were the dominant population throughout the first year in this study. In our previous study of pediatric and young adult patients, this peripheral expansion was not observed,3 perhaps because the level of T-cell depletion was more severe than in the FLAC/Taxol study. The number of mature, peripheral CD4 cells present (or administered) posttranplantation has been observed to play an important role in the rate of CD4 recovery. T-cell recovery is delayed in T-depleted transplants.21 Patients receiving an infusion of autologous peripheral blood stem cells (PBSC) recover lymphocyte populations faster than those receiving autologous marrow, perhaps because of the more sizeable population of lymphocytes included in the PBSC.22,23 In adult patients receiving dose-intensive chemotherapy or transplantation without such an infusion, an extended period is required for recovery of the CD4 T-cell populations critical for immune function.21 Hence, the early expansion of CD4 cells may be dependent on the size of the residual CD4 population.
CD4 reconstitution dependent upon mature CD45RO+ expansion has significant consequences for immune function postchemotherapy. When thymectomized mice were reconstituted with limited numbers of mature CD4 cells, the resultant population was limited in number and did not reach the levels found in intact mice.6,24 More important, the expansion was antigen-driven and prone to skewing and loss of portions of the T-cell repertoire.24 Following autologous and allogeneic transplantation, T-cell recovery dependent on expansion of existing mature cells has similarly resulted in a skewed oligoclonal repertoire.21 25-27 A limited repertoire could contribute to prolonged immune deficits, particularly in response to neoantigens.
CD4 populations following chemotherapy or autologous transplantation have several elements in common with those in HIV-infected individuals. We have observed prolonged deficits in IL-2 production in reponse to mitogens in postchemotherapy patients (data not shown); immune deficits continue for the first year following autologous transplantation,28 and accompany even asymptomatic HIV infection.29 In both chemotherapy and transplantation, an early T-cell expansion is followed by rapid decline21,23; recent evidence suggests a continuous high level of CD4 expansion in HIV patients, as well.30 All populations show a predominance of CD45RO+ CD4 cells and an increased frequency of cells bearing activation markers such as HLA-DR and CD38.4,31 CD4:CD8 ratios may decrease or even become inverted due to both loss of CD4 cells and more rapid production of CD8 cells. The T-cell receptor repertoire shows evidence of oligoclonal expansion or loss of repertoire diversity.21,26 27 These diverse elements may have in common the rapid expansion of depleted peripheral T-cell populations in response to antigenic stimulation. Increased susceptibility to apoptosis in these systems may therefore be a regulatory response, a consequence of the increased level of activation in the CD4 population.
The evidence that postchemotherapy lymphocytes are susceptible to apoptosis on stimulation provides a new perspective on postchemotherapy immune dysfunction. This susceptibility may be responsible for the immune deficiency that has often been observed in vitro in the first year after chemotherapy or transplantation. If a sizeable percentage of the cells were to undergo apoptosis when stimulated, then a variety of common functional assays (such as mitogen-stimulated proliferation and cytokine production) would be depressed as stimulated cells were eliminated. Furthermore, the susceptibility to apoptosis may be responsible for the decline in CD4 numbers observed in many of the patients. An immune response weighted toward apoptosis would result in T-cell decline rather than expansion when environmental pathogens were encountered. This susceptiblity to apoptosis could also result in a loss of relevant T-cell repertoire. If this were to occur, then attempts at immunotherapy involving administration of tumor antigen could result in deletion from the T-cell repertoire of the very cells that were targeted for activation and expansion. Thus, the susceptibility to apoptosis should be considered in the design of immune therapies in the postchemotherapy period.
ACKNOWLEDGMENT
The authors thank Gene Shearer and Ray Bergan for advice, discussions, and review of this manuscript.
Address reprint requests to Frances T. Hakim, MD, National Cancer Institute, National Institutes of Health, Bldg 10, Room 12N226, Bethesda, MD 20892.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal