Abstract
Use of the CD28/B7 costimulatory signal for T-cell activation was analyzed in granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood mononuclear cells (G-PBMCs) and in peripheral blood mononuclear cells obtained before administration of G-CSF (preG-PBMCs). CTLA4Ig inhibition of OKT3-stimulated proliferation was significantly lower in G-PBMCs compared with preG-PBMCs (39.9% ± 5.6% and 72.2% ± 5.4%, respectively; P < .001). Furthermore, as shown in electrophoretic mobility-shift assays, the inducible level of the T-cell transcription factor CD28 responsive complex (CD28RC) was suppressed in CD4 cells derived from G-PBMC. However, depletion of CD14 cells from G-PBMCs restored CD28RC induction to normal levels. Taken together, these findings suggest that the large number of CD14 monocytes in G-PBMCs may limit T-cell responsiveness by suppressing the induction of the CD28RC.
GRANULOCYTE colony-stimulating factor (G-CSF) mobilized peripheral blood mononuclear cells (G-PBMCs) have been used increasingly to reconstitute hematopoiesis after myeloablative therapy. Although G-PBMC grafts contain at least 10 times more T cells than standard marrow grafts, the incidence and severity of acute graft-versus-host disease (aGVHD) is not higher than observed with allogeneic marrow.1-7 Hypothetically these clinical observations could be explained by a direct effect of G-CSF on T-cell function.8 Alternatively, G-PBMCs may contain cells that can suppress donor T-cell responsiveness.
Previously we have reported that G-PBMC leukapheresis products contain a large number of CD14+ monocytes that suppress donor T-cell proliferation in a dose-dependent fashion.9-11Normal CD14 cells when used in comparable numbers could also suppress T-cell responsiveness, suggesting that G-PBMCs differed from normal marrow or PBMCs in the number of CD14 cells. However, our data also suggested that G-PBMC–derived CD14 cells expressed significantly lower levels of the costimulatory molecule B7-2 (CD86).9
Given that the engagement of the T-cell receptor in vitro in the absence of costimulatory molecules can result in a state of antigen-specific anergy,12-21 we hypothesized that the large number of CD14+/B7-2lo cells in G-PBMCs may contribute to low T-cell responsiveness by providing suboptimal amounts of costimulatory signals. The potential relevance of costimulatory signals for the activation of donor T cells and the development of GVHD has been shown in vivo by blocking the interaction of B7-1 and B7-2 with CD28 on T cells using CTLA4Ig, a soluble fusion protein of human CTLA-4 and IgG1 Fc region.22 This treatment reduced lethal aGVHD after allogeneic marrow transplantation in mice.23 24
Recently, a CD28-responsive element (CD28RE) with promoter activity in the interleukin-2 (IL-2) gene has been identified17 and nuclear transcription factors that bind to CD28RE were found and classified as members of the nuclear factor (NF) κB family (CD28 responsive complex [CD28RC]).25 26 In the present study, we have analyzed the role of CD28/B7 costimulation in T-cell activation by measuring the inducible levels of CD28RC. We show in CTLA4Ig inhibition studies that CD4 cells in G-PBMCs (G-CD4 cells) use the CD28/B7 costimulatory pathway to a lesser degree compared with CD4 cells in preG-PBMCs (preG-CD4 cells). Further, the induction of CD28RC in G-CD4 cells appears to be suppressed by the presence of CD14 cells in the G-PBMCs.
MATERIALS AND METHODS
Donors, G-CSF mobilization, and PBMC processing.
Samples were collected from normal volunteers or peripheral blood stem cell donors after written informed consent was obtained as approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (Seattle, WA). Heparinized peripheral blood samples were obtained before the first administration of G-CSF (preG-PBMC). Peripheral blood stem cell donors were administered recombinant human G-CSF (rhG-CSF; Amgen, Inc, Thousand Oaks, CA) by subcutaneous injection at a dose of 8 μg/kg twice daily for 4 to 7 days. Leukapheresis was performed using a continuous flow blood cell separator (Cobe Laboratories, Lakewood, CO) on 2 consecutive days beginning on day 4 of rhG-CSF administration, and G-PBMCs were obtained from the first leukapheresis. PreG-PBMCs were isolated over Ficoll (Accu-Prep; Accurate Chemicals, Westbury, NY; 1.077-g/mL gradients) and washed with Hanks' balanced salt solution (HBSS)/1% bovine serum albumin (BSA). G-PBMCs were suspended in HBSS/1% BSA and centrifuged at 200 g for 10 minutes to remove platelets and then hemolysed in hemolysis buffer (150 mmol/L ammonium chloride; 12 mmol/L sodium bicarbonate). All samples were cryopreserved to allow simultaneous testing, and paired preG- and G-PBMC samples from the same donor were used in some experiments as indicated.
Mixed lymphocyte culture (MLC).
Responder PBMCs (25 to 100 × 103) were cultured with 100 × 103 irradiated (30 Gy), allogeneic PBMC stimulators in 200 μL of RPMI 1640 supplemented with 10% fetal calf serum (FCS), 0.4 μg/mL L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in round-bottom 96-well plates (Corning, New York). After 5 days incubation at 37°C in 5% CO2, cultures were pulsed with 1.0 μCi/well 3H-thymidine for the final 16 hours. Cells were harvested and 3H-thymidine incorporation was measured by scintillation counting. For CTLA4Ig (kindly provided by Bristol-Myers Squibb, Princeton, NJ)19inhibition of T-cell proliferation in MLC, both responder and irradiated stimulator cells were preincubated with 10 μg/mL of CTLA4Ig for 30 minutes at 37°C.
Polyclonal T-cell stimulation assays using immobilized OKT3 monoclonal antibody.
Ninety-six–well flat-bottomed microtiter plates (Costar, Cambridge, MA) were preincubated with 6.25 to 100 ng/mL OKT3 monoclonal antibody (Ortho, Raritan, NJ)27 in 100 mmol/L Tris-HCl buffer (pH 9.5) for 16 hours at 4°C. For CTLA4Ig inhibition of T-cell responsiveness, cells were preincubated with 0.1 to 30 μg/mL CTLA4Ig for 30 minutes at 37°C before seeding into OKT3-coated wells. PreG-PBMCs and G-PBMCs were suspended in RPMI 1640 medium supplemented with 10% FCS at indicated concentrations, cultured at 37°C for 3 days, and pulsed with 3H-thymidine for the final 16 hours of culture.
Percent CTLA4Ig-mediated suppression was calculated using the following formula: [3H-thymidine uptake after OKT3 stimulation without CTLA4Ig (cpm) − OKT3 stimulation with CTLA4Ig (cpm)]/OKT3 stimulation without CTLA4Ig (cpm) × 100.
To analyze induction of nuclear transcription factor CD28RC in preG-PBMCs and G-PBMCs, 1 × 106/mL cells were cultured in OKT3-coated (25 ng/mL) T75 culture flasks (Costar) for 4 hours with or without CTLA4Ig (10 μg/mL).
Immunofluorescent staining and flow cytometric analysis.
Cells were stained with Leu-3a (anti-CD4–phycoerythrin [PE]; Becton Dickinson, San Jose, CA) plus Leu-28 (anti-CD28–fluorescein isothiocyanate [FITC], Becton Dickinson) and with LeuM3 (anti-CD14–PE, Becton Dickinson) plus MAB104 (anti-CD80 [B7/BB1]-FITC, Immunotech, Boston, MA) or FUN-1 (anti-CD86 [B70/B7-2]-FITC, Pharmingen, San Diego, CA). Isotype-matched antibodies of irrelevant specificity were used as negative controls. Samples were analyzed with the use of a FACS Calibur (Becton Dickinson) flow cytometer.
Immunomagnetic cell sorting for CD4 enrichment and CD14 depletion.
Enriched CD4 T cells were obtained by positive selection using Leu-3a anti-CD4–FITC, antihuman-mouse IgG1 (Becton Dickinson) as primary antibody and rat-antimouse IgG1 conjugated to magnetic microbeads as the secondary antibody. Flow cytofluorometric analysis of enriched populations indicated that they contained >90% CD4+cells. CD14 cell-depleted fractions containing <1% CD14+cells were obtained by negative selection using LeuM3 anti-CD14–PE, antihuman-mouse IgG2a (Becton Dickinson) as primary antibody and rat-antimouse IgG2a+b conjugated to magnetic microbeads as the secondary antibody according to the manufacturer's instructions (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Electrophoretic mobility-shift assay (EMSA).
Unfractionated preG- or G-PBMCs were cultured for 4 hours on immobilized OKT3 antibody. CD4 cells were then purified by immunomagnetic enrichment as described previously, and nuclear extracts were isolated from purified CD4 cells as described previously.28 29 In brief, cells were suspended in 400 μL of cold hypotonic buffer containing 10 mmol/L HEPES-KOH pH 7.9, 10 mmol/L KCI, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol (DTT), and 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF). The cells were allowed to swell on ice for 20 minutes. Thereafter, 25 μL of 10% NonidentP-40 (Sigma, St Louis, MO) was added and the contents were vigorously mixed. After centrifugation, the nuclear pellet was resuspended in 50 μL of cold extraction buffer (20 mmol/L HEPES-KOH, pH 7.9, 400 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, and 1 mmol/L PMSF) and vigorously rocked at 4°C for 60 minutes. Nuclear extracts were cleared by centrifugation. Protein concentration was assayed using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). The oligonucleotide 5′-AAAGAAATTCCAAAGAAAAGAAATTCCAAAGA-3′28 was used as a CD28RC recognition sequence after 32P-end labeling by T4 polynucleotide kinase (GIBCO-BRL, Gaithersburg, MD). Each reaction mixture contained about 100 fmol of double-stranded32P-end labeled probe and 1 μg of protein from nuclear extracts. Binding reactions were performed in binding buffer (25 mmol/L HEPES-KOH, pH 7.9, 30% [vol/vol] Glycerol, 10 mmol/L MgCl2, 50 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L DTT, and 0.5 μg of poly[dI-dC]) in a final volume of 20 μL at room temperature for 20 minutes. Complexes of nuclear protein and 32P-end labeled probe were electrophoresed on 6% polyacrylamide gels in TBE buffer (22 mmol/L Tris, 22 mmol/L borate, and 0.4 mmol/L EDTA) for about 2 hours (100 constant voltage [CV]). Gels were dried and analyzed using ImageQuant3.3 software (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Proliferative responsiveness of preG- and G-PBMCs in MLC.
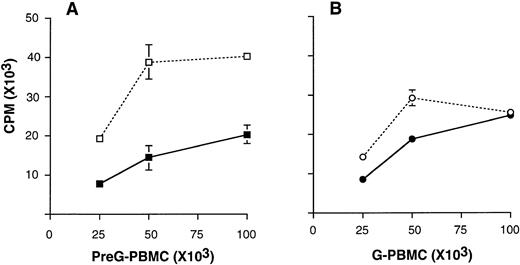
Unfractionated G-PBMC showed lower proliferative responses in MLC compared with equivalent numbers of unfractionated preG-PBMCs obtained from the same donor (Fig 1). Hyporesponsiveness of G-PBMCs in MLC may be caused by the lower percentage of T cells and the higher percentage of suppressive CD14 cells in G-PBMCs compared with preG-PBMCs, as reported previously.9 In addition, CTLA4Ig-mediated inhibition in MLC was more pronounced at all responder cell concentrations when preG-PBMCs compared with G-PBMCs were used as responders.
Mixed lymphocyte cultures using increasing numbers of preG-PBMCs (A) and G-PBMCs (B) cultured with 100 × 103irradiated, allogeneic PBMC stimulators in the presence (solid line) or absence (dashed lines) of 10 μg/mL CTLA4Ig. Values for3H-thymidine incorporation represent the mean counts per minute (cpm) ± the standard error of the mean (SEM) from triplicate cultures. Results from one of three representative experiments are shown.
Mixed lymphocyte cultures using increasing numbers of preG-PBMCs (A) and G-PBMCs (B) cultured with 100 × 103irradiated, allogeneic PBMC stimulators in the presence (solid line) or absence (dashed lines) of 10 μg/mL CTLA4Ig. Values for3H-thymidine incorporation represent the mean counts per minute (cpm) ± the standard error of the mean (SEM) from triplicate cultures. Results from one of three representative experiments are shown.
PreG- and G-PBMC proliferation in response to immobilized OKT3 monoclonal antibody.
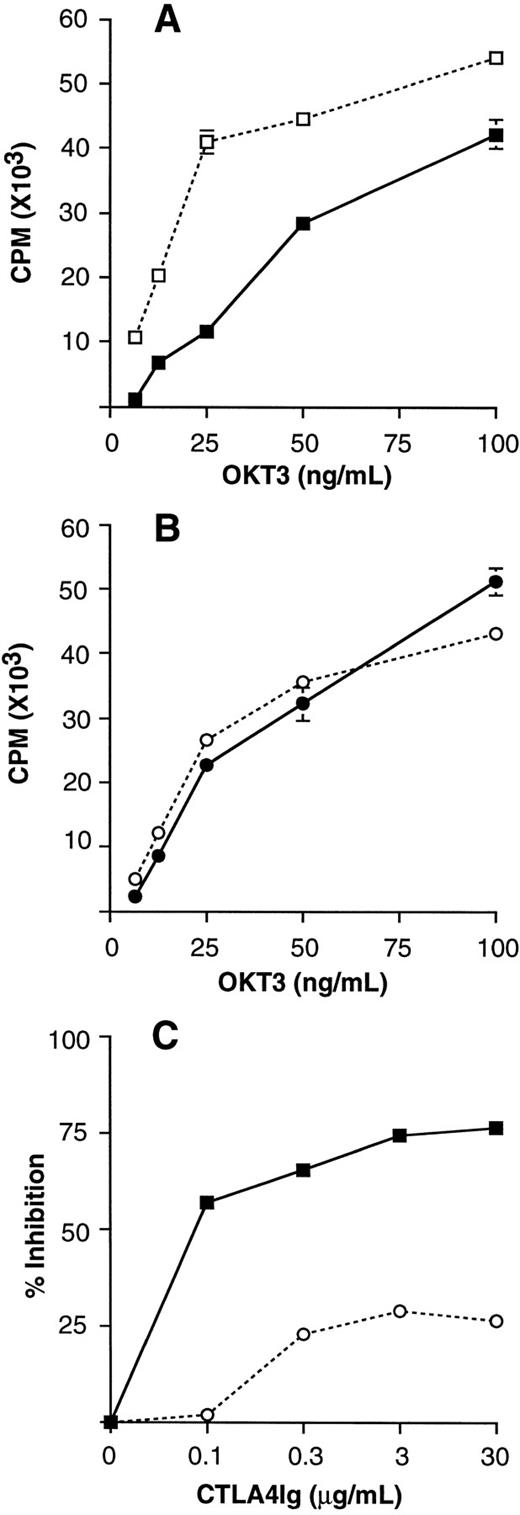
Unfractionated preG- and G-PBMCs obtained from the same donor were stimulated with immobilized OKT3 monoclonal antibody at different concentrations (Fig 2
A and B). OKT3 monoclonal antibody immobilized at a concentration of 25 ng/mL provided suboptimal stimulation, which could be largely inhibited by 10 μg/mL CTLA4Ig in preG-PBMCs. CTLA4Ig-mediated inhibition was less effective at higher OKT3 concentrations. Therefore, an OKT3 concentration of 25 ng/mL was chosen for subsequent polyclonal stimulation assays.
Polyclonal T-cell stimulation assays using immobilized OKT3 monoclonal antibody. Unfractionated preG-PBMCs (A) and G-PBMCs (B) (100 × 103 cells/200 μL) were cultured in the presence (solid line) or absence (dashed line) of 10 μg/mL CTLA4Ig. Values for 3H-thymidine incorporation represent the mean cpm ± SEM from triplicate cultures. (C) Dose-dependent CTLA4Ig-mediated inhibition of immobilized OKT3 stimulated preG-PBMCs (solid line) and G-PBMCs (dashed line) obtained from the same donor. Cells were stimulated by immobilized OKT3 (25 ng/mL) in the absence or presence of CTLA4Ig at the concentrations indicated. The percent CTLA4Ig-mediated inhibition was calculated as described in Materials and Methods. Results from one of three representative experiments are shown.
Polyclonal T-cell stimulation assays using immobilized OKT3 monoclonal antibody. Unfractionated preG-PBMCs (A) and G-PBMCs (B) (100 × 103 cells/200 μL) were cultured in the presence (solid line) or absence (dashed line) of 10 μg/mL CTLA4Ig. Values for 3H-thymidine incorporation represent the mean cpm ± SEM from triplicate cultures. (C) Dose-dependent CTLA4Ig-mediated inhibition of immobilized OKT3 stimulated preG-PBMCs (solid line) and G-PBMCs (dashed line) obtained from the same donor. Cells were stimulated by immobilized OKT3 (25 ng/mL) in the absence or presence of CTLA4Ig at the concentrations indicated. The percent CTLA4Ig-mediated inhibition was calculated as described in Materials and Methods. Results from one of three representative experiments are shown.
CTLA4Ig-mediated inhibition of immobilized OKT3-stimulated preG- and G-PBMC proliferation.
The role of CD28/B7 costimulation in preG- and G-PBMCs was compared by measuring T-cell proliferation in response to OKT3 stimulation in the presence and absence of different concentrations of CTLA4Ig. Comparing paired samples from the same donor, CTLA4Ig consistently had a greater inhibitory effect on the preG- compared with the G-PBMC proliferative response. As shown in Fig 2C, maximum inhibition of preG-PBMC proliferative response was >70% at a CTLA4Ig concentration of 3 μg/mL. In contrast, maximum inhibition of G-PBMC proliferation was <30% even at a CTLA4Ig concentration of 30 μg/mL.
In larger series of experiments, CTLA4Ig at 10 μg/mL was used to evaluate the role of B7 costimulation in OKT3-stimulated proliferation in both paired and unpaired samples of preG- and G-PBMCs. Using all samples the inhibition of proliferation by CTLA4Ig in preG-PBMCs (n = 11) was 72.2% ± 5.4%, which was significantly higher than the 39.9% ± 5.6% (P < .001) observed in G-PBMCs (n = 15). When the five paired samples were analyzed separately, CTLA4Ig inhibition in preG-PBMCs was also significantly higher than that seen in G-PBMCs (62.9% ± 10.0% v 25.4% ± 11.6%; P < .002; data not shown).
Expression of costimulatory molecules in preG- and G-PBMCs.
Expression levels for CD28/B7 were compared between CD4 and CD14 cells derived from preG- and G-PBMCs. As reported previously,9CD28 was equally expressed on preG- and G-CD4 cells. In addition, preG- and G-CD14 cells had comparably low expression of B7-1 (CD80). However, expression levels of B7-2 (CD86), a ligand for CD28, were reduced by 60% to 70% on G-CD14 cells compared with preG-CD14 cells (data not shown).
Detection of CD28RC in EMSA.
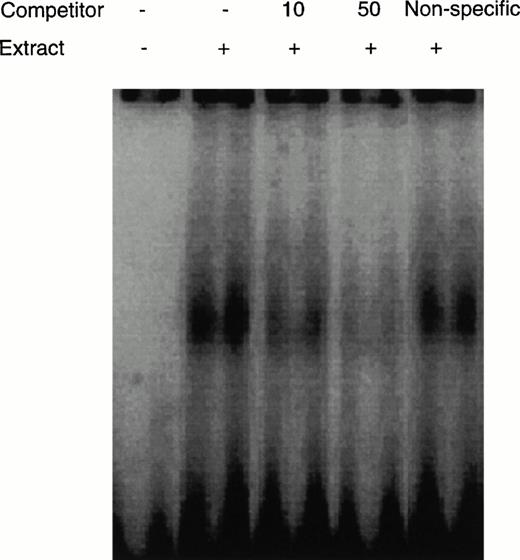
CD28RC was detectable in the nuclear protein fraction extracted from CD4 T cells derived from OKT3-stimulated preG-PBMCs by this assay (Fig3). The addition of unlabeled CD28RE inhibited the detection of CD28RC in a dose-dependent manner. However, the addition of a nonspecific probe did not interfere with the detection of CD28RC, indicating that this assay was specific for CD28RC.
Detection of CD28RC in EMSA. The nuclear extract from CD4 cells derived from OKT3-stimulated preG-PBMCs was incubated with a32P-labeled CD28RE. Extracts were electrophoresed on a 6% polyacrylamide gel and complexes of nuclear proteins and CD28RE were visualized using ImageQuant3.3 software (Molecular Dynamics). Unlabeled CD28RE (10 and 50 pmol) as a specific competitor or negative regulatory element A (NRE-A) as a nonspecific competitor were added as indicated.
Detection of CD28RC in EMSA. The nuclear extract from CD4 cells derived from OKT3-stimulated preG-PBMCs was incubated with a32P-labeled CD28RE. Extracts were electrophoresed on a 6% polyacrylamide gel and complexes of nuclear proteins and CD28RE were visualized using ImageQuant3.3 software (Molecular Dynamics). Unlabeled CD28RE (10 and 50 pmol) as a specific competitor or negative regulatory element A (NRE-A) as a nonspecific competitor were added as indicated.
Comparison of CD28RC induction in CD4 T cells derived from OKT3-stimulated preG- and G-PBMCs.
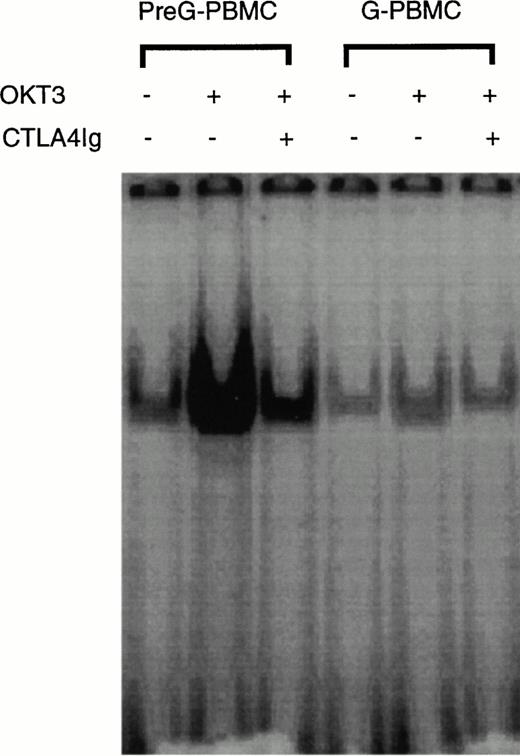
Because of functional differences seen in the CTLA4Ig inhibition experiments, we analyzed CD28RC expression in CD4 cells derived from unfractionated preG- and G-PBMCs after 4 hours of stimulation on immobilized OKT3 monoclonal antibody. The purity of CD4 cells after immunomagnetic enrichment and before nuclear protein extraction usually exceeded 90% as assessed by flow cytometric analysis. In preG-CD4 cells, CD28RC expression was upregulated by OKT3 stimulation and suppressed in the presence of CTLA4Ig (Fig4). In contrast, CD28RC expression in G-CD4 cells was not significantly increased in responses to OKT3 stimulation. In addition, the inhibitory effect of CTLA4Ig on CD28RC expression was considerably less pronounced in G-CD4 compared with preG-CD4 cells. Thus, the inducible expression of CD28RC appeared to be suppressed in G-CD4 cells.
EMSA of CD28RC in OKT3-stimulated preG- and G-CD4 cells. 1 × 106/mL unfractionated preG-PBMCs and G-PBMCs were cultured for 4 hours in OKT3-coated T75 culture flasks in the presence or absence of CTLA4Ig. One microgram of nuclear extract isolated from CD4 cells purified by immunomagnetic enrichment was used for the EMSA. Results from one of four experiments are shown.
EMSA of CD28RC in OKT3-stimulated preG- and G-CD4 cells. 1 × 106/mL unfractionated preG-PBMCs and G-PBMCs were cultured for 4 hours in OKT3-coated T75 culture flasks in the presence or absence of CTLA4Ig. One microgram of nuclear extract isolated from CD4 cells purified by immunomagnetic enrichment was used for the EMSA. Results from one of four experiments are shown.
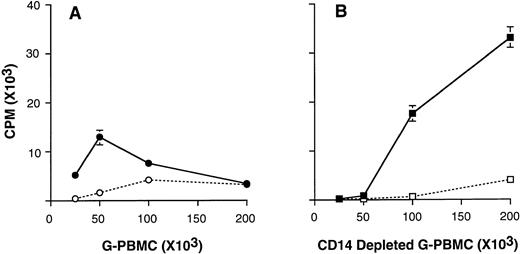
Proliferation of CD14-depleted G-PBMCs in response to stimulation with immobilized OKT3.
We have reported previously that G-PBMCs contain a large number of CD14+ monocytes that suppress T-cell proliferation in MLC in a dose-dependent manner.9 To analyze whether CD14 cells can also suppress OKT3-stimulated T-cell proliferation, we compared unfractionated and CD14-depleted G-PBMCs. As shown in Fig5, CD14-depleted G-PBMCs (<1% CD14 cells) responded better than unfractionated G-PBMCs to OKT3 stimulation. In addition to the improved responsiveness, CD14-depleted G-PBMCs were more susceptible to CTLA4Ig-mediated inhibition. In contrast, OKT3-stimulated proliferation of unfractionated G-PBMCs decreased at cell concentrations exceeding 100 × 103/200 μL.
3H-thymidine incorporation by OKT3-stimulated G-PBMCs. Unfractionated G-PBMCs (A) and CD14 depleted G-PBMCs (B) were cultured at various cell concentrations in the absence (solid lines) or in the presence (dashed lines) of CTLA4Ig. Values for3H-thymidine incorporation represent the mean cpm ± SEM from triplicate cultures. Results from one of four representative experiments are shown.
3H-thymidine incorporation by OKT3-stimulated G-PBMCs. Unfractionated G-PBMCs (A) and CD14 depleted G-PBMCs (B) were cultured at various cell concentrations in the absence (solid lines) or in the presence (dashed lines) of CTLA4Ig. Values for3H-thymidine incorporation represent the mean cpm ± SEM from triplicate cultures. Results from one of four representative experiments are shown.
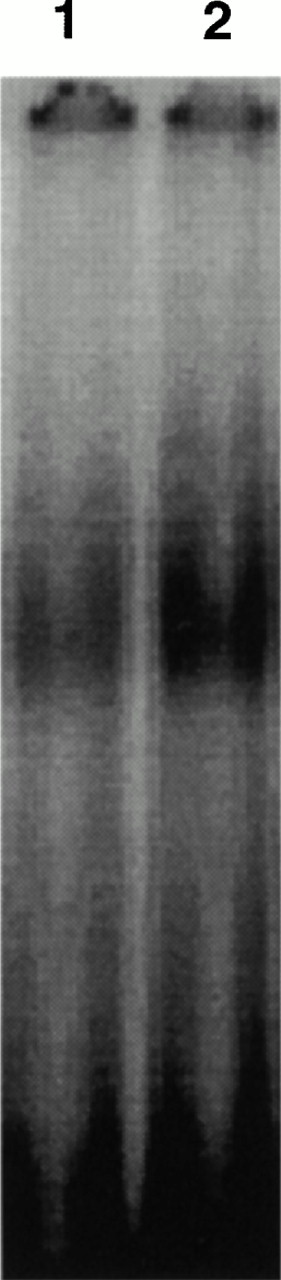
CD28RC induction in G-CD4 cells derived from unfractionated and CD14 depleted G-PBMCs in response to stimulation with immobilized OKT3.
To test whether CD14+ monocytes in G-PBMCs could suppress OKT3-induced expression of CD28RC, CD4 cells derived from unfractionated and CD14-depleted G-PBMCs were analyzed for CD28RC expression. As shown in Fig 6, this transcription factor was expressed at considerably higher levels in CD4 cells derived from CD14-depleted compared with unfractionated G-PBMCs.
EMSA of CD28RC in OKT3-stimulated CD4 cells derived from unfractionated or CD14-depleted G-PBMCs. A total of 1 × 106/mL unfractionated G-PBMCs and CD14 depleted G-PBMCs were cultured in OKT3-coated T75 culture flasks for 4 hours. One microgram of nuclear extract isolated from CD4 cells derived from either unfractionated (lane 1) or CD14-depleted (lane 2) G-PBMCs was used for EMSA. Results shown are generated from the same sample used in the experiment shown in Fig 5.
EMSA of CD28RC in OKT3-stimulated CD4 cells derived from unfractionated or CD14-depleted G-PBMCs. A total of 1 × 106/mL unfractionated G-PBMCs and CD14 depleted G-PBMCs were cultured in OKT3-coated T75 culture flasks for 4 hours. One microgram of nuclear extract isolated from CD4 cells derived from either unfractionated (lane 1) or CD14-depleted (lane 2) G-PBMCs was used for EMSA. Results shown are generated from the same sample used in the experiment shown in Fig 5.
DISCUSSION
The present study investigates the role of the CD28/B7-costimulatory pathway in T-cell activation in G-PBMCs compared with normal peripheral blood. This study was prompted by the observations that unfractionated G-PBMCs are hyporesponsive to alloantigen stimulation in vitro and that the monocytes that comprise 25% to 50% of the cells in G-PBMCs express reduced levels of B7-2 (CD86), a critical costimulatory molecule for T-cell activation.9 Using OKT3 stimulation and CTLA4Ig inhibition experiments, we now report that in comparison to normal PBMCs, G-PBMCs are less susceptible to CTLA4Ig-mediated suppression of OKT3-stimulated proliferation. This suggests that CD28/B7 interactions may not contribute significantly to T-cell activation in G-PBMCs. In keeping with this observation, G-CD4 cells have decreased levels of inducible CD28RC. However, the inducible levels of this DNA binding protein increased after G-PBMCs were depleted of CD14 cells. These data suggest that the large number of CD14+/B7-2lo cells in G-PBMCs may be interfering with CD28 signal transduction.
B7-1 and B7-2 molecules expressed on antigen-presenting cells (APCs) bind to CD28 and CTLA-4 on T cells, thereby providing a costimulus for T-cell activation and clonal expansion.12-14,17 Antigen presentation by APCs to T cells without costimulation results in a state of antigen-specific anergy. It has been shown in vitro that blocking the interaction between CD28 and B7 can result in T-cell clonal anergy.18-21 In addition, in vivo data suggest that blockade of the CD28/B7 costimulatory pathway with CTLA4Ig, which specifically blocks B7-1 and B7-2 on the APC side, may reduce lethal aGVHD after allogeneic marrow transplantation in mice.23,24Therefore, costimulatory molecules may play an important role in regulating T-cell function and the development of GVHD in human marrow and blood stem cell transplantation.30 The unexpectedly low incidence and severity of aGVHD in patients receiving G-PBMCs despite the transfer of at least 10 times more T cells compared with marrow transplantation may be a consequence of the large number of CD14+/B7-2lo cells suppressing CD28-mediated signal transduction.
Whether the relatively low levels of B7-2 expressed by these cells contributes directly to this suppressive effect is not clear at this time. Monocytes, in addition to their role as APCs,31,32can also inhibit T-cell function by several mechanisms.33-36 Soluble monocyte products have been shown to downregulate nuclear transcription factors required for T-cell activation and proliferation. IL-10, for example, inhibits NF-κB/Rel activity and prostaglandin E2 inhibits AP-1 and NF-AT induction in human T cells.37 38 Therefore, in addition to the potential relevance of reduced B7-2 levels, factors produced and secreted by monocytes may play a role in downregulation of CD28RC in G-CD4 cells. There also remains the possibility that G-CSF treatment might directly alter T-cell function by altering signaling events downstream of the CD28 receptor.
These considerations notwithstanding, our data suggest a decreased use of the CD28/B7 costimulatory pathway by stimulated CD4 T cells in G-PBMC leukapheresis products which, in turn, appears to be influenced by the large number of CD14/B7-2lo cells present. Whether these findings influence either the incidence or severity of aGVHD observed after peripheral blood stem cell transplantation remains speculative. However, they may stimulate interest in investigating the potential use of manipulated cell populations to prevent or treat graft-versus-host reactions.
ACKNOWLEDGMENT
We thank Gretchen Johnson, Ludmila Golubev, and Laura Bolles for their technical assistance and Harriet Childs for typing the manuscript.
Supported in part by grants DK51417 and CA18221 awarded by the National Institutes of Health, DHHS, Bethesda, MD. J.T. is supported by the Ministry of Education, Science and Culture, Tokyo, Japan.
Address reprint requests to Junji Tanaka, MD, PhD, Fred Hutchinson Cancer Research Center, 1124 Columbia Street, M318, Seattle, WA 98104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal