Abstract
Mammalian 15-lipoxygenases, which have been implicated in the differentiation of hematopoietic cells are commonly regarded as cytosolic enzymes. Studying the interaction of the purified rabbit reticulocyte 15-lipoxygenase with various types of biomembranes, we found that the enzyme binds to biomembranes when calcium is present in the incubation mixture. Under these conditions, an oxidation of the membrane lipids was observed. The membrane binding was reversible and led to an increase in the fatty acid oxygenase activity of the enzyme. To find out whether such a membrane binding also occurs in vivo, we investigated the intracellular localization of the enzyme in stimulated and resting hematopoietic cells by immunoelectron microscopy, cell fractionation studies and activity assays. In rabbit reticulocytes, the 15-lipoxygenase was localized in the cytosol, but also bound to intracellular membranes. This membrane binding was also reversible and the detection of specific lipoxygenase products in the membrane lipids indicated the in vivo activity of the enzyme on endogenous substrates. Immunoelectron microscopy showed that in interleukin-4 –treated monocytes, the 15-lipoxygenase was localized in the cytosol, but also at the inner side of the plasma membrane and at the cytosolic side of intracellular vesicles. Here again, cell fractionation studies confirmed the in vivo membrane binding of the enzyme. In human eosinophils, which constitutively express the 15-lipoxygenase, the membrane bound share of the enzyme was augmented when the cells were stimulated with calcium ionophore. Only under these conditions, specific lipoxygenase products were detected in the membrane lipids. These data suggest that in hematopoietic cells the cytosolic 15-lipoxygenase translocates reversibly to the cellular membranes. This translocation, which increases the fatty acid oxygenase activity of the enzyme, is calcium-dependent, but may not require a special docking protein.
LIPOXYGENASES (LOX) are lipid peroxidizing enzymes that are categorized according to their positional specificity of arachidonic acid oxygenation.1 5-LOXs are involved in the biosynthesis of leukotrienes that constitute important mediators of anaphylactic and inflammatory diseases.1 In contrast to 5-LOXs, which strongly prefer free arachidonic acid as substrate, mammalian 15-LOXs are capable of oxygenating not only free, but also esterified polyenoic fatty acids,2-4 and even more complex lipid protein assemblies such as biomembranes2,4,5and lipoproteins.6 The enzyme is constitutively expressed in reticulocytes of various species,7,8 in airway epithelial cells, and in eosinophilic granulocytes.8 Human peripheral monocytes do not express the 15-LOX, but when the cells are cultured in the presence of interleukin-4 (IL-4) or IL-13, the synthesis of the enzyme is induced.9,10 Although the biological role of 15-LOXs is still under discussion, there is a substantial body of experimental evidence for its implication in cell development and differentiation.11,12 In rabbit reticulocytes the expression of the enzyme parallels the maturational decline of the cytochrome C oxidase, a marker enzyme of mitochondrial respiration.12 At this stage of red blood cell development, specific LOX products can be detected in the lipid extracts of mitochondrial membranes and, to a lesser extent, in the extracts of the plasma membranes indicating the in vivo activity of the enzyme.13 Because the expression of the 15-LOX parallels the inactivation of respiratory enzymes and the loss of cell membrane receptors during the time course of reticulocyte maturation, an involvement of the enzyme in the programmed breakdown of mitochondria and in the remodeling process of the plasma membrane was suggested.11,12 This hypothesis was later supported by experiments on the in vitro maturation of rabbit reticulocytes in the presence of 15-LOX inhibitors.12 A similar role of the enzyme in remodeling of intracellular structures may be assumed for monocyte/macrophage transition.9 11
For the 5-LOX of polymorphonuclear leukocytes and of alveolar macrophages, which is involved in the synthesis of mediators of inflammatory reactions,14,15 it has been shown that the cytosolic enzyme binds to the cellular membranes when the cells were stimulated with calcium ionophore16,17 suggesting a calcium-dependent membrane association. Later on it was shown that the enzyme translocates preferentially to the nuclear envelope18,19 where it interacts with a special docking protein, the five lipoxygenase activating protein (FLAP), which appears to enhance the availability of the substrate fatty acid.20In rat basophilic leukemia cells21,22 and alveolar macrophages,23,24 the 5-LOX was localized inside the nucleus and may also translocate to the nuclear envelope on cell stimulation.24 In contrast to the well investigated intracellular localization of the 5-LOX,16-24 little is known about the subcellular distribution of the 15-LOX. The enzyme is commonly referred to as cytosolic protein because it can be purified from the cytosol of various mammalian cells7 and because there is no obvious membrane binding sequence in its primary structure. On the other hand, its capability of directly oxygenating biomembranes2-5 and the recent finding that in more mature reticulocytes the 15-LOX was predominantly detected in the stroma fraction25 suggested a maturation-dependent membrane association of the enzyme.
The lack of information on the subcellular localization of the 15-LOX in mammalian cells prompted us to investigate the membrane association behavior of this enzyme in reconstituted in vitro systems and in intact cells. We found that in various hematopoietic cells the 15-LOX is localized in the cytosol, but also bound to cellular membranes. Membrane binding of the enzyme is calcium-dependent and may not require a special docking protein.
MATERIALS AND METHODS
Chemicals.
The chemicals used were from the following sources: linoleic acid (9Z,12Z-octadecadienoic acid) from Serva (Heidelberg, Germany); human recombinant IL-4 from UBI (Lake Placid, NY); phosphate-buffered saline (PBS) from Sigma (Deisenhofen, Germany); immunochemicals from Dianova (Hamburg, Germany); Percoll from Pharmacia (Freiburg, Germany); Dextran 200 from Serva; authentic standards of hydroxy fatty acids from Cayman Chem (Alexis Deutschland GmbH, Grünberg, Germany); 3,3'-diaminobenzidine tetrahydrochloride from Sigma (St Louis, MO); osmic acid from Paesel (Frankfurt am, Germany); araldite from Serva; uranyl acetate and lead citrate from Sigma. For the immunostainings, a polyclonal antirabbit 15-LOX antibody (fast protein liquid chromatography preparation of the IgG fraction), which was raised in guinea pigs, was used. This antibody strongly cross-reacted with the human 15-LOX and with the porcine leukocyte 12-LOX, but not with the human platelet 12-LOX and with the human 5-LOX.
Preparations and incubations.
The 15-LOX was purified to apparent electrophoretic homogeneity from a reticulocyte rich blood cell suspension obtained from anemic rabbits by a three-step purification procedure, which comprised fractionated ammonium sulfate precipitation, hydrophobic interaction chromatography, and anion exchange chromatography.6 Submitochondrial particles (SMP), which constitute vesicles of mitochondrial membranes were prepared from beef heart homogenates.26
For quantification of membrane binding, the pure 15-LOX was incubated for 5 minutes at room temperature with EDTA washed SMP or with EDTA washed erythrocyte ghosts in the presence or absence of 0.5 mmol/L calcium. The membrane fraction was sedimented by ultracentrifugation (100,000g), the supernatant was removed and the pellet was resuspended in water (original volume). The resuspended membranes and the supernatants were dialyzed overnight against water and the samples were dried down in the Speedvac centrifuge. The protein residues were redissolved in electrophoresis sample buffer (60 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate [SDS], 10% glycerol, 5% mercaptoethanol) and aliquots were applied to SDS electrophoresis. After semidry blotting, the blots were probed with the anti–15-LOX antibody.
Cell culture.
Reticulocytosis was induced in rabbits by daily removal of about 30 to 50 mL of blood for a period of 5 days. The hematocrit was checked every day and if it dropped below 20%, bleeding was skipped that day. When the reticulocyte content reached values between 20% to 25% (sixth or seventh day of bleeding), blood was collected and the red blood cells were prepared for immunoelectron microscopy and for the cell fractionation studies. The cellular 15-LOX activity was checked by incubating 0.1 mL of packed cells with 0.1 mmol/L arachidonic acid at 37°C for 15 minutes. After lipid extraction the 15-LOX products (15-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid [15-HETE]) were quantified by reverse phase high performance liquid chromatography (RP-HPLC) (see below).
Human peripheral monocytes were prepared from buffy coats obtained from the local blood bank by density gradient centrifugation and adherence to plastic dishes9 or by elutriation using a Beckman elutriation system (Munich, Germany). The cells were cultured in RPMI 1640 medium containing 10% fetal calf serum in the absence or presence of 670 pmol/L IL-4. After 3 to 4 days in culture, the cells were scraped off, washed twice with isotonic saline, and prepared for immunoelectron microscopy.
Human eosinophils were purified from the blood of patients with a high degree of eosinophilia (more than 10% eosinophilic granulocytes in the differential blood counts). A total of 10 to 40 mL of heparinized blood was withdrawn and the erythrocytes were sedimented through an isotonic dextrane 200 solution. Afterwards the eosinophils were prepared by centrifugation on a discontinuous Percoll density gradient.27 The final cell preparations contained more than 90% eosinophils as indicated by Giemsa staining.
Immunoelectron microscopy.
The subcellular localization of the 15-lipoxygenase in vivo was visualized by preembedding immunoperoxidase staining or by postembedding immunogold labeling. For preembedding stainings, the cells were fixed with Nakane's fixative for 10 minutes at room temperature. After washing in phosphate-buffered saline (PBS), the cells were incubated with the anti–15-LOX antibody for 2 hours at 4°C and subsequently with a peroxidase conjugated mouse antiguinea pig IgG antibody (2 hours at 4°C). Positive reactions were visualized with 3,3′-diaminobenzidine tetrahydrochloride reaction. After immunostaining, the cells were postfixed in 1.3% osmic acid (dissolved in PBS) for 2 hours at room temperature, dehydrated in graded ethanol series, and embedded in Araldite. Ultrathin sections were stained with uranyl acetate/lead citrate. Specificity of the staining was checked using a nonimmune rabbit IgG preparation instead of the anti–15-LOX antibody.
For immunogold stainings, the postembedding technique was used. For this purpose, rabbit reticulocytes and human eosinophils were fixed in Nakane's fixative for 10 minutes at room temperature. After washing in PBS, the cells were dehydrated in graded ethanol series and afterwards embedded in LR White. Ultrathin sections were mounted on nickel grids and were incubated for 2 hours with the anti–15-LOX antibody at 20°C. After washing, the sections were incubated with a gold (particle diameter 20 μm) conjugated mouse antiguinea pig IgG antibody. The sections were then dried and stained with uranyl acetate/lead citrate. Here again, specificity of the staining was checked using a nonimmune rabbit IgG preparation instead of the anti–15-LOX antibody. Examination of the sections stained with both the pre- and post-embedding method was performed with a Zeiss EM9S or a EM412 electron microscope (Oberkochen, Germany).
Subcellular fractionation and immunoblot analysis.
Reticulocyte-rich blood was obtained from rabbits as described above. The cells were spun down and the buffy coat was removed. After washing twice in 5 vol of PBS, the cells were lysed by suspending them in 10 vol of ice-cold water containing dithioerythreitole (1 mmol/L), phenylmethylsulfonyl fluoride (32 mg/L), and variable concentrations of calcium chloride. To achieve complete cell disruption, the suspension was sonicated with a microtip sonifier (Braun, Melsungen, Germany) for 15 seconds at 40 W. The homogenate was divided into two portions. Both were centrifuged for 1 hour at 100,000g. The supernatant and the pellet of the first portion were immediately prepared for immunoblot analysis. The pellet of the second portion was resuspended in the lysis buffer containing the appropriate calcium concentration and the suspension was centrifuged again for 1 hour at 100,000g. Afterwards the pellet (washed membrane pellet) and the supernatant (washing supernatant) of the second centrifugation were prepared for immunoblot analysis. For SDS-electrophoresis, the membrane pellets (unwashed and washed pellets) were dissolved in electrophoresis sample buffer (60 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% mercaptoethanol) and boiled for 2 minutes. The supernatants were dried down in a Speedvac centrifuge, the remaining proteins were dissolved in the electrophoresis sample buffer, the samples were boiled for 2 minutes, and applied to SDS-polyacrylamide gel electrophoresis (PAGE).
Human peripheral monocytes cultured for 3 to 4 days in the presence of 670 pmol/L IL-4 were scraped off the plastic dishes and washed twice with PBS. Cell fractionation and preparation of the samples for electrophoresis was performed as described for the reticulocytes.
After SDS-PAGE, the proteins were transferred to a Immobilon P membrane by a semidry blotting procedure and the blot was incubated with the anti–15-LOX antibody. After extensive washing, the membrane was incubated with a peroxidase-labeled mouse antiguinea pig IgG antibody and the blot was stained with 3,3′-diaminobenzidine. As a reference compound, the pure rabbit reticulocyte enzyme was used.
Lipoxygenase activity assays.
Linoleate oxygenase activity was measured recording the increase in absorbance at 235 nm. The assay mixture consisted of a 0.1 mol/L sodium phosphate buffer, pH 7.4 containing 0.2% sodium cholate and 0.250 mmol/L linoleic acid as substrate. Membrane oxygenase activity of the 15-LOX was assayed oxygraphically using a Gilson oxygraph (Middelton, WI).
Quantification and structural elucidation of the oxygenated polyenoic fatty acids formed during the in vitro and in vivo interaction of the 15-LOX with biomembranes was performed by three sequential steps of HPLC on various types of columns. For the in vitro interaction of the 15-LOX with biomembranes, the following protocol was applied. After the incubation period, the hydroperoxy lipids formed were reduced to the corresponding hydroxy derivatives by the addition of sodium borohydride (Serva). The lipids were extracted,28 hydrolyzed under alkaline conditions, and the resulting free fatty acid derivatives were analyzed by RP-HPLC on a Nucleosil C-18 column (Macherey/Nagel, KS-system, 250 × 4 mm, 5 μm particle size) with a solvent system of methanol/water/acetic acid (85/15/0.1; by vol) and a flow rate of 1 mL/minute. The absorbances at 235 nm (detection of oxygenated fatty acids) and at 210 nm (detection of polyenoic fatty acids) were recorded simultaneously. For further structure elucidation of the oxygenated fatty acids, the compounds comigrating with an authentic standard of 13S-hydroxy-9Z,11E-octadecadienoic acid (13S-HODE) were prepared and further analyzed by straight phase (SP)-HPLC and chiral phase (CP)-HPLC. SP-HPLC, which separates the positional isomers of hydroxy fatty acids, was performed on a Zorbax SIL column (DuPont, Wilmington, DE; 250 × 4.6 mm, 5 μm particle size) with a solvent system of n-hexane/2-propanol/acetic acid (100/2/0.1, by vol) and a flow rate of 1 mL/minute. The enantiomer composition of the hydroxy fatty acids was analyzed by CP-HPLC using a Chiralcel OD column (Z.T. Baker, Deventer, The Netherlands; 250 × 4.6 mm, 5 μm particle size) with a solvent system of hexane/2-propanol/acetic acid (100/5/0.1, by vol) and a flow rate of 1 mL/minute. To quantify the in vivo action of the 15-LOX in rabbit reticulocytes and human eosinophils, the total lipids of the membrane preparations were extracted under reducing conditions (presence of triphenylphosphine) and the extracts were subjected to alkaline hydrolysis. After acidification to pH 3, the hydrolysis mixture was injected to RP-HPLC (see above) and the hydroxy fatty acid/polyenoic fatty acid ratio was calculated as suitable measure for the degree of oxidation of the membrane lipids. For more detailed information on the structure of the oxidized lipids, SP-HPLC and CP-HPLC was also performed.
Calcium concentrations in the incubation mixtures were determined by atomic absorption spectroscopy.
RESULTS
Membrane association of the 15-LOX in vitro.
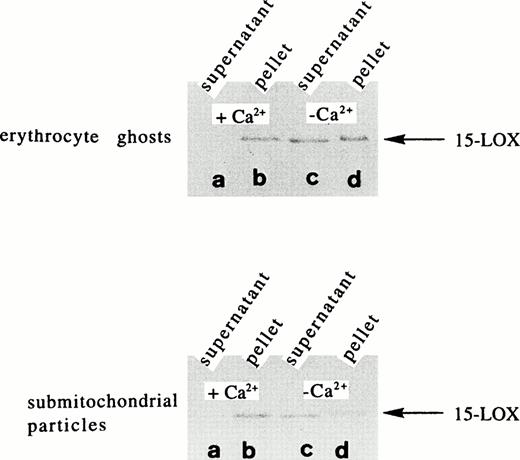
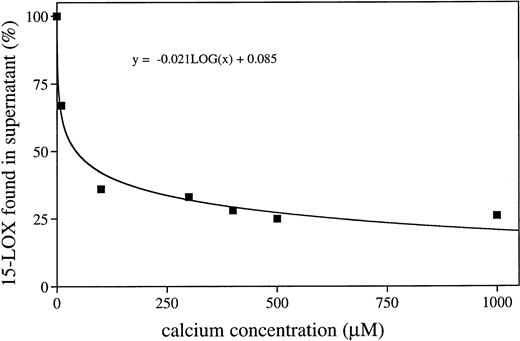
It has been reported2-5 that mammalian 15-LOXs are capable of oxidizing biomembranes in vitro and in vivo suggesting that the enzyme is capable of binding to the membranes. To find out whether calcium is of importance for this membrane binding, we incubated the purified rabbit 15-LOX with submitochondrial membranes (SMP) and erythrocyte ghosts for 5 minutes in the presence and absence of exogenously added calcium. After incubation, the membranes were spun down and aliquots of the 100,000g pellet and of the supernatant were analyzed by immunoblotting. From Fig1, it can be seen that in the presence of 0.5 mmol/L calcium the enzyme was detected mainly in the membrane fraction. Only small amounts of the enzyme (5% to 15%, three different sets of experiments) were recovered in the supernatant. In the absence of exogenously added calcium, the majority of the enzyme was detected in the 100,000g supernatant. Nevertheless, even under these conditions, a considerable share of the enzyme remained membrane bound. This result may be due to the fact that the SMP did contain significant amounts of calcium, which cannot be removed completely, even by washing with EDTA. In fact, when the sample to which no exogenous calcium was added was checked for endogenous calcium, a concentration of 10.4 μmol/L was measured. When the binding assays were performed in the presence of 1 mmol/L EDTA, which complexes the calcium ions, more than 95% of the enzyme was found in the supernatant (not shown). These data suggest that membrane binding in the absence of calcium is minimal and that calcium-dependent membrane association is a graded phenomenon. From Fig2, it can be seen that a gradual increase in the calcium concentration was accompanied by an increase in the share of membrane bound enzyme pool. It appears to be of particular importance that at calcium concentrations already in the lower μmol/L range (10.4 μmol/L), more than 30% of the enzyme was bound to the membranes. Such calcium concentrations may be reached in the cytosol of activated cells and, thus, membrane association of the 15-LOX may be an integral part of cell activation.
Calcium-dependent membrane binding of the rabbit reticulocyte lipoxygenase in vitro. The purified rabbit 15-LOX (30 nanokatals/mL) was incubated for 5 minutes with erythrocyte ghosts (2 mg/mL) and SMP (1 mg/mL) in the presence (0.5 mmol/L) and absence of calcium chloride. Sample work-up, electrophoresis, and immunoblotting are described in Materials and Methods. (A) Supernatant in the presence of calcium, (B) membrane pellet in the presence of calcium, (C) supernatant in the absence of calcium, (D) membrane pellet in the absence of calcium.
Calcium-dependent membrane binding of the rabbit reticulocyte lipoxygenase in vitro. The purified rabbit 15-LOX (30 nanokatals/mL) was incubated for 5 minutes with erythrocyte ghosts (2 mg/mL) and SMP (1 mg/mL) in the presence (0.5 mmol/L) and absence of calcium chloride. Sample work-up, electrophoresis, and immunoblotting are described in Materials and Methods. (A) Supernatant in the presence of calcium, (B) membrane pellet in the presence of calcium, (C) supernatant in the absence of calcium, (D) membrane pellet in the absence of calcium.
Membrane bound share of the 15-LOX depends on the calcium concentration in the incubation mixture. The purified rabbit 15-LOX (30 nkat/mL) was incubated for 5 minutes with SMP (1 mg/mL) in the presence of different calcium concentrations. The membranes were spun down and the 15-LOX was determined in the supernatant by immunoblot analysis. The blots were quantified with a Phoretix 1D Standard program. The LOX content in the control incubation (no membranes) was set at 100%.
Membrane bound share of the 15-LOX depends on the calcium concentration in the incubation mixture. The purified rabbit 15-LOX (30 nkat/mL) was incubated for 5 minutes with SMP (1 mg/mL) in the presence of different calcium concentrations. The membranes were spun down and the 15-LOX was determined in the supernatant by immunoblot analysis. The blots were quantified with a Phoretix 1D Standard program. The LOX content in the control incubation (no membranes) was set at 100%.
Because membrane binding of the enzyme is a prerequisite for oxygenation of the membrane lipids, the membrane oxygenase activity of the 15-LOX in the presence and absence of calcium was assayed (Table1). When submitochondrial particles were incubated with the 15-LOX, an oxygen uptake of about 13 nmol/mg protein was measured. When EDTA was present in the assay mixture, an almost 50% inhibition of the membrane oxygenase activity was observed. This result may also be explained by the fact that small concentrations of calcium were present in the assay buffer. Under the conditions of maximal membrane binding (0.5 mmol/L calcium), the membrane oxygenase activity was strongly stimulated. Similar results were obtained when the formation of oxygenated fatty acids and the disappearance of polyenoic fatty acids were assayed. Interestingly, when the lipids of submitochondrial particles were extracted and used as 15-LOX substrate, there was no stimulation of the oxygenase activity by calcium; even at millimolar concentrations, we did not detect any increase in the oxygen uptake and in the formation of specific LOX products (data not shown).
Calcium Stimulates the Membrane Oxygenase Activity of the Purified Rabbit 15-Lipoxygenase
| Sample . | Lipid Extracts* Oxygen Uptake (nmol O2) . | SMP Oxygen Uptake (nmol O2/mg protein) . | Disappearance of PUFA (nmol/mg protein) . | Formation of Oxygenated PUFA (nmol/mg protein) . |
|---|---|---|---|---|
| SMP | 77.5 | 13.0 | 1.8 | 0.7 |
| SMP + 1 mmol/L EDTA | 73.8 | 7.3 | 1.5 | 0.6 |
| SMP + 1 mmol/L Ca2+ | 76.8 | 81.0 | 12 | 8.2 |
| Sample . | Lipid Extracts* Oxygen Uptake (nmol O2) . | SMP Oxygen Uptake (nmol O2/mg protein) . | Disappearance of PUFA (nmol/mg protein) . | Formation of Oxygenated PUFA (nmol/mg protein) . |
|---|---|---|---|---|
| SMP | 77.5 | 13.0 | 1.8 | 0.7 |
| SMP + 1 mmol/L EDTA | 73.8 | 7.3 | 1.5 | 0.6 |
| SMP + 1 mmol/L Ca2+ | 76.8 | 81.0 | 12 | 8.2 |
SMP were washed in PBS containing 1 mmol/L EDTA. After resuspending the membranes in PBS, aliquots (0.05 mL) of this membrane suspension were added as substrate stock to 1.8 mL assay mixture used as substrate. Oxygen uptake was measured with a Clark-type oxygen electrode. Disappearance of PUFA and formation of oxygenated PUFAs was quantified by RP-HPLC (see Materials and Methods). Similar results were obtained with three different enzyme preparations. A representative experiment is shown.
Abbreviations: SMP, submitochondrial particles; PUFA, polyenoic fatty acid.
For these experiments, the lipids were extracted from SMP and used as LOX substrates. The final concentration of phospholipids in this assay was the same as in the incubation with SMP.
Summarizing these data one may conclude that membrane binding and oxygenation of the membrane lipids by the purified 15-LOX requires calcium and that membrane proteins and/or an intact membrane structure are necessary for the stimulatory effects of calcium.
Besides activation of the membrane oxygenase activity, calcium-mediated membrane binding of the 15-LOX leads to an activation of the free fatty acid oxygenase activity of the enzyme. When the oxygenation of linoleic acid was assayed, we observed a strong increase in the formation of conjugated dienes (13-HPODE) when biomembranes and calcium were present (Table 2). Under the conditions of maximal membrane binding of the enzyme (0.5 mmol/L calcium in the incubation mixture), its linoleic acid oxygenase activity was eightfold increased. Because calcium alone (absence of biomembranes) only slightly augmented the rate of linoleic acid oxygenation, the increase in the linoleic acid oxygenase activity may be due to a calcium-mediated membrane/enzyme interaction. Although the mechanism of this phenomenon has not been investigated, it might be possible that membrane association of the enzyme leads to a better availability of the free fatty acid substrate.
Activation of Fatty Acid Oxygenase Activity of 15-LOX by Membrane Binding
| Sample . | Lipoxygenase Activity ΔE235/min . |
|---|---|
| 15-LOX, no SMP | 0.015 |
| 15-LOX, no SMP + Ca2+ | 0.019 |
| 15-LOX + SMP, no Ca2+ | 0.017* |
| 15-LOX + SMP + Ca2+ | 0.124 |
| Sample . | Lipoxygenase Activity ΔE235/min . |
|---|---|
| 15-LOX, no SMP | 0.015 |
| 15-LOX, no SMP + Ca2+ | 0.019 |
| 15-LOX + SMP, no Ca2+ | 0.017* |
| 15-LOX + SMP + Ca2+ | 0.124 |
The pure 15-LOX (0.5 mg/mL) was preincubated in 0.9 mL 50 mol/L Tris-HCl buffer, pH 7.4 for 3 minutes with the different additives listed in the table. The lipoxygenase reaction was started by the addition of a linoleic stock solution (final substrate concentration 0.26 mmol/L). Calcium was added as calcium chloride stock reaching a final concentration of 0.5 mmol/L. The concentration of SMP used as model membranes was adjusted to 53 μg/mL assay mixture. When the enzyme was incubated with SMP in the absence of linoleic acid (no linoleic acid, control 1) and when linoleic acid was incubated with SMP (no enzyme, control 2), no increase in absorbance at 235 nm was observed. One of two experiments performed with different LOX charges is shown. Both of them gave similar results, but are not strictly comparable.
Presence of 1 mmol/L EDTA.
Intracellular localization of 15-LOX in hematopoietic cells.
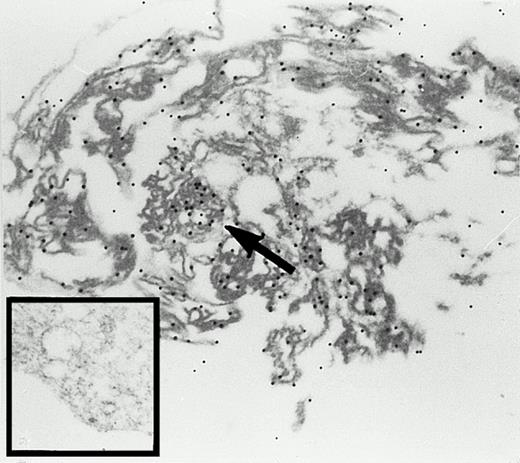
In rabbit reticulocytes the 15-LOX is expressed at a very high level. It has been estimated that 1 mL of packed cells contain about 4 mg of the enzyme.7 Recent cell fractionation studies suggested a membrane association of the enzyme in mature reticulocytes.25 We performed immunogold electron microscopy studies of rabbit reticulocytes (Fig3) and found that the enzyme is localized in the cytosol and at the cytoplasmic side of intracellular membranes. A strong 15-LOX labeling was consistently observed at the mitochondria (arrow). Because mammalian reticulocytes do not contain a nucleous, we could not check a hypothetical localization of the enzyme at the chromatin or at the nuclear envelope.
Immunogold labeling of the 15-lipoxygenase in rabbit reticulocytes. Cells were prepared and stained as described in the Materials and Methods section, 24,300-fold magnification. Inset, staining with a nonimmuno-IgG preparation; 27,100-fold magnification.
Immunogold labeling of the 15-lipoxygenase in rabbit reticulocytes. Cells were prepared and stained as described in the Materials and Methods section, 24,300-fold magnification. Inset, staining with a nonimmuno-IgG preparation; 27,100-fold magnification.
For independent evidence of the intracellular localization of the 15-LOX, cell fractionation studies and immunoblot analyses were performed. When the cells were lysed at different calcium concentrations and the membrane and cytosol fractions were separated by centrifugation, we consistently found the enzyme in both cellular compartments (Fig 4). If the cells were lysed at a calcium concentration of 10−3 mol/L, an almost equal distribution of the enzyme between the membranes and the cytosol were observed (Fig 4, lanes A and B). At lower calcium concentrations, the cytosolic shares were more pronounced (Fig4, lanes E and F, I and K). To check the reversibility of the membrane binding, the membrane pellet was washed at various calcium concentrations. When washing was performed at 10−3mol/L calcium, no enzyme could be removed from the membranes (Fig4, lanes C and D). However, at lower calcium concentrations (10−6 mol/L), small but detectable amounts of the enzyme were washed away (Fig 4, lanes G and H). Decreasing the calcium concentrations to a level which represents the cytosolic calcium concentrations in most resting mammalian cells (Fig 4, lanes L and M), a significant share of the enzyme was removed from the membrane pellet. It must be stressed that the buffer used for the fractionation studies was not completely free of calcium; the endogenous calcium concentration varied between 0.1 and 0.5 μmol/L in the different sets of experiments. Thus, the real calcium concentration for this experiment was in this range. These data suggest that membrane binding of the 15-LOX is at least in part reversible and that the shares of membrane bound and cytosolic 15-LOX may be variable depending on the intracellular calcium concentration. Considering the fact that calcium-mediated membrane binding of the 15-LOX increases both the membrane oxygenase activity and the free fatty acid oxygenase activity of the enzyme, the cytosolic calcium concentration may be regarded as endogenous regulator of the intracellular 15-LOX activity.
Cell fractionation studies of rabbit reticulocytes at various calcium concentrations. Cell preparation, hemolysis, sample work-up, and immunoblotting are described in the Materials and Methods section. (A) Cytosol fraction; (B) membrane pellet; (C) supernatant of washing (washed at 10−3 mol/L calcium); (D) washed membrane pellet (washed at 10−3 mol/L calcium); (E) cytosol fraction; (F) membrane pellet; (G) supernatant of washing (washed at 10−6 mol/L calcium); (H) washed membrane pellet (washed at 10−6 mol/L calcium); (I) cytosol; (K) membrane pellet; (L) supernatant of washing (washed at 5 × 10−8 mol/L calcium); (M) washed membrane pellet (washed at 5 × 10−8 mol/L calcium).
Cell fractionation studies of rabbit reticulocytes at various calcium concentrations. Cell preparation, hemolysis, sample work-up, and immunoblotting are described in the Materials and Methods section. (A) Cytosol fraction; (B) membrane pellet; (C) supernatant of washing (washed at 10−3 mol/L calcium); (D) washed membrane pellet (washed at 10−3 mol/L calcium); (E) cytosol fraction; (F) membrane pellet; (G) supernatant of washing (washed at 10−6 mol/L calcium); (H) washed membrane pellet (washed at 10−6 mol/L calcium); (I) cytosol; (K) membrane pellet; (L) supernatant of washing (washed at 5 × 10−8 mol/L calcium); (M) washed membrane pellet (washed at 5 × 10−8 mol/L calcium).
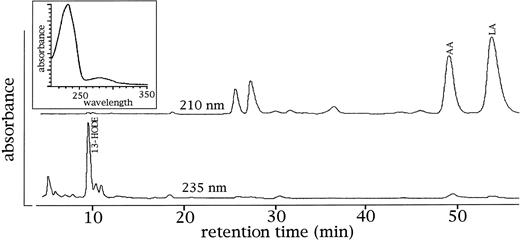
To find out whether the in vivo membrane binding of the 15-LOX is associated with an intracellular action of the enzyme, in vivo activity assays were performed. It has been reported13 that the membranes of rabbit reticulocytes contain specific lipoxygenase products indicating the in vivo activity of the enzyme. We confirmed these data in the present study (Fig 5) and calculated a hydroxy fatty acid/polyenoic fatty acid ratio of the membrane lipids of 0.8% suggesting that in the membranes one of 100 polyenoic fatty acid residues was present as oxygenated derivative. In rabbit erythrocytes, which lack a 15-LOX, this ratio was about 100-fold lower (0.01%). Because an increase in the intracellular calcium concentration was supposed to increase the share of membrane bound enzyme, an augmentation of the oxygenated polyenoic fatty acids in the membrane lipids was expected. To test this hypothesis, the following experiment was performed. Young rabbit reticulocytes (sixth and seventh day of a bleeding anemia) were prepared and divided into three portions. One sample was hemolyzed with 2 vol of water and the membranes were recovered by centrifugation. The other two samples were incubated in PBS (containing calcium and magnesium) in the presence or in the absence of 4 μmol/L calcium ionophore A23187. After 4 hours, the cells were lysed and the membranes were prepared. After extraction of the membrane lipids, transmethylation was performed and the resulting fatty acid methyl esters were analyzed by RP-HPLC. From Table3, it can be seen that incubation of the cells in the absence of A23187 did not lead to an increase in the hydroxy fatty acid content of the membrane. However, if ionophore was present, a 100% increase in the content of lipoxygenase products was observed. These data suggest that an increase in the cytosolic calcium level stimulates the 15-LOX activity on the membrane ester lipids.
15-LOX products occur in the membranes of rabbit reticulocytes. Reticulocyte-rich blood cell suspensions were prepared from a rabbit on the seventh day of the bleeding period. The cells were lysed with two vol of distilled water, the membranes were spun down, and the membrane lipids were extracted. After transmethylation with sodium methoxide, the resulting fatty acid methylesters were analyzed by RP-HPLC with a solvent system methanol/water/acetic acid (85/15/0.1, by vol) and a flow rate of 1 mL/min. Upper trace: recording at 210 nm (detection of polyenoic fatty acids), lower trace: recording at 235 nm (detection of oxygenated polyenoic fatty acids. Each trace was normalized with respect to the highest peak of the partial chromatogram. Inset: ultraviolet (UV)-spectrum of the products comigrating with an authentic standard of 13-HODE. In the membranes of rabbit erythrocytes, which were analyzed as controls, these products were not detected (data not shown). 13-HODE, 13-hydroxy-9Z,11E-octadecadienoic acid; LA, linoleic acid; AA, arachidonic acid.
15-LOX products occur in the membranes of rabbit reticulocytes. Reticulocyte-rich blood cell suspensions were prepared from a rabbit on the seventh day of the bleeding period. The cells were lysed with two vol of distilled water, the membranes were spun down, and the membrane lipids were extracted. After transmethylation with sodium methoxide, the resulting fatty acid methylesters were analyzed by RP-HPLC with a solvent system methanol/water/acetic acid (85/15/0.1, by vol) and a flow rate of 1 mL/min. Upper trace: recording at 210 nm (detection of polyenoic fatty acids), lower trace: recording at 235 nm (detection of oxygenated polyenoic fatty acids. Each trace was normalized with respect to the highest peak of the partial chromatogram. Inset: ultraviolet (UV)-spectrum of the products comigrating with an authentic standard of 13-HODE. In the membranes of rabbit erythrocytes, which were analyzed as controls, these products were not detected (data not shown). 13-HODE, 13-hydroxy-9Z,11E-octadecadienoic acid; LA, linoleic acid; AA, arachidonic acid.
Increased Intracellular Calcium Concentrations Facilitate the Intracellular 15-LOX Activity With Endogenous Substrates
| Incubation Conditions . | No. . | Hydroxy Fatty Acid/Polyenoic Fatty Acid Ratio (%) . | Significance P . |
|---|---|---|---|
| Sample 1 | |||
| No incubation | 4 | 0.80 ± 0.28 | |
| .825* | |||
| Sample 2 | |||
| 4 hours without ionophore | 4 | 0.75 ± 0.30 | |
| .032† | |||
| Sample 3 | |||
| 4 hours with ionophore | 4 | 1.41 ± 0.35 |
| Incubation Conditions . | No. . | Hydroxy Fatty Acid/Polyenoic Fatty Acid Ratio (%) . | Significance P . |
|---|---|---|---|
| Sample 1 | |||
| No incubation | 4 | 0.80 ± 0.28 | |
| .825* | |||
| Sample 2 | |||
| 4 hours without ionophore | 4 | 0.75 ± 0.30 | |
| .032† | |||
| Sample 3 | |||
| 4 hours with ionophore | 4 | 1.41 ± 0.35 |
Rabbit reticulocytes (sixth and seventh day of a bleeding anemia) were prepared and divided into three samples. One sample was hemolyzed with 2 vol of water and the membranes were recovered by centrifugation. The other two samples were incubated in PBS (containing calcium and magnesium) in the presence or in the absence of 4 μmol/L calcium ionophore A23187. After 4 hours, the cells were lysed as described above, the membranes were prepared and the lipids were extracted. Transmethylation of the ester lipids was performed by incubating the lipids with sodium methoxide for 1 hour under argon atmosphere. Aliquots of the transmethylation mixture were subjected to RP-HPLC analysis as described in Materials and Methods. The chromatograms obtained were quantified by peak areas. For calculation of the hydroxy fatty acid/polyenoic fatty acid ratio, the peaks of 13-HODE, linoleic acid, and arachidonic acid (see Fig 4) were quantified.
Comparison of sample 1 and 2.
Comparison of sample 2 and 3. When shorter incubation periods (15 minutes) were used, no significant differences were observed.
Human peripheral monocytes do not express the 15-LOX.9,10However, if the cells are cultured in the presence of IL-4 or IL-13, the enzyme is induced as indicated by immunohistochemical staining, activity assays, and Western blot analysis.8,9 In our hands, human monocytes cultured for 4 days in the presence of IL-4, exhibited a linoleic acid oxygenase activity of 2.2 μg 13S-HODE formation/106 cells. Immunohistochemical staining with an anti–15-LOX antibody indicated the expression of the enzyme in IL-4–treated cells and its absence in untreated monocytes (data not shown). Interestingly, we consistently observed that only a subpopulation (10% to 40%) of the monocytes was stained lipoxygenase positive. The reasons for this inhomogeneity are unclear, but a maturation dependence of the lipoxygenase expression may be assumed. Ultrastructural examinations of peripheral monocytes kept in culture for 4 days in the absence of IL-4 showed typical monocyte characteristics including a round-shaped nucleus and an organized cytoplasm containing small lysosomes (Fig6A). As expected from earlier studies,9 no specific staining with an anti–15-LOX antibody was observed (not shown). In contrast, staining of IL-4–treated monocytes with this antibody indicated a strong positive reaction at the cytosolic side of the cell membrane and at the membranes of intracellular vacuoles (Fig 6B). We did not detect major amounts of the 15-LOX at the nuclear envelope or inside the nucleus. Microscopic inspections of several monocyte preparations led to the impression that there is a decreasing outside-inside gradient of 15-LOX distribution in the cytoplasm of IL-4–treated monocytes. The highest concentration of the enzyme was consistently observed just underneath the plasma membrane, whereas the enzyme was virtually indetectable in the surrounding nucleus.
Intracellular distribution of the 15-lipoxygenase in IL-4–treated monocytes. Cell preparation, immunoperoxidase staining, and electron microscopy are described in Materials and Methods. (A) Control monocytes (no IL-4 treatment) incubated with a nonimmune rabbit IgG preparation; 10,500-fold magnification; (B) IL-4–treated monocyte, 9,200-fold magnification. Here the cells were stained with a polyclonal anti–15-LOX antibody (IgG fraction). Arrows indicate the localization of the enzyme at the membranes of subcellular vesicles. Arrowheads indicate the localization at the cytosolic side of the plasma membrane.
Intracellular distribution of the 15-lipoxygenase in IL-4–treated monocytes. Cell preparation, immunoperoxidase staining, and electron microscopy are described in Materials and Methods. (A) Control monocytes (no IL-4 treatment) incubated with a nonimmune rabbit IgG preparation; 10,500-fold magnification; (B) IL-4–treated monocyte, 9,200-fold magnification. Here the cells were stained with a polyclonal anti–15-LOX antibody (IgG fraction). Arrows indicate the localization of the enzyme at the membranes of subcellular vesicles. Arrowheads indicate the localization at the cytosolic side of the plasma membrane.
Cell fractionation studies at high calcium concentrations (10−6 mol/L) indicated an approximate 1:1 distribution of the 15-LOX between the 100,000g supernatant and the pellet (Fig 7, lanes A and B). Quantification of the immunoblots showed that 42% of the enzyme was localized in the cytosol, whereas 58% was found at the membranes. Lysing the cells at lower calcium concentrations (5 × 10−8 mol/L) led to an increase in the cytosolic share of the 15-LOX (42% at 10−6 mol/L v 63% at 5 × 10-8 mol/L) and to a concomitant drop of the membrane bound share (Fig 7, lanes E and F). Washing the membrane pellets with the lysis buffer at both calcium concentrations (Fig 7, lanes C and D, as well as G and H) removed the enzyme partly from the membranes indicating the reversibility of the membrane binding. These data suggest that modification of the cytosolic calcium concentrations in the range between 10−6mol/L and 5 × 10−8 mol/L may regulate the relative shares of membrane bound and cytosolic 15-LOX. To test this hypothesis in a whole cell system, we attempted to estimate the membrane bound share of the 15-LOX in IL-4–treated monocytes that were incubated for 5 minutes in the presence of the calcium ionophore A23187 by immunoelectron microscopy. Unfortunately, the cells became fragile and did not survive the work-up procedure.
Cell fractionation studies of IL-4–treated monocytes at various calcium concentrations. Cell preparation, sample work-up, and immunoblotting are described in Materials and Methods. Cell lysis was performed at two calcium concentrations (10−6 mol/L for A through D and 5 × 10−8 mol/L for E through H). After lysis, the membranes were spun down at 100,000g. The membrane pellet and the supernatant of the 100,000g centrifugation, which was considered the cytosol, were prepared for electrophoresis as described in the Materials and Methods section. (A) Membrane pellet; (B) cytosol; (C) washing supernatant (washed at 10−6mol/L calcium); (D) washed membrane pellet (washed at 10−6 mol/L calcium); (E) membrane pellet; (F) cytosol; (G) washing supernatant (washed at 5 × 10−8 mol/L calcium); (H) washed membrane pellet (washed at 5 × 10−8 mol/L calcium).
Cell fractionation studies of IL-4–treated monocytes at various calcium concentrations. Cell preparation, sample work-up, and immunoblotting are described in Materials and Methods. Cell lysis was performed at two calcium concentrations (10−6 mol/L for A through D and 5 × 10−8 mol/L for E through H). After lysis, the membranes were spun down at 100,000g. The membrane pellet and the supernatant of the 100,000g centrifugation, which was considered the cytosol, were prepared for electrophoresis as described in the Materials and Methods section. (A) Membrane pellet; (B) cytosol; (C) washing supernatant (washed at 10−6mol/L calcium); (D) washed membrane pellet (washed at 10−6 mol/L calcium); (E) membrane pellet; (F) cytosol; (G) washing supernatant (washed at 5 × 10−8 mol/L calcium); (H) washed membrane pellet (washed at 5 × 10−8 mol/L calcium).
As third cellular system, human eosinophils were used to study the intracellular localization of the 15-LOX. From Fig8A, it can be seen that the enzyme is localized at the cytosolic site of the plasma membrane and in a rather diffuse pattern in the cytoplasm. After stimulation of the cells with calcium ionophore, an increased 15-LOX labeling was found (Fig 8B and C). This increase, which was consistently observed in the eosinophils of different preparations, may be explained by the fact that ionophore leads to an enrichment of the enzyme at the intracellular membranes and thus the antigen is picked up more efficiently by the antibody.
Intracellular localization of the 15-LOX in human eosinophilic granulocytes. Cell preparation and immunogold labeling was performed as described in the Materials and Methods section. (A) Unstimulated eosinophilic granulocyte (12,700-fold magnification); (B) unstimulated eosinophilic granulocyte (30,400-fold magnification); (C) eosinophilic granulocyte stimulated with 4 μmol/L calcium ionophore A 23187 (29,600-fold magnification).
Intracellular localization of the 15-LOX in human eosinophilic granulocytes. Cell preparation and immunogold labeling was performed as described in the Materials and Methods section. (A) Unstimulated eosinophilic granulocyte (12,700-fold magnification); (B) unstimulated eosinophilic granulocyte (30,400-fold magnification); (C) eosinophilic granulocyte stimulated with 4 μmol/L calcium ionophore A 23187 (29,600-fold magnification).
If ionophore treatment increases the membrane bound share of the 15-LOX in eosinophils, it should be possible to detect higher amounts of specific LOX products in the membrane lipids. To check this assumption, we extracted the membrane lipids of human eosinophils treated with and without calcium ionophore, hydrolyzed the lipid extracts and analyzed the resulting free fatty acid derivatives by HPLC. As shown in Fig9, products which comigrate with authentic standards of 15-HETE were detected in RP-HPLC in the membranes of ionophore-treated cells. These products were characterized by a conjugated diene chromophore with an absorbance maximum at 235 nm (Fig9). In contrast, the hydrolyzed lipid extracts obtained from cells that were not treated with ionophore did not contain oxygenated fatty acids. Analysis of the enantiomer composition (Fig 9, inset) showed as strong preponderance of 15S-HETE over its 15R-enantiomer indicating that the products are formed via 15-LOX reaction and not by nonenzymatic lipid peroxidation. These data are consistent with the finding that calcium ionophore may increase the membrane bound share of 15-LOX in human eosinophils.
HPLC analysis of oxygenated lipids present in the cellular membranes of human eosinophilic granulocytes. After preparation (see Materials and Methods), human eosinophils were incubated for 10 minutes in the absence and presence of 4 μmol/L calcium ionophore (extracellular calcium concentration of 2 mmol/L). The cells were spun down, the membrane lipids were extracted, and the extracts were hydrolyzed under alkaline conditions. HPLC analysis of the resulting free fatty acid derivatives was performed as described in the Materials and Methods section. Inset: enantiomer composition of 15-HETE isolated from the membrane lipids determined by CP-HPLC.
HPLC analysis of oxygenated lipids present in the cellular membranes of human eosinophilic granulocytes. After preparation (see Materials and Methods), human eosinophils were incubated for 10 minutes in the absence and presence of 4 μmol/L calcium ionophore (extracellular calcium concentration of 2 mmol/L). The cells were spun down, the membrane lipids were extracted, and the extracts were hydrolyzed under alkaline conditions. HPLC analysis of the resulting free fatty acid derivatives was performed as described in the Materials and Methods section. Inset: enantiomer composition of 15-HETE isolated from the membrane lipids determined by CP-HPLC.
DISCUSSION
Mammalian 15-LOX, which can be prepared from natural and recombinant sources, have been well characterized with respect to their protein chemical, enzymatic,7,29-33 and molecular biologic34,35 properties. In contrast, little is known about the subcellular localization of the enzyme. 15-LOXs have been regarded as cytosolic enzymes because they do not contain any obvious membrane binding structural element and because they can be prepared from the cytosol of rabbit reticulocytes7 and human eosinophils.36 However, recent cell fractionation studies and concomitant activity assays suggested that in mature rabbit reticulocytes, the 15-LOX can also be detected in the membrane fraction.25 The immunoelectron microscopical data presented here indicate that in intact cells, too, a cytosolic and a membrane bound pool of the 15-LOX can be differentiated. Moreover, we found a similar distribution of the enzyme between the cytosol and the membranes for other 15-LOX–expressing cells such as IL-4–treated monocytes and human eosinophils.
Our data on the calcium-dependent membrane association of the 15-LOX in the reconstituted in vitro system suggested that in intact cells the enzyme may behave similarly. It is, however, very difficult to show directly that the 15-LOX is redistributed from the cytosol to the membranes in intact cells when the cytosolic calcium concentration is increased. Neither the immunogold technique nor the immunoperoxidase staining can be quantified exactly. However, there is indirect evidence suggesting a membrane translocation of the enzyme. In freshly prepared eosinophils, we did not detect any 15-LOX products in the membrane lipids. However, when the cells were incubated with ionophore, such products were found. Considering the fact that the enzyme itself is not activated by calcium, one may conclude that the formation of these products is due to an enhanced intracellular binding of the enzyme to the membranes. A similar conclusion may be drawn from the data reported in Table 3 for rabbit reticulocytes.
It is of particular importance for the regulation of the intracellular 15-LOX activity that membrane translocation of the enzyme activates its fatty acid oxygenase activity. Thus, calcium-mediated membrane binding may be regarded as an additional regulatory element of the intracellular LOX activity. Other elements of regulation of cellular 15-LOX activity have also been reported. For instance, human peripheral monocytes do not express the enzyme, but when cultured in the presence of IL-4, large amounts of 15-LOX can be detected.9 However, in these cells, the intracellular activity of the enzyme on endogenous substrates, such as the membrane phospholipids, is rather limited.9 Moreover, we found that in 15-LOX–transfected human promonocytic cells (U937) and in a 15-LOX–transfected rabbit smooth muscle cell line, no 15-LOX products can be detected in the cellular membranes, suggesting that the enzyme, which is expressed in large quantities, may not be active on endogenous ester lipid substrates (unpublished data). Thus, one may conclude that the intracellular activity of 15-LOXs does not necessarily parallel the expression of the enzyme. Most probably, the expressed enzyme requires additional intracellular activation. Although the in vivo activation mechanisms are not clear and may differ from cell to cell, calcium-dependent membrane association may be involved. As indicated in our in vitro studies, membrane binding of the 15-LOX is of dual consequences: (1) it enhances the specific activity with free fatty acids as substrate and (2) it is a necessary precondition for the membrane oxygenase activity. In mature reticulocytes in which the intracellular calcium concentration is in the micromolar range,37 38 a large share of the enzyme is membrane bound and specific lipoxygenase products were detected in the membrane lipids.
It should be stressed that membrane binding alone may not be sufficient for the activation of the intracellular 15-LOX. It has been reported before that the native ferrous 15-LOX is enzymatically silent and requires oxidation to a ferric enzyme to become catalytically active. In vitro studies on various 15-LOX indicated that trace amounts of hydroperoxy lipids are sufficient to initiate the oxidation of the enzyme to the activated ferric form.39,40 Thus, in addition to the intracellular calcium concentrations, the cellular peroxide tone may be regarded as modulator of the cellular 15-LOX activity.11
For the human 5-LOX, it has been reported that the enzyme translocates to the nuclear envelope18,19 when polymorphonuclear leukocytes were stimulated with calcium ionophore A23187. There the enzyme interacts with the five lipoxygenase activating protein and thus becomes activated.20,41,42 In resting rat basophilic leukemia cells and alveolar macrophages, the 5-LOX was detected in the euchromatin region of the nucleus and on ionophore stimulation, it also translocates to the nuclear envelope.21 22 Because of these findings, we specifically examined the nucleus, and in particular, the nuclear envelope of IL-4–treated monocytes for the occurrence of the 15-LOX, but were not able to detect the enzyme there. The same conclusion was drawn when the electron micrographs of eosinophilic granulocytes were evaluated. Even after ionophore stimulation, we did not detect the enzyme at the nuclear envelope. In contrast, our findings suggest a calcium-dependent translocation of the 15-LOX from the cytosol to the cellular and to subcellular membranes. According to our knowledge, the nuclear envelope constitutes a major side of the synthesis of eicosanoids involved in allergy and inflammatory reactions. Because the 15-LOX is not present at the nuclear envelope, it may not be involved in the formation of inflammatory mediators.
As mentioned above, translocation of the 5-LOX to the nuclear envelope requires the special anchoring protein, five lipoxygenase activating protein (FLAP). In contrast, we suppose that such a docking protein may not be implicated in the membrane binding of the 15-LOX because of two experimental findings: (1) In vitro, the 15-LOX binds to various kinds of biomembranes (rat liver and beef heart mitochondrial membranes, rat liver endoplasmic membranes, human inside-out and right side-out erythrocyte ghosts). If a special docking protein were involved in membrane binding of the enzyme, it would have to be present in all types of biomembranes. This, however, appears to be unlikely. (2) To test a specific 15-LOX/docking protein interaction, we performed cross-linking studies. After loading SMPs with 15-LOX in the presence of 1 mmol/L calcium, we attempted to cross-link the 15-LOX with a hypothetical docking protein. However, in immunoblots, we were unable to detect a distinct high molecular weight band that cross-reacted with the anti-15-LOX antibody.
The molecular mechanism of the translocation process of the 15-LOX to biomembranes remains to be investigated. This study suggests that membrane binding is reversible and requires calcium. Preliminary calcium binding studies on the purified 15-LOX indicated that the enzyme is capable of binding calcium (unpublished data). However, databank searches in which the amino acid sequence of the 15-LOX was compared with the sequences of calcium binding proteins did not show typical calcium binding motifs in the primary structure of the 15-LOX. In particular, we found that 15-LOXs lack any obvious calcium-binding domain.43,44 On the other hand, computer-assisted modeling of the three-dimensional structure of the rabbit 15-LOX, which was based on the x-ray coordinates of the soybean enzyme,45 46showed several putative calcium binding structures at the surface of the enzyme. Whether these structures are required for calcium-associated membrane binding is currently under investigation. Because a specific docking protein appears not to be involved in membrane binding of the 15-LOX, calcium ions may bridge unspecifically between negatively charged residues (lipids or proteins) of the membrane and acidic amino acid residues at the surface of the LOX protein.
Supported in part by research grants from Deutsche Forschungsgemeinschaft Ku 961/1-2 (Bonn, Germany).
Address reprint requests to Hartmut Kühn, MD, OSci, Institute for Biochemistry, University Clinics (Charité), Humboldt University, Hessische Str. 3-4, 10115 Berlin, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal