Abstract
Interleukin-4 (IL-4) is a cytokine that induces both proliferation and differentiation and suppresses apoptosis of B cells. Although IL-4 has been shown to activate the phosphatidylinositol 3′ (PI3)-kinase pathway, the role of PI3 kinase in the IL-4 receptor (IL-4R) signaling remains unclear. In this study, we demonstrated that c-Cbl proto-oncogene product is inducibly phosphorylated on tyrosine residues and is associated with the p85 subunit of PI3-kinase by IL-4 stimulation. Overexpression of c-Cbl enhances the PI3-kinase activity and, at the same time, mitogenic activity and survival of cells in the presence of IL-4. However, these effects of c-Cbl were abolished by wortmannin, a specific inhibitor for the PI3 kinase pathway, or by a point mutation at tyrosine 731 of c-Cbl, which is a major binding site for p85. These results indicate that c-Cbl plays a role in linking IL-4R with the PI3 kinase pathway and thus enhancing the mitogenic and survival signals.
INTERLEUKIN-4 (IL-4) is a cytokine that possesses various biological activities. First of all, IL-4 induces proliferation and differentiation of B cells.1 It induces expression of class II major histocompatibility complex molecules on resting B cells and enhances both secretion and cell surface expression of IgE and IgG1. Furthermore, IL-4 upregulates the expression of CD23, the low-affinity Fc receptor for IgE.2 IL-4 is also shown to act not only on B cells, but also on T cells, mast cells, macrophages, and even nonhematopoietic cells.3 The activation of intracellular signaling by IL-4 depends on ligand binding to the IL-4 receptor (IL-4R) complex, composed of IL-4Rα subunit and γc subunit. IL-4Rα subunit is a 140-kD protein, which is sufficient to permit high affinity binding of the IL-4 ligand.4,5 On the other hand, γc subunit is a common component of receptors for IL-2, IL-7, IL-9, and IL-15.6-9Mutations in the γc subunit have been shown to result in X-linked severe combined immunodeficiency (X-SCID).10 The binding of IL-4 to IL-4R induces activation of JAK1 and JAK3 tyrosine kinases, leading to phosphorylation of STAT6.11-14 Another signaling pathway activated by IL-4 is the phosphatidylinositol 3′ (PI3)-kinase pathway. IL-4 induces tyrosine phosphorylation of IRS-1 and/or IRS-2/4PS and their association with the p85 subunit of the PI3 kinase, resulting in activation of the PI3 kinase pathway.15-17 However, the role of the PI3-kinase pathway in the IL-4R signaling pathway remains unclear.
We report in this study that c-Cbl proto-oncogene product is a substrate for the IL-4–induced intracellular signaling. The c-Cbl proto-oncogene was originally identified as a cellular homologue of v-Cbl oncogene, which was cloned from the Cas NS-1 murine leukemia virus.18 The c-Cbl gene product is a 120-kD protein, which contains an NH2-terminal domain with a nuclear localization signal, followed by a RING finger motif.19 The COOH-terminal half of the protein contains a proline-rich domain, which has been shown to function as a ligand for the SH3 domains of several signaling molecules.20-26 Although many growth factors induce tyrosine phosphorylation of c-Cbl,24,25,27-30 the role of this molecule is not well-understood. As far as we know, phosphorylation of c-Cbl is closely related to activation of the Ras signaling pathway. Recent studies have demonstrated that Sli-1, Caenorhabditis elegans(C elegans) homologue of c-Cbl, is a negative regulator of the let-60–mediated signaling pathway, a C eleganscounterpart of the Ras pathway.31,32 However, it has been demonstrated that IL-4 cannot activate the Ras pathway, at least in hematopoietic tissues.33 Therefore, our data showing that IL-4 phosphorylates c-Cbl is somewhat surprising. Thus, we were very interested in the role of phosphorylation of c-Cbl in IL-4R signaling. In the present study, we analyzed the role of c-Cbl in the IL-4R signaling pathway by using various mutants of c-Cbl and obtained data that it enhances the PI3-kinase activity, which is responsible for transmitting mitogenic and antiapoptotic signals in the IL-4R signaling pathway.
MATERIALS AND METHODS
Cells and antibodies.
Ba/F3 cells were cultured in RPMI 1640 containing 10% fetal calf serum (FCS) and 0.25 ng/mL of murine interleukin-3 (mIL-3). Anti–c-Cbl antibody, antiphosphotyrosine antibody 4G10, anti-α subunit of IL-4R and antiinfluenza hemagglutinin (HA) epitope tag antibody 12CA5 were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA), Upstate Biotechnology Inc (Lake Placid, NY), Genzyme Inc (Cambridge, MA), and Bohehringer Mannheim Inc (Mannheim, Germany), respectively. Anti-p85 antibody was a gift from Y. Fukui (University of Tokyo, Tokyo, Japan).
Construction of c-Cbl mutant cDNAs and transfection.
The human c-cbl cDNA epitope-tagged with a nine amino acid HA peptide (YPYDVPDYA) from the human influenza virus was a kind gift from W.Y. Langdon (University of Western Australia, Nedlands, Australia). The construction of deletion mutants of c-Cbl, ΔRING-Cbl, and ΔPD-Cbl were described previously.34 The tyrosine 731-phenylalanine mutation of c-Cbl was generated by site-directed mutagenesis with the Chameleon Site-Directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instruction. Retrovirus vector was used to transfect cDNAs of c-Cbl and its mutants into Ba/F3 cells as described previously.35
Immunoprecipitation and immunoblotting.
Before stimulation, cells were starved in RPMI 1640 containing 0.5% FCS for 12 hours. Cells were then stimulated with 10 ng/mL of mIL-4 (PEPRO TECH EC LTD, London, UK) for 5 minutes at 37°C, washed twice with ice-cold phosphate-buffered saline (PBS), and lysed in Triton lysis buffer (0.5% (vol/vol) Triton X-100, 50 mmol/L Tris-HCl pH 7.4, 2 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 U/mL aprotinin, 1 mmol/L sodium orthovanadate, 1 mmol/L EDTA). Cell lysates were collected and subjected to immunoprecipitation and immunoblotting as described previously.36 The immunoblots were developed with the ProtoBlot system (Promega, Madison, WI) or the ECL system (Amersham, Arlington Heights, IL).
Measurement of PI3-kinase activity.
The kinase activity of PI3-kinase was measured as described previously.37 Briefly, lysates from cells were immunoprecipitated with indicated antibodies. The immunoprecipitates were washed four times in PI3 kinase buffer (20 mmol/L, Tris-HCl pH 7.5, 100 mmol/L NaCl, 0.5 mmol/L EGTA). Then crude brain phosphoinositides (Sigma, St Louis, MO, P-6023) were added to the immunoprecipitates at a final concentration of 0.2 mg/mL. Adenosine triphosphate (ATP), [γ- 32P] ATP and MgCl2 were added and incubated for 20 minutes at room temperature. The lipids were extracted into a chloroform/methanol mixture (1:1, vol/vol). The lipids-containing organic phase was resolved on thin layer chromatography (TLC) plates (Silica Gel 60, Merck, Darmstadt, Germany), developed in chloroform/methanol/ammonia solution (28%)/water (215:190:25:35 mixture) and visualized by autoradiography.
The [3H]thymidine incorporation assay.
Cells were deprived of mIL-3 for 24 hours. Then 1 ng/mL of mIL-4 was added to the medium and incubated for 12 hours. The cells were then labeled with 1 mCi of [3H]thymidine for 3 hours after growth factor stimulation. The amount of nucleotide incorporated into DNA was quantitated by scintillation counting. Each experiment was repeated at least three times and a growth rate was expressed as a ratio over the basal value at no mIL-4 stimulation.
Analysis of chromosomal DNA fragmentation.
To extract the fragmented DNA observed during apoptotic death, 5 × 106 cells were collected and lysed with 400 μL of lysis buffer (10 mmol/L Tris-HCl, pH 7.5, 10 mmol/L EDTA, 0.2 % Triton X-100). The lysate was kept on ice for 10 minutes and centrifuged at 15,000 rpm for 10 minutes. The supernatant was subjected to extraction with phenol/chloroform/isoamylalcohol (25:24:1) followed by ethanol precipitation. The precipitate was dissolved in 20 μL of Tris-EDTA (TE) and incubated for 1 hour with RNase A (2 μg/mL ) at 37°C. DNA fragments were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
RESULTS
IL-4 induces tyrosine phosphorylation of c-Cbl proto-oncogene product in Ba/F3 cells.
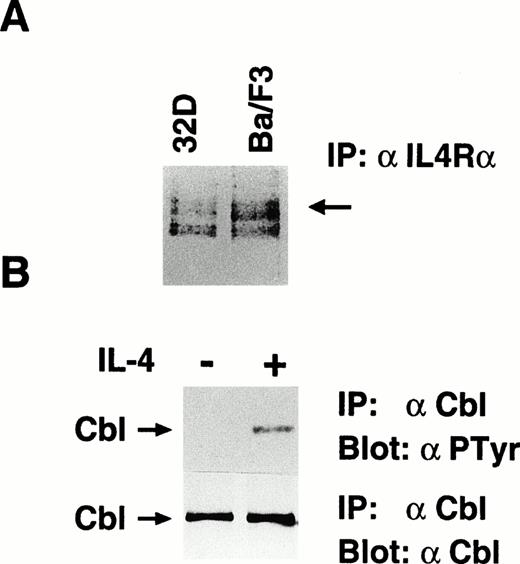
We examined whether c-Cbl is involved in the IL-4R signaling pathway. First, we tested whether stimulation with IL-4 results in phosphorylation of c-Cbl. We confirmed that Ba/F3 cells, a mIL-3–dependent pro-B cell line, express endogenous IL-4R by methionine labeling of cells followed by immunoprecipitation with anti–IL-4R antibody. Because 32D cells are known to express endogenous IL-4R, we used them as a positive control (Fig1A). We then stimulated Ba/F3 cells with mIL-4, lysed, and immunoprecipitated with anti–c-Cbl antibody. The immunoprecipitates were subjected to the antiphosphotyrosine immunoblotting. In this experiment, we found that c-Cbl was inducibly phosphorylated on tyrosine residues by IL-4 stimulation (Fig 1B). This result indicates that c-Cbl is one of the target molecules in the IL-4R signaling pathway.
IL-4 phosphorylates c-Cbl on tyrosine residues. (A) Ba/F3 cells express endogenous IL-4R. Methionine-labeled Ba/F3 and 32D cells were lysed, immunoprecipitated with anti-α subunit of IL-4R. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by autoradiography. An arrow indicates the 140-kD α subunit of IL-4R. (B) Tyrosine phosphorylation of c-Cbl by IL-4. Ba/F3 cells were serum-starved for 10 hours and stimulated with murine IL-4 (10 ng/mL) for 5 minutes at 37°C, lysed, and immunoprecipitated with anti–c-Cbl antibody. The immunoprecipitates were subjected to immunoblotting with antiphosphotyrosine antibody, 4G10 (upper panel) or anti–c-Cbl antibody (lower panel). An arrow indicates c-Cbl.
IL-4 phosphorylates c-Cbl on tyrosine residues. (A) Ba/F3 cells express endogenous IL-4R. Methionine-labeled Ba/F3 and 32D cells were lysed, immunoprecipitated with anti-α subunit of IL-4R. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by autoradiography. An arrow indicates the 140-kD α subunit of IL-4R. (B) Tyrosine phosphorylation of c-Cbl by IL-4. Ba/F3 cells were serum-starved for 10 hours and stimulated with murine IL-4 (10 ng/mL) for 5 minutes at 37°C, lysed, and immunoprecipitated with anti–c-Cbl antibody. The immunoprecipitates were subjected to immunoblotting with antiphosphotyrosine antibody, 4G10 (upper panel) or anti–c-Cbl antibody (lower panel). An arrow indicates c-Cbl.
Overexpression of c-Cbl enhances the survival effects and the mitogenic signal of IL-4 in Ba/F3 cells, which are abolished by wortmannin.
To examine the roles of c-Cbl in the signaling pathway through IL-4R, we introduced cDNA of c-Cbl into Ba/F3 cells by the retroviral vector and established a stable line, HCbl cells, which overexpress c-Cbl. We confirmed by the anti–c-Cbl immunoblot and, as an internal control, the anti-p85 immunoblot that HCbl cells express c-Cbl 10 times more than parental Ba/F3 cells (Fig 2A). IL-4 is known to generate antiapoptotic and growth signals in IL-4R expressing cell lines38-40 and, as expected, the survival of Ba/F3 cells was prolonged in the presence of IL-4, even if IL-3 was depleted (Fig 2B). However, when HCbl cells were cultured in the presence of IL-4, transient proliferation and survival of cells was significantly enhanced when compared with those of mock cells. Interestingly, both HCbl and mock cells rapidly fell into apoptotic death in the presence of wortmannin, a specific inhibitor of PI3 kinase (Fig 2B). The low molecular weight DNAs were extracted from HCbl and parental Ba/F3 cells cultured in the presence or absence of wortmannin and electrophoresed in an agarose gel. Notably, the ladder pattern typical for apoptotic death was observed for DNAs extracted from cells cultured in the presence of wortmannin and IL-4 for 48 hours (Fig 2C). We performed the thymidine incorporation assay to assess the mitogenic activities of c-Cbl overexpressing cell lines treated with IL-4. Compared with mock cells, the thymidine incorporation into HCbl cells in the presence of IL-4 was significantly enhanced, but in the presence of wortmannin, that into HCbl cells was reduced to the level without IL-4 (Fig 2D). From these data, we can conclude that survival and mitogenic signals were enhanced by overexpression of c-Cbl and that activation of the PI3 kinase pathway is required for generation of these signals.
The effects of c-Cbl in IL-4R signaling. (A) Establishment of HCbl cells that overexpress c-Cbl. Lysates from HCbl and mock cells were subjected to the immunoblotting with anti–c-Cbl antibody (upper panel) and anti-p85 antibody (lower panel). Arrows indicate c-Cbl and p85. We think that bands located below p85 are nonspecific bands. (B) Overexpression of c-Cbl enhances transient growth and elongates survival of Ba/F3 cells in the presence of IL-4. HCbl and mock cells were cultured in RPMI containing 5% FCS and IL-4 (1 ng/mL) in the presence (+WT, 50 nmol/L) or absence of wortmannin. (C) Survival effects of IL-4 enhanced by c-Cbl is sensitive to wortmannin. Low molecular weight DNA extracts from HCbl and mock cells cultured with or without IL-4 (1 ng/mL) plus wortmannin (WT, 50 nmol/L) for 48 hours were electrophoresed on an agarose gel (2%). (D) HCbl and mock cells were deprived of IL-3 for 24 hours. IL-4 (1 ng/mL) and wortmannin (WT, 50 nmol/L) were then added to the medium, incubated for 12 hours, and subjected to the [3H]thymidine incorporation assay. The growth rate was expressed as a ratio over the basal values at no IL-4 stimulation.
The effects of c-Cbl in IL-4R signaling. (A) Establishment of HCbl cells that overexpress c-Cbl. Lysates from HCbl and mock cells were subjected to the immunoblotting with anti–c-Cbl antibody (upper panel) and anti-p85 antibody (lower panel). Arrows indicate c-Cbl and p85. We think that bands located below p85 are nonspecific bands. (B) Overexpression of c-Cbl enhances transient growth and elongates survival of Ba/F3 cells in the presence of IL-4. HCbl and mock cells were cultured in RPMI containing 5% FCS and IL-4 (1 ng/mL) in the presence (+WT, 50 nmol/L) or absence of wortmannin. (C) Survival effects of IL-4 enhanced by c-Cbl is sensitive to wortmannin. Low molecular weight DNA extracts from HCbl and mock cells cultured with or without IL-4 (1 ng/mL) plus wortmannin (WT, 50 nmol/L) for 48 hours were electrophoresed on an agarose gel (2%). (D) HCbl and mock cells were deprived of IL-3 for 24 hours. IL-4 (1 ng/mL) and wortmannin (WT, 50 nmol/L) were then added to the medium, incubated for 12 hours, and subjected to the [3H]thymidine incorporation assay. The growth rate was expressed as a ratio over the basal values at no IL-4 stimulation.
The ΔPD mutant of c-Cbl is not tyrosine-phosphorylated by IL-4, whereas the ΔRING mutant is tyrosine-phosphorylated.
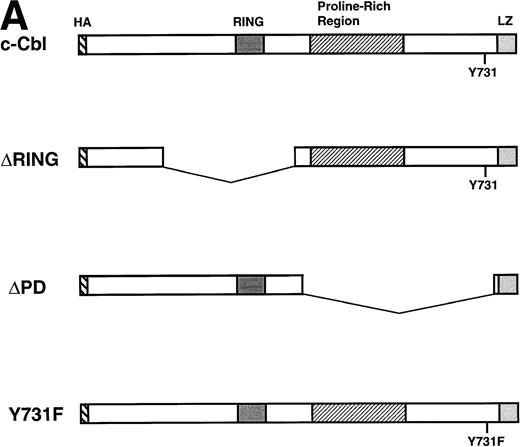
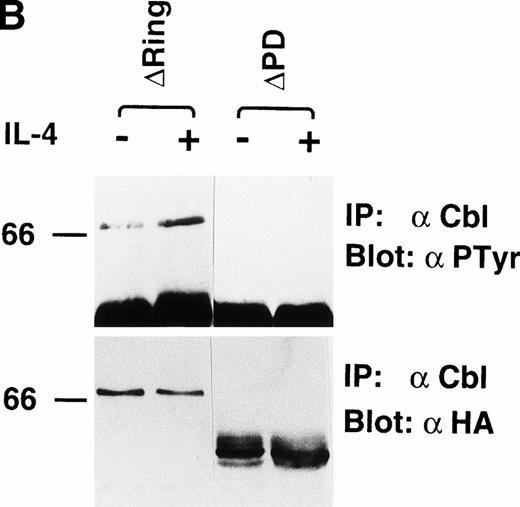
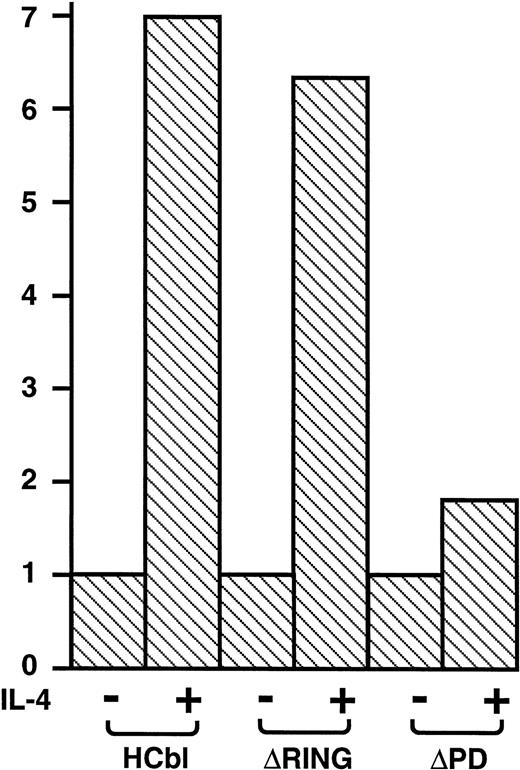
To examine the mechanism of how c-Cbl enhances the survival and mitogenic signals transmitted by IL-4R, we first tested which domain of c-Cbl is phosphorylated on tyrosine residues by IL-4 treatment. We constructed deletion mutants of c-Cbl, ΔRING-Cbl, which lacks amino acids (aa) 193-491 including the RING finger domain and ΔPD-Cbl, which lacks aa 492-877 including the proline-rich domain of c-Cbl (Fig3A) and introduced them into Ba/F3 cells by the retroviral vector and established stable transfectants, ΔRING and ΔPD cells. The cDNAs for wild-type c-Cbl and both deletion constructs were epitope-tagged with an HA peptide and therefore distinguishable from endogenous c-Cbl protein (Fig 3B). We examined if these deletion mutants of c-Cbl were phosphorylated by IL-4 treatment, finding that the ΔPD mutant of c-Cbl was not tyrosine-phosphorylated by IL-4, whereas the ΔRING mutant was tyrosine-phosphorylated (Fig 3B). This result indicates that it is within aa 497-877 of c-Cbl, which is phosphorylated by IL-4 stimulation. Next, HCbl, ΔRING, ΔPD, and mock cells were cultured in the presence of IL-4 and subjected to the thymidine incorporation assay. We found in this experiment that while the levels of thymidine incorporated into HCbl and ΔRING cells treated with IL-4 were approximately the same, that of ΔPD cells was significantly decreased (Fig 4), suggesting that the region within aa 497-877 of c-Cbl is required for generating mitogenic signals.
IL-4 phosphorylates the region within aa 492-877 of c-Cbl. (A) Constructs of cDNAs for wild-type and deletion mutants of c-Cbl epitope-tagged with an HA peptide. ▿RING-Cbl lacks aa 193-491 including the RING finger domain and ▿PD-Cbl lacks aa 492-877 including the proline-rich domain of c-Cbl. HA, a nine amino acid HA peptide (YPYDVPDYA) from the human influenza virus. RING, the RING finger motif. LZ, the leucine zipper motif. (B) Expression and tyrosine phosphorylation of deletion mutants of c-Cbl by IL-4 stimulation. The cDNAs encoding deletion mutants, ▿RING-Cbl and ▿PD-Cbl, were introduced into Ba/F3 cells by the retroviral vector, and stable transfectants, ▿RING and ▿PD cells were established. These cells were stimulated with IL-4 (10 ng/mL) for 5 minutes at 37°C, lysed, and immunoprecipitated with anti–c-Cbl antibody. The immunoprecipitates were then subjected to the immunoblotting with antiphosphotyrosine antibody (upper panel) or anti-HA monoclonal antibody, 12CA5 (lower panel).
IL-4 phosphorylates the region within aa 492-877 of c-Cbl. (A) Constructs of cDNAs for wild-type and deletion mutants of c-Cbl epitope-tagged with an HA peptide. ▿RING-Cbl lacks aa 193-491 including the RING finger domain and ▿PD-Cbl lacks aa 492-877 including the proline-rich domain of c-Cbl. HA, a nine amino acid HA peptide (YPYDVPDYA) from the human influenza virus. RING, the RING finger motif. LZ, the leucine zipper motif. (B) Expression and tyrosine phosphorylation of deletion mutants of c-Cbl by IL-4 stimulation. The cDNAs encoding deletion mutants, ▿RING-Cbl and ▿PD-Cbl, were introduced into Ba/F3 cells by the retroviral vector, and stable transfectants, ▿RING and ▿PD cells were established. These cells were stimulated with IL-4 (10 ng/mL) for 5 minutes at 37°C, lysed, and immunoprecipitated with anti–c-Cbl antibody. The immunoprecipitates were then subjected to the immunoblotting with antiphosphotyrosine antibody (upper panel) or anti-HA monoclonal antibody, 12CA5 (lower panel).
The thymidine incorporation assay of deletion mutants of c-Cbl in the presence of IL-4. HCbl, ▿RING and ▿PD cells were subjected to the thymidine incorporation assay. The growth rates were expressed as in Fig 2D. The data for HCbl cells are the same as presented in Fig 2D.
Tyrosine 731 of c-Cbl is required for transmission of mitogenic signal in IL-4R signaling.
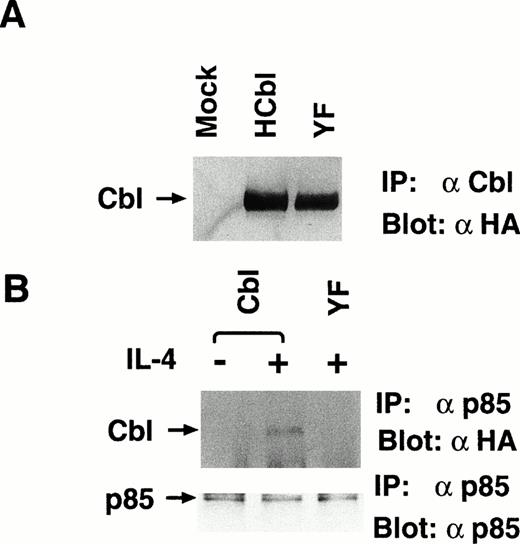
We hypothesized from the results presented above that the PI-3 kinase pathway is required for transmission of survival mitogenic signals enhanced by c-Cbl. The SH2 domain of p85 subunit of PI3 kinase is known to bind to c-Cbl phosphorylated by T-cell receptor activation,23 but the binding site on c-Cbl has not been identified. There are two potential binding sequences for the SH2 domain of p85 on aa 371-374 and aa 731-734 of c-Cbl, which are known as YXXM motifs.41 42 Because, as indicated above, the region phosphorylated by IL-4R is located within aa 492-877 of c-Cbl, it is most likely that the binding site for the SH2 domain of p85 is tyrosine 731. To confirm this hypothesis, we introduced a tyrosine 731-phenylalanine mutation into the c-Cbl cDNA and established a stable Ba/F3 cell line, YF, which expresses the Y731F mutant of c-Cbl (Fig5A). To confirm that tyrosine 731 of c-Cbl is the major binding site for p85, HCbl and YF cells stimulated with IL-4 were lysed and immunoprecipitated with anti-p85 antibody. The immunoprecipitates were subjected to immunoblotting with anti-HA antibody, which recognizes introduced c-Cbl and its mutants, but not endogenous c-Cbl protein. In this experiment, while p85 could coprecipitate wild-type c-Cbl, it could not coprecipitate the Y731F mutant of c-Cbl (Fig 5B). These data support that the binding site for p85 on c-Cbl is tyrosine 731. We also performed immunoprecipitation with anti-HA followed by immunoblotting with anti-p85 antibody and could observe that p85 was inducibly coimmunoprecipitated with the wild-type c-Cbl, but not with the Y731F mutant of c-Cbl (data not shown).
The Y731F mutant of c-Cbl cannot associate with p85 by IL-4 stimulation. (A) The establishment of YF cells, which stably express Y731F mutant of c-Cbl. Lysates from mock, HCbl, and YF cells were immunoprecipitated with anti–c-Cbl antibody and subjected to the immunoblotting with anti-HA antibody, 12CA5. The construct of Y731F mutant of c-Cbl is presented in Fig 3A. An arrow indicates c-Cbl. (B) Y731F c-Cbl mutant cannot associate with p85. Lysates from HCbl and YF stimulated with IL-4 (10 ng/mL) for 5 minutes at 37°C were immunoprecipitated with anti-p85 antibody, followed by the immunoblotting with antiphosphotyrosine antibody (upper panel) or anti-p85 antibody (lower panel). An arrow indicates c-Cbl.
The Y731F mutant of c-Cbl cannot associate with p85 by IL-4 stimulation. (A) The establishment of YF cells, which stably express Y731F mutant of c-Cbl. Lysates from mock, HCbl, and YF cells were immunoprecipitated with anti–c-Cbl antibody and subjected to the immunoblotting with anti-HA antibody, 12CA5. The construct of Y731F mutant of c-Cbl is presented in Fig 3A. An arrow indicates c-Cbl. (B) Y731F c-Cbl mutant cannot associate with p85. Lysates from HCbl and YF stimulated with IL-4 (10 ng/mL) for 5 minutes at 37°C were immunoprecipitated with anti-p85 antibody, followed by the immunoblotting with antiphosphotyrosine antibody (upper panel) or anti-p85 antibody (lower panel). An arrow indicates c-Cbl.
The PI3 kinase activation by IL-4 treatment was enhanced in HCbl cells, but not in YF-Ba/F3 cells.
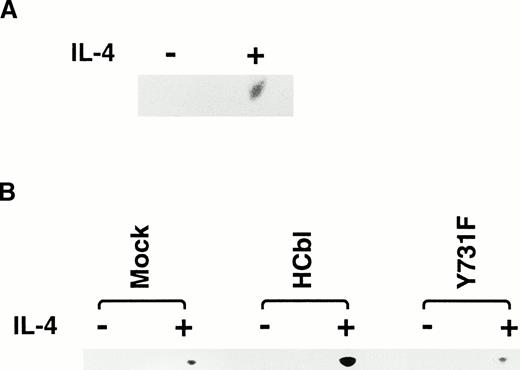
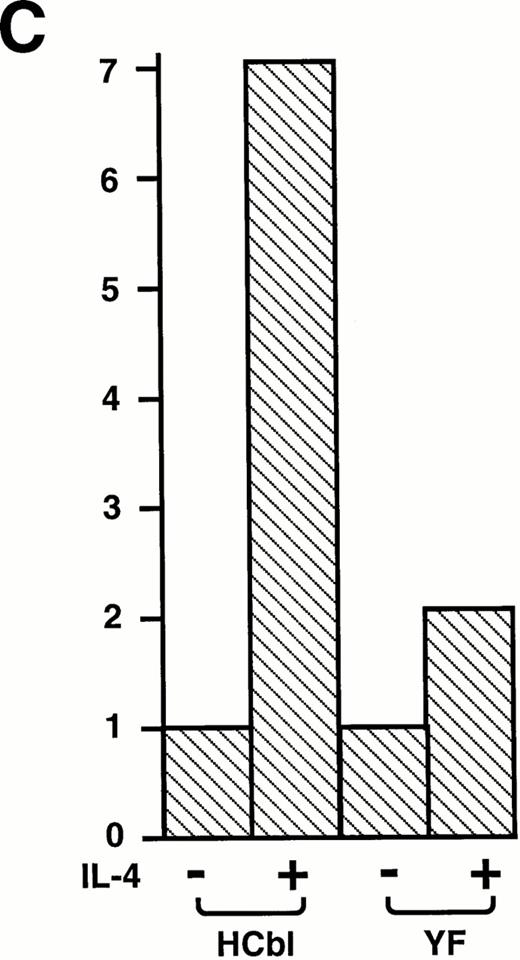
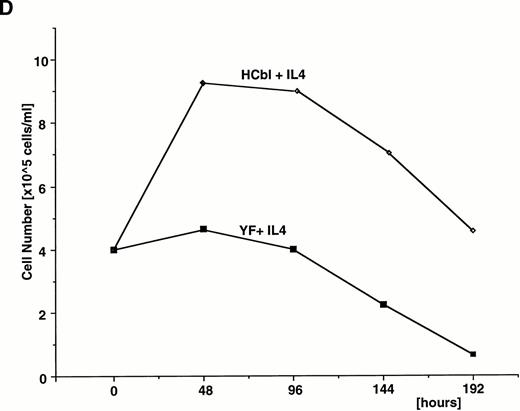
First, we confirmed that IL-4 simulation enhances c-Cbl–associated PI3 kinase activity. Ba/F3 cells treated with or without IL-4 were lysed and immunoprecipitated with anti–c-Cbl antibody. The immunoprecipitates were subjected to the PI3 kinases assay. As expected, enhancement of c–Cbl-associated PI3 kinase activity by IL-4 treatment was observed (Fig 6A). This result indicates that c-Cbl can connect IL-4R with the PI3 kinase pathway. If this is the case, overexpression of c-Cbl should enhance the PI-3 kinase activity in Ba/F3 cells by IL-4 treatment and, to the contrary, the Y731F mutant of c-Cbl, which cannot bind to p85, could not. To examine this, HCbl, YF, and mock cells stimulated with IL-4 were lysed and immunoprecipitated with antiphosphotyrosine antibody. The immunoprecipitates were then subjected to the PI3 kinase assay. As expected, PI3 kinase activity in HCbl was significantly enhanced when compared with that of mock cells, while that of YF cells was approximately equal to the level of mock cells (Fig 6B). We next performed the thymidine incorporation assay to assess the mitogenic activities of HCbl and YF cells and found that the Y731F-Cbl mutant could not enhance the DNA synthesis as did the wild-type c-Cbl (Fig6C). If the c–Cbl-PI3 kinase pathway is responsible for enhancing transient growth and survival effects of IL-4 observed in Fig 2, we can expect that growth and survival of YF cells in the presence of IL-4 should not be enhanced as HCbl cells. As expected, growth of YF cells in the presence of IL-4 was similar to that of parental Ba/F3 cells (Fig 6D). All of these results indicate that tyrosine 731 of c-Cbl, which is a binding site for p85, is critical for transmitting mitogenic signal of IL-4R, suggesting that the PI3-kinase pathway plays a role in enhancing DNA synthesis, transient growth, and survival of cells.
Tyrosine 731 of c-Cbl is critical for enhancing PI3 kinase activity. (A) IL-4 activates c-Cbl–associated PI3 kinase in Ba/F3 cells. Lysates from Ba/F3 cells stimulated with or without IL-4 were immunoprecipitated with anti–c-Cbl antibody. The immunoprecipitates were subjected to the PI3 kinase assay. (B) The PI3 kinase assay of mock, HCbl, and YF cells stimulated with or without IL-4. Lysates from mock, HCbl and YF cells stimulated with or without IL-4 were immunoprecipitated with antiphosphotyrosine antibody. The immunoprecipitates were then subjected to the PI-3 kinase assay. (C) The thymidine incorporation assay of HCbl and YF cells in the presence or absence of IL-4 (10 ng/mL). The growth rates were expressed as in Fig 2D. The data for HCbl cells are the same as presented in Fig 2D. (D) Growth of HCbl and YF cells in the presence of IL-4. HCbl and mock cells were cultured in RPMI containing 5% FCS and IL-4 (1 ng/mL), but without IL-3. The data for HCbl cells are the same as presented in Fig 2B.
Tyrosine 731 of c-Cbl is critical for enhancing PI3 kinase activity. (A) IL-4 activates c-Cbl–associated PI3 kinase in Ba/F3 cells. Lysates from Ba/F3 cells stimulated with or without IL-4 were immunoprecipitated with anti–c-Cbl antibody. The immunoprecipitates were subjected to the PI3 kinase assay. (B) The PI3 kinase assay of mock, HCbl, and YF cells stimulated with or without IL-4. Lysates from mock, HCbl and YF cells stimulated with or without IL-4 were immunoprecipitated with antiphosphotyrosine antibody. The immunoprecipitates were then subjected to the PI-3 kinase assay. (C) The thymidine incorporation assay of HCbl and YF cells in the presence or absence of IL-4 (10 ng/mL). The growth rates were expressed as in Fig 2D. The data for HCbl cells are the same as presented in Fig 2D. (D) Growth of HCbl and YF cells in the presence of IL-4. HCbl and mock cells were cultured in RPMI containing 5% FCS and IL-4 (1 ng/mL), but without IL-3. The data for HCbl cells are the same as presented in Fig 2B.
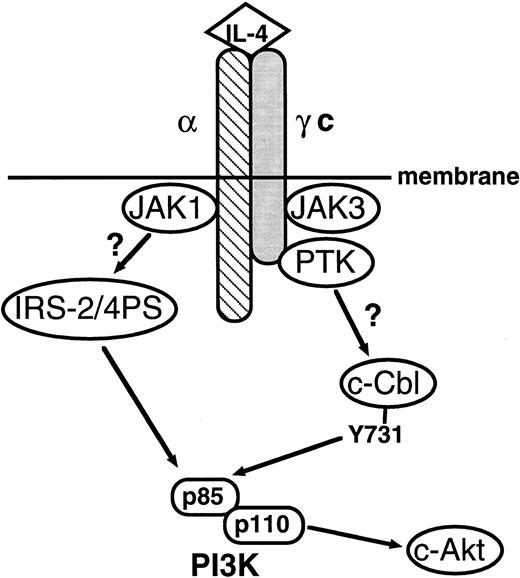
The signaling pathway of IL-4R is summarized in Fig7. In this study, we demonstrated that, like IRS-1 and IRS-2, c-Cbl plays a role in linking IL-4R with the PI3 kinase pathway, which enhances mitogenic and antiapoptotic signals of IL-4R.
Schematic representation of the signaling pathway of IL-4R. In this study, we showed that c-Cbl serves as a second pathway linking IL-4R with the PI3 kinase pathway. α, the α subunit of IL-4R; γc, the γ subunit of IL-4R; c-Akt, c-Akt serine/threonine kinase.49
Schematic representation of the signaling pathway of IL-4R. In this study, we showed that c-Cbl serves as a second pathway linking IL-4R with the PI3 kinase pathway. α, the α subunit of IL-4R; γc, the γ subunit of IL-4R; c-Akt, c-Akt serine/threonine kinase.49
DISCUSSION
In this study, we presented data showing that c-Cbl is a substrate for IL-4R signaling. IL-4 phosphorylates c-Cbl on tyrosine residues and then c-Cbl enhances mitogenic and survival signals evoked by IL-4R. These effects of c-Cbl are considered to be generated by linking IL-4R with the PI3 kinase pathway, because wortmannin, a specific inhibitor for the PI3 kinase pathway, abolished the effects of c-Cbl. It is well-known that IL-4 stimulation of IL-4R–expressing cells phosphorylates IRS-1 and IRS-2 on tyrosine residues, resulting in their association with the p85 subunit of PI3 kinase. Thus, one of the roles of IRS protein in the IL-4R signaling pathway is to link IL-4R with the PI3 kinase pathway. Our data demonstrated in the present study indicate that c-Cbl functions as a second molecule that links IL-4R with the PI3 kinase pathway.
A recent study on c-Cbl demonstrated that the SH3 domain of p85 constitutively binds to the proline rich domain of c-Cbl, but this interaction is weak. On tyrosine-phosphorylation of c-Cbl, the SH2 domain of p85 also binds to c-Cbl, achieving a more tight association.43 There are two YXXM motifs, which are potential binding motifs for the SH2 domain of p85 on human c-Cbl protein, at aa 371-374 and aa 731-734. From the data that IL-4 did not phosphorylate the ΔPD-Cbl mutant, which lacks aa 492-877, we can speculate that the binding site of c-Cbl for the SH2 domain of p85 should be tyrosine 731. Therefore, we constructed tyrosine-731 phenylalanine mutant of c-Cbl. As expected, IL-4–induced association between p85 and c-Cbl was considerably reduced by introducing the Y731F mutation. Moreover, the Y731F mutant of c-Cbl could not enhance PI3 kinase activity.
The next question is what is the role of the PI3 kinase pathway in the IL-4R signaling pathway. Interestingly, Yao et al44reported that the PI3 kinase pathway is responsible for generating survival signals activated by nerve growth factor (NGF) and platelet-derived growth factor (PDGF) in PC12 cells. Moreover, recent studies indicate that c-Akt serine/threonine kinase, which is a direct target of PI-3 kinase, regulates neuronal survival.45-47 Because IL-4 is known to suppress apoptosis of B cells,38-40 it is likely that the PI-3 kinase-c–Akt pathway plays a critical role in enhancing the survival signal through IL-4R. Combined with our data that c-Cbl–overexpressing cells showed enhanced PI-3 kinase activity in the presence of IL-4, it is likely that c-Cbl contributes to the survival effects of IL-4 by connecting with the PI3 kinase-c-Akt pathway (Fig 7). Moreover, transient elevation of DNA synthesis was observed in c-Cbl–overexpressing cells when treated with IL-4, which was abolished in the presence of wortmannin or by generating the Y731F mutation into c-Cbl. These data indicate that the PI3 kinase pathway contributes to the mitogenic signal of IL-4.
We do not know yet the tyrosine kinase, which phosphorylates c-Cbl or which region of IL-4R is required for phosphorylation of c-Cbl. Because IL-4 is known to activate JAK1 and JAK3 tyrosine kinases,11-13 it is possible that c-Cbl is phosphorylated by JAK kinases, but we have not obtained any supporting data. By using various IL-4R mutants, Deutsch et al48 reported that distinct regions of IL-4R differentially regulate apoptosis inhibition and cell growth. They demonstrated that the proline- rich motif (PRM) (P242-K264) and the acidic region (S330-S365) are required for both growth and apoptosis inhibition effects.48 These regions are distinct from the NPXY motif, which is required for IRS protein phosphorylation. Because our data in the present study indicate that c-Cbl enhances both growth and survival signals of IL-4, the PRM or the acidic region on IL-4R might be involved in c-Cbl phosphorylation. Currently, the mechanism of how IL-4R phosphorylates c-Cbl and what differential roles c-Cbl and IRS protein play are under investigation.
ACKNOWLEDGMENT
The human c-Cbl cDNA was a kind gift of W.Y. Langdon. We thank Y. Fukui for the anti-p85 antibody. We also thank O.N. Witte for the expression vector.
Supported in part by grants-in-aid from the Ministry of Education, Science, and Culture of Japan and from the Ministry of Health and Welfare of Japan, Tokyo, Japan.
Address reprint requests to Hisamaru Hirai, MD, Third Department of Internal Medicine, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. The effects of c-Cbl in IL-4R signaling. (A) Establishment of HCbl cells that overexpress c-Cbl. Lysates from HCbl and mock cells were subjected to the immunoblotting with anti–c-Cbl antibody (upper panel) and anti-p85 antibody (lower panel). Arrows indicate c-Cbl and p85. We think that bands located below p85 are nonspecific bands. (B) Overexpression of c-Cbl enhances transient growth and elongates survival of Ba/F3 cells in the presence of IL-4. HCbl and mock cells were cultured in RPMI containing 5% FCS and IL-4 (1 ng/mL) in the presence (+WT, 50 nmol/L) or absence of wortmannin. (C) Survival effects of IL-4 enhanced by c-Cbl is sensitive to wortmannin. Low molecular weight DNA extracts from HCbl and mock cells cultured with or without IL-4 (1 ng/mL) plus wortmannin (WT, 50 nmol/L) for 48 hours were electrophoresed on an agarose gel (2%). (D) HCbl and mock cells were deprived of IL-3 for 24 hours. IL-4 (1 ng/mL) and wortmannin (WT, 50 nmol/L) were then added to the medium, incubated for 12 hours, and subjected to the [3H]thymidine incorporation assay. The growth rate was expressed as a ratio over the basal values at no IL-4 stimulation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.46/3/m_blod4010602a.jpeg?Expires=1769997496&Signature=klBQiTe2BpIfk2l3GHW~F4PXvRHvmXRet6hMmKFJEEdZwgFSgvNjbq9~sJONqUuYcigkKSg1ma1EwhJGxuSHqwfEmcObihyWj91dTQ9CDvAaWuJ9dNZahG-Z4yXTVfAnYSHc3EnpuKlK2iPQlNtsqiQdTIri9~7-KcKjdXP87LuGV~la~h79B3FW1MS7oqKR-u453~i7tzEtuertxuQ4SAnw6B0~DYFBNfouATHnL7~y-GbcTtAGQ8ETlccm~-zx6ypfUoHgUW6i8C4nA2WVPTJwtiOTBqaspXxQJSMQbVmXWtBXMPvlEnrT-Mwxylsy1DwiNXWxTbbuVf4-yVipZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. The effects of c-Cbl in IL-4R signaling. (A) Establishment of HCbl cells that overexpress c-Cbl. Lysates from HCbl and mock cells were subjected to the immunoblotting with anti–c-Cbl antibody (upper panel) and anti-p85 antibody (lower panel). Arrows indicate c-Cbl and p85. We think that bands located below p85 are nonspecific bands. (B) Overexpression of c-Cbl enhances transient growth and elongates survival of Ba/F3 cells in the presence of IL-4. HCbl and mock cells were cultured in RPMI containing 5% FCS and IL-4 (1 ng/mL) in the presence (+WT, 50 nmol/L) or absence of wortmannin. (C) Survival effects of IL-4 enhanced by c-Cbl is sensitive to wortmannin. Low molecular weight DNA extracts from HCbl and mock cells cultured with or without IL-4 (1 ng/mL) plus wortmannin (WT, 50 nmol/L) for 48 hours were electrophoresed on an agarose gel (2%). (D) HCbl and mock cells were deprived of IL-3 for 24 hours. IL-4 (1 ng/mL) and wortmannin (WT, 50 nmol/L) were then added to the medium, incubated for 12 hours, and subjected to the [3H]thymidine incorporation assay. The growth rate was expressed as a ratio over the basal values at no IL-4 stimulation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.46/3/m_blod4010602b.jpeg?Expires=1769997496&Signature=u3neOwsX-mr7GKOVmwiSBTTbRwTa-yEV59TCenhJGkVN~iMSgk2A5v18WDCuP-xltNyRbu0iEc85zJtbp3GX~wspQ7YyMxLTMa13ivnyOO5vr0eKG5LWJNjBSEluOMhXKoASDjuhHr2zhtY9fBsxzaxJYeesYyVlq6Y~-bueh2EjsVGgfawhtYjnI8WWBK9TnwoKWK9hV4iK4CAn4AXoNHwDgxboj8~HHU9yKbvZvhJKa3VFhtjF9HNcyCiNKkD0~85znm2pt5haCE3ckAz~4LjhPXxS-hz2j4Ql54P77P8uOm~a2wyVxdgto5NGiiKhX4VWSuVtv7M3EQ96rV~Zyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. The effects of c-Cbl in IL-4R signaling. (A) Establishment of HCbl cells that overexpress c-Cbl. Lysates from HCbl and mock cells were subjected to the immunoblotting with anti–c-Cbl antibody (upper panel) and anti-p85 antibody (lower panel). Arrows indicate c-Cbl and p85. We think that bands located below p85 are nonspecific bands. (B) Overexpression of c-Cbl enhances transient growth and elongates survival of Ba/F3 cells in the presence of IL-4. HCbl and mock cells were cultured in RPMI containing 5% FCS and IL-4 (1 ng/mL) in the presence (+WT, 50 nmol/L) or absence of wortmannin. (C) Survival effects of IL-4 enhanced by c-Cbl is sensitive to wortmannin. Low molecular weight DNA extracts from HCbl and mock cells cultured with or without IL-4 (1 ng/mL) plus wortmannin (WT, 50 nmol/L) for 48 hours were electrophoresed on an agarose gel (2%). (D) HCbl and mock cells were deprived of IL-3 for 24 hours. IL-4 (1 ng/mL) and wortmannin (WT, 50 nmol/L) were then added to the medium, incubated for 12 hours, and subjected to the [3H]thymidine incorporation assay. The growth rate was expressed as a ratio over the basal values at no IL-4 stimulation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.46/3/m_blod4010602c.jpeg?Expires=1769997496&Signature=CkTVRfwAsNV3Cj-yCS5PIM2ZOD~m4SbljxjpizOwYItvitTozAsIa9MERN1YCoI1pKOGhOJlpqq~k-XuKAlr6AiYoZHnmVLHzI~aO5NRK75DmHCfDntUTMBkpky20QXJrT9Je1MHqSUO7D-0~km6w9NkFNHPXpo6-Uj8ZnBlo66kkItKVFZdFzYOdw-kiYV~ujo6rXFKnDvItfzbo~zcOwTFryz0Yr4vPua7ofkpHHprCOabQzjZcN7F9zxWk0SIoiRZg5NqJbRBFGf25UXORC5PZ6WXHvQ5UYRVp7H13apTTlUmobpBsK--tTIBdm8-M6d6v4yndlj1OcXvfEqBNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. The effects of c-Cbl in IL-4R signaling. (A) Establishment of HCbl cells that overexpress c-Cbl. Lysates from HCbl and mock cells were subjected to the immunoblotting with anti–c-Cbl antibody (upper panel) and anti-p85 antibody (lower panel). Arrows indicate c-Cbl and p85. We think that bands located below p85 are nonspecific bands. (B) Overexpression of c-Cbl enhances transient growth and elongates survival of Ba/F3 cells in the presence of IL-4. HCbl and mock cells were cultured in RPMI containing 5% FCS and IL-4 (1 ng/mL) in the presence (+WT, 50 nmol/L) or absence of wortmannin. (C) Survival effects of IL-4 enhanced by c-Cbl is sensitive to wortmannin. Low molecular weight DNA extracts from HCbl and mock cells cultured with or without IL-4 (1 ng/mL) plus wortmannin (WT, 50 nmol/L) for 48 hours were electrophoresed on an agarose gel (2%). (D) HCbl and mock cells were deprived of IL-3 for 24 hours. IL-4 (1 ng/mL) and wortmannin (WT, 50 nmol/L) were then added to the medium, incubated for 12 hours, and subjected to the [3H]thymidine incorporation assay. The growth rate was expressed as a ratio over the basal values at no IL-4 stimulation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.46/3/m_blod4010602d.jpeg?Expires=1769997496&Signature=SGDLc5l-dx1Jo7hB7CRYyc21nV5KAgPDdD6Tb8HSbHaPnPe0y~oZwcSp4VslOG1asKeSkxecJFEFwUUPS1lDq8DAE07mvl91xi3eVXYGZctn6JdJF9GlK~KVSrMX7lVieZE89KPix~9FZg1-TEXutJdn1JJ~diU2ggWT9ivfAMCM5he3zaAXdiQ5JnPaLsVTHKts1X0Ivhz5yU51buZzUgkGjmslLoCGUp3tb~Iwr0PgFsTAVzG~aoDLQU1mlR82bV3jNq1K-pAXJpFCSTNlc3dPKclOA5VhqEQnVeNg22X1VDmNhjDIge0kCDj-SwY~aD5ehf8VIMeQXHz8QN53IQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal