MULTIPLE MYELOMA (MM) is a clonal B-cell neoplasm that affects terminally differentiated B cells (ie, plasma cells) and may proceed through different phases: an inactive phase in which tumor cells are nonproliferating mature plasma cells, an active phase with a small percentage (<1%) of proliferating plasmablastic cells, and a fulminant phase with the frequent occurrence of extramedullary proliferation and an increase in plasmablastic cells. During the past years, considerable progress has been made in identifying some of the critical components of neoplastic transformation in MM. This review intends to propose a model of a stepwise malignant transformation during MM pathogenesis. Both diagnostic and therapeutic implications of this model will be discussed.
IMMORTALIZATION
Normal Plasma Cell Development
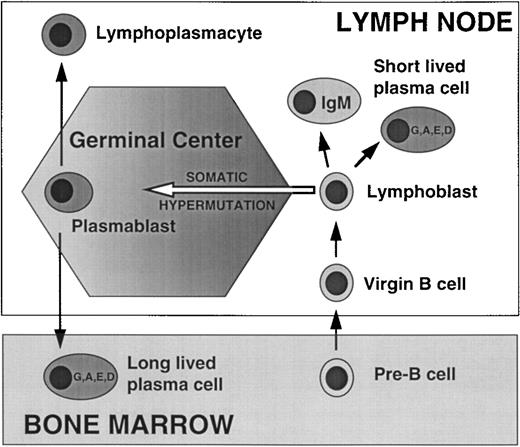
Productive stimulation of mature B cells with antigen results in proliferation and differentiation into memory B cells and plasmablasts. The plasmablasts terminally differentiate into short-lived plasma cells that remain in the local site and die within 3 days; the Ig secreted by short-lived plasma cells is not somatically hypermutated, and is often IgM, although switching to other isotypes (G, A, D, E) can occur. Alternatively, activated B cells enter germinal centers where they are stimulated to actively hypermutate rearranged Ig V sequences but subject to programmed cell death unless rescued by antigen selection. The resultant plasmablasts that undergo IgH switch to another isotype typically migrate to the BM, where they interact with stromal cells and differentiate into long-lived plasma cells that survive for about 30 days (Fig 1).
Normal plasma cell development. Functional V(D)J rearrangements of IgH and IgL genes in pre-B cells in the BM generate an immature B cell that expresses a functional Ig on the cell surface, which then exits the BM as a virgin (mature) B cell, and homes to the secondary lymphoid tissues. Early in the immune response productive interaction with antigen stimulates formation of a lymphoblast which differentiates into a short-lived nonswitched (IgM), or switched (IgG, IgA, IgE, or IgD) PC. Later in the primary response or in a secondary response, the lymphoblast generated by productive interaction with antigen enters a germinal center, where it undergoes somatic hypermuation of its IgH and IgL genes, and antigen selection of cells with high affinity Ig receptor. A germinal center plasmablast that undergoes productive IgH switch recombination typically homes to the BM where it differentiates into a long-lived plasma cell (cf. myeloma cell).
Normal plasma cell development. Functional V(D)J rearrangements of IgH and IgL genes in pre-B cells in the BM generate an immature B cell that expresses a functional Ig on the cell surface, which then exits the BM as a virgin (mature) B cell, and homes to the secondary lymphoid tissues. Early in the immune response productive interaction with antigen stimulates formation of a lymphoblast which differentiates into a short-lived nonswitched (IgM), or switched (IgG, IgA, IgE, or IgD) PC. Later in the primary response or in a secondary response, the lymphoblast generated by productive interaction with antigen enters a germinal center, where it undergoes somatic hypermuation of its IgH and IgL genes, and antigen selection of cells with high affinity Ig receptor. A germinal center plasmablast that undergoes productive IgH switch recombination typically homes to the BM where it differentiates into a long-lived plasma cell (cf. myeloma cell).
The Malignant Myeloma Cell Corresponds to a Long-lived Plasma Cell
The malignant plasma cells in MM are localized to the bone marrow (BM) in close association with stromal cells, and are rarely found in other locations. They are long-lived cells with a very low labeling index (LI = 1% to 2%). The rearranged Ig genes are extensively somatically hypermutated in a manner compatible with antigen selection,1 with no evidence that the process of hypermutation is continuing. However, myeloma cells have a significantly lower rate of Ig secretion than normal plasma cells. Thus, it appears that the critical oncogenic events in MM cells either occur after or do not interfere with most of the normal differentiation process involved in generating a long-lived plasma cell. In the BM, the myeloma cells and stromal cells secrete cytokines and interact through adhesion molecules, activating the stromal cells (including osteoclasts) that further support the growth and survival of the myeloma cells and lead to the complications associated with MM.2 3
Karyotypic Abnormalities
By conventional analyses, karyotypic abnormalities are detected at a frequency of 30% to 50% in large studies of myeloma tumors.4-15 The frequency and extent of karyotypic abnormalities correlates with the stage, prognosis, and response to therapy, eg, approximately 20% abnormal in stage I, 60% in stage III, and greater than 80% for extramedullary tumor. This analysis is dependent on obtaining reliable metaphase preparations and greatly underrepresents the extent of DNA alterations in these infrequently dividing cell populations. By interphase fluorescence in situ hybridization (FISH) analysis using probes for 5 or 10 different chromosomes, respectively, two studies report that at least one chromosome is trisomic in 96% or 89% of myeloma.16,17Although conventional karyotypes are not reported for monoclonal gammopathy of undetermined significance (MGUS), it appears that a substantial fraction of MGUS plasma cells are aneuploid as well. By FISH analysis using only 4 chromosome probes, the incidence of trisomy for at least one chromosome was 43% and 53% in two studies of MGUS cells; in the former case 61% of the cells had an aneuploid DNA content by image analysis.16 18 Despite the limited analyses available for MGUS, it appears that processes leading to karyotypic instability begin in MGUS, progress substantially in frankly malignant MM, and continue to progress throughout the entire course of the disease.
The characteristic numerical abnormalities are monosomy 13, and trisomies of chromosome 3, 5, 7, 9, 11, 15, and 19. Nonrandom structural abnormalities most frequently involve chromosome 1 with no apparent locus specificity; 14q32(IgH locus) in 20% to 40%; 11q13(bcl-1 locus) in about 20% but mostly translocated to 14q32; 13q14 interstitial deletion in 15%; and 8q24 in about 10%, with about half of these involved in a translocation.
Low Incidence of Karyotypically Detectable Translocations to Ig Loci in MM
The hallmark genetic lesion in many B-lymphocyte tumors involves dysregulation of an oncogene as a consequence of a translocation involving the IgH locus (14q32.3), or, less frequently, variant translocations involving one of the IgL loci (2p12, κ or 22q11, λ).19-23 From conventional karyotypic analyses, translocations involving 14q32 appear to occur in about 20% to 40% of myeloma tumors with an abnormal karyotype (references as above). The incidence of these translocations is significantly higher at the extramedullary phase of the disease and in cell lines, perhaps due to a higher number of metaphase spreads that can be examined. In about 30% of these translocations, the partner chromosomal locus is 11q13 (bcl-1, cyclin D1) but in most cases the partner is not identified (14q32+). Other recurrent partner loci have been identified infrequently, eg, 8q24(c-myc) in less than 5%, 18q21(bcl-2), 11q23(MLL-1), 6p21.1. Variant translocations involving 8q24(c-myc) have been detected in a few percent of myeloma tumors.6,9,11,13 14
Translocations Involving the IgH Locus Are Essentially Invariant in Myeloma Cell Lines
Recently, by combining conventional karyotypic analysis with a comprehensive Southern blot assay that detects translocations involving IgH switch regions, it has become apparent that most (19 of 21) myeloma cell lines and the one primary tumor fully examined have IgH translocations that mainly involve IgH switch regions.24-26Notably, seven of nine cell lines with no karyotypically detectable 14q32 translocation have a translocation involving an IgH switch region using this assay. For four of the cell lines it was possible to examine primary tumor material and show the presence of the translocation breakpoint, indicating that the translocations are not an artifact of cell culture. In six cases, there is translocation involving 11q13 and overexpression of cyclin D1. The four cloned translocation breakpoints are into or near an IgH switch region, whereas the same translocation in mantle cell lymphoma invariably involves JH regions.19Five additional lines and the tumor have been determined to have an IgH switch translocation breakpoint involving the telomeric end of chromosome 4 (ie, 4p16.3, near the tip of 4p), despite the fact that no karyotypic translocation was detected in 5 of 6 of these samples.27 The apparent oncogene dysregulated by the 4;14 translocation is the fibroblast growth factor receptor 3 (FGFR3) gene. The cloning of three other translocation breakpoints identified four different chromosome loci (6, 8q24, 16q23, and 21q22).24
The t(6;14) breakpoint was also independently cloned from the same myeloma cell line (SK-MM1) and found to map to 6p25 near IRF4 (MUM1/ICSAT/LSIRF), a member of the interferon regulatory factor (IRF) family of transcription factors.28 The level of IRF4 mRNA was significantly over-expressed in the SK-MM1 cell line compared to the basal level in 10 other MM cell lines, suggesting that juxtaposition of this gene to IgH regulatory elements may have deregulated its expression.28 IRFs are involved in the transcriptional regulation of interferon (IFN) and the IFN-stimulated genes through recognition of the IFN-stimulated response element. IRF4 is a lymphoid specific gene that is rapidly induced by T- and B-cell receptor cross-linking, and is basally expressed in most B, but not T, tumor cell lines.29 Mice deficient in IRF4 have profound hypogammaglobulinemia and cannot mount detectable antibody responses, indicating a critical role of this gene in normal plasma cell development.30
IgH Translocations Involving a Promiscuous Array of Partner Chromosomes: Possibly a Universal and Early Event in MM
Based on the results presented above, it appears that translocations to the IgH or one of the IgL loci may be a nearly universal event in MM despite the apparent low incidence by karyotypic analysis. These translocations are usually into IgH switch regions. They involve two nonrandom loci, ie, 11q13 (bcl-1, cyclin D1) in 25% and 4p16.3 (FGFR3) in 25%. The remaining 50% or so of cases involve a promiscuous array of chromosome partners, some of which have been identified in at least two unrelated tumors [8q24(c-myc), 18q21(bcl-2), 6p21.1 (?), 11q23 (MLL-1)], and others of which have been identified in only one tumor thus far [1p13, 1q21, 3p11, 6p25, 7q11, 12q24, 16q23, and 21q22]. Considering the developmental pathway for long-lived plasma cells (Fig1), the timing of normal IgH switching, and the shared productive switch for paired MGUS and myeloma cells, we hypothesize that this translocation affects the nonproductive Ig allele, and may be one of the earliest molecular events in the pathogenesis of MM, although this remains to be confirmed.
Oncogenes Dysregulated by Recurrent IgH Translocations in Myeloma
c-myc.
c-myc, the cellular homologue of the transforming gene v-myc from the oncogenic avian retrovirus, appears to play a central role in controlling proliferation, differentiation, and apoptosis.31-33 In BALB/c plasmacytomas and rat immunocytomas, activation of c-myc by chromosome translocation into a switch region of the IgH locus (or by a variant translocation to one of the IgL loci) is a universal event.22,34,35 In human MM, by contrast, a t(8;14) occurs in less then 5% of cases, and variant translocations involving 8q24 are reported in only a few percent of tumors (references above). Karyotypic abnormalities of 8q24 other than translocation to one of the Ig loci have also been identified in a small fraction of myeloma samples.9 DNA rearrangement of c-myc has been detected by Southern blot in a few patients.3,36-38 Rearrangements have also been seen in the MLVI-4 locus located 20 kb downstream of c-myc.38,39Infrequently, DNA amplification of c-myc has also been reported.3 36
Adding together Ig translocations and other karyotypic abnormalities involving 8q24, DNA rearrangements involving c-myc or the MLVI-4 locus, and DNA amplification of c-myc, structural genetic abnormalities near c-myc may be present in 10% to 20% of tumors. Both a normal and a polymorphic c-myc allele were found in six myeloma cell lines, but no apparent karyotypic abnormality, DNA rearrangement, or amplification involving c-myc. The c-myc mRNA was expressed from only one of the two alleles, consistent with cis-dysregulation in every informative line.40 However, there is evidence that elevated expression of c-myc and selective expression of one c-myc allele may occur frequently in myeloma,37,40-43 even though structural genetic changes near c-myc have been identified in only 10% to 20% of tumors. Moreover, expression of the c-Myc protein is frequently increased in MM cells44 and cell lines45independently of the c-myc mRNA levels. The elevated c-Myc expression might be caused by mutations in the 5′ untranslated region of the c-myc gene leading to a deregulated translational control of c-myc gene expression.46
bcl-1/PRAD-1/cyclin D1.
The bcl-1 locus at 11q13 is involved in recurrent translocations to 14q32 in chronic lymphocytic leukemia (CLL), lymphomas, and myeloma. This translocation is almost invariant in mantle cell lymphoma, where the breakpoints on 11q13 are clustered predominantly in one region (called MTC, for major translocation cluster), and the breakpoints in the IgH locus are in the JH regions. Rearrangements involving the MTC are rare in myeloma [0 of 58 unselected; and 0 of 13 with t(11;14)].19,39,47-51 The first t(11;14) breakpoints have recently been cloned from myeloma samples and in contrast to mantle zone lymphoma, the MTC region was not involved, and the breakpoints in the IgH locus are frequently into switch regions.24,26 The cyclin D1 gene is approximately 120 kb telomeric to these breakpoints. Cyclin D1 together with CDK4 phosphorylates (and inactivates) pRB and allows for progression through the G1 phase of the cell cycle.52 Unlike other genes identified in this region of 11q13, there is a close association between t(11;14) and enhanced expression of cyclin D1.53Furthermore, a variant translocation (ie, involving a light chain gene) just telomeric to cyclin D154 and a translocation breakpoint within the 3′ untranslated region of cyclin D1 (resulting in a 3′ truncated mRNA)51 serve to effectively define cyclin D1 as the gene targeted by the t(11;14), although given the distance of the breakpoints from cyclin D1, it is possible that other genes may also be involved.
Cyclin D1 has been studied extensively in lymphoma and found to be expressed almost exclusively, and universally in mantle zone lymphoma.55 The expression of cyclin D1 has not been extensively studied in myeloma. In 21 myeloma cell lines, 6 of which have t(11;14), there is a 1:1 correlation between the presence of the translocation and over-expression of cyclin D1 protein.24,26 Expression has been studied in 19 bone and 3 head and neck plasmacytomas using a monoclonal antibody (MoAb), and 1 of 22 have been found to be reactive.55 It should be noted that the presence of the t(11;14)(q13;q32) translocation in myeloma is associated with a lymphoplasmacytic cell morphology as well as with more aggressive disease and a poorer prognosis.27,56 57
FGFR3.
Recently the fibroblast growth factor receptor 3 gene at 4p16.3 was identified as dysregulated by t(4;14)(p16;q32) in 5 of 21 myeloma cell lines and 3 of 11 primary tumors.27 This novel, karyotypically silent translocation has not been described before and may be unique to myeloma, although it has not yet been examined in other tumors, nor has it been extensively studied in primary tumor samples. Because it involves the telomere of 4p16 this translocation generally is not identified by conventional karyotypes, and more sensitive techniques (eg, fluorescent in situ hybridization or Southern blot) are required to detect it. FGFR3 is normally expressed in the lung, kidney, and the chondrocytes at the ends of growing bones. Different germline mutations of FGFR3 result in distinct dwarfing conditions of increasing severity: hypochondroplasia, achondroplasia, and thanatophoric dysplasia.58-63 FGFR3 is expressed at a high level only in those myeloma cell lines with t(4;14). Activating mutations of FGFR3 occur frequently in myeloma cell lines and primary tumors with 4;14 translocations (in 3 of 6 cell lines and 1 of 3 primary tumor samples). The same mutations have been described previously in patients with the most severe form of dwarfism, thanatophoric dysplasia, and they are thought (for some mutations proven) to be activating mutations.64 65 In addition, although both alleles are present in the genomic DNA, in each case only the mutant allele is expressed, indicating that the translocation has caused cis-dysregulation, ie, selective expression of this allele.
We postulate that myeloma cells with dysregulated expression of FGFR3 as a result of t(4;14) receive an FGFR3-mediated signal from FGF produced by stromal cells in the BM micro-environment. Presumably the continuous signal in this environment interferes with their terminal differentiation and apoptosis, and may also stimulate growth. In addition, it seems likely that the activating mutations occurred at a later time than the translocation, and resulted in progression of the tumor to survival and/or growth in the absence of an FGF ligand.
ESTABLISHMENT
The Role of Growth Factors and the Bone Marrow (BM) Microenvironment
The proliferation, differentiation, and function of lympho-hematopoietic cells is regulated by a complex network of lympho-hematopoietic growth factors and cell surface molecules which establish a fine-tuned communication between stroma cells and lympho-hematopoietic precursors in the BM.66-68 These growth factors bind to specific cell-surface receptors that belong to different families, the receptor tyrosine kinases and the hematopoietic cytokine receptors.69-72 The nature of the biological response to any growth factor is defined by the tissue or lineage distribution of growth factor receptors and by distinct transmembrane signaling events in which tyrosine kinases play a pivotal role.73 74
The pathogenesis of MM depends on the presence of some of these growth factors which support the survival, proliferation, and differentiation of MM cells in the BM during the different disease stages.75 76 These cytokines involved in MM pathogenesis are similar to those mediating the proliferation of normal early plasma cells (plasmablastic cells), and their differentiation to mature plasma cells (plasmacytic cells). Interleukin-6 (IL-6) is of particular importance during this process.
IL-6 is a cytokine that has pleiotropic effects on hematopoietic and nonhematopoietic cells.71,77-82 It induces purified B cells to differentiate into Ig-secreting plasma cells.77,78,81,82It acts also as a growth factor for MM (see below).75,83 84Thus, IL-6 mediates the expansion of plasmablastic cells, but also of their malignant counterparts.
The evidence that IL-6 is involved in MM pathogenesis was established by the following experimental and clinical findings: (1) IL-6 could induce in vitro growth of myeloma cells freshly isolated from patients, (2) myeloma cells spontaneously produced IL-6 and expressed IL-6R, (3) anti-IL6 antibodies inhibited the growth of MM cells or cell lines in vitro, and (4) treatment of MM patients with MoAbs to IL-6 showed some antitumor effect.76 83-85
IL-6 supports the survival and/or expansion of MM cells not only by stimulating cell division, but also by preventing programmed cell death (apoptosis) which can be induced by serum starvation,86 or by treatment with compounds like vitamin D3 or its derivative EB1089,87dexamethasone,86,88,89 anti-Fas antibodies,90or suramin.88 Retinoid acid (RA) induces programmed cell death in MM cell lines by downmodulating the IL-6R expression.91 Similarly IL-6R antagonists, which block the activation of MM cells by IL-6, act as pro-apoptotic factors for MM cells.92
There is still some controversy about the source of IL-6 provided as a MM growth factor during disease progression. Some investigators have found IL-6 to be produced by the tumor itself in an autocrine manner,84 but stronger evidence supports the notion of a paracrine IL-6 secretion by the tumor microenvironment in the BM.76,83 IL-6 is probably produced in large amounts by BM stroma cells (BMSC), osteoblasts, and osteoclasts.93-95 The IL-6 secretion by BMSCs seems to be regulated by cytokines like IL-1β secreted by the tumor, although the experimental proof for this hypothesis has been hampered by the difficulty to generate pure myeloma cell samples.76 Antibodies to IL-1 or the use of the IL-1 receptor antagonists were able to block the IL-6 production in short-term cultures of BM cells from MM patients.76 Colony-stimulating factor-1 (CSF-1) is also produced by MM cells, but a role in stimulating IL-6 secretion has not been shown so far.96
Viral IL-6 (vIL-6) produced by BM dendritic cells infected with Kaposi's sarcoma–associated herpesvirus (KSHV) is an alternative source for IL-6 in MM pathogenesis.97 KSHV has been detected consistently in human immunodeficiency (HIV)-related and HIV-unrelated cases of Kaposi's sarcoma (KS), pleural effusion lymphoma, and multicentric Castleman's disease.98 A homolog to human IL-6 has recently been identified in the KSHV genome which has biological properties of human IL-6 in that it supports the growth of IL-6–dependent cell lines. Using a sensitive polymerase chain reactive (PCR) assay, Rettig et al97 detected KSHV DNA in the BM dendritic cells of 15 out of 15 MM patients but not in malignant plasma cells or BM dendritic cells from normal individuals, or patients with other malignancies. In addition, the virus was detected in the BM dendritic cells of 2 of 8 MGUS patients. vIL-6 was found to be transcribed in the myeloma BM dendritic cells.97 These data indicate that KSHV infection of BM dendritic cells and vIL-6 may be involved in transformation from MGUS to myeloma and perpetuate the growth of malignant plasma cells.
Stimulation of cells by IL-6 requires binding to the IL-6 receptor (IL-6R) composed of at least two subunits with apparent molecular weights (m.w.) of 80 and 130 kD, the IL-6R α (IL-6Rα) and β chain (IL-6Rβ or gp130). Binding of IL-6 to IL-6Rα induces the tyrosine phosphorylation and association with gp130, the signal transducing subunit of the IL-6R, and the subsequent formation of gp130 homodimers.99 gp130 is the common β subunit shared by the receptors for ciliary neurotropic factor (CNTF), oncostatin M (OSM), leukemia inhibitory factor (LIF), IL-11, cardiotrophin 1, and IL-6 and is essential for transmitting their respective signals.71Accordingly, these six cytokines share some biological functions with IL-6, and some MM cell lines respond to LIF, OSM, or CNTF if the appropriate receptor α chain is expressed.100 In some MM patients, IL-11 levels may also be elevated in the BM, but the effects of IL-11 and of the other three cytokines on the growth of MM cells in vivo remain uncertain and have not been studied with the same scrutiny as the effects of IL-6.76
Another potential mechanism contributing to the growth and expansion of MM is the agonistic effect of the soluble IL-6Rα which enhances the sensitivity of myeloma cell lines to the effects of IL-6.101 These soluble IL-6Rα are generated by receptor shedding from the cell membrane or by alternative RNA splicing.102,103 High serum levels of IL-6 were shown to predict a poor prognosis or to reflect an active disease in MM by many studies.101,104-111 Serum IL-6 receptor levels may less reflect the disease activity in MM.101 However, decreases of both parameters have been reported to accompany the response to treatment in MM.112
Additional Growth Factors for MM
Granulocyte colony-stimulating factor (G-CSF) is a hematopoietic growth factor with structural homology to IL-6.113,114 Moreover, the G-CSF receptor shares also some homology with gp130.115Both G-CSF and IL-6 induce activation of NF-IL-6, a transcription factor involved in the synthesis of IL-6.116 G-CSF is a potent growth factor for freshly explanted myeloma cells, and MM cell lines may respond to G-CSF.76 The mechanism of action by which G-CSF mediates MM growth is unknown. However, the data caution that treatment of MM patients with G-CSF after high-dose chemotherapy or for stem cell harvest might eventually enhance proliferation of the tumor,76 although this has never been shown in clinical practice.
Interferon-α (IFN-α) is another growth factor for MM in vitro, although growth inhibitory effects have been reported as well (see below). IFN-α stimulates the proliferation of fresh myeloma cells from 16% to 50% of patients.117-119 IFN-α also stimulates the growth of IL-6–dependent MM cell lines.120This effect seems to be mediated by an enhancement of the autocrine IL-6 production by MM cell lines.120 These results may explain why adjuvant IFN-α treatment has little, if any beneficial effect in MM: despite encouraging results in an early clinical trial with adjuvant IFN-α treatment after chemotherapy,121 more recent studies have failed to demonstrate a benefit for IFN-α maintenance.122-125
IL-10 is also a growth factor for MM cells, because it enhances the proliferation of freshly explanted myeloma cells in short-term BM culture.126 Moreover, the growth of myeloma cell lines is also supported by IL-10.76 IL-10 has inhibitory effects on the production of IL-6 by MM cells; therefore, its effects are probably not mediated by IL-6. More likely, IL-10 enhances the responsiveness of MM cells by regulating the expression of other cytokines and cytokine receptors. IL-10 might increase the responsiveness of some myeloma cells to IL-11 by upregulating the expression of IL-11 receptors.127 Moreover, IL-10 induces an autocrine OSM loop in human myeloma cells, probably by increasing the expression of the LIF receptor which forms with gp130 a receptor for OSM.128
Granulocyte-macrophage-CSF (GM-CSF), IL-3, stem cell factor (SCF), tumor necrosis factor-α (TNF-α), hepatocyte growth factor (HGF), and insulin-like growth factor 1 and 2 (IGF-1 and IGF-2) are also potential MM growth factors, because they were shown to stimulate growth and/or specific intracellular signaling events of MM cells or cell lines in vitro, often in a synergistic manner with IL-6.129-138
Factors Inhibiting the Growth of MM Cells
Interferon-γ (IFN-γ) was reported to inhibit IL-6–dependent proliferation of fresh MM cells.139 It did not affect the endogenous IL-6 production, but seemed to act directly on MM cells. It is possible that IFN-γ interferes with IL-6 transmembrane signaling, resulting in enhanced apoptosis. Interestingly, IFN-γ also inhibits cytokine-mediated bone resorption, which is a relevant clinical problem in MM.76 TNF-α and transforming growth factor-β (TFG-β) are other potential inhibitors of the proliferative effects of IL-6.140
IFN-β and IFN-α were both shown to inhibit the proliferation of the MM cell line U266.141-143 These effects are mediated, at least in part, by a downmodulation of the IL-6R.141,143 As a consequence, IFN-β reduces the IL-6–dependent tyrosine phosphorylation and activation of several signaling proteins, including Ras.142 These observations are in contrast to the above described stimulatory effects of IFN-α on MM cell proliferation. It is possible that the effects of type I interferons (IFN-β and IFN-α) vary in different MM cell lines or patients. Although a therapeutic modulation of the BM microenvironment by type I interferons has been well documented in chronic myeloid leukemia,144-146 this has not been demonstrated for MM.
Stimulation of the Fas antigen (alternatively termed APO-1/CD95), a transmembrane molecule of the TNF nerve growth factor (NGF) receptor superfamily, induces programmed cell death in different cell types, including MM cells.147-150 Fas is expressed on almost all MM tumors and cell lines.151 However, activation of Fas induces MM apoptosis only in some patients' samples.151Activation of MM cells by IL-6 was shown to antagonize the cellular effects of Fas, including the activation of a stress activated protein kinase (SAPK), thus preventing Fas-triggered apoptosis.90This antagonism of IL-6 and Fas may serve as a paradigm for the many interactions that regulate MM growth in a fine-tuned network of cytokines and growth factors in the BM microenvironment.
Taken together, a variety of growth factors promote the growth of myeloma cells in vitro and/or in vivo: IL-6, IL-11, OSM, LIF, G-CSF, SCF, IFN-α (?), IL-10, TNF-α (?), IGF-1, and IGF-2. In contrast, stimulation of myeloma cells with anti-Fas antibodies and IFN-γ seems to inhibit cell growth. It is difficult to clarify the functional relevance of these redundant growth factor effects on MM cells. Moreover, it seems important to emphasize that the expression of the various cytokine receptors may vary among individual patients, and also among different tumor cells in a given patient. Nevertheless, IL-6 clearly seems to be the most important stimulatory factor of these cytokines, because the biologically active IL-6 concentrations found in vitro and in vivo are 500- to 5,000-fold higher than the concentrations of other MM growth factors.76
Adhesion Molecules and Other Cell-Surface Antigens
The differentiation of lymphoid precursor cells into mature B lymphocytes is accompanied by characteristic changes of cell-surface antigens. The application of high resolution, multiparameter flow cytometry has been used to identify and characterize normal plasma cells in the human BM. Plasma cells exist in at least two different subpopulations, early lymphoplasmacytoid plasma cells and late mature plasma cells.152 These two populations appeared phenotypically different, but both strongly express CD38. In distinction to mature plasma cells, early lymphoplasmacytoid cells express CD22, CD35, and surface IgE receptors, and intracytoplasmatic Ig light chain only at low density. Subpopulations of mature, normal plasma cells show a very heterogeneous immunophenotype in that they can express early B-cell antigens (CD19, CD20, CD10), myeloid antigens (CD13, CD33), HLA-DR, common hematopoietic antigens (CD45), and adhesion molecules (CD11b, CD11c).153
The neoplastic cells of MM probably follow this maturational pattern. Although the MM “stem cell” is unknown, there is evidence for cell clones which show the immunophenotype of early B-cell precursors which express CD38 at high levels, as well as CD10, CD34, CD19, CD20, and CD24.154,155 Immature myeloma cells (CD38++CD45− or CD38++CD49E+) appear to express the same clonal idiotype as mature cells (CD38++CD45− or CD38++CD49E+). Whether CD34 is expressed on MM cells remains controversial. In a recent study, 74% to 94% of individual sorted CD34+19+ B cells from four different MM patients were shown to express clonotypic IgH mRNA, as detected by in situ reverse transcriptase (RT)-PCR with patient-specific primers.156 Other studies failed to detect CD34 on myeloma cells157 or did not find myeloma cells in anti-CD34 MoAb-enriched stem cell harvests.158 Myeloma cells show a similar heterogeneity in their immunophenotype as their normal counterparts, according to their differentiation stage. It is believed that myeloma cells originate outside the BM and give rise to plasma cells upon migration, using expression of unique adhesion molecules to interact with the marrow microenvironment.159Finally, costimulatory molecules such as CD28 and B7-1 or B7-2 seem to be expressed by some immature myeloma cells, but may be lacking in mature MM or in MM cell lines.160 161 This may prevent activation of T cells against the tumor.
Despite the similarity of most antigens expressed on myeloma cells and normal plasma cells, some of the antigens detectable on myeloma cells are rather unique. For example, the adhesion molecule CD56, which is the 140-kD isoform of N-CAM, is highly expressed on some myeloma but not on normal mature plasma cells.129,162 It seems to mediate adhesion of myeloma cells to each other (homotypic adhesion). Some MM cells or cell lines lack expression of the B-lineage–specific antigen CD19 antigen which is a key member of a B-cell surface signal transduction complex which includes TAPA-1, Leu 13, and CD21, and initiates multiple intracellular signaling cascades, either through CD19 directly or through other members of the complex.163 164
The expression of adhesion molecules and other cell-surface molecules is important for the communication of MM cells with the BM micro-environment. In a murine BM metastasis model, expression of at least three adhesion molecules, CD44, VLA-4, and ICAM-1, was important for mediating adherence of B9/BM myeloma cells to stromal cells.165 Adhesion of MM cells triggers IL-6 secretion by normal and MM BM stroma cells (BMSCs).166 Adhesion of MM cells to BMSCs induces IL-6 gene transcription in BMSCs which is conferred by NF-κB binding to the IL-6 promoter.167 One of the cell-surface molecules triggering IL-6 secretion in MM cells is CD40, a member of the TNF receptor superfamily that is expressed on various lymphoid malignancies.168-171 Activation of CD40 induces the clonogenic growth and enhances the survival of MM cells in vitro.172 Stimulation of CD40 by its ligand, CD40L, stimulates the secretion of IL-6 from MM cells and cell lines, suggesting the possibility for induction of IL-6–mediated autocrine MM cell growth.173,174 However, the source of CD40 stimulation in the BM stroma remains unknown; expression of CD40L seems to be restricted to T lymphocytes, but its expression on BMSCs has not been investigated. Some of the remaining questions on the homing and adhesion of MM cells in the BM may now be addressed using in vivo models, because human MM cell lines seem to engraft in the human bone marrow of severe combined immunodeficient-human (SCID-hu) mice and maintain the characteristics of clonal MM tumors.175
Mechanisms of Signal Transduction
IL-6 receptor.
Because IL-6 is of central importance in the pathogenesis of MM, the intracellular signaling events elicited by this cytokine are also of potential relevance. Recently, some progress has been made in understanding the biochemical events involved in IL-6 transmembrane signaling at a molecular level (Fig 2).Binding of IL-6 to IL-6Rα induces the formation of a hexameric receptor complex composed of 2 IL-6Rα, 2 gp130, and 2 IL-6 molecules. Subsequently, receptor-associated tyrosine kinases mediate the phosphorylation of gp130, the signal transducing subunit of the IL-6R.99 This receptor complex further activates tyrosine kinases including members of the Janus kinase (JAK) and Src kinase families which mediate the phosphorylation of cytosolic phosphoproteins.176-181 Because both IL-6Rα and gp130 do not possess any apparent kinase domain, their association with nonreceptor tyrosine kinases like JAKs or Src kinases is critical for mediating the effects of IL-6.
Transmembrane signaling of IL-6, which is a major growth factor for MM. Binding of IL-6 to the α chain (gp80) of the IL-6 receptor (IL-6R) causes the formation of multimeric complexes composed of 2 IL-6R α chains, 2 β chains (IL-6Rβ or gp130), and 2 IL-6 molecules. Subsequently, tyrosine kinases (JAKs and Src kinases, in particular Hck) which are bound constitutively to the IL-6Rβ, become activated and (trans)phosphorylate the receptor. This creates specific docking sites for several signaling proteins including STAT1, STAT3, and Shc (?) on the IL-6Rβ, allowing further phosphorylation of these proteins by receptor-associated kinases. Activation of Shc recruits Grb2/Sos1 to the cell membrane. Sos1, a Ras-GDP/GTP exchange factor activates Ras; this activates a signaling cascade of several serine/threonine kinases including Raf-1, MKK, MAPK. Finally, MAPK phosphorylates substrates like c-Myc, c-Jun, c-Fos, RSK, and these events eventually enhance MM proliferation or prevent apoptosis. Upon phosphorylation, STAT1 and STAT3 form homodimers and heterodimers that are translocated to the nucleus and act as transcription factors for IL-6–induced promoters. Although the Ras-MAPK signaling cascade is believed to promote MM growth, no such function has yet been reported for the JAK-STAT pathway, believed to trigger rather metabolic events.
Transmembrane signaling of IL-6, which is a major growth factor for MM. Binding of IL-6 to the α chain (gp80) of the IL-6 receptor (IL-6R) causes the formation of multimeric complexes composed of 2 IL-6R α chains, 2 β chains (IL-6Rβ or gp130), and 2 IL-6 molecules. Subsequently, tyrosine kinases (JAKs and Src kinases, in particular Hck) which are bound constitutively to the IL-6Rβ, become activated and (trans)phosphorylate the receptor. This creates specific docking sites for several signaling proteins including STAT1, STAT3, and Shc (?) on the IL-6Rβ, allowing further phosphorylation of these proteins by receptor-associated kinases. Activation of Shc recruits Grb2/Sos1 to the cell membrane. Sos1, a Ras-GDP/GTP exchange factor activates Ras; this activates a signaling cascade of several serine/threonine kinases including Raf-1, MKK, MAPK. Finally, MAPK phosphorylates substrates like c-Myc, c-Jun, c-Fos, RSK, and these events eventually enhance MM proliferation or prevent apoptosis. Upon phosphorylation, STAT1 and STAT3 form homodimers and heterodimers that are translocated to the nucleus and act as transcription factors for IL-6–induced promoters. Although the Ras-MAPK signaling cascade is believed to promote MM growth, no such function has yet been reported for the JAK-STAT pathway, believed to trigger rather metabolic events.
JAK-STAT pathway.
As indicated above, IL-6 binding to IL-6Rα results in the formation of a hexameric complex consisting of 2 IL-6, 2 IL-6Rα, and 2 gp130 molecules. Subsequently, Janus kinases JAK-1, JAK-2, and Tyk2, which are constitutively associated with gp130, become phosphorylated and activated.182-185 This results in the activation of a recently described signaling cascade named JAK-STAT pathway.186-188 JAKs are protein tyrosine kinases that associate with various cytokine receptors and induce phosphorylation of the receptor and of cellular substrates called STAT (signaltransducer andactivation oftranscription) proteins.178,184STATs were identified on the search for transcription factors binding to IFN-responsive promoters.189,190 STATs are present in a latent form in the cytoplasm and become phosphorylated on a single tyrosine within minutes after ligand binding. Phosphorylated STATs dimerize and translocate to the nucleus.191,192 Many growth factors activate the JAK-STAT pathway.186 IL-6 activates STAT3 and STAT1 via distinct cytoplasmic domains of the IL-6 signal transducer gp130.183,184,186,193-196 However, the role of the JAK-STAT pathway for the pathogenesis of MM remains uncertain. Both STAT1 and STAT3 seem constitutively active in IL-6 responsive and nonresponsive MM cells, and stimulation of cells by IL-6 does not increase the activity of both STATs.197 198 Therefore, and in contrast to the Ras-MAPK pathway described below, STATs seem less involved in MM pathogenesis.
Ras-MAP kinase signaling pathway.
The three proteins encoded by the human ras genes (H-ras, K-ras, N-ras) are membrane-associated guanosine triphosphatases (GTPases) with a m.w. of 21 kD.199 These three proteins are usually referred to as Ras or p21ras. The essential feature of Ras is its ability to bind the guanine nucleotides GTP and guanosine diphosphate (GDP). Ras is active in the GTP-bound form, and inactive in the GDP-bound form. Ras functions as an important mediator of many biological responses stimulated by tyrosine kinases. Activation of Ras lies downstream of receptor and nonreceptor tyrosine kinases and upstream of a kinase cascade which includes other important signaling intermediates such as Raf-1 and MAPK (see below and Fig 2).200
More recently, some novel mammalian proteins have been identified that are involved in the regulation and activation of Ras by tyrosine kinases. Shc (Srchomology and collagen), Grb2 (growth factorreceptor bound protein 2), and Sos1 (Son ofsevenless) are members of a group of mammalian proteins involved in the regulation and activation of p21ras by tyrosine kinases. Shc is a SH2 domain containing protein which binds itself to the SH2 domain of Grb2; Grb2 is a small protein with one SH2 domain flanked by two SH3 domains; Sos1 is a guanine nucleotide releasing factor for p21ras.200-210 Activation of p21rasrequires the coordinate interaction of these proteins and is regulated by tyrosine phosphorylation. A model of p21ras activation has been proposed from studies on epidermal growth factor (EGF) and insulin receptors (EGFR, IR).200 After ligand-induced receptor activation and homodimerization, the SH2 domains of Grb2 bound in a complex with Sos1, become attached to specific tyrosine residues of the receptor, thus relocating Sos1 to the plasma membrane. Subsequently, Sos1 induces an exchange of GDP for GTP on membrane-bound p21ras, thereby activating p21ras.200,202 Activated p21ras is later shut off by hydrolysis of GTP to GDP; this reaction is enhanced by GTPase activating proteins such asrasGAP.202,211 In contrast to the EGFR, stimulation of p21ras by the insulin receptor (IR) involves two additional proteins, Shc and insulin receptor substrate-1 (IRS-1). Upon stimulation with insulin, both proteins become activated and bind to Grb2 to recruit Grb2/Sos-1 complexes to the cell membrane.210
Different events with critical importance for cell growth and/or malignant transformation activate Shc: stimulation with growth factors, activation of the T-cell receptor/CD3 complex and the B-cell antigen receptor, as well as cellular transformation with v-src, v-fps, orbcr/abl-oncogenes.210,212-223 IL-6 and other cytokines known to act through gp130 (CNTF, LIF, OSM) induce the activation and complex formation of Shc and Grb2 in a time- and concentration-dependent manner in human MM cells.185,194,224,225 This may enable Shc to interact with the adaptor protein Grb2 and with Sos1 to activate p21ras.226 Thereafter, the other signaling intermediates of the Ras signaling pathway like Raf-1 and MAPK become activated.185
The tyrosine kinase(s) that phosphorylate and activate Shc and Grb2 in response to IL-6 are unknown. JAKs may be involved, because OSM was shown to induce activation of JAK2, and its subsequent binding to the SH2 domain of Grb2.224 IL-6–induced activation of the Src-family kinases Hck, Lyn, or Fyn might also stimulate the Ras pathway (M. Hallek, G. de Vos, C. Neumann, M. Schäffer, unpublished results, August 1997).180,181,227 In MM cell lines, IL-6 activates the Src-kinases Hck, Lyn, and Fyn. Hck is associated with gp130.181 The overexpression of a constitutively active Hck kinase induces similar effects as constitutively active v-Ha-Ras, providing further support for the hypothesis that the Ras signaling pathway involves Hck.227
Vav, a multifunctional signaling protein expressed exclusively in hematopoietic cells and trophoblasts,228-230 also associates with the membrane-distal region of gp130 and becomes phosphorylated on tyrosine residues in response to IL-6 and to IGF-1 in the MM cell line U266.231,232 Vav coimmunoprecipitates with MAPK and Grb2.231 Therefore, Vav might be another protein regulating the Ras-MAPK signaling cascade in MM cells.
The activation of p21ras usually induces the subsequent phosphorylation and activation of downstream signaling proteins like Raf-1 and MAPK; this signaling pathway has therefore been termed Ras or MAPK signaling pathway (Fig 2). MAPKs (alternatively namedextracellular signalregulatedkinases, ERKs) are serine-threonine kinases present at low abundance and in different isoforms of 39 to 45 kD in many cell types that are enzymatically activated by tyrosine and threonine phosphorylation in response to various extracellular stimuli.233,234 The best-characterized MAPKs are the 42-kD and 44-kD isoforms (p42MAPK and p44MAPK; ERK2 and ERK1). They are activated by different growth factors and are involved in cell-cycle progression.233 MAPKs are an important integration point in the signal transduction machinery, because they interact with a variety of substrates, including a 90-kD cell-cycle regulatedS6 protein kinase (RSK or pp90RSK), cytosolic phospholipase A2, c-Jun, c-Myc, c-Fos, NF-IL-6, myelin basic protein (MBP), TAL-1 and Raf-1.74,233-236 MAPKs are activated by at least two MAP kinase kinases, MKK1 and MKK2.237,238 MKKs are activated by the serine-threonine kinase Raf-1, although this is not the only way of activation.237
Like many lympho-hematopoietic growth factors, IL-6 activates MAPK and its substrates.73,185,233,239 The functional consequence of the IL-6 triggered Ras-MAPK activation for MM pathogenesis is becoming better understood. Studies with IL-6–responsive and IL-6–nonresponsive cell lines suggest that a lack of Sos1 activation is observed only in IL-6–nonresponsive cells, and that Sos1 seems to be constitutively complexed with Grb2 in some of the IL-6–nonresposive cells.197 The activation of the Ras-MAPK signaling cascade by IL-6 but not the JAK-STAT pathway (STAT1, STAT3) correlates with the proliferative response of MM cells to IL-6.240 Treatment with MAPK antisense oligonucleotides inhibits the proliferation of cells induced by IL-6.240 Activation of MAPK by IL-6 causes an increase of the transcriptional activity of NF-IL-6 by phosphorylation on threonine residue 235.241 This effect is similar to oncogenic p21ras, which also increases the transcriptional activity of NF-IL-6 by activation of MAP kinases.241 Taken together, these data support the view that a (constitutive) activation of MAPK by IL-6, oncogenic p21ras, or by other mechanisms is important to sustain MM growth.
ESCAPE: ACTIVATION OF GROWTH FACTOR INDEPENDENT GROWTH, OR PREVENTION OF PROGRAMMED CELL DEATH
Chromosomal aberrations increase with disease progression in MM,242 indicating that the transformation from MGUS to MM or plasma cell leukemia requires additional mutations to enable the malignant cell clone to survive and proliferate in the absence of the marrow microenvironment.
Ras Mutations
The above-described studies on IL-6 signaling strongly suggest a critical role for p21ras in the pathogenesis of MM. This hypothesis is further nourished by the finding that rasmutations occur in about 39% of newly diagnosed MM patients (Table1). Interestingly, the frequency ofras mutations increases with disease progression: Mutations of N- and K-ras are rarely detected in solitary plasmacytoma and monoclonal gammopathies of undetermined significance (MGUS), but more frequently in MM (in 9% to 30%), and in the majority of terminal disease or plasma cell leukemia patients (63.6% to 70%).243-246 N-ras codon 61 mutations seem more frequent than N-ras codon 12 and 13, or K-ras mutations (Table1).243-245
Ras Point Mutations in MM
| First Author . | N12 . | N13 . | N61 . | K12 . | K13 . | K61 . | Total . |
|---|---|---|---|---|---|---|---|
| Neri, patients at diagnosis244 | 2 | 2 | 9 | 2 | 1 | 0 | 12/43 |
| Neri, patients at relapse244 | 0 | 1 | 3 | 2 | 0 | 0 | 6/13 |
| Matozaki247 | 2 | 3 | 2 | 4/13 | |||
| Portier246 | 4 | 1 | 4 | 3 | 0 | 3 | 14/30 |
| Tanaka245 | 1 | 2 | 2 | 0 | 0 | 0 | 5/10 |
| Paquette248 | 2 | 2/17 | |||||
| Corradini243 | 2 | 1 | 7 | 1 | 0 | 0 | 11/90 |
| Yasuga249 | 0 | 2 | 2/45 | ||||
| Liu250 | 6/129 | 5/129 | 50/346 | 16/139 | 7/139 | 63/160 | |
| Total | 17/373 | 15/328 | 81/607 | 24/325 | 8/325 | 3/186 | |
| 4.5% | 4.6% | 13.3% | 7.4% | 2.5% | 1.6% | 34% |
| First Author . | N12 . | N13 . | N61 . | K12 . | K13 . | K61 . | Total . |
|---|---|---|---|---|---|---|---|
| Neri, patients at diagnosis244 | 2 | 2 | 9 | 2 | 1 | 0 | 12/43 |
| Neri, patients at relapse244 | 0 | 1 | 3 | 2 | 0 | 0 | 6/13 |
| Matozaki247 | 2 | 3 | 2 | 4/13 | |||
| Portier246 | 4 | 1 | 4 | 3 | 0 | 3 | 14/30 |
| Tanaka245 | 1 | 2 | 2 | 0 | 0 | 0 | 5/10 |
| Paquette248 | 2 | 2/17 | |||||
| Corradini243 | 2 | 1 | 7 | 1 | 0 | 0 | 11/90 |
| Yasuga249 | 0 | 2 | 2/45 | ||||
| Liu250 | 6/129 | 5/129 | 50/346 | 16/139 | 7/139 | 63/160 | |
| Total | 17/373 | 15/328 | 81/607 | 24/325 | 8/325 | 3/186 | |
| 4.5% | 4.6% | 13.3% | 7.4% | 2.5% | 1.6% | 34% |
The number of mutations of N-ras codon 12 (N12), codon 13 (N13), and codon 61 (N61), and of K-ras codon 12 (K12), codon 13 (K13), and codon 61 (K61) detected in MM patients in various studies is summarized.
Overexpression of H-ras or N-ras oncogenes in Epstein-Barr virus (EBV)-infected human B lymphoblasts induces the malignant transformation and plasmacytoid differentiation of these cells.251 The introduction of a constitutively active N-ras cDNA containing a glutamine to arginine (CAA-CGA) amino acid substitution at codon 61 into the IL-6–dependent myeloma cell line ANBL6 resulted in significant IL-6–independent growth, as well as augmentation of growth at suboptimal concentrations of IL-6.252 Furthermore, mutant N-ras expression decreased the percentage of cells undergoing apoptosis in the absence of IL-6.252 This suggests that activating mutations of the ras oncogenes may result in growth factor independence and suppression of apoptosis in MM. Taken together, these findings support the following scenario: in early disease where myeloma cell growth seems strictly dependent on the presence of IL-6, p21ras is (constitutively) activated by the paracrine secretion of IL-6 in the BM microenvironment. At later disease stages, activating mutations of N- or K-ras replace this function, thus allowing an IL-6–independent tumor expansion and dissemination outside the marrow. This hypothesis awaits its verification by a careful analysis of the p21ras function at different MM stages.
Inhibitors of Programmed Cell Death: p53 and bcl-2
Bcl-2 and related proteins.
Apoptosis or programmed cell death is a process of pivotal importance during normal development, for immunoselection against autoreactive T and B cells, or in the elimination of old or damaged cells. Apoptosis is also induced by a variety of drugs, heat shock, or by growth factor deprivation. The membrane protein Bcl-2 is a highly conserved, ubiquitous membrane protein associated with the outer-membrane of mitochondria and nuclei, and with the endoplasmatic reticulum which regulates apoptosis.253 Overexpression of bcl-2 in cancer cells can result in chemoresistance and blocks apoptosis. Recent work has shown the existence of several Bcl-2–related proteins that can inhibit (Bcl-XL, Mcl-1, NR-13, A1, Bcl-W) or enhance (Bax, Bcl-XS, Bak, Bad) apoptosis.254 255 Bcl-2 forms inactivating or activating heterodimers with other proteins encoded by these genes of the bcl-2 superfamily.
Expression of bcl-2 is thought to play an important role in B-cell malignancies, in particular in follicular lymphoma, where more than 80% of the patients have the translocation t(14;18), which results in overexpression of Bcl-2.256 This translocation occurs at lower frequency (0% to 15%) in MM, but despite this an overexpression of Bcl-2 is seen in the majority of MM and in MM cell lines.257-259 High levels of Bcl-2 protein are likely to mediate the resistance of MM cells to apoptosis induced by dexamethasone, IL-6 deprivation, staurosporine, or other drugs.260-262 In a murine myeloma cell line, Bcl-XL showed a predominant role in preventing apoptosis in response to cycloheximide treatment or IL-6 withdrawal.263Similarly, overexpression of Bcl-2 or Bcl-XL could prevent apoptosis induced by IL-6 withdrawal in the IL-6–dependent cell line B-9.262
p53.
The tumor suppressor gene p53 has many effects on cell growth and differentiation and is often viewed as a gate-keeper to enter the cell cycle. Recently, is has been found that p53 binds to response elements on bcl-2 and bax genes, resulting in the downregulation of bcl-2 and in an upregulation of bax. Therefore, it was postulated that p53 induces the synthesis of bax and reduces the synthesis of bcl-2, thereby affecting the balance between cell growth and death.264
In MM cell lines, p53 mutations occur frequently but without apparent correlation with autonomous, IL-6–independent cell growth.265 Overexpression of wildtype p53 can suppress the autocrine IL-6 production and proliferation of U266 cells.266 p53 mutations are infrequent in MM, and a late event in the disease. p53 mutations occur in about 5% of inactive MM, and in 20% to 40% of acute plasma cell leukemia.246,249 267-270 Thus, p53 mutations might cause a block of plasmablastic apoptosis and differentiation at the final stages.
Retinoblastoma (Rb) Gene and Other Cell-Cycle Regulatory Genes
Rb.
The retinoblastoma tumor suppressor gene product (pRb) is involved in cell growth and differentiation. pRb is a nuclear phosphoprotein that suppresses the G1 → S transition in the cell cycle by inhibiting E2F-mediated transactivation of a variety of genes involved in initiating DNA synthesis, such as c-myc, b-myb, cdc2, dihydrofolate reductase, and thymidine kinase.271-276 pRb function is regulated by phosphorylation: hypophosphorylated or dephosphorylated pRb is activated and binds E2F, thereby inducing cell-cycle arrest; in contrast, phosphorylated pRb is inactivated and cannot bind E2F, thereby promoting entry of cells into S phase.271 Mutations of the Rb gene contribute to cellular transformation in many types of malignancies. To date, mutations of the Rb gene or abnormalities in pRb have been described in up to 70% of MM patients and 80% of MM-derived cell lines.267,277,278 MM cells may show a very strong expression of pRb,279 mostly in its phosphorylated form.280
As noted above, monosomies of chromosome 13 have been identified in approximately 30% of abnormal MM karyotypes. By Southern blot or interphase FISH analysis, monoallelic deletion of Rb (loss of one copy of chromosome 13 or interstitial deletions encompassing the 13q12-14 region) has been reported in about 50% to 60% of MM tumors and cell lines, independent of the disease stage. Bi-allelic deletion of Rb was found in 1 of 22 MM cell lines and in 1 of 10 primary tumors.267,277,278,281 The monoallelic lesions were not associated with any effect on the pRb expression, and no mutations or rearrangements of Rb have been described. These findings suggest that a bi-allelic loss of the Rb gene is infrequent in MM and, like in CLL, there may be a tumor suppressor gene other than Rb (DMB, deleted in B-cell malignancies?; BRCA2?) on 13q12-14 that is activated in MM.282
Transforming growth factor (TGFβ) does not suppress pRb phosphorylation and proliferation of MM cells, in contrast to normal B cells, consistent with the notion that pRb may contribute to MM cell growth.283 Incubation of MM cells with Rb antisense oligonucleotides triggers IL-6 secretion and cell proliferation.280 Overexpression of wild-type pRb can suppress the autocrine IL-6 production and proliferation of U266 cells.266 In IL-6–responsive MM cells, stimulation with IL-6 further shifts pRb from its dephosphorylated to its phosphorylated form, thereby promoting MM cell growth.280 This suggests that IL-6–induced signaling through pRb may have two effects, the phosphorylation (inactivation) of pRb and the upregulation of IL-6 secretion, which both augment proliferation of MM cells. Taken together, both molecular studies and the occasional observation of pRb inactivation in MM cells indicate that the loss of pRb function may contribute to MM transformation.
Inhibitors of cyclin-dependent kinases.
The p16INK4A (p16) protein is an inhibitor of cyclin-dependent kinases (CDK) 4 and CDK6. It is expressed in some MM cells or cell lines, and a higher expression correlates with IL-6 responsiveness of more mature MM tumors (VLA-5+, MPC+).284,285 In contrast, expression of cyclin D1 is more frequent in immature MM tumors (VLA-5−, MPC−).285 The p16 gene is frequently hypermethylated in MM.286 This hypermethylation occurs more frequently in advanced disease (plasma cell leukemia [PCL]) and in MM cell lines.287 Treatment with the demethylating agent 5-deoxyazacytidine restores the p16 protein expression and induces G1 growth arrest in patient PCL cells and in MM cell lines.287 This suggests that inactivation of the p16 gene by hypermethylation may be associated with decreased growth control and with the development of PCL. Homozygous deletions of genes encoding for the CDK inhibitors p15INK4B, p16INK4A, and p18INK4Cmay also occur in some patients with MM.288
p21WAF1.
p21WAF1 (p21) inhibits cell proliferation by both p53-dependent and -independent mechanisms. It is believed that p21 protects from (p53 dependent) apoptosis by induction of cell-cycle arrest and subsequent DNA repair.289,290 In the majority of MM cells, p21 seems to be constitutively expressed, while it is not detected in normal B cells.291 IL-6 downregulates and IFN-γ upregulates p21 expression, and this effect correlates with G1 → S transition induced by IL-6 in MM cells.291 Taken together, p21 overexpression in MM may induce resistance to apoptosis (by chemical agents or radiation), and slow clinical progression of disease.
MDM2.
The murine double minute 2 (MDM2) gene product facilitates the G1 → S transition by direct DNA binding and enhancing transcription of genes associated with proliferation, or by activation of E2F-1-DP1; it also abrogates tumor suppressive functions of wild-type p53. Recently, the overexpression of the MDM2 protein has been reported in MM cell lines and PCL cells from patients.292 MDM2 protein was found to be constitutively bound to wild-type or mutant p53 in MM cells.292 Treatment with MDM2 antisense oligonucleotides seemed to induce G1 arrest.292 This suggests that MDM2 enhances cell-cycle progression in MM.
Multiple Drug Resistance (MDR) Gene
The MDR gene encodes a 170-kD p-glycoprotein which is responsible for the resistance of tumor cells to a variety of antineoplastic drugs. The amplification of MDR gene expression can be detected in myeloma cells, and its expression is correlated with a resistance to doxorubicin and vincristine (VAD regimen).293-295 Recently, clinical trials have been activated to overcome this treatment refractoriness with drug-resistance modifiers like cyclosporin A and verapamil.295
THERAPEUTIC IMPLICATIONS
The central role of IL-6 as a growth factor for MM cells suggests that strategies to block its effects could be exploited therapeutically. This effect may be achieved either by conventional therapeutic agents or by compounds specifically designed to block the action of IL-6. Among the conventional agents is IFN-γ, which may inhibit the growth of IL-6–dependent MM cell lines by a downregulation of the IL-6R expression on MM cells.139,296 All-trans retinoic acid (ATRA) inhibits the IL-6–dependent growth of freshly isolated MM cells and MM cell lines, decreases the IL-6 production by MM cells and BMSCs and downregulates IL-6R and gp130.91,297,298 IFN-α may downregulate IL-6R and gp130, thereby inhibiting MM growth.141 Glucocorticoids (GC), which are very common components of MM therapy, were also reported to repress IL-6 gene transcription and production and to induce apoptosis of MM cells.89,299 IL-4 has inhibitory effects on the in vitro growth of MM cells and suppresses the IL-6 gene transcription in BMSCs.300 301
More specific antagonists of IL-6–dependent growth are anti–IL-6 MoAbs or anti–IL-6R MoAbs that disrupt autocrine or paracrine IL-6R stimulation. Both strategies have been shown to suppress the growth of MM or PCL cells in vivo and in vitro.302-304 For repeated administration, humanization of these MoAbs seems critical to decrease their immunogenicity in vivo.302,305 Similarly, toxic fusion proteins targeting the IL-6R on MM cells may be used for treatment. Recombinant proteins consisting of IL-6 fused toPseudomonas exotoxin (IL-6-PE4E) or to diphtheria toxin were successfully used to kill MM cells.306,307 These compounds may be particularly helpful for ex vivo purging of autologous BM or peripheral blood stem cells (PBSCs) contaminated with tumor cells.307 Antisense oligonucleotides that inhibit the expression of IL-6 or IL-6R genes may be used to suppress MM growth, and the principal feasibility of this approach has been shown in vitro.308 A very elegant method of blocking the effects of IL-6 on MM growth may be the use of recombinant chimeric human/murine IL-6 proteins which introduce biologically inactive murine residues into the human IL-6 protein, thereby blocking the interaction with gp130.309 The resulting IL-6 molecules were inactive on human myeloma cells and in addition completely inhibited wild-type IL-6 activity on these cells. A similar approach is the molecular modeling of IL-6 superantagonists that contain variant binding motifs with higher affinity to the IL-6Rα chain, but impair efficient gp130 dimerization by antagonistic amino acid substitutions in sites critical for the contact with gp130.310-312 Some of these IL-6 superantagonists are potent inducers of apoptotic cell death in MM cell lines and inhibit intracellular signaling.92 Chemically synthesized peptides may also block the IL-6Rα/gp130 interaction.313 Such antagonists will be tested as specific inhibitors of IL-6 activity in vivo.
Novel strategies may also target the signaling intermediates or oncogene products involved in MM pathogenesis. The above-described experiments with antisense oligonucleotides used to block the Ras-MAPK pathway are another potential way to antagonize some critical components of neoplastic plasma cell growth.240 Of equal importance are efforts to create specific inhibitors of tyrosine kinases and their substrates. Unfortunately, most tyrosine kinase inhibitors available (genistein, staurosporin, erbstatin) are not specific and therefore too toxic. The recently discovered inhibitors of Ras farnesyltransferases, which block the action of Ras, may act more specifically and may be particularly helpful in MM patients.314 Because the cellular mechanisms of (proto-)oncogene activation are inherently linked with signaling pathways of MM growth factors, the ongoing research in this field will certainly contribute to the development of new molecular therapies for MM.
Because growth of MM seems to depend critically on the support by the BM environment and on a defective antitumor immune response, novel therapeutic strategies might also act here. For example, bisphosphonates like pamidronate which reduce skeletal complications and improve survival of MM patients, were shown to suppress the IL-6 production by BMSCs.315 Thus, pamidronate might inhibit MM growth more specifically than originally thought. It has also been shown that the gene transfer of costimulatory molecules B7-1 and B7-2 into B7-negative MM cells is able to induce a cytolytic T-cell response against MM cells in vitro.161 Thus, adjuvant gene transfer modified tumor vaccines might add to the novel, molecular therapeutic arsenal against this disease.
SUMMARY
Based on the information described above, we propose a working multi-step model of the molecular pathogenesis of myeloma (Fig3). The cell that gives rise to myeloma appears to have passed through the pathway that generates the long-lived plasma cell which has a phenotype similar to myeloma cells. Thus, the oncogenic events in myeloma either occur after or do not interfere with the normal maturation process that generates long-lived plasma cells. Like a long-lived plasma cell, a myeloma cell has undergone three developmentally regulated changes in the DNA structure of the IgH and IgL loci, including productive V(D)J recombination of its IgH and IgL genes, somatic hypermutation of the IgH and IgL V regions, and productive IgH switch recombination to another IgH isotype. It is attractive to speculate that errors in one or more of these processes has caused genetic changes that contribute to the malignant process.
Progressive genetic events in MM. Although not every stage is discernible in each patient, there appears to be an ordered progression from a normal plasma cell; to MGUS where the cells are immortalized, but not transformed, and do not progressively accumulate or cause bone destruction; to intra-medullary myeloma, where the cells are confined to the BM micro-environment, accumulate and cause bone destruction; to extra-medullary myeloma, where the cells proliferate more rapidly and grow in the blood (plasma cell leukemia) or other extra-medullary sites; to a myeloma cell line, where the cells may be propagated in vitro. This model summarizes the possible timing of genetic events in relation to clinical progression. When the event may occur at a discrete time, we have indicated this with an arrow. When it is clearly associated with a defined clincal stage we have indicated this with a solid line. When the timing is unclear, we have used a dashed line. We suspect that the 14q32 translocation may be an early event, concordant with isotype switch recombination, so that it may precede MGUS. Some translocations [eg, t(11;14)] may lead more rapidly to fulminant disease. There is evidence of karyotypic instability in MGUS that continues throughout all stages of tumour progression. Monosomy 13 is present in intramedullary myeloma, independent of stage, but there is no evidence as to whether or not it is present in MGUS. Dysregulation of c-myc appears to be common, but the timing is unclear. In patients with ectopic FGFR3 expression caused by t(4;14), a mutation of FGFR3 could lead to ligand independence and clinical progression, as is suggested in one analyzed example. Mutations of N- and K-ras are not present in MGUS, but are present in intramedullary myeloma, with an increasing incidence as the disease progresses. Mutations of p53 are a late event associated with aggressive extra-medullary myeloma.
Progressive genetic events in MM. Although not every stage is discernible in each patient, there appears to be an ordered progression from a normal plasma cell; to MGUS where the cells are immortalized, but not transformed, and do not progressively accumulate or cause bone destruction; to intra-medullary myeloma, where the cells are confined to the BM micro-environment, accumulate and cause bone destruction; to extra-medullary myeloma, where the cells proliferate more rapidly and grow in the blood (plasma cell leukemia) or other extra-medullary sites; to a myeloma cell line, where the cells may be propagated in vitro. This model summarizes the possible timing of genetic events in relation to clinical progression. When the event may occur at a discrete time, we have indicated this with an arrow. When it is clearly associated with a defined clincal stage we have indicated this with a solid line. When the timing is unclear, we have used a dashed line. We suspect that the 14q32 translocation may be an early event, concordant with isotype switch recombination, so that it may precede MGUS. Some translocations [eg, t(11;14)] may lead more rapidly to fulminant disease. There is evidence of karyotypic instability in MGUS that continues throughout all stages of tumour progression. Monosomy 13 is present in intramedullary myeloma, independent of stage, but there is no evidence as to whether or not it is present in MGUS. Dysregulation of c-myc appears to be common, but the timing is unclear. In patients with ectopic FGFR3 expression caused by t(4;14), a mutation of FGFR3 could lead to ligand independence and clinical progression, as is suggested in one analyzed example. Mutations of N- and K-ras are not present in MGUS, but are present in intramedullary myeloma, with an increasing incidence as the disease progresses. Mutations of p53 are a late event associated with aggressive extra-medullary myeloma.
A chromosome translocation to the IgH locus, most frequently into a switch region, is a nearly universal event in myeloma cell lines, and it appears to be equally frequent in primary tumor samples. We postulate that these translocations occur at the time of isotype switch recombination, on the nonproductive allele. Because MGUS and myeloma share the same legitimate switch recombination on the productive allele, they may also share the same illegitimate switch recombination on the nonproductive allele. The result of these translocations is to dysregulate the expression of an oncogene (eg, c-myc) by juxtaposing it to the strong regulatory sequences of the IgH locus, resulting in immortalization of the malignant clone. The presence of intraclonal heterogeneity in the Ig hypervariable regions in MGUS indicates that these cells are still subject to somatic hypermutation.316After immortalization, the malignant cell establishes a supportive environment in the BM with a network of cytokines, adhesion molecules, and other costimulatory interactions with the stromal cells. Later additional mutations which might be caused by a spillover of the hypermutational process onto ras or a tumor suppressor gene on chromosome 13, result in the selection of a single clone for malignant expansion. Further mutations, like p53, lead to stroma-independent growth, and escape from the BM micro-environment. Further understanding of this transformation process in MM will allow the development of new therapies defined at the molecular level.
ACKNOWLEDGMENT
P.L.B. would like to acknowledge the equal contribution of Michael Kuehl to our shared results and ideas presented here.
M.H. is supported by in part by grants from the Deutsche Forschungsgemeinschaft (Ha 1680/2-2) and the Deutsche Krebshilfe (no. 10-1094-Ha I).
Address reprint requests to Michael Hallek, MD, Medizinische Klinik, Klinikum Innenstadt, Universität München, Ziemssenstrasse 1, D-80336 München, Germany.


![Fig. 3. Progressive genetic events in MM. Although not every stage is discernible in each patient, there appears to be an ordered progression from a normal plasma cell; to MGUS where the cells are immortalized, but not transformed, and do not progressively accumulate or cause bone destruction; to intra-medullary myeloma, where the cells are confined to the BM micro-environment, accumulate and cause bone destruction; to extra-medullary myeloma, where the cells proliferate more rapidly and grow in the blood (plasma cell leukemia) or other extra-medullary sites; to a myeloma cell line, where the cells may be propagated in vitro. This model summarizes the possible timing of genetic events in relation to clinical progression. When the event may occur at a discrete time, we have indicated this with an arrow. When it is clearly associated with a defined clincal stage we have indicated this with a solid line. When the timing is unclear, we have used a dashed line. We suspect that the 14q32 translocation may be an early event, concordant with isotype switch recombination, so that it may precede MGUS. Some translocations [eg, t(11;14)] may lead more rapidly to fulminant disease. There is evidence of karyotypic instability in MGUS that continues throughout all stages of tumour progression. Monosomy 13 is present in intramedullary myeloma, independent of stage, but there is no evidence as to whether or not it is present in MGUS. Dysregulation of c-myc appears to be common, but the timing is unclear. In patients with ectopic FGFR3 expression caused by t(4;14), a mutation of FGFR3 could lead to ligand independence and clinical progression, as is suggested in one analyzed example. Mutations of N- and K-ras are not present in MGUS, but are present in intramedullary myeloma, with an increasing incidence as the disease progresses. Mutations of p53 are a late event associated with aggressive extra-medullary myeloma.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.3/3/m_blod4014403.jpeg?Expires=1769087605&Signature=WtOnDuWHBSq3zfuMpHK2qhZN4~JYOK7AcUBaqfod3T642bPCmqofQaEsGtqfeKz9GlfIXi0E0pRGGHOUPi7lv~ON3TMi28jYSRFYuGuU9-AURxeu4gE-beADbwEG8R96SBkAhSExpeTmA4rGeMoUJ2QKAd9l3juCHjdBYmXkotGRDefe81Nm-kF0zbTAay25LSZtE~JCUhKqJPZG7U5yqBWK~WSWI6X0r3acx5FOv9IYR-KXR~mpN6HbgcrV~hTYXxNAjzvxBQpsnpEEKnuIhgE18CHpYRUxkqFAm3QBU2N7Q4k~5idfQGhKt4CPdXHyTOYwYefmE6alMKxsyf1mLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal