Abstract
We have previously described a kindred with factor VII (FVII) deficiency whose members exhibited reduced procoagulant activity relative to FVII antigen concentration. In this report, the molecular genetic basis of the FVII defect has been determined to be a heterozygous substitution of Asp for Asn at position 57 in the first epidermal growth factor (EGF) domain. Recombinant FVII (N57D) cDNA was created by site-directed mutagenesis and transiently expressed in human 293 cells. The transfected cells synthesized an immunoprecipitable protein with an apparent molecular weight of 50 kD. Quantitation of expression by FVII enzyme-linked immunosorbent assay indicated that mutant protein yields were consistently low, typically 10% to 30% of wild-type FVII. FVII (N57D) protein did not accumulate intracellularly, and Northern blot analysis indicated equivalent FVII mRNA levels in 293 cells expressing either wild-type FVII or FVII (N57D). Secreted FVII (N57D) protein did not bind tissue factor, exhibited no procoagulant activity, and failed to bind a conformation-dependent monoclonal antibody specific for the first EGF domain of FVII. Molecular modeling of the first EGF domain of FVII predicted that the N57D amino acid substitution would disrupt tertiary bonding structure. We conclude that the N57D mutation affects folding of the first EGF domain of FVII resulting in decreased cellular secretion of a mutant FVII molecule, which is unable to bind tissue factor and is therefore biologically inactive.
HUMAN FACTOR VII (FVII) is a 406-amino acid, 50-kD plasma protein that is essential for the initiation of the extrinsic pathway of blood coagulation. The FVII gene consists of nine exons spanning 12.8 kb of DNA in the q34 region of chromosome 13.2,3 Synthesized in hepatocytes, FVII circulates in normal human plasma at levels of 4 to 12 nmol/L, approximately 98% of which is in the zymogen form.4 Blood clotting is initiated when FVII binds in a calcium-dependent reaction to its cofactor, the transmembrane protein tissue factor (TF). This interaction occurs on exposure of plasma FVII to either subendothelial cells, which constitutively express TF,5-7 or to monocytes and endothelial cells, which transiently express TF in response to various stimuli, eg, bacterial endotoxin.8
Several epitopes of FVII important to the interaction between FVII and TF have been previously identified by biochemical analysis.9-13 Crystallization of the activated FVII (FVIIa)/TF complex has extended these observations and directly identified three main contact points between FVIIa and TF.14 One contact point exists within the γ-carboxyglutamic acid (GLA) domain of FVIIa, a second is in the first epidermal growth factor domain (EGF-1) module of FVIIa, and the last spans both the second epidermal growth factor domain (EGF-2) module and the catalytic domain of FVIIa. Initially, both EGF modules of FVII were identified as being equally important for TF binding.15 Subsequent studies using a monoclonal antibody (MoAb) specific for the FVII EGF-1 domain16 and analysis of the EGF-1 mutant R79Q11,17-19 have provided strong evidence for a critical role of the FVII EGF-1 module in TF binding, observations which have been corroborated by the FVIIa/TF crystallization data.14
MATERIALS AND METHODS
Materials.
FVII cDNA in the vector pCMV5 was the generous gift of Dr Katherine High (Children's Hospital, Philadelphia, PA). COS-1 (ATCC CRL 1650) and 293 cells (ATCC CRL 1573) were obtained from the American Type Culture Collection, Rockville, MD. Rabbit anti-human FVII polyclonal antibody was purchased from Diagnostica Stago (Wellmark Diagnostics, Guelph, Ontario, Canada). Human FVII EGF-1 specific murine MoAb 231-7 and FVII-depleted plasma were prepared as previously described.16 The FVII-specific MoAb E.A.8.1 was the generous gift of Dr George Broze (Jewish Hospital of St Louis, St Louis, MO). β-Actin cDNA probe for Northern hybridizations was the kind gift of Dr Carl Richards (McMaster University). Recombinant TF apoprotein was the kind gift of Dr Robert Kelley (Genentech, San Francisco, CA) and was relipidated with phosphatidylcholine-phosphatidylserine vesicles as previously described.17 21 Immulon II 96-well polystyrene microtitre plates were purchased from Dynatech Laboratories Inc (Chantilly, VA). Purified human FVII was obtained from Enzyme Research Laboratories Inc (Southbend, IN). Factor Xa chromogenic substrate S-2222 was obtained from Helena Laboratories (Mississauga, Ontario). Goat anti-rabbit IgG conjugated to alkaline phosphatase was obtained from Jackson Laboratories (West Grove, PA). Tween 20, bovine serum albumin fraction V, and disodium-p-nitrophenyl phosphate were purchased from Sigma Chemical Company (St Louis, MO). All other chemical reagents were of the highest quality available.
DNA analysis.
After purification of genomic DNA from peripheral blood mononuclear cells, each of the nine exons of the FVII gene was isolated using exon-specific EcoRI-containing oligonucleotide primers and the polymerase chain reaction (PCR) technique essentially as previously described.11 Products of the PCR reactions were purified after agarose gel electrophoresis and subcloned into the EcoRI site of the vector pGEM3Z (Promega, Madison, WI). DNA sequence analysis of multiple cloned samples from each exon was performed on both strands using a commercial version of the Sanger dideoxy technique (Pharmacia-Biotec, Uppsala, Sweden).
Site-directed mutagenesis.
Oligonucleotide site-directed mutagenesis (Clontech, Palo Alto, CA) was performed on FVII cDNA in the vector pGEM7Z (Promega) to create rFVII (N57D) cDNA as per the method of Deng and Nickoloff.22 The selection primer used was the 26 mer 5′-GAATTGGGCCCGTCGACGCATGCTCC-3′ converting anAatII site in the polylinker region to anSal I site. The mutagenic primer was the 27 mer 5′-CAAGTCCATGCCAGGATGGGGGCTCCT-3′ converting the A at the FVII cDNA nucleotide position 330 to G. The cDNA was then subcloned into the EcoRI-HindIII site of the mammalian expression vector pCMV5.23 rFVII (R79Q) cDNA was prepared as previously described.11
Cell culture and transfection.
Wild-type rFVII (WT), rFVII (R79Q), and rFVII (N57D) cDNA in the vector pCMV5 were transfected into either 293 or COS-1 cells by liposome-mediated transfer using Lipofectin reagent (GIBCO-BRL, Gaithersberg, MD) as previously described.24 Both 293 and COS-1 cells were routinely maintained in an equal mixture of Dulbecco's Modified Eagles-Ham's F12 tissue culture media containing 10% fetal calf serum and 100 ng/mL vitamin K. Cell culture conditioned media were collected for analysis 72 hours posttransfection.
FVII antigen determination.
Total rFVII antigen levels were determined in conditioned media by solid-phase immunoassay using a commercially available enzyme-linked immunosorbent assay (ELISA) from Diagnostica Stago (Wellmark Diagnostics). Briefly, the ELISA incorporated rabbit anti-human FVII Fab fragments as the trapping antibody and rabbit anti-human FVII conjugated to horseradish peroxidase as the detecting antibody. Serial dilutions of purified human FVII in tissue culture media were used to generate a standard curve for relative FVII antigen determination in the recombinant samples.
FVII-TF binding assay.
rFVII proteins secreted by transfected 293 and COS-1 cells were assayed for binding to TF essentially as described by Sridhara et al.17 Briefly, relipidated, recombinant TF was coated onto 96-well microtiter plates, nonspecific binding blocked, and the wells overlaid with conditioned media containing FVII protein. Rabbit anti-human FVII IgG conjugated to biotin was used as the primary antibody, followed by streptavidin-alkaline phosphatase as the secondary detecting component. Serial dilutions of purified human FVII in tissue culture media were used to generate a standard curve. A405nm was directly proportional to FVII bound to immobilized TF in the range 1 to 20 ng FVII/mL.17 All rFVII test samples were diluted in pCMV5 mock-transfected conditioned media to maintain total protein levels and uniformity among samples.
FVII-MoAb binding assay.
rFVII proteins secreted by transfected 293 cells were assayed for binding to purified IgG of FVII-specific murine MoAb according to the method of Ofosu et al.25
Amidolytic/prothrombin time assay.
Northern blot analysis.
Total RNA was isolated from 293 cells transfected with FVII cDNA in pCMV5 using a commercial RNA isolation kit (Qiagen, Chatsworth, CA). Total RNA was electrophoresed in formaldehyde-agarose gels and transferred to a nylon membrane (Zetaprobe 30; Biorad, Mississauga, Ontario, Canada) as described by the manufacturer. Radioactive32P-α-dATP–labeled probes used for the quantitation of FVII mRNA were synthesized by the random primer27 method (GIBCO-BRL) using either gel-purified FVII cDNA insert or human β-actin cDNA insert as templates for DNA synthesis.
Molecular modeling analysis.
Molecular modeling of the EGF-1 domain of FVII was accomplished using Insight II (version 2.3) and Homology (version 2.3) software (Biosym/MSI, San Diego, CA) on an SGI INDIGO 2 workstation (SiliconGraphics, Mississanga, Ontario, Canada). The x-ray crystallographic coordinates of the FIX EGF-1 domain28 and the FVIIa-sTF complex14 used to generate the FVII models were graciously provided by Dr David Stuart and Dr David Banner, respectively.
Other methods.
RESULTS
FVII antigen levels and procoagulant activity of (N57D) kindred.
To confirm the status of the extrinsic pathway of coagulation in the affected kindred,1,20 both the FVII antigen concentration and procoagulant activity assays were conducted by two separate laboratories using fresh, unfrozen plasma samples from each family member (Table 1). Normal pooled plasma control FVII antigen levels are defined as 100% (450 ng/mL), with a procoagulant value of 1 U/mL. The plasma of the propositus (III3, Table 1), the most severely affected clinically, had a mean FVII antigen level of 62% and a mean procoagulant activity of 0.35 U/mL. Individual II2, a nonaffected family member, exhibited a mean FVII antigen level of 110% and procoagulant activity of 1.56 U/mL. Individual III5, an affected family member (Fig 1), was unavailable for repeat FVII antigen and activity determinations. Mean FVII antigen levels in the four affected family members investigated were 84% and 85% as determined by each lab independently, values that are within the normal range of 60% to 140%.31 Similarly, the mean FVII procoagulant activity in plasmas from the four affected family members were determined to be 0.58 and 0.59 U/mL by each laboratory, respectively. Antigen to procoagulant activity ratio (Ag/C), a measure of procoagulant activity normalized to 100% FVII antigen concentration, was 0.56 for the propositus and 0.68 for the affected family members collectively. Thus, we initially concluded that this kindred was affected by a type 2 FVII deficiency.20
Coagulation and FVII Antigen Indices of the FVII (N57D) Kindred
| Subject . | Exon 4 Genotype . | Laboratory 1* . | Laboratory 2* . | ||
|---|---|---|---|---|---|
| FVII:Ag (%) . | FVII:C (U/mL) . | FVII:Ag (%) . | FVII:C (U/mL) . | ||
| II1 | H | 96 | 0.75 | 97 | 0.71 |
| III2 | H | 102 | 0.67 | 92 | 0.66 |
| III3† | H | 51 | 0.32 | 72 | 0.37 |
| IV3 | H | 86 | 0.57 | 80 | 0.60 |
| Mean‡ | 84 | 0.58 | 85 | 0.59 | |
| Subject . | Exon 4 Genotype . | Laboratory 1* . | Laboratory 2* . | ||
|---|---|---|---|---|---|
| FVII:Ag (%) . | FVII:C (U/mL) . | FVII:Ag (%) . | FVII:C (U/mL) . | ||
| II1 | H | 96 | 0.75 | 97 | 0.71 |
| III2 | H | 102 | 0.67 | 92 | 0.66 |
| III3† | H | 51 | 0.32 | 72 | 0.37 |
| IV3 | H | 86 | 0.57 | 80 | 0.60 |
| Mean‡ | 84 | 0.58 | 85 | 0.59 | |
Abbreviation: H, heterozygous.
Experiments were performed on both identical fresh plasma samples and a single lot of normal pooled plasma by two independent laboratories. All values are compared to normal pooled plasma which was assigned values of 100% FVII:Ag and 1 unit/ml FVII:C activity.
Propositus.
The mean of FVII antigen and coagulant activities from affected family members.
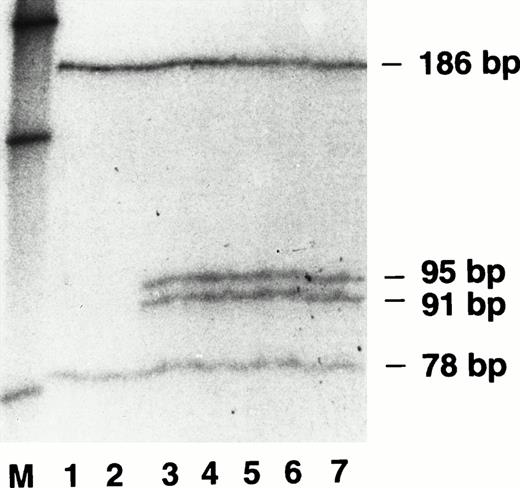
Electrophoresis of Fok I-digested PCR-amplified exon 4 DNA samples from the FVII (N57D) kindred. Lanes 1 to 7 are radiolabeled, PCR-amplified DNA samples from a control and individuals II2, II1, III5, III2, III3, and IV3, respectively (see Table 1). Lane M contained radiolabeled DNA molecular weight markers of 220 bp, 154 bp, and 75 bp (top to bottom) derived from a HinfI digest of pBR322.
Electrophoresis of Fok I-digested PCR-amplified exon 4 DNA samples from the FVII (N57D) kindred. Lanes 1 to 7 are radiolabeled, PCR-amplified DNA samples from a control and individuals II2, II1, III5, III2, III3, and IV3, respectively (see Table 1). Lane M contained radiolabeled DNA molecular weight markers of 220 bp, 154 bp, and 75 bp (top to bottom) derived from a HinfI digest of pBR322.
Genetic basis of FVII deficiency.
DNA sequencing of multiple samples from each exon of the propositus identified a single base pair heterozygous substitution of adenosine for guanine at nucleotide 5989 in exon 4 of the FVII gene. This mutation predicted the amino acid change N57D in the EGF-1 domain of the FVII protein and the creation of a second Fok I restriction endonuclease site in the 264 bp PCR product of the mutant allele of exon 4. For determination of the existence of the second Fok I restriction endonuclease site, we amplified exon 4 of each family member using a standard PCR reaction mix11 with added 10 μCi 32P-α-dATP. After digestion withFok I, the radiolabeled PCR products were separated on a DNA sequencing gel containing 6% polyacrylamide/8mol/L urea and the dried gel was analyzed by autoradiography. As shown in Fig 1(lanes 1 and 2) exon 4 DNA from a normal control and the unaffected family member II2 had one Fok I restriction site yielding the predicted bands at 186 bp and 78 bp. Lanes 3 through 7 in Fig 1 confirmed that the five affected family members, including the propositus (III3), were heterozygous for the A to G mutation and therefore exhibited bands of 186 bp and 78 bp from the normal allele plus two additional bands at 95 bp and 91 bp, a result of the new Fok I restriction site in the 186-bp DNA fragment of the mutant allele.
Expression of rFVII (N57D) in mammalian cells.
In view of the technical difficulties expected in the purification of FVII (N57D) from the plasma of family members, we chose to express and analyze rFVII (N57D). The recombinant cDNA encoding FVII (N57D) was created by site-directed mutagenesis. After confirmation of the fidelity of the entire FVII (N57D) cDNA by DNA sequence analysis, it was subcloned into the mammalian expression vector pCMV5 and transfected into both COS and 293 cells. SDS-PAGE and autoradiographic analysis of 35S-methionine–labeled proteins immunoprecipitated from 293 cell conditioned media with a FVII-specific polyclonal antibody showed a specific 50-kD band in both the rFVII (N57D) and rFVII (WT) samples (Fig 2).Three days after transfection into both COS and 293 cells, total FVII antigen in the conditioned media was quantitated by an ELISA using a polyclonal FVII-specific antibody (Fig 3).In cultures containing equal numbers (5 × 105) of 293 cells, rFVII (WT) antigen levels routinely (n = 5) exceeded 30 ng/mL of conditioned media, whereas rFVII (N57D) antigen levels varied from 1.5 to 10 ng/mL of FVII protein. This latter result is not a general characteristic of mutants of the FVII EGF-1 domain as the previously described variant rFVII (R79Q)11 18 was secreted at levels equivalent to that of rFVII (WT). Levels of rFVII (N57D) secreted by transfected COS cells (n = 2) were similar to those obtained from 293 cells, with rFVII (N57D) protein concentration being approximately 20% of rFVII (WT). To eliminate the possibility of vector bias, rFVII (WT) cDNA and rFVII (N57D) cDNA were excised from their respective pCMV5 vectors by EcoRI-HindIII restriction digestion, and the gel purified cDNA inserts were resubcloned into the opposite pCMV5 host vector and transfected into 293 cells with no change in secretion levels of either rFVII (WT) or rFVII (N57D) protein. After lysis of the 293 cells by sonication the intracellular concentration of rFVII (N57D) protein (4.1 ± 0.1 ng per 35-mm plate) was found to be comparable to the concentration of rFVII (WT) protein (6.5 ± 0.1 ng per 35-mm plate) in an equivalent cell number, indicating that sequestration of rFVII (N57D) protein was not occurring within the secretory pathway. Lastly, to eliminate the possibility that low rFVII (N57D) antigen expression was caused by poor efficiency of either transfection or transcription of the rFVII (N57D) cDNA, Northern blot analysis of total RNA from 293 cells transfected with either rFVII (N57D) cDNA or rFVII (WT) cDNA was performed. Repeated experiments (n = 3, data not shown) indicated that FVII mRNA expression was equivalent in 293 cells transfected with either rFVII (WT) or rFVII (N57D) cDNA when FVII mRNA levels in the two cell populations were normalized to the concentration of the constitutively expressed β-actin gene.
PAGE analysis of radiolabeled, immunoprecipitated rFVII (N57D) protein. Lanes 1 to 3 contain immunoprecipitated protein from 293 cells expressing FVII (WT), mock transfected control, and FVII (N57D), respectively. Protein molecular weight markers ranging from 106 kD to 18.5 kD are indicated on the right.
PAGE analysis of radiolabeled, immunoprecipitated rFVII (N57D) protein. Lanes 1 to 3 contain immunoprecipitated protein from 293 cells expressing FVII (WT), mock transfected control, and FVII (N57D), respectively. Protein molecular weight markers ranging from 106 kD to 18.5 kD are indicated on the right.
Quantitation of rFVII proteins after transient expression in mammalian cells. (□) FVII antigen concentration in conditioned media from transfected 293 cells; (▧) FVII antigen expressed by COS cells. FVII (R79Q) was expressed in 293 cells only. Error bars represent the standard error of the mean.
Quantitation of rFVII proteins after transient expression in mammalian cells. (□) FVII antigen concentration in conditioned media from transfected 293 cells; (▧) FVII antigen expressed by COS cells. FVII (R79Q) was expressed in 293 cells only. Error bars represent the standard error of the mean.
TF binding of rFVII (N57D).
Figure 4 shows the TF binding of equal quantities of rFVII (WT), FVII (N57D), and FVII (R79Q) proteins derived from 293 cell conditioned media. As previously reported11 18 rFVII (R79Q) exhibited reduced binding to TF as compared with rFVII (WT) protein. rFVII (N57D) protein also showed reduced ability to bind TF compared with that seen with rFVII (WT). The ability of rFVII (N57D) to bind TF was also significantly reduced as compared with rFVII (WT) after expression in COS cells (data not shown). Mixed samples of rFVII (WT) and rFVII (N57D) in equimolar concentrations in 293 cell conditioned media showed an approximate 50% decrease in TF binding (data not shown), indicating that there was no apparent interaction between the two FVII molecular populations.
TF binding of rFVII proteins. TF binding of FVII (WT), FVII (N57D), and FVII (R79Q) at a FVII protein concentration of 3 ng/mL in conditioned media from transfected 293 cells. Error bars represent the standard error of the mean.
TF binding of rFVII proteins. TF binding of FVII (WT), FVII (N57D), and FVII (R79Q) at a FVII protein concentration of 3 ng/mL in conditioned media from transfected 293 cells. Error bars represent the standard error of the mean.
Prothrombin time/amidolytic activity of rFVII (N57D).
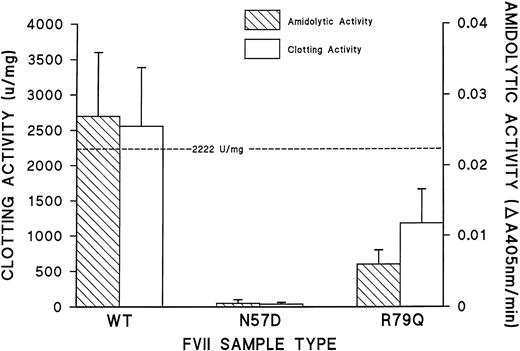
Figure 5 illustrates the procoagulant and amidolytic activities of rFVII (WT), rFVII (N57D), and rFVII (R79Q) proteins derived from 293 cell conditioned media. The rFVII (WT) protein synthesized by transfected 293 cells exhibited a procoagulant activity similar to plasma-derived FVII. The procoagulant activity of rFVII (R79Q) was approximately 60% of rFVII (WT), whereas rFVII (N57D) protein had no procoagulant activity. Similarly, the activity of 60 pmol/L rFVII (WT) and rFVII (R79Q) proteins in the coupled, TF-dependent S-2222 amidolytic assay for FVII26 was determined to be 0.027 and 0.006 A405nm U/min, respectively. The rFVII (N57D) protein exhibited no amidolytic activity.
Procoagulant and amidolytic activity of rFVII proteins. Prothrombin time and amidolytic activity of the rFVII proteins were measured at a rFVII concentration of 3 ng/mL in tissue culture media. The dashed line indicates the specific activity of plasma-derived FVII (2,222 U/mg). Error bars represent the standard error of the mean.
Procoagulant and amidolytic activity of rFVII proteins. Prothrombin time and amidolytic activity of the rFVII proteins were measured at a rFVII concentration of 3 ng/mL in tissue culture media. The dashed line indicates the specific activity of plasma-derived FVII (2,222 U/mg). Error bars represent the standard error of the mean.
Antibody recognition of rFVII (N57D).
Figure 6 illustrates the binding of equal concentrations of rFVII(WT), rFVII (N57D), and rFVII (R79Q) to two human FVII-specific antibodies in ELISA assay. All three rFVII molecular populations were bound equally by a FVII-specific polyclonal antibody previously shown to react with both native and denatured FVII (Fig 6A). In contrast, the FVII EGF-1 conformation-specific MoAb 231-716 did not bind rFVII (N57D) but exhibited normal binding to both rFVII (WT) and another mutant of the EGF-1 domain rFVII (R79Q) (Fig 6B). The binding of MoAb 231-7 is dependent on the integrity of the disulphide bonds and overall conformation of residues 51-88 of the first EGF module of FVII (unpublished data).16,25 As an additional test of the conformation of rFVII(N57D), we determined that the FVII light chain-specific MoAb E.A.8.1 isolated by Broze et al32 did not bind to rFVII(N57D), whereas rFVII(WT) was bound normally (data not shown).
Binding of rFVII proteins to polyclonal and monoclonal FVII-specific antibodies. Antibody binding of FVII (WT), FVII (N57D), and FVII (R79Q) in 293 cell conditioned media to a rabbit anti-human FVII-specific polyclonal antibody (A) and the human FVII-specific MoAb 231-7 (B). Error bars represent the standard error of the mean.
Binding of rFVII proteins to polyclonal and monoclonal FVII-specific antibodies. Antibody binding of FVII (WT), FVII (N57D), and FVII (R79Q) in 293 cell conditioned media to a rabbit anti-human FVII-specific polyclonal antibody (A) and the human FVII-specific MoAb 231-7 (B). Error bars represent the standard error of the mean.
DISCUSSION
Reexamination of the plasma FVII antigen and procoagulant activities in a kindred with combined FVII and FXI coagulation defects confirmed that FVII antigen concentration in affected family members was low but remained in the normal range, whereas FVII coagulant activity was abnormally low (Table 1).1 Detailed molecular genetic analyses of FVII DNA from the propositus (individual III3) showed that she did not encode the R353Q polymorphism known to affect FVII antigen levels in plasma33 but revealed that she, as well as four other affected family members, were heterozygous for a G → A substitution at nucleotide 5989 in exon 4 of the FVII gene (Fig 1). This mutation predicted the amino acid change N57D in the EGF-1 domain of the FVII protein, but the mechanism whereby the N57D substitution might affect FVII coagulant activity was not readily apparent because initial studies of TF binding by kindred plasma FVII appeared to be normal.1 20 The combined clinical and molecular genetic data suggested that plasma FVII protein in affected individuals was a mixture of wild-type and mutant FVII molecules. Because functional analyses of the FVII in kindred plasma samples would be difficult to interpret, homogeneous FVII (N57D) protein was generated by recombinant means.
Transient expression of rFVII (N57D) in both COS and 293 cells showed reduced synthesis of rFVII (N57D) protein versus rFVII (WT) protein (Figs 2 and 3). Northern blot analysis indicated that the rFVII (N57D) cDNA was transfected and transcribed at levels equivalent to rFVII (WT) cDNA. In addition, the intracellular levels of rFVII (N57D) protein were low and similar to rFVII (WT) protein, suggesting that the mutant protein was not being sequestered in the protein secretory pathway as has been reported for the FVII (T359M) variant.34 Inclusion of a protease inhibitor in the culture medium failed to increase rFVII (N57D) protein levels in the media, providing evidence that the mutant protein was not being proteolytically digested by trypsin-like enzymes outside the cell. Collectively, the evidence suggested that the majority of the rFVII (N57D) protein synthesized was being degraded before secretion into the extracellular space, a result also observed for the FVII heavy chain variant FVII Mie.35 Secreted rFVII (N57D) was dysfunctional in both TF binding and enzymatic activity (Figs 4 and 5), a result in accord with the approximately 10% coagulant activity of the analogous mutant factor IX N58K36(mutation UK373 in the hemophilia B database,http://www.ebi.ac.uk/pub/database/ haemb/). Thus, both rFVII (N57D) and another EGF-1 domain mutant (R79Q) exhibit sharply decreased binding to TF, a result consistent with previous evidence that the FVII EGF-1 module is essential for TF binding.11 16-18
Both rFVII (WT) and rFVII (R79Q) bound the conformation-dependent, EGF-1 domain–specific MoAb 231-7 in a dose-dependent manner (Fig 6B). In contrast rFVII (N57D) did not bind to either of the EGF-1–specific MoAbs tested, suggesting that the proper folding of the rFVII (N57D) EGF-1 module was compromised. In most cases, proteins that fail to fold correctly are not released from the endoplasmic reticulum (ER)37-39 and are cleared rapidly from the ER by way of lysosomal or ER-mediated protein degradation pathways. This interpretation is compatible with the reduced secretion levels observed with rFVII (N57D).
Molecular modeling of the effect of the N57D substitution on the structure of the EGF-1 domain of FVII was performed initially utilizing the x-ray crystallographic coordinates of the FIX EGF-128module as a template. The high structural homology between the EGF domains of the blood coagulation proteins40-43 combined with the greater than 60% sequence homology between the FIX and FVII EGF-1 modules make the FIX EGF-1 module an excellent prototype for the FVII model. This initial model predicted that the nitrogen of the secondary amino group of residue N57 would be .298 nm from the carbonyl atom of residue C81, a distance ideal for intramolecular hydrogen bonding (data not shown). The prediction was confirmed by an updated model of the FVII EGF-1 domain (Fig7) constructed using the x-ray crystallographic coordinates of FVIIa.14 In this model the atomic distance between the side chains of N57 and C81 was .283 nm (Fig7). Notably, the importance of an interface between the amino and carboxyl regions of human EGF for its three dimensional structure has recently been established.44 Furthermore, the integrity of this interface is required for receptor binding by both human EGF and transforming growth factor α.44 Thus, a nuclear magnetic resonance model of the structure of human EGF is compatible with the concept that an interface between the amino and carboxyl regions of EGF-1, mediated by a hydrogen bond between N57-C81, is of structural and functional significance in human coagulation FVII.
Molecular modeling of the human FVII EGF-1 module. The figure depicts the FVII EGF-1 loop containing N57 and shows the .283-nm distance from the side chain nitrogen (blue) of N57 to the main chain carbonyl oxygen (red) of C81. Carbon atoms are colored green and sulphur atoms are colored yellow. The main chain is shown as a solid orange ribbon.
Molecular modeling of the human FVII EGF-1 module. The figure depicts the FVII EGF-1 loop containing N57 and shows the .283-nm distance from the side chain nitrogen (blue) of N57 to the main chain carbonyl oxygen (red) of C81. Carbon atoms are colored green and sulphur atoms are colored yellow. The main chain is shown as a solid orange ribbon.
In conclusion, we have shown that there are three important consequences of the N57D mutation in the FVII EGF-1 domain. Firstly, there is a marked decrease in secretion of the FVII (N57D) protein. Secondly, the FVII (N57D) protein that is secreted possesses no procoagulant activity and does not bind either TF or a conformation-dependant, EGF-1–specific MoAb. Thirdly, molecular modeling data support the hypothesis that the effects of this mutation are a result of misfolding of the EGF-1 module of FVII caused by the loss of an important intramolecular hydrogen bond. Although recent crystallization and molecular modeling of the FVIIa/sTF complex by Banner et al14 has indicated that N57 is not located at the FVIIa/sTF binding interface, the critical role of a structurally intact EGF-1 domain for FVII function is confirmed11 16-18 and extended by this report.
ACKNOWLEDGMENT
The authors thank Dr Robert Kelley, Genentech Inc, for generously providing recombinant human TF apoprotein, Dr David Stuart for the x-ray crystallography coordinates of the isolated factor IX EGF-1 domain, and Dr David Banner for both the x-ray crystallography coordinates of factor VII and helpful discussions. The authors also gratefully acknowledge the Heart and Stroke Foundation of Ontario (B.J.C.) and the Canadian Red Cross Society (F.A.O. and M.A.B.) for their support.
Address reprint requests to Bryan J. Clarke, PhD, Department of Pathology, HSC 4N65, McMaster University, 1200 Main St West, Hamilton, Ontario, L8N 3Z5, Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal