Abstract
Administration of macrophage colony-stimulating factor (M-CSF) to mice (2 to 8 mg/kg/d × 5d) produced dose-dependent thrombocytopenia, which reached its nadir on days 4 to 5, followed by rapid recovery. Surprisingly, when administration of M-CSF was prolonged, the thrombocytopenia completely resolved, despite continued treatment. Splenectomy did not prevent the thrombocytopenia. Readministration of M-CSF after various intervals continued to produce the thrombocytopenic effect, even after 35 days. Measurements of Meg-CFC and megakaryocyte ploidy during the periods of M-CSF treatment and recovery of normal platelet levels showed no evidence of bone marrow suppression. Platelet survival was markedly decreased after 5 days of M-CSF (at the platelet count nadir) and after 9 days of continued M-CSF treatment, when the platelet count had returned to normal. Platelets from M-CSF–treated donors demonstrated normal survival when transfused into normal recipients. We concluded that thrombocytopenia produced by M-CSF was not due to suppression of thrombopoiesis, but to increased activity of the monocyte/macrophage system, which caused shortened platelet survival, and that subsequently, increased platelet production compensated for ongoing platelet destruction and resulted in normal platelet levels.
MACROPHAGE colony-stimulating factor (M-CSF; CSF-1) is essential for the survival, growth, and development of the monocyte/macrophage cell lineage.1-5 Experiments in normal6 and M-CSF–deficient (op/op) mice7,8suggest that M-CSF has a major regulatory role in the development and maintenance of mononuclear phagocytes in liver, spleen, and kidney. Most circulating M-CSF is cleared by binding to its receptor on the macrophages of the liver and spleen, with subsequent endocytosis of the receptor-ligand complex and intracellular degradation.9,10M-CSF has been shown to acutely increase monocyte levels when administered to various animals and man. The magnitude of reported increases has been variable, but dose/response relationships have been demonstrated.5,6,11-14 Some investigations also have reported a return toward normal of elevated monocyte levels with continued M-CSF administration.12,13,15,16 Bone marrow monocyte production has been shown to be increased12; therefore, the decrease in circulating monocytes during prolonged M-CSF administration may be due to increased movement of monocytes into tissues.17 M-CSF administration has been shown to cause increases in the weights of livers and spleens, with both livers and spleens exhibiting increased infiltrates of macrophages.18,19 No consistent changes in the total white blood cell (WBC), lymphocyte, or neutrophil counts are produced by M-CSF, but occasionally increases in these cells are reported.12,15,16 These effects may be secondary to the induction of other cytokines produced by the responding macrophages.20 A puzzling and major adverse effect of M-CSF administration has been the production of thrombocytopenia in all species studied,12,15,16,21,22 which sometimes resolves despite continued M-CSF administration.16,22-24Thrombocytopenia was dose-related, but was not sufficiently severe to cause bleeding. Several reports document a temporal relationship between the nadir of the platelet count and the maximum increase in monocyte counts.12,13,15,20,24 25 In this investigation, we have characterized the thrombocytopenic effect of M-CSF and have sought to determine the mechanism of this unpredicted physiologic result and its resolution despite continued administration of M-CSF.
MATERIALS AND METHODS
General techniques.
Female Swiss Webster (SW) mice, 27 to 30 g, and female C57BL/6N (C57BL) mice, 22 to 25 g, (Simonsen Laboratories, Gilroy, CA) were used for these studies. Uninjected mice from the same shipments were used as normal controls. Mice were housed in an American Association for Accreditation of Laboratory Animal Care approved facility in filter cages and fed standard rodent chow and tap water ad libitum. All experimental protocols were approved by the Committee for Animal Experimentation of the VAMC. In conducting research using animals, the investigators adhered to the “Guide for the Care and Use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (National Academy Press, Washington, DC, 1996). Blood samples were obtained from the retroorbital venous plexus, with the use of 70 μL heparinized EDTA-coated glass capillary tubes (Drummond Scientific Co, Broomall, PA), on the days indicated, immediately before M-CSF injection. Splenectomy was performed under anesthesia with methoxyflurane vapor (Metofane; Pitman-Moore, Inc, Mundelein, IL). Mice were allowed to recover from surgery for at least 1 month before experimentation. Mice were killed by cervical dislocation.
Reagents.
Recombinant human macrophage colony-stimulating factor (M-CSF; CSF-1) was a generous gift from the Cetus and Chiron Corporations, Emeryville, CA. Dilutions were made in pyrogen-free 0.9% saline for injection (Abbott Laboratories, Inc, North Chicago, IL). M-CSF was administered by intraperitoneal (IP) injection twice daily, 8 hours apart, at the doses indicated, in volumes of approximately 0.3 mL, beginning on day 1.
Blood cell counts.
Platelet counts, total WBC counts, and hematocrit values were determined in whole blood diluted 1:2 (vol/vol) in isotonic saline solution (Hematall, Fisher Scientific Co, Pittsburgh, PA) and analyzed with an automated flow cytometric whole blood counter (Technicon H-1 System, Technicon Instruments, Tarrytown, NY), as previously described.26
The small size of rodent white blood cells makes the differentiation between atypical lymphocytes and monocytes imprecise by Wright's stain, the most common method reported. To unambiguously quantify the monocytes, we established a new method based on the receptor for M-CSF, which among cells of the blood is present only on cells of the monocyte/macrophage lineage.27 Differential WBC counts in peripheral blood were determined after incubating buffy coat cells for 1 hour at 4°C with 0.1 mL of supernatant from an M-CSF–producing cell line (Rat2 pAPtag1 MCSF clone C5) (a generous gift of Drs Larry Rohrschneider and Gary Myles, Fred Hutchinson Cancer Research Center, Seattle, WA). The supernatant contained a secreted fusion protein that consisted of alkaline phosphatase fused in frame to amino acids 33-180 of rmM-CSF,28 prepared according to the method of Flanagan and Leder.29 Cells were then cytofuged and fixed for 30 seconds in a solution of 4% paraformaldehyde, 1.4 mmol/L Na2HPO4, 7.3 mmol/L KH2PO4, and 45% acetone, pH 6.6, at 4°C. An Alkaline Phosphatase Substrate Kit IV (BCIP/BNT) (Vector Laboratories, Burlingame, CA) was used to produce a blue precipitated reaction product, that indicated M-CSF binding to its receptor on monocytes. Cells were counterstained using 0.5% neutral red, dehydrated, and mounted. One thousand nucleated blood cells per animal were enumerated to obtain the differential cell count. Lymphocytes and neutrophils were identified by standard morphologic criteria, and monocytes were identified by the presence of a blue precipitate in or on cells.
Proplatelet quantification.
Platelet morphology was quantified as previously described.30 Briefly, blood was obtained by cardiac puncture and anticoagulated with acid-citrate dextrose containing prostaglandin E1, pH 6.7. Platelet-rich plasma (PRP) was prepared by centrifugation. Platelet morphology was observed by phase-contrast microscopy. Differential counts of platelet forms were performed with platelets drifting slowly between the coverslip and an ordinary glass slide using 400x magnification.
Tissue weights.
At the end of selected experiments, livers, spleens, and lungs were removed from normal or M-CSF–treated mice and tissue weights were recorded.
Cell culture.
Soft agar cultures of spleen and bone marrow cells from normal and M-CSF–treated mice were prepared for quantification of granulocyte-macrophage colony-forming cells (GM-CFC) and megakaryocyte colony-forming cells (Meg-CFC), as previously described,31except for the following modifications: 20% horse serum was used instead of fetal calf serum and 0.1 mL (instead of 0.2 mL) of pokeweed mitogen spleen cell conditioned medium was used as the source of growth factors in each 1-mL culture. Control values were bone marrow: GM-CFC, 116 colonies/5 × 104 cells and Meg-CFC, 16 colonies/5 × 104 cells; spleen: GM-CFC, 62 colonies/106 cells, and Meg-CFC, 52 colonies/106 cells.
DNA measurements.
The ploidy distribution (DNA content) of megakaryocytes from the bone marrow of C57BL mice was measured using two-color flow cytometry, as previously described,32 with the following modifications: a FACScan with Lysis II software (Becton-Dickinson, Inc, San Jose, CA) was used for analyses. C57BL mice were used because normal SW mice demonstrate a ploidy distribution that is too variable for precise studies of changes in ploidy (J. Levin, unpublished observation, March 1992).
Platelet survival.
Normal SW mice, or SW mice treated with M-CSF, were used as platelet donors and recipients for these studies. Platelet survival studies were performed as previously described.33 Briefly, platelets pooled from donor mice were fluorescently labeled with 5-chloromethylfluorescein diacetate (CMFDA) (Molecular Probes, Eugene, OR) and injected into the tail veins of recipient mice. After infusion of labeled platelets, blood samples were obtained from the retroorbital venous plexus at 2, 4, and 6 hours and then approximately every 12 hours for the next 4 days. Blood samples were analyzed by flow cytometry, using a FACScan, to determine the proportion of labeled platelets present at each time point. Survival curves were constructed, and the circulating half-life (T1/2) of the labeled platelets was determined graphically. In addition, platelet survival times were determined by using the multiple hit model (gamma function)34,35 and the best fit estimate, derived from the use of both linear and exponential sum of squares calculations.33
Carbon clearance.
The rate of disappearance of carbon particles from the circulating blood of normal or M-CSF–treated mice was measured as previously described.36 Briefly, a solution of India Ink (Difco Laboratories, Detroit, MI) was injected intravenously into either normal or M-CSF–treated mice. Blood samples were obtained at various times from 1 to 11 minutes after injection. Blood was lysed, and duplicate aliquots of each sample were read spectrophotometrically to determine the absorbance at 620 nm. The background values, obtained from lysed blood from mice not injected with carbon particles, were subtracted. Resultant values were used to determine the disappearance rate (T1/2) of carbon particles from the blood.
Levels of M-CSF and anti–M-SF antibodies in serum.
Serum samples were obtained 4 and 8 hours after administration of 4 mg/kg/d M-CSF on days 1, 3, and 5 for determination of circulating M-CSF levels. Samples were stored at −70°C until assayed. Radioimmunoassays were performed in duplicate in a two-step procedure as previously described by Stanley37 with modifications.38 39
For detection of murine anti-human M-CSF antibodies, 4 mg/kg/d M-CSF was administered for 5 days. Serum samples were obtained on days 5, 10, 12, and 15, and stored at −70°C. Samples were assayed using conditions described for the M-CSF radioimmunoassay37 with 10% normal rabbit serum replacing anti-CSF–antiserum.
Statistical analysis.
Statistical analyses were performed with a two-tailed Student'st-test, using StatView (Abacus Concepts, Berkeley, CA).
RESULTS
Effect of M-CSF on platelet levels.
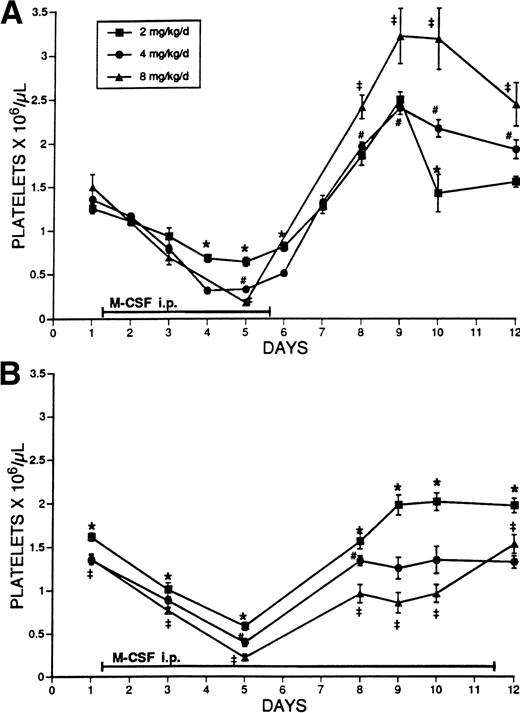
The effect of different doses of M-CSF on the platelet counts of normal mice was examined. M-CSF was administered to SW mice for 5 days, in 2 daily IP injections, at doses of 2, 4, or 8 mg/kg/d. The platelet counts gradually decreased in a dose-dependent manner during the period of administration, reached a nadir on days 4 to 5, and immediately began to increase to above normal levels on discontinuation of treatment (Fig 1A). Blood sampling alone had no effect on the platelet count (data not shown).
(A) The effect of administration for 5 days of different doses of M-CSF on the platelet counts of SW mice. M-CSF was administered to SW mice at the indicated total daily dosages, given in two IP injections, 8 hours apart, for 5 days. On days 1 to 5, platelet counts were obtained immediately before the morning M-CSF injection. The mean platelet count ± 1 SE is shown. The number of determinations at each time point was at least 3, and usually ranged from 5 to 28 (mean, 13). The symbols indicate significant differences between the values (P < .05) (★, 2 mg/kg/d v 4 mg/kg/d; ‡, 2 mg/kg/d v 8 mg/kg/d; #, 4 mg/kg/d v 8 mg/kg/d). (B) The effect of administration for 11 days of different doses of M-CSF on the platelet counts of SW mice. M-CSF was administered to SW mice at the indicated dosages, given in two daily IP injections, 8 hours apart, for 11 days. Platelet counts were obtained immediately before the morning M-CSF injection. The mean platelet count ± 1 SE is shown. The number of determinations ranged from 5 to 21 at each time point (mean, 11). The symbols indicate significant differences between the values (P < .05) (★, 2 mg/kg/d v 4 mg/kg/d; ‡, 2 mg/kg/d v 8 mg/kg/d; #, 4 mg/kg/d v 8 mg/kg/d).
(A) The effect of administration for 5 days of different doses of M-CSF on the platelet counts of SW mice. M-CSF was administered to SW mice at the indicated total daily dosages, given in two IP injections, 8 hours apart, for 5 days. On days 1 to 5, platelet counts were obtained immediately before the morning M-CSF injection. The mean platelet count ± 1 SE is shown. The number of determinations at each time point was at least 3, and usually ranged from 5 to 28 (mean, 13). The symbols indicate significant differences between the values (P < .05) (★, 2 mg/kg/d v 4 mg/kg/d; ‡, 2 mg/kg/d v 8 mg/kg/d; #, 4 mg/kg/d v 8 mg/kg/d). (B) The effect of administration for 11 days of different doses of M-CSF on the platelet counts of SW mice. M-CSF was administered to SW mice at the indicated dosages, given in two daily IP injections, 8 hours apart, for 11 days. Platelet counts were obtained immediately before the morning M-CSF injection. The mean platelet count ± 1 SE is shown. The number of determinations ranged from 5 to 21 at each time point (mean, 11). The symbols indicate significant differences between the values (P < .05) (★, 2 mg/kg/d v 4 mg/kg/d; ‡, 2 mg/kg/d v 8 mg/kg/d; #, 4 mg/kg/d v 8 mg/kg/d).
To further investigate the relationship of dosage and duration of M-CSF administration to thrombocytopenia, 2, 4, or 8 mg/kg/d M-CSF was administered to normal SW mice for a period of 11 days. Platelet levels decreased in a dose-dependent manner up to day 5, after which levels began to increase despite continued administration (Fig 1B). The increase of platelet levels during the latter period of M-CSF administration also was dose-related; the largest dose allowed the least platelet recovery. At these doses, rebound thrombocytosis did not occur during the treatment period. To rule out the production of a neutralizing antibody against M-CSF as a potential cause of refractoriness to M-CSF, serum was obtained on days 5, 10, 12, and 15 from mice that had been treated with M-CSF for 5 days and analyzed for the presence of antibodies to M-CSF. No antibodies against M-CSF were detected after 5 days of M-CSF administration. Although anti–M-CSF antibodies subsequently became detectable 10 days after initiation of M-CSF administration in most animals, the titers were low.
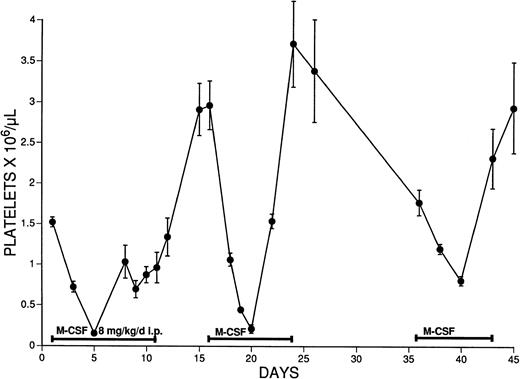
To determine the effect of long-term administration and repeated exposure to M-CSF, mice received a dose of 8 mg/kg/d for 11 days (Fig2). Platelet levels increased after reaching their nadir on day 5, but rebound thrombocytosis did not occur during this first course of treatment until after M-CSF administration was discontinued. Following various lengths of time between repeat challenges of M-CSF, despite the detection of low levels of anti–M-CSF antibodies 10 to 15 days after the initial injection, thrombocytopenia still occurred within 5 days of M-CSF treatment, and rebound thrombocytosis now occurred during administration of M-CSF.
The effect of multiple courses of M-CSF administration on the platelet counts of SW mice. M-CSF, 8 mg/kg/d, was administered to SW mice in two daily IP injections, 8 hours apart. Mice initially received M-CSF for 11 days, were rested for 4 days, and then received M-CSF on days 16 to 24 and 36 to 43. The mean platelet count ± 1 SE is shown, n = 3 to 4.
The effect of multiple courses of M-CSF administration on the platelet counts of SW mice. M-CSF, 8 mg/kg/d, was administered to SW mice in two daily IP injections, 8 hours apart. Mice initially received M-CSF for 11 days, were rested for 4 days, and then received M-CSF on days 16 to 24 and 36 to 43. The mean platelet count ± 1 SE is shown, n = 3 to 4.
Effect of M-CSF on circulating blood cell levels.
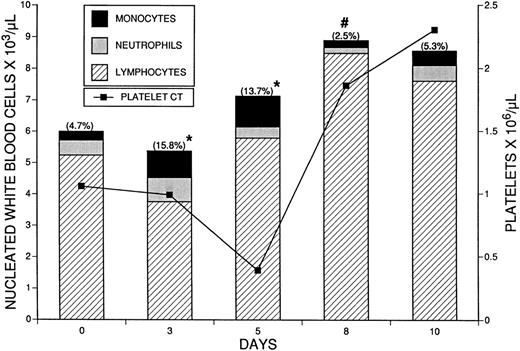
The effect of M-CSF, 4 mg/kg/d, on the blood cell counts of normal mice was documented. Blood samples were obtained on days 3 and 5 of M-CSF treatment, and days 8 and 10, after completion of the 5 days of M-CSF treatment. Total nucleated WBC counts, platelet counts, and hematocrit values were obtained. Differential cell counts were performed on cytocentrifuged buffy coat preparations in which monocytes had been specifically stained (see Materials and Methods) (Fig3), and neutrophils and lymphocytes were identified by standard morphologic characteristics. Total monocytes were significantly increased over control levels of 0.28 ± 0.4 × 103/μL (mean ± 1 standard error [SE]) on days 3 (0.85 ± 0.17 × 103/μL) (P < .05) and 5 (0.99 ± 0.25 × 103/μL) (P < .05) of M-CSF treatment (Fig 4). The platelet count was significantly and maximally decreased from normal levels at the time of maximum absolute monocytosis. The total WBC count was significantly increased only on day 8, from the control level of 6.0 ± 0.3 × 103/μL to 8.9 ± 0.8 × 103/μL (P < .05). The hematocrit fell from an initial value of 52.0% ± 0.7% to a nadir of 44.6% ± 1.3% on day 5 of M-CSF treatment, and then gradually returned to normal.
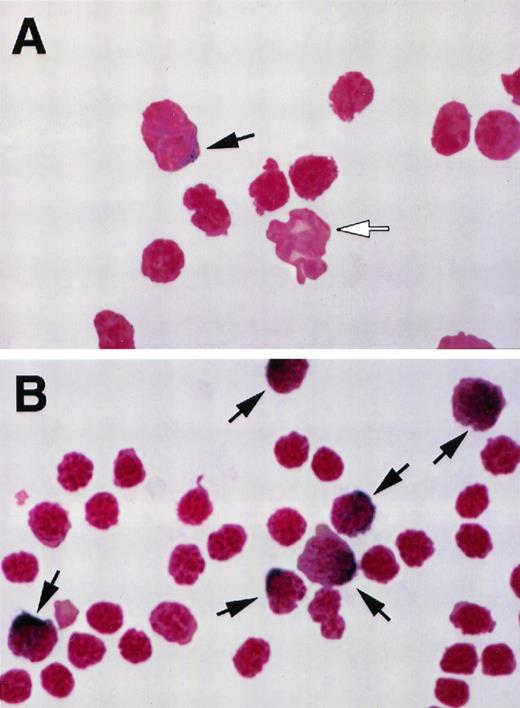
(A) Representative field from a cytospin preparation of buffy coat from a normal mouse, stained as described in Materials and Methods. Black arrow indicates a monocyte identified by the dark blue precipitate. White arrow indicates a neutrophil nucleus (final magnification × 1,000). (B) Representative field from a cytospin preparation of buffy coat from a mouse treated with M-CSF, 4 mg/kg/d, for 4 days. Cells were stained as described in Materials and Methods. Black arrows indicate six monocytes identified by the dark blue precipitate. Note the relative increase in the number of monocytes (final magnification × 1,000).
(A) Representative field from a cytospin preparation of buffy coat from a normal mouse, stained as described in Materials and Methods. Black arrow indicates a monocyte identified by the dark blue precipitate. White arrow indicates a neutrophil nucleus (final magnification × 1,000). (B) Representative field from a cytospin preparation of buffy coat from a mouse treated with M-CSF, 4 mg/kg/d, for 4 days. Cells were stained as described in Materials and Methods. Black arrows indicate six monocytes identified by the dark blue precipitate. Note the relative increase in the number of monocytes (final magnification × 1,000).
The effect of M-CSF on the differential WBC counts of SW mice. M-CSF, 4 mg/kg/d, was administered to SW mice for 5 days, on days 1 to 5. Blood samples were obtained and the total WBC and platelet counts were determined. Percentages of monocytes, neutrophils, and lymphocytes were determined from buffy coat cytofuge preparations. Neutrophils and lymphocytes were identified by standard morphologic characteristics. Monocytes were identified by histochemical staining, using M-CSF conjugated to alkaline phosphatase to bind to the M-CSF receptor. Absolute total numbers of nucleated WBC are indicated by the height of the bars. The total WBC count was significantly increased only on day 8 (#, P < .05). The percentages of monocytes on each day are indicated in parentheses at the top of each bar. On day 3 (n = 6) and day 5 (n = 5), the total numbers of monocytes were significantly different from the control (n = 4) (★, P < .05). On day 8 (n = 6) and day 10 (n = 6), total numbers of monocytes were not different from the control.
The effect of M-CSF on the differential WBC counts of SW mice. M-CSF, 4 mg/kg/d, was administered to SW mice for 5 days, on days 1 to 5. Blood samples were obtained and the total WBC and platelet counts were determined. Percentages of monocytes, neutrophils, and lymphocytes were determined from buffy coat cytofuge preparations. Neutrophils and lymphocytes were identified by standard morphologic characteristics. Monocytes were identified by histochemical staining, using M-CSF conjugated to alkaline phosphatase to bind to the M-CSF receptor. Absolute total numbers of nucleated WBC are indicated by the height of the bars. The total WBC count was significantly increased only on day 8 (#, P < .05). The percentages of monocytes on each day are indicated in parentheses at the top of each bar. On day 3 (n = 6) and day 5 (n = 5), the total numbers of monocytes were significantly different from the control (n = 4) (★, P < .05). On day 8 (n = 6) and day 10 (n = 6), total numbers of monocytes were not different from the control.
Effect of splenectomy on M-CSF–induced thrombocytopenia.
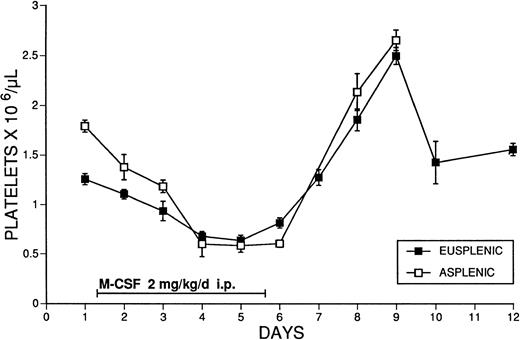
The possible role of splenic platelet sequestration in the production of thrombocytopenia after M-CSF administration was examined. Splenectomized animals were given M-CSF, 2 mg/kg/d, and the platelet counts were compared with those of intact animals treated with the same dosage. Platelet counts fell to equivalent levels in intact and splenectomized SW mice during M-CSF treatment, and splenectomy did not affect either the degree of the thrombocytopenia or subsequent rebound thrombocytosis produced (Fig 5).
The effect of M-CSF administration on the platelet counts of eusplenic and asplenic SW mice. M-CSF, 2 mg/kg/d, was administered for 5 days, in two daily IP injections, 8 hours apart, to normal mice, or mice that had been splenectomized at least 4 weeks previously. Blood samples were obtained immediately before the morning M-CSF injection on days 1 to 5. The mean ± 1 SE is shown. The number of determinations at each time point was at least 3, and usually ranged from 4 to 28 (mean, 12).
The effect of M-CSF administration on the platelet counts of eusplenic and asplenic SW mice. M-CSF, 2 mg/kg/d, was administered for 5 days, in two daily IP injections, 8 hours apart, to normal mice, or mice that had been splenectomized at least 4 weeks previously. Blood samples were obtained immediately before the morning M-CSF injection on days 1 to 5. The mean ± 1 SE is shown. The number of determinations at each time point was at least 3, and usually ranged from 4 to 28 (mean, 12).
Effect of M-CSF on colony-forming cells.
Soft agar cultures were performed to determine the total number of GM-CFC and Meg-CFC in spleen and bone marrow. M-CSF, 2 mg/kg/d, was administered to mice for up to 5 days, and total colony numbers were determined on days 2, 4, 5, 6, and 8. Both GM and Meg colony numbers remained normal on day 2 and then were increased twofold to sixfold over controls in the spleens of M-CSF–treated mice on days 4 to 8. The maximum increase in the spleen occurred on days 5 and 6. However, there were no differences between the total numbers of detectable GM-CFC or Meg-CFC present in the bone marrows of M-CSF–treated and normal mice (data not shown).
Effect of M-CSF on DNA levels in megakaryocytes.
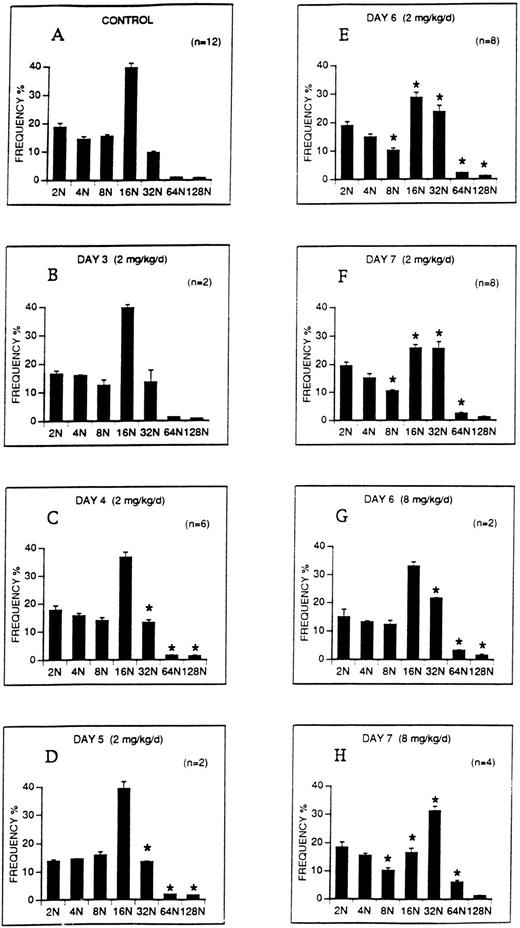
The effect of M-CSF on the ploidy distribution of megakaryocytes in C57BL mice was analyzed. M-CSF, 2 mg/kg/d, was administered on days 1 to 5. DNA content (ploidy) of bone marrow megakaryocytes was analyzed on days 3 to 7 (Fig 6). On days 4 to 7, the proportion of 32N, 64N, and 128N megakaryocytes was significantly increased over control levels (Fig 6C through F). However, although the proportion of 16N megakaryocytes decreased on days 6 and 7 as the higher ploidy megakaryocytes were increasing, 16N megakaryocytes remained the modal class. A higher dose of M-CSF, 8 mg/kg/d, which produced more severe thrombocytopenia, also significantly increased the proportion of 32N, 64N, and 128N classes on days 6 and 7 (Fig 6G through H), and in addition, 32N became the modal class on day 7.
The ploidy distribution of bone marrow megakaryocytes after M-CSF administration. M-CSF was administered at the indicated doses to C57BL mice, starting on day 1, for up to 5 days. Bone marrow was harvested on the morning of the indicated days, before M-CSF administration. DNA levels (ploidy) of megakaryocytes were determined by flow cytometry. Each panel shows the DNA distribution (ploidy class) on the abscissa and the mean frequency ± 1 SE of each ploidy class as a percentage of all megakaryocytes on the ordinate. One million bone marrow cells were analyzed from each animal. The number of determinations at each time point, representing individual animals, is shown on each panel. (A) Illustrates the ploidy distribution of normal C57BL mice. (B through F) Demonstrate the ploidy distributions on the indicated days following administration of 2 mg/kg/d M-CSF. (G through H) Demonstrate the ploidy distributions on days 6 and 7 following the administration of 8 mg/kg/d M-CSF. The asterisks in panels C through H indicate that the frequencies of these ploidy classes were significantly different from control (P < .05).
The ploidy distribution of bone marrow megakaryocytes after M-CSF administration. M-CSF was administered at the indicated doses to C57BL mice, starting on day 1, for up to 5 days. Bone marrow was harvested on the morning of the indicated days, before M-CSF administration. DNA levels (ploidy) of megakaryocytes were determined by flow cytometry. Each panel shows the DNA distribution (ploidy class) on the abscissa and the mean frequency ± 1 SE of each ploidy class as a percentage of all megakaryocytes on the ordinate. One million bone marrow cells were analyzed from each animal. The number of determinations at each time point, representing individual animals, is shown on each panel. (A) Illustrates the ploidy distribution of normal C57BL mice. (B through F) Demonstrate the ploidy distributions on the indicated days following administration of 2 mg/kg/d M-CSF. (G through H) Demonstrate the ploidy distributions on days 6 and 7 following the administration of 8 mg/kg/d M-CSF. The asterisks in panels C through H indicate that the frequencies of these ploidy classes were significantly different from control (P < .05).
Effect of M-CSF on platelet survival.
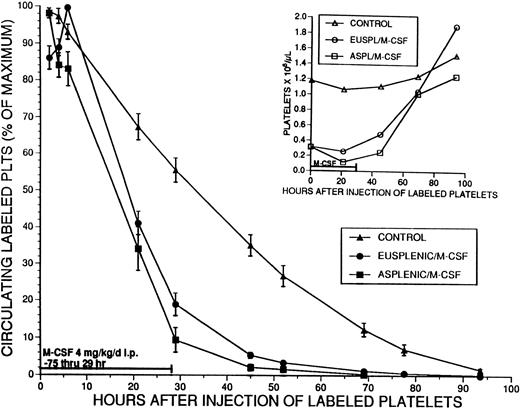
To more thoroughly investigate the possible cause of thrombocytopenia and the role of the spleen, platelet survival in M-CSF–treated eusplenic and splenectomized mice was determined. M-CSF, 4 mg/kg/d, was administered to groups of eusplenic and asplenic SW mice starting on day 1. On day 4, pooled platelets harvested from normal mice and labeled with CMFDA were injected intravenously into the treated mice and a control group. M-CSF injections continued through day 5, and platelet survival was measured for 93 hours after injection of labeled platelets (Fig 7). The circulating half-life (T1/2) of the labeled platelets in the control group was 32.9 ± 2.3 hours, and platelet survival, as measured by the multiple hit model (gamma function) was 2.58 ± 0.18 days. The T1/2 of the labeled platelets was markedly reduced to 19.0 ± 0.7 hours in the eusplenic M-CSF–treated mice, and to 16.3 ± 1.7 hours in the asplenic mice (for both,P < .001 v control). Platelet survival was significantly shorter in M-CSF–treated recipient animals (1.19 ± 0.07 days in eusplenics and 1.06 ± 0.15 days in asplenics [for both, P < .001 v control]). There was no difference between the circulating half-life (T1/2) or platelet survival in eusplenic and asplenic animals. Platelet survival also was examined in animals in which M-CSF, 4 mg/kg/d, was administered for 8 days before the injection of labeled platelets and was continued throughout the experiment. The T1/2 of the labeled platelets in these animals was 26.2 ± 3.1 hours, and the platelet survival was 2.07 ± 0.43 days (P < .01 v control) (data not shown). Similar results were obtained for all these experimental groups when platelet survival estimates also were calculated using the best fit method (data not shown).
Effect of M-CSF on platelet survival. Eusplenic SW mice, or SW mice that had been splenectomized at least 4 weeks previously, were treated with M-CSF, 4 mg/kg/d for 3 days. On the fourth day, M-CSF was administered in the morning, and 3 hours later approximately 2.5 × 108 platelets that had been fluorescently labeled with CMFDA were injected into the tail veins of these mice. A control group of mice that had not received M-CSF also was injected with the same number of platelets. The percent of the maximum number of circulating labeled platelets was serially determined for 93 hours by flow cytometry, and the means ± 1 SE are shown by the closed symbols. The mean T1/2 for platelet survival in the control animals (▴) was 32.9 hours (n = 5). The T1/2 in the eusplenic M-CSF treated animals (•) (19.0 hours; n = 7) and in the asplenic M-CSF–treated animals (▪) (16.3 hours; n = 7) were both significantly shorter than that of the controls (P < .001). The T1/2 values for platelet survival in the 2 groups of M-CSF–treated animals were not significantly different from each other. Serial platelet counts for each group were obtained and are shown with the corresponding open symbols in the inset graph.
Effect of M-CSF on platelet survival. Eusplenic SW mice, or SW mice that had been splenectomized at least 4 weeks previously, were treated with M-CSF, 4 mg/kg/d for 3 days. On the fourth day, M-CSF was administered in the morning, and 3 hours later approximately 2.5 × 108 platelets that had been fluorescently labeled with CMFDA were injected into the tail veins of these mice. A control group of mice that had not received M-CSF also was injected with the same number of platelets. The percent of the maximum number of circulating labeled platelets was serially determined for 93 hours by flow cytometry, and the means ± 1 SE are shown by the closed symbols. The mean T1/2 for platelet survival in the control animals (▴) was 32.9 hours (n = 5). The T1/2 in the eusplenic M-CSF treated animals (•) (19.0 hours; n = 7) and in the asplenic M-CSF–treated animals (▪) (16.3 hours; n = 7) were both significantly shorter than that of the controls (P < .001). The T1/2 values for platelet survival in the 2 groups of M-CSF–treated animals were not significantly different from each other. Serial platelet counts for each group were obtained and are shown with the corresponding open symbols in the inset graph.
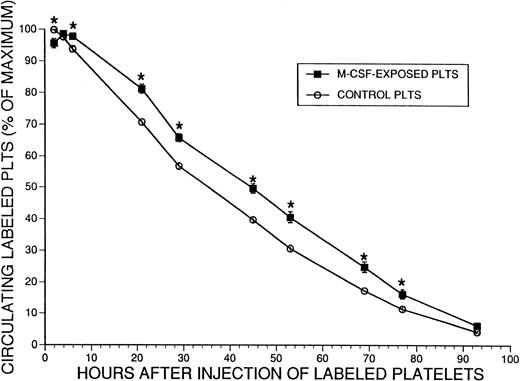
These results indicated faster clearance of platelets from the circulation in mice that had received M-CSF, that the spleen was not the primary mediator of this process, and that with prolonged administration of M-CSF, platelet survival remained significantly shortened, at a time when the platelet counts had returned to normal levels (Fig 1B). However, it was unclear whether the normal donor platelets were modified by exposure to M-CSF present in the circulation of the recipient mice, and thus had demonstrated shortened survival. Therefore, we performed additional platelet survival studies, using platelets from M-CSF–treated donors or normal donors. Donor mice received M-CSF, 4 mg/kg/d, for 2 days. On the morning of the third day, a dose of 2 mg/kg M-CSF was administered, and 2 hours later platelets were harvested from these and a group of untreated mice. Each pool of platelets was labeled with CMFDA and injected into normal mice, and survival of the untreated and M-CSF exposed platelets was compared (Fig8). The M-CSF–exposed donor platelets exhibited a normal or slightly prolonged T1/2 in normal mice in comparison to untreated platelets, indicating that the M-CSF–treated platelets had not been altered by exposure to M-CSF in a manner that rendered them more susceptible to clearance.
Survival of normal or M-CSF-exposed CMFDA labeled platelets in normal SW mice. SW mice received M-CSF, 4 mg/kg/d, administered in two daily IP injections, 8 hours apart. Two hours after the fifth dose (on the third morning), platelets were harvested from these animals and from additional nontreated control animals. The two pools of platelets were then fluorescently labeled with CMFDA and approximately 2.5 × 108 platelets were injected into the tail veins of two groups of normal recipient mice (n = 9 for the control group and 10 for the M-CSF group). The percent of the maximum number of circulating labeled platelets was serially determined for 93 hours by flow cytometry. The mean ± 1 SE is shown for each time point. The mean T1/2 for control platelet survival was 35.3 ± 0.9 hours ( ± 1 SE) and for M-CSF–exposed platelet survival was 45.0 ± 1.4 hours ( ± 1 SE) (P < .0001). The asterisks indicate a significant difference in the percent of circulating labeled platelets at each indicated time point (P < .05).
Survival of normal or M-CSF-exposed CMFDA labeled platelets in normal SW mice. SW mice received M-CSF, 4 mg/kg/d, administered in two daily IP injections, 8 hours apart. Two hours after the fifth dose (on the third morning), platelets were harvested from these animals and from additional nontreated control animals. The two pools of platelets were then fluorescently labeled with CMFDA and approximately 2.5 × 108 platelets were injected into the tail veins of two groups of normal recipient mice (n = 9 for the control group and 10 for the M-CSF group). The percent of the maximum number of circulating labeled platelets was serially determined for 93 hours by flow cytometry. The mean ± 1 SE is shown for each time point. The mean T1/2 for control platelet survival was 35.3 ± 0.9 hours ( ± 1 SE) and for M-CSF–exposed platelet survival was 45.0 ± 1.4 hours ( ± 1 SE) (P < .0001). The asterisks indicate a significant difference in the percent of circulating labeled platelets at each indicated time point (P < .05).
Effect of M-CSF on the monocyte/macrophage (reticuloendothelial) system.
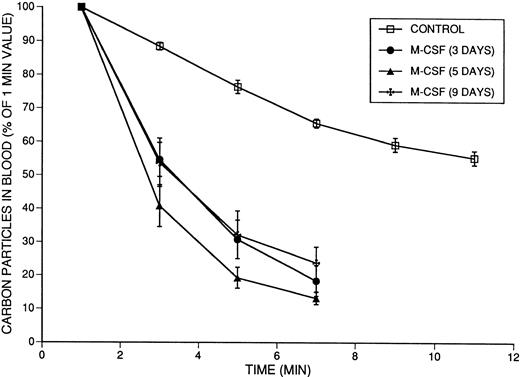
To evaluate the effect of M-CSF on the monocyte/macrophage system, carbon clearance experiments were performed in mice that had been treated with M-CSF, 4 mg/kg/d, for various lengths of time. Absorbance (at 620 nm) due to the presence of carbon particles was monitored beginning at 1 minute after bolus injection to ensure homogeneous intravascular distribution of particles. Clearance of intravenously injected India Ink particles was significantly faster in mice that had been treated with M-CSF for 3, 5, or 9 days (Fig9). The T1/2 of the carbon particles in mice treated for 3 days was 3.7 ± 0.4 minutes; for 5 days, 2.8 ± 0.2 minutes; and for 9 days, 3.9 ± 0.5 minutes, compared with the control value of 12.0 ± 0.7 minutes (for all,P < .0001). Furthermore, the measured 1-minute absorbance values (A620) were 1.091 for the control, 0.717 for the 3-day group, 0.566 for the 5-day group, and 0.637 for the day 9 group, indicating that there was greater clearance of carbon particles during the first minute in M-CSF–treated mice than in controls. Carbon clearance remained faster than normal in mice treated for 9 days with M-CSF, indicating that the monocyte/macrophage system remained hyperactive despite recovery of the platelet count.
The effect of 3, 5, or 9 days of M-CSF administration on carbon particle clearance in SW mice. Mice were injected with 4 mg/kg/d M-CSF, administered in two daily IP injections, 8 hours apart, starting on day 1. Mice received the final injection of M-CSF on the morning of the indicated day, approximately 2 hours before the injection of the carbon particles on day 3, 5, or 9. The mean absolute absorbance of lysed blood at 620 nm at 1 minute was 1.091 (n = 12) for the control animals, 0.717 (n = 6) for mice treated with 3 days, 0.566 (n = 6) for mice treated with 5 days, and 0.637 (n = 6) for mice treated with 9 days of M-CSF. These values were designated as 100%, and subsequent results were calculated as a percentage of the 1-minute values. The means ± 1 SE are shown. The T1/2 values obtained after 3 days (3.7 ± 0.4 minutes), 5 days (2.8 ± 0.2 minutes), and 9 days (3.9 ± 0.5 minutes) of M-CSF treatment were significantly shorter (faster clearance) than the T1/2 in the control mice (12.0 ± 0.7 minutes) (P < .0001), but were not significantly different from each other.
The effect of 3, 5, or 9 days of M-CSF administration on carbon particle clearance in SW mice. Mice were injected with 4 mg/kg/d M-CSF, administered in two daily IP injections, 8 hours apart, starting on day 1. Mice received the final injection of M-CSF on the morning of the indicated day, approximately 2 hours before the injection of the carbon particles on day 3, 5, or 9. The mean absolute absorbance of lysed blood at 620 nm at 1 minute was 1.091 (n = 12) for the control animals, 0.717 (n = 6) for mice treated with 3 days, 0.566 (n = 6) for mice treated with 5 days, and 0.637 (n = 6) for mice treated with 9 days of M-CSF. These values were designated as 100%, and subsequent results were calculated as a percentage of the 1-minute values. The means ± 1 SE are shown. The T1/2 values obtained after 3 days (3.7 ± 0.4 minutes), 5 days (2.8 ± 0.2 minutes), and 9 days (3.9 ± 0.5 minutes) of M-CSF treatment were significantly shorter (faster clearance) than the T1/2 in the control mice (12.0 ± 0.7 minutes) (P < .0001), but were not significantly different from each other.
Effect of M-CSF on the liver, spleen and lung weights.
In selected experiments, livers, spleens, and lungs were removed from M-CSF–treated animals after sacrifice. After administration of M–CSF, 4 mg/kg/d for 5 days, the livers and spleens of treated mice were significantly larger than those of the control animals. On day 5, liver weights were increased to 2.0 g (n = 14), from the control value of 1.3 g (n = 19) (P < .001), but by day 9, they were 1.4 g, almost normal. Spleen weights were increased to 250 mg on day 5 (n = 14), in contrast to the control value of 136 mg (n = 19) (P < .001) and remained increased (208 mg) on day 9 (n = 9). Total lung weights were not significantly increased on day 5 or day 9 from the control value of 157 mg (n = 8). After 9 days of administration of the same dose of M-CSF, liver weights were comparably increased to 2.0 g (n = 4), and spleen weights were increased further to 289 mg (n = 4).
Effect of M-CSF on proplatelet formation.
Proplatelet formation was quantified as a potential indicator of stimulation of platelet production. M–CSF, 4 mg/kg/d, was administered for up to 5 days, and platelet differentials were performed on days 3, 5, and 9. At these times, when platelet levels were either falling (day 3), at their nadir (day 5), or increasing (day 9), the proportion of proplatelets was not increased (Table 1). However, the absolute numbers of proplatelets circulating on day 5 were significantly decreased from control levels, and conversely, were significantly increased on day 9 (Table 1).
Platelet Morphology After Administration of M-CSF
| Platelet Form . | Control (n = 8) . | Day 3 (n = 3) . | Day 5 (n = 3) . | Day 9 (n = 3) . |
|---|---|---|---|---|
| Discoid (%) | 91.6 ± 1.1 | 89.3 ± 1.3 | 94.0 ± 1.4 | 91.7 ± 0.7 |
| Elongated (%) | 6.9 ± 1.0 | 9.4 ± 1.3 | 4.5 ± 1.3 | 6.9 ± 0.9 |
| Beaded (%) | 1.6 ± 0.4 | 1.2 ± 0.1 | 1.6 ± 0.7 | 1.5 ± 0.1 |
| Total percent nondiscoid (proplatelets) | 8.5 ± 1.1 | 10.6 ± 1.3 | 6.1 ± 1.4 | 8.4 ± 0.7 |
| Proplatelets × 10−3/μL blood | 102.1 ± 13.0 | 63.0 ± 10.1 | 11.1 ± 5.0* | 199.8 ± 9.7* |
| Platelet count × 10−6/μL blood | 1.208 ± 0.028 | 0.587 ± 0.029 | 0.160 ± 0.055 | 2.413 ± 0.093 |
| Platelet Form . | Control (n = 8) . | Day 3 (n = 3) . | Day 5 (n = 3) . | Day 9 (n = 3) . |
|---|---|---|---|---|
| Discoid (%) | 91.6 ± 1.1 | 89.3 ± 1.3 | 94.0 ± 1.4 | 91.7 ± 0.7 |
| Elongated (%) | 6.9 ± 1.0 | 9.4 ± 1.3 | 4.5 ± 1.3 | 6.9 ± 0.9 |
| Beaded (%) | 1.6 ± 0.4 | 1.2 ± 0.1 | 1.6 ± 0.7 | 1.5 ± 0.1 |
| Total percent nondiscoid (proplatelets) | 8.5 ± 1.1 | 10.6 ± 1.3 | 6.1 ± 1.4 | 8.4 ± 0.7 |
| Proplatelets × 10−3/μL blood | 102.1 ± 13.0 | 63.0 ± 10.1 | 11.1 ± 5.0* | 199.8 ± 9.7* |
| Platelet count × 10−6/μL blood | 1.208 ± 0.028 | 0.587 ± 0.029 | 0.160 ± 0.055 | 2.413 ± 0.093 |
Mice were injected with M-CSF, 4 mg/kg/d, on days 1 to 5. On days 3, 5, and 9, blood was obtained by cardiac puncture using ACDE1 as anticoagulant. PRP was prepared by centrifugation, and the platelets immediately examined at 400× magnification using phase-contrast microscopy. Platelets were grouped according to Tong, Seth, and Penington30 into the following different classes of morphologic forms by phase-contrast microscopy: (1) discoid; (2) elongated-length three times greater than the width; or (3) beaded forms showing two or more well-formed cytoplasmic masses separated by slender cytoplasmic threads. The mean percentages ± 1 SE of each morphologic form are shown. n = the number of individual mice examined. Platelets examined per animal: 390 ± 21 (mean ± 1 SE); range, 186-562. There were no significant differences in the frequency of the various forms of platelets between the control group and any M-CSF treated group.
Indicates that the absolute concentrations of circulating proplatelets were significantly different from control, P < .05.
DISCUSSION
Our studies have confirmed previous reports that M-CSF can produce thrombocytopenia.11,12,15,16,21,22 In addition, we have attempted to define the mechanisms of the production of thrombocytopenia by M-CSF and of the recovery of platelet levels during its continued administration. Treatment with M-CSF for 5 days produced dose-dependent thrombocytopenia, with rapid recovery of normal platelet levels and subsequent development of rebound thrombocytosis occurring after discontinuation of treatment. However, in experiments in which M-CSF was administered over a longer period, platelet levels surprisingly began to recover after 5 days, despite continued administration of M-CSF, strongly suggesting that thrombocytopenia was not the result of suppression of platelet production. Additional evidence for this hypothesis was that the ploidy distribution of megakaryocytes in the bone marrow remained essentially normal during the period of falling platelet levels, which reached their nadir on days 4 to 5. In contrast, in a model of bone marrow damage, produced by administration of 5-fluorouracil (5-FU) to mice, the proportion of higher ploidy megakaryocytes decreased initially, with 8N becoming the modal class on day 4.40 After M-CSF, we did not observe a decrease in the proportion of 16N and 32N megakaryocytes, but rather observed a slight right shift in ploidy. No changes were observed in the total GM-CFC and Meg-CFC in the bone marrow following M-CSF, in contrast to the marked increases observed during recovery from bone marrow damage produced by 5-FU.41 The rapidity of the recovery to normal or above normal platelet levels after termination of M-CSF treatment also argues against bone marrow suppression.
Additionally, we saw evidence of delayed stimulation of platelet production. The increased frequency of high ploidy megakaryocytes on days 4 to 7 is consistent with previous observations that ploidy levels shift upward in response to different degrees of thrombocytopenia produced by peripheral destruction of platelets, and that maximal changes in ploidy occur 48 to 72 hours after the stimulus of thrombocytopenia, preceding the recovery of normal platelet levels.42 The delayed increase in splenic Meg-CFC (and GM-CFC) is also consistent with the previously reported response of normal murine hematopoiesis to the stimulus of peripheral platelet destruction.31 43 Preliminary data have indicated that the mean platelet volume is increased after 3 or 5 days of M-CSF administration, consistent with stimulation of thrombopoiesis. The occurrence of rebound thrombocytosis during recovery of platelet levels, as well as the less severe nadir of thrombocytopenia observed after repeated exposure to M-CSF are consistent with the development of an expanded pool of megakaryocytes that resulted from this stimulation.
There was a gradual decrease in maximum observed M-CSF levels during the treatment period; however, M-CSF remained detectable after 5 days of administration, and no anti-M–CSF antibodies were present at this time. Clearance rates increased after repeated exposure to M-CSF, in agreement with previous reports.11,13,14,44 These observations are consistent with an increased monocyte/macrophage population and the proposed mechanism of monocyte/macrophage-mediated clearance of M-CSF.11 However, M-CSF remains efficacious after continued administration. Increased macrophage activity was still present after prolonged administration of M-CSF, as evidenced by our platelet survival and carbon clearance data. The ability to repeatedly produce thrombocytopenia during multiple courses of M-CSF administration further indicated that development of neutralizing antibody against M-CSF was not responsible for the phenomenon of recovery of platelet levels during short-term M-CSF administration.
The most likely cause for the thrombocytopenia appeared to be either peripheral destruction or platelet sequestration. We have documented a maximum increase in the level of circulating monocytes after M-CSF administration at the time of the platelet nadir, an inverse relationship that has been previously reported.12,13,25Evidence for increased removal of platelets from the circulation is the observation that platelet survival was decreased in M-CSF–treated mice. The role of the spleen in the sequestration or destruction of platelets in this model, though, is minimal or absent, because asplenic animals exhibited the same degree of thrombocytopenia as eusplenic mice and equivalently shortened platelet survival after M-CSF administration. However, in humans, M-CSF administration resulted in shortened platelet survival with marked platelet uptake occurring in the spleen.25 Because platelet survival remained shortened during prolonged M-CSF administration, at a time when platelet levels were increasing, it appeared that increased platelet production, stimulated by thrombocytopenia, had compensated for the increased platelet clearance. Exposure of platelets to M-CSF did not appear to produce a platelet defect, because platelets obtained from M-CSF–treated donors did not exhibit shorter survival than nonexposed platelets prepared in the same manner, after transfusion into normal mice.
M-CSF treatment increased the size of the liver and spleen in treated animals, a common result of stimulation of the monocyte/macrophage system, which may have contributed to the observed thrombocytopenia. Our detection of increased rates of carbon particle clearance after M-CSF administration is consistent with a mechanism for the development of thrombocytopenia that includes the production of activated macrophages. There is precedence for a nonimmune process of platelet removal by macrophages. In the reactive hemophagocytic syndrome, activated macrophages (designated histiocytes) have been shown to phagocytose blood cells, including platelets.45,46Interestingly, M-CSF levels are markedly increased in at least some patients with this syndrome.47 Following administration of M-CSF, macrophages from bone marrow aspirates in patients25and circulating macrophage-like cells in rabbits15 have been reported to demonstrate phagocytosis of platelets. We attempted to determine if Kupffer cells were responsible for the clearance of platelets, by injecting mice with carrageenan, because these cells can be selectively blocked with carrageenan.10 However, this reagent cannot be used to evaluate the role of Kupffer cell activity in production of thrombocytopenia, because we confirmed that carrageenan itself causes acute, severe thrombocytopenia.48
Administration of other cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF),49,50G-CSF,51,52 interleukin (IL)-1,53,54IL-2,55,56 and stem cell factor (SCF)57,58 also has been shown to produce dose-dependent thrombocytopenia, which has been reported in some studies to resolve during continued administration,50,51,54,57 indicating that this is a phenomenon not unique to M-CSF. Although the mechanism(s) of thrombocytopenia associated with these other cytokines has not been established, descriptions of normal bone marrow function after cytokine treatment,49,51,55,59 data suggesting peripheral destruction of platelets,49-51,55 and reports of large platelets or increased mean platelet volume (MPV) at the platelet count nadir51,59 suggest that suppression of megakaryocytopoiesis is probably not the mechanism of the thrombocytopenia. In a single dog, GM-CSF–induced thrombocytopenia was not prevented by splenectomy, similar to our finding in mice, but thrombocytopenia persisted for 4 to 5 weeks after discontinuation of treatment,50 in contrast to our results in mice. However, the thrombocytopenia produced by IL-1 administration to mice was prevented by splenectomy,53 suggesting a different role for the spleen in that model.
We have concluded that thrombocytopenia produced by M-CSF was due to increased activity of the monocyte/macrophage system and that increased removal of platelets from the circulation, primarily due to phagocytosis by the liver (and perhaps bone marrow) macrophages, occurred as a result of some as yet unidentified nonimmune process. The return to normal platelet levels despite continued administration of M-CSF suggested that increased platelet production, stimulated by thrombocytopenia, was able to compensate for the increased rate of removal of platelets from the circulation. Further experiments are needed to determine whether M-CSF–induced thrombocytopenia can be prevented in normal or bone marrow damaged individuals by prior or concurrent administration of a stimulator of thrombopoiesis, such as thrombopoietin.60 61 This might eliminate the dose-limiting toxicity of M-CSF and permit further investigation of its clinical potential.
ACKNOWLEDGMENT
The authors thank Dr Jolanda Schreuers (formerly of Chiron Corp) for helpful discussions and assistance in making these studies possible, and Dr E. Richard Stanley, Albert Einstein College of Medicine, whose laboratory determined the M-CSF levels and anti-M–CSF antibody titers.
Supported in part by US Army Medical Research, Development, Acquisition, and Logistics Command, Fort Detrick, MD, Research Contract MIPR No. MM4585HL7. Also supported in part by the Department of Veterans Affairs, Washington, DC.
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Army.
Address reprint requests to Jack Levin, MD, Veterans Administration Medical Center (111 H2), 4150 Clement St, San Francisco, CA 94121.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal