Abstract
The product of the SCL gene is a basic helix-loop-helix (bHLH) transcription factor that is essential for the development of hematopoietic stem cells in both the embryo and the adult. However, once the stem cell compartment is established, the function of SCL in subsequent differentiation and commitment events within normal hematopoietic cells remains undefined. The aim of the current study was to investigate this role using purified normal human hematopoietic CD34+ cells. An SCL retrovirus was used to transduce CD34+ cells isolated from human bone marrow, peripheral blood, and umbilical cord blood. Enforced expression of SCL increased by a median of twofold the number of erythroid colonies, with an increase in both colony size and the rate of hemoglobinization. Unexpectedly, enforced expression in CD34+ cells also significantly increased the number of megakaryocyte colonies, but with no impact on the size of colonies. There was no consistent effect on the number nor size of granulocyte-macrophage (GM) colonies. The proliferative effect of enforced SCL expression on erythroid cells was attributed to a shortened cell cycle time; the self-renewal capacity of erythroid or GM progenitors was unchanged, as was survival of cells within colonies. These results demonstrate a role for SCL in determining erythroid and megakaryocyte differentiation from normal human hematopoietic CD34+ cells.
HEMATOPOIESIS requires the differentiation of a pluripotent stem cell into at least 8 distinct cell lineages representing diverse functional phenotypes; however, the mechanisms involved in regulation of hematopoietic differentiation are poorly understood. The basic helix-loop-helix (bHLH) family of transcription factors plays a critical role in regulating differentiation of many cell types.1,2 MyoD for instance, is a master regulator gene3 that plays an important role in the control of mammalian myogenesis,4,5 and NeuroD plays a similar role in neuronal differentiation.6 However, the role of bHLH proteins in regulating hematopoietic stem cell differentiation is unclear.
The SCL (also known as TAL-1) proto-oncogene encodes a member of the bHLH family that is predominantly expressed in hematopoietic cells.7 Aberrant expression of SCL is implicated in the development of 20% to 60% of cases of T-cell acute lymphoblastic leukemia (T-ALL),8-12 and SCL is oncogenic when expressed in T lymphocytes.13-16 Within the hematopoietic compartment, SCL is normally present in cells of the erythroid, mast, and megakaryocyte (MK) cell lineages, in some early myeloid cell lines, and in committed (CD34+/CD38+) progenitor cells.7,17-22 A crucial role for SCL has been demonstrated in the development of hematopoietic stem cells: mice homozygous for a null mutation of the SCL gene die in utero at embryonic day 9 due to failure of primitive hematopoiesis.23,24 In addition, studies using SCL null embryonic stem (ES) cells also demonstrate an essential role for SCL in the development of hematopoietic stem cells in the adult.25 26
The contribution of SCL to normal hematopoietic differentiation once the hematopoietic stem cell has been established has not been investigated. However, SCL expression during erythroid and macrophage differentiation in a number of hematopoietic cell lines suggests that, in addition to its essential role in hematopoietic stem cell development, SCL may be an important regulator of normal hematopoietic differentiation. SCL mRNA and protein levels increase throughout erythroid differentiation, with a late decrease in SCL protein levels.17,19,27-29 Conversely, levels progressively decrease during myeloid differentiation.30-33 These results are supported by studies demonstrating enforced expression of SCL increases the rate of erythroid differentiation,27,33 but perturbs monocytic and granulocytic differentiation.31-34
The aim of the current study was to investigate the function of SCL in normal hematopoiesis using primary human CD34+ cells, a population of cells representing a range of cell types from most primitive to committed progenitor cells. We show that enforced expression of SCL results in enhanced differentiation of these hematopoietic stem cells towards the erythroid and MK lineages.
MATERIALS AND METHODS
Construction of the LNC(SCL) retrovirus.
The LNC(SCL) retroviral vector was constructed by cloning a 1.88-kbHindIII-Xba I fragment from human SCL cDNA7containing the full-length coding region into the Hpa I site of the LNCX vector.35 This retrovirus expressed SCL from an internal cytomegalovirus (CMV) promoter and the viral LTR controlled expression of the neomycin phosphotransferase (NeoR) gene, conferring resistance of infected cells to G418.35 A high-titer amphotropic producer cell line was generated by infection of PA317 cells with viral supernatant from GP+E-86 cells electroporated with LNC(SCL). A high-titer clonal cell line was identified using RNA slot blot analysis36 and standard NIH3T3 assay.37 NIH3T3 assay was used to routinely monitor the titer of the retroviruses. SCL mRNA expression and protein production from the LNC(SCL) retrovirus within PA317 cells was confirmed by Northern blot and Western blot analysis, respectively (data not shown). The control cell line used throughout these studies was PA317/LNL6, a cell line that produces a retrovirus expressing the NeoR gene alone.38 39 Stocks of equivalent titer of both the PA317/LNL6 (4.4 × 106 ± 2 × 106infectious units [IU]/ ml, n=6 NIH3T3 assays) and PA317 / LNC(SCL) (3.1 × 106 ± 1.5 × 106 IU/mL, n = 6) cell lines were established and frozen. In each series of experiments, retroviral preparations were confirmed to be of comparable titer. These preparations were then used for a maximum of 4 to 6 weeks when comparable titer was again confirmed. To further ensure that experiments were comparable, if the retroviral transduction efficiency of CD34+ cells was greater than or equal to twofold discordant between LNC(SCL) and the controls (LNL6-infected CD34+ cells), the results were disregarded.
CD34+ cell selection and retroviral transduction.
Clinical samples were bone marrow (BM) or peripheral blood progenitor cells (PBPC) mobilized by granulocyte colony-stimulating factor (G-CSF) alone or G-CSF in combination with stem cell factor (SCF) using clinical protocols previously described40,41: there was no difference between BM-derived and PBPC-derived CD34+cells in response to enforced expression of SCL (data not shown). Mononuclear cells (MNC) were isolated by centrifugation on Ficoll density gradient, and cells were selected based on expression of CD34, a surface glycoprotein expressed on developmentally early hematopoietic stem and progenitor cells (for review see Krause et al,42Huang and Terstappen,43 Schmitt et al,44 Murray et al,45 and Graf and Torok-Storb46). The CD34+ cells were isolated using the Miltenyi MiniMacs Immunomagnetic Separation system and the CD34+progenitor cell isolation kit (Becton Dickinson, Knoxfield, Victoria, Australia). These cell preparations were confirmed ≥95% pure CD34+ cells by reanalysis, as previously described.40
CD34+-purified cells (2.5 × 105 to 5 × 105 cells) were cocultivated for 3 days (37°C in 5% CO2) in 60-mm dishes (Falcon; Becton Dickinson, Lincoln Park, NJ) with 106 irradiated (3,000 rad) PA317/LNL6 or PA317/LNC(SCL) retrovirus producer cells using previously optimized conditions.40 Retroviral transductions were performed in 5 mL Iscove's modified Dulbecco's medium (IMDM)/10% FCS in the presence of human interleukin-3 (huIL-3; 100 ng/mL; unless otherwise stated, all cytokines used throughout this study were purified recombinant human proteins provided by Agen, Thousand Oaks, CA), recombinant murine (r mu) Flt3/Flk-2 ligand (FL; 100 ng/mL; produced as previously described47 48), and 4 μg/mL polybrene (Sigma, Gymea, New South Wales, Australia). For all experiments, mock cocultivations were performed using the packaging cell line PA317. After retroviral transduction, nonadherent cells were collected and efficiency of infection determined by progenitor cell assay in the presence of 1.5 mg/mL active concentration G418 (Life Technologies, Melbourne, Australia). This concentration was selected based on titrations of G418 on human CD34+ cells (data not shown). In all experiments, mock-transduced CD34+ cells were assayed in the presence of G418 and confirmed effective killing of all cells that did not express the NeoR gene. Retroviral transductions using LNL6 and LNC(SCL) were performed in parallel and under identical conditions. Within each experiment, the titers of the retroviruses were equivalent.
Expression of retroviral SCL mRNA in G418-resistant (G418R) colonies (both granulocyte-macrophage [GM] and erythroid) was confirmed by performing reverse transcriptase-polymerase chain reaction (RT-PCR) on mRNA extracted from separate pools of 100 G418R GM and 100 G418R erythroid colonies. mRNA was extracted using Trizol (Life Technologies), samples were DNase-treated (Boehringer Mannheim, Castle Hill, New South Wales, Australia), and oligo(dT)-and random-primed cDNA was synthesized from the total RNA in 60 μL. Ten microliters of each sample was PCR-amplified with primers to amplify endogenous human SCL (forward CAAGTAAGAGGCTGGAGTTG and reverse GTGATGTCGAGGAGTTGAAG). To detect retrovirally expressed SCL cDNA sequences, the oligonucleotide primer pair consisted of the above-noted forward primer for human SCL and an antisense primer complementary to a sequence within the provirus (CTAGCTTGCCAAACCTACAG). Thirty-five cycles of PCR amplification were performed in 50 μL reaction mixtures and 30 μL of the product was separated by agarose gel electrophoresis, transferred to HybondN+ (Amersham), and hybridized with an internal SCL oligonucleotide (GCCTATGTTGATCCATCCAG). Relative mRNA abundance was estimated by densitometric analysis using a Stratagene Eagle Eye II video system and the Stratagene (La Jolla, CA) ZERO-Dscan software. In control experiments, endogenous SCL expression was detected in GM and erythroid colonies. Within the limits of this PCR technique, retroviral SCL sequences were detected in GM and erythroid colonies at levels up to 22-fold (mean, 9-fold; n = 6 patient samples) above that observed for endogenous SCL in these colonies.
Progenitor cell assays.
Progenitor cells were grown in agar culture prepared by adding 1 vol of double-strength AIMDM and 1 vol of pretested FCS to 2 vol of 0.6% agar (Difco Laboratories, Detroit, MI) in a final volume of 1 mL.49 Cultures were stimulated by granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 ng/mL), G-CSF (500 U/mL), and SCF (100 ng/mL) for growth of GM colony-forming cells (GM-CFC) and eosinophil progenitor cells (Eo-CFC). Colonies were stimulated with GM-CSF (100 ng/mL), IL-3 (100 ng/mL), IL-6 (100 ng/mL), SCF (100 ng/mL), and erythropoietin (EPO; 2 U/mL) for erythroid progenitors (burst-forming unit-erythroid [BFU-E]), as previously described.41 Cultures were established using 1 × 103 cells/mL in the absence of G418. Because of the interpatient variability in both the transduction and cloning efficiencies (see below), in the presence of G418, a range of CD34+ cell concentrations (from 1 × 103to 5 × 104 cells/mL) was always examined. To accurately quantitate progenitor cells, multiple replicate cultures were examined; results shown for colonies grown in G418 are the mean of a minimum of nine replicate cultures. All other results are the mean of triplicate cultures. Cultures were incubated in a fully humidified atmosphere of 5% CO2 in air at 37°C for 14 days. Replicate cultures were scored for colonies (>40 cells) after 14 days using a dissection microscope at 35× magnification. Throughout this study, hematopoietic colonies derived from LNL6- or LNC(SCL)-transduced CD34+ cells will be referred to as LNL6 or SCL colonies, respectively.
Retroviral transduction efficiency, as determined by the percentage of G418R colonies after culture in agar, was a median of 9% (range, 1% to 16%; n = 13). Consistent with our previous findings,40a wide interpatient variation in transduction efficiency was observed. A broad interpatient variation was also apparent in the frequency of clonogenic cells within the CD34+ cell population (see Results). Only patient samples were included in which a minimum of 20 G418R colonies/104 CD34+ cells cultured were observed after transduction with LNC(SCL).
Megakaryocyte cultures.
MK colonies were stimulated and identified in agar culture as previously described.50 Briefly, cultures were established using 0.3% agar, IMDM, 25% FCS, and 1 × 103CD34+ cells/mL in the absence of G418 and 1 × 103 and 3 × 103 CD34+cells/mL in the presence of G418 (1.5 mg/mL). Cultures were stimulated with G-CSF (500 U/mL), GM-CSF (100 ng/mL), SCF (100 ng/mL), IL-3 (100 ng/mL), IL-6 (100 ng/mL), EPO (2 U/mL), and pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF; 200 ng/mL). After incubation for 10 days at 37°C in 5% CO2 in air, cultures were dehydrated and MK identified using an alkaline phosphatase anti-alkaline phosphatase method of staining whole agar cultures in situ. MK colonies were scored using an inverted microscope (×100) and were defined as containing 3 or more cells.
Morphology of myeloid colonies.
Agar cultures stimulated with GM-CSF (100 ng/mL), G-CSF (500 U/mL), and SCF (100 ng/mL) were fixed at day 14 in 2.5% (vol/vol) glutaraldehyde (TAAB Laboratories, Reading, UK) in phosphate-buffered saline (PBS) and stained with Luxol-Fast blue to identify eosinophils.51Colonies were scored under light microscope (×100).
Colony size and hemoglobinization.
To determine the mean colony size for erythroid and GM colonies, 30 to 50 colonies were plucked from agar culture into 150 μL PBS, the number of cells was counted, and the result was divided by the number of colonies pooled. Cell counts were performed after culture in agar for 7, 10, and 14 days.
Hemoglobinization of erythroid colonies in situ was determined by staining with 2,7-diaminofluorene (DAF) as previously described.52 Results presented are the number of DAF-positive colonies expressed as a percentage of the total number of colonies present in EPO-containing cultures in the presence of G418 (1.5 mg/mL) for 7, 10, and 14 days. To allow for the variable cloning and transduction efficiencies, cultures were established using a range of CD34+ cell numbers (1 × 103 to 5 × 104 CD34+ cells/mL).
Isolation and retroviral transduction of human cord blood (CB) CD34+ cells.
Ficoll-separated/red blood cell-lysed human umbilical CB cells were enriched for CD34+ cells by immunomagnetic negative selection using a cocktail of iron-conjugated lineage antibodies and the StemSep device as described by the manufacturer (Stem Cell Technologies Inc, Vancouver, British Columbia, Canada). In preparation for retroviral infection, CD34-enriched/lineage-depleted cells were resuspended in IMDM supplemented with 2.5% FCS, IL-3 (50 ng/mL), IL-6 (20 ng/mL), SCF (50 ng/mL), FL (50 ng/mL; Immunex Corp, Seattle, WA), and G-CSF (20 ng/mL) and prestimulated for 16 hours on 35-mm petri dishes precoated with the C-terminal fibronectin fragment, CH-296 (supplied by Takara Shuzo Co Ltd, Otsu, Japan). After prestimulation, virus-containing medium from a confluent T75 cm2 flask of either PA317/LNL6 or PA317/LNC(SCL) retroviral producer cells supplemented with the aforementioned cytokines was used to infect the CD34-enriched/lineage-depleted cells at 12-hour intervals for 48 hours. Cells were then collected and used to establish methylcellulose cultures as previously described53 using 1,000 and 333 CD34+ cells/mL in the presence or absence of 1.5 mg/mL G418. Colonies were scored on day 14 using a dissection microscope (magnification ×35), and morphology of colonies was determined by May-Grünwald Giemsa staining of cytospin preparations.
Recloning experiments.
In recloning experiments, approximately 100 randomly selected G418R erythroid and GM colonies derived from LNL6- or LNC(SCL)-transduced cells were isolated, resuspended, and recloned into agar culture in the same stimuli (without G418) as the original cultures. The number of secondary clones (>15 cells) and colonies (>50 cells) was enumerated after a further 14 days.
Analysis of cell death.
To determine the number of dying cells in colonies derived from retrovirally transduced cells after 7, 10, and 14 days in agar culture, 40 G418R erythroid colonies were plucked from agar into 150 μL PBS and a single cell suspension was prepared. Cells were then examined either by eosin uptake or propidium iodide (PI) staining. The ability of cells to exclude eosin was compared with the total number of cells as quantitated by staining with crystal violet as previously described.49 For experiments performed using PI, 1 μL of PI (stock 100 μg/mL in PBS; Sigma) was added to 100 μL containing cells from the pooled colonies. Twenty microliters was then dropped onto a slide, coverslipped, and examined with an oil immersion lens and a light microscope containing a UV lamp. Fluorescently stained, nonviable cells were quantitated under UV light and compared with the total number of cells observed under phase contrast. Using both protocols, the proportion of dying cells per G418R colony was determined.
Statistical analyses.
Unless stated otherwise, all statistical analyses were performed using the Student's t-test.
RESULTS
Enforced expression of SCL increased both the number and size of erythroid colonies.
Purified CD34+ cells from adult BM and blood were incubated with irradiated cells producing a retrovirus containing the full-length human SCL cDNA [LNC(SCL) retrovirus]. In control experiments, CD34+ cells were transduced with the LNL6 retrovirus, and CD34+ cells were mock-transduced by incubation with PA317 cells for 3 days. There was no difference between groups in the number of cells recovered at the end of this 3-day period (130% to 150% cell recovery in 9 experiments), at which time agar cultures were established.
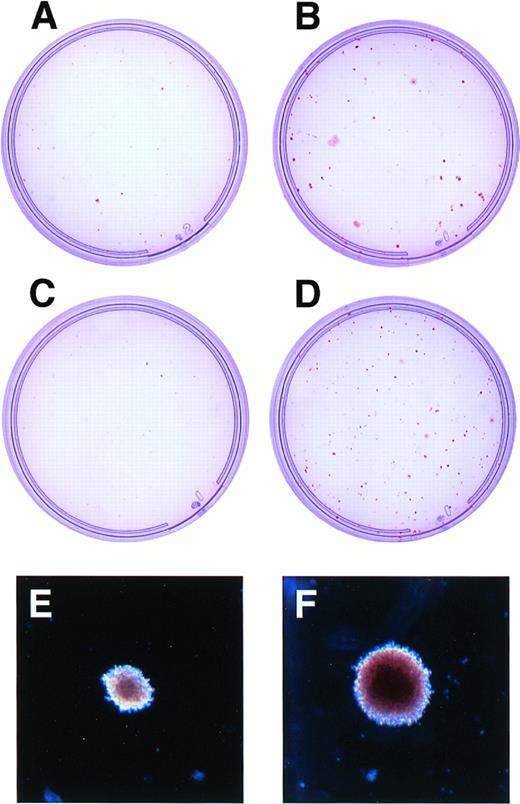
There was a median 1.5-fold increase in the absolute number of clonogenic cells after transduction with the LNC(SCL) retrovirus when compared with the mock-transduced and LNL6-control infected cells after agar culture for 14 days (P < .005, paired t-test, n = 13 samples). This increased frequency of clonogenic cells was due to increased numbers of erythroid colonies and was often evident macroscopically (Fig 1A and B). The increase in erythroid colony number was also evident with and without applying G418 selection (Fig 1C and D). This indicated that the differences observed were not solely due to more efficient expression of the NeoR gene in erythroid progenitor cells transduced with the LNC(SCL)-retrovirus compared with those transduced with LNL6. In addition to the striking effects on erythroid colony number, there was also an increase in the size of erythroid colonies (Fig 1E and F and see below). The enhanced erythroid colony formation, in the absence of G418, is consistent with studies demonstrating that the transduction efficiency of CD34+ cells is substantially greater than that assessed by colony formation in the presence of G418.40,54,55 We have previously reported PCR on DNA extracted from individual GM and erythroid colonies as an independent indicator of transduction efficiency40: NeoR gene was detected in 39%, 68%, and 79% of colonies grown in cultures without G418 (total of 90 colonies examined). In comparison, gene transfer, as assessed by colony formation in the presence of G418, was much lower, at 0.1%, 7%, and 3%, respectively. An explanation to account for such a difference has not yet been elucidated.55
Growth of erythroid colonies in agar culture after transduction of human CD34+ cells with LNL6 or LNC(SCL). (A) through (D) show agar culture plates of LNL6-transduced (A and C) or LNC(SCL)-transduced (B and D) CD34+ cells stimulated for erythroid colonies in the presence (A and B) or absence (C and D) of G418. CD34+ cells were cultured at 1,000 cells/mL in the absence of G418 (C and D) and at 104 cells/mL in the presence of G418 (A and B). Photographs were taken at day 13 of culture in agar. (E) and (F) show morphology of individual erythroid colonies (original magnification ×40) grown in the presence of G418 after transduction of CD34+ cells with LNL6 (E) or LNC(SCL) (F). The control erythroid colony shown in (E) was the largest colony in the culture, whereas that in (F) represents a typical erythroid colony. Colonies were photographed at day 13.
Growth of erythroid colonies in agar culture after transduction of human CD34+ cells with LNL6 or LNC(SCL). (A) through (D) show agar culture plates of LNL6-transduced (A and C) or LNC(SCL)-transduced (B and D) CD34+ cells stimulated for erythroid colonies in the presence (A and B) or absence (C and D) of G418. CD34+ cells were cultured at 1,000 cells/mL in the absence of G418 (C and D) and at 104 cells/mL in the presence of G418 (A and B). Photographs were taken at day 13 of culture in agar. (E) and (F) show morphology of individual erythroid colonies (original magnification ×40) grown in the presence of G418 after transduction of CD34+ cells with LNL6 (E) or LNC(SCL) (F). The control erythroid colony shown in (E) was the largest colony in the culture, whereas that in (F) represents a typical erythroid colony. Colonies were photographed at day 13.
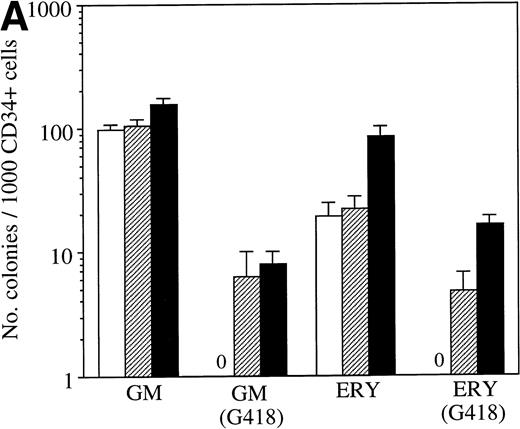
Figure 2A shows a typical experiment with the number of erythroid and GM colonies derived from LNC(SCL)-infected versus control CD34+ cells. In this experiment there was a fourfold increase in the total number of erythroid colonies in the presence or absence of G418. The increase in erythroid colonies was consistently observed and was a median of twofold in 13 experiments. The increase in erythroid colony formation therefore accounted for the increase in absolute number of colonies generated (Fig 2B). As discussed below, there was no consistent change in numbers of GM colonies. Despite the heterogeneity between different patients, analysis of the results from 13 patient samples showed a significant enhancement of erythroid colony formation as a result of enforced expression of SCL (Fig 2C, P < .001, paired t-test).
(A) Typical experiment showing the number of progenitor cells after retroviral transduction of CD34+ cells with PA317 alone (□), LNL6 (▨), or LNC(SCL) (▪). GM and erythroid colonies in the presence and absence of G418 (1.5 mg/mL active concentration) are shown. Agar cultures were established using 1,000 CD34+ cells and GM and erythroid colonies were quantitated in independent cultures using the appropriate growth factor combinations. Data points are the mean ± SD of triplicate cultures (in absence of G418) or 9-replicate cultures (in presence of G418). (B) Total number of G418R colonies per 1 × 104CD34+ cells for 5 representative patient samples. Cultures were established in the presence of G418 (1.5 mg/mL) using a range of cell concentrations (1 × 103 to 5 × 104 CD34+ cells) after transduction with LNL6 (N) or LNC(SCL) (S). (▪) The number of GM colonies; (▨) the number of erythroid colonies. GM and erythroid colonies were quantitated in independent cultures. Each bar represents the mean from 9-replicate cultures. (C) The proportion of erythroid colonies grown in agar culture as a function of the total number of colonies obtained after the transduction of CD34+ cells with LNL6 or LNC(SCL). Each point represents an individual patient sample.
(A) Typical experiment showing the number of progenitor cells after retroviral transduction of CD34+ cells with PA317 alone (□), LNL6 (▨), or LNC(SCL) (▪). GM and erythroid colonies in the presence and absence of G418 (1.5 mg/mL active concentration) are shown. Agar cultures were established using 1,000 CD34+ cells and GM and erythroid colonies were quantitated in independent cultures using the appropriate growth factor combinations. Data points are the mean ± SD of triplicate cultures (in absence of G418) or 9-replicate cultures (in presence of G418). (B) Total number of G418R colonies per 1 × 104CD34+ cells for 5 representative patient samples. Cultures were established in the presence of G418 (1.5 mg/mL) using a range of cell concentrations (1 × 103 to 5 × 104 CD34+ cells) after transduction with LNL6 (N) or LNC(SCL) (S). (▪) The number of GM colonies; (▨) the number of erythroid colonies. GM and erythroid colonies were quantitated in independent cultures. Each bar represents the mean from 9-replicate cultures. (C) The proportion of erythroid colonies grown in agar culture as a function of the total number of colonies obtained after the transduction of CD34+ cells with LNL6 or LNC(SCL). Each point represents an individual patient sample.
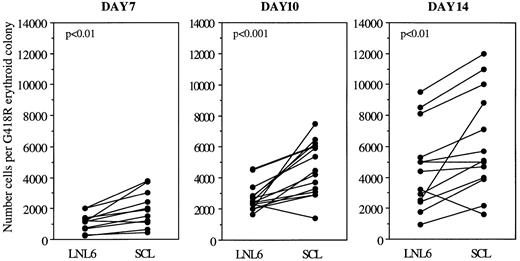
As well as an increase in erythroid colony number, enforced SCL expression resulted in increased size of erythroid colonies. This was quantitated by documenting the number of cells per G418R colony (Fig 3). After 7 days in agar culture, erythroid colonies were selected based on their typical colony morphology rather than on their red appearance. However, at days 10 and 14, erythroid colonies were clearly hemoglobinized. Erythroid colonies derived from LNC(SCL)-infected cells (SCL-erythroid colonies) were up to 3.8 times (median, 1.6-fold) larger than colonies from LNL6-transduced control cells at all three timepoints. The increase in colony size was most apparent at days 7 and 10, at which the median fold increase was 1.8 (P < .01) and 1.6 (P < .001). The increase at day 14 was 1.3-fold (P < .01).
Number of cells per G418R erythroid colony after transduction of CD34+ cells with LNL6 or LNC(SCL). Each point represents an individual patient sample. The mean colony size for each patient sample was determined by pooling 30 to 50 colonies in 150 μL PBS, the number of cells was counted, and the result was divided by the number of colonies pooled. Cell counts were performed after culture in agar for 7 (n = 11 experiments), 10 (n = 15 experiments), and 14 days (n = 13 experiments). Statistics were performed using the paired t-test.
Number of cells per G418R erythroid colony after transduction of CD34+ cells with LNL6 or LNC(SCL). Each point represents an individual patient sample. The mean colony size for each patient sample was determined by pooling 30 to 50 colonies in 150 μL PBS, the number of cells was counted, and the result was divided by the number of colonies pooled. Cell counts were performed after culture in agar for 7 (n = 11 experiments), 10 (n = 15 experiments), and 14 days (n = 13 experiments). Statistics were performed using the paired t-test.
Enforced expression of SCL enhanced hemoglobinization of erythroid colonies.
While examining these cultures, it appeared that SCL-erythroid colonies were hemoglobinized earlier than control colonies. To quantitate this more objectively, colonies were stained with DAF. After 7 days in agar culture, approximately half the control, LNL6 colonies assessed as erythroid based on typical colony morphology were stained positively with DAF. Consistent with the earlier hemoglobinization of SCL-erythroid colonies, DAF staining corresponded well with the colony morphology of SCL-erythroid colonies (data not shown). However, to overcome any investigator bias, results are presented as a percentage of the total number of colonies observed. Approximately 70 colonies were evaluated at each time point for each sample. After 7 days in culture, 45% of all SCL colonies stained positive for DAF. This finding compared with 20% of LNL6 colonies (n = 9 experiments,P < .001). Similarly, at day 10 of agar culture, 78% of all SCL colonies stained positive for DAF, compared with 52% of LNL6 colonies (n = 11 experiments, P < .001). At this timepoint, all colonies of typical erythroid morphology in SCL cultures were DAF positive. This was not the case in control cultures. As expected, at day 14 of culture, all colonies of typical erythroid morphology were visibly hemoglobinized and DAF positive. This represented 88% of all the SCL colonies grown under conditions favoring erythroid growth and 72% of LNL6 colonies (n = 7 experiments, P < .001).
There was no evidence for SCL-induced growth factor independence of hematopoietic cells; agar cultures established in the absence of cytokines did not result in hematopoietic colonies and the erythroid colonies formed in response to enforced expression of SCL remained absolutely EPO-dependent (data not shown). Together, these results demonstrated that enforced expression of the SCL gene in freshly isolated human CD34+ cells increased the number of erythroid colonies and the number of cells present within these colonies and hastened the hemoglobinization of colonies. Thus, this documented an action for SCL to enhance clonogenicity, proliferation, and differentiation within the adult erythroid compartment.
Enhanced erythroid colony formation in CD34+cells derived from CB.
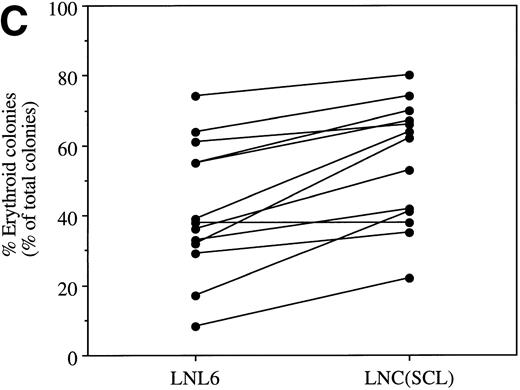
To evaluate the role of SCL in fetal hematopoietic cells, CD34+ cells were isolated from human CB and transduced with LNL6 or LNC(SCL) retroviruses (Table 1). For all 3 CB samples, the size of the erythroid colonies was greater after LNC(SCL) transduction compared with LNL6. In two of three CB experiments, an increase in the number of SCL erythroid colonies was observed. Consistent with the results described above, hemoglobinization was almost twofold greater after enforced expression of SCL compared with NeoR alone. In this case, the difference in hemoglobinization at day 7 existed despite no increase in the number of erythroid colonies at day 14. As with PBPC- and BM-derived CD34+ cells, and somewhat surprisingly, the total number and morphology of myeloid colonies was unchanged. Therefore, CB-derived CD34+ cells behaved in a similar manner to CD34+ cells from adult blood and BM. In addition to the different cellular source, the similar effect of enforced SCL expression was despite the different methodology used to isolate and transduce the CD34+ cells. Thus, enforced SCL expression enhanced erythroid colony formation in cells from both adult and fetal tissues.
Growth of Progenitor Cells in Methylcellulose After Retroviral Transduction of CD34+ Cells Isolated From Human Cord Blood
| Experiment No. . | Cell Source . | Percentage of G418R Progenitors . | BFU-E Ratio (S/N) . | No. of Cells per BFU-E (ratio S/N) . | % DAF+ Colonies-150 . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BFU-E . | GM . | G . | M . | Mix . | |||||||||||
| N . | S . | N . | S . | N . | S . | N . | S . | N . | S . | N . | S . | ||||
| 1 | CB1 | 34 | 62 | 2.3 | 1.6 | 33 | 20 | 30 | 16 | 1.0 | 0 | 1.8 | 2.0 | ND | ND |
| CB2 | 44 | 59 | 1.7 | 1.0 | 22 | 18 | 33 | 22 | 0 | 0 | 1.3 | 1.8 | ND | ND | |
| 2 | CB | 62 | 65 | 1.0 | 1.0 | 22 | 26 | 14 | 8.3 | 0.5 | 0 | 1.1 | 1.3 | 23 | 40 |
| Experiment No. . | Cell Source . | Percentage of G418R Progenitors . | BFU-E Ratio (S/N) . | No. of Cells per BFU-E (ratio S/N) . | % DAF+ Colonies-150 . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BFU-E . | GM . | G . | M . | Mix . | |||||||||||
| N . | S . | N . | S . | N . | S . | N . | S . | N . | S . | N . | S . | ||||
| 1 | CB1 | 34 | 62 | 2.3 | 1.6 | 33 | 20 | 30 | 16 | 1.0 | 0 | 1.8 | 2.0 | ND | ND |
| CB2 | 44 | 59 | 1.7 | 1.0 | 22 | 18 | 33 | 22 | 0 | 0 | 1.3 | 1.8 | ND | ND | |
| 2 | CB | 62 | 65 | 1.0 | 1.0 | 22 | 26 | 14 | 8.3 | 0.5 | 0 | 1.1 | 1.3 | 23 | 40 |
Retroviral transduction efficiency as determined by G418R was equivalent within each experiment. Three experiments were established using CB. Results are presented as the percentage of G418R colonies established from different progenitor cells (viz, BFU-E, GM-CFC, G-CFC, M-CFC, and Mix-CFC) after transduction with LNL6 (N) or LNC(SCL) (S). A minimum of 40 colonies were scored to obtain the various percentages.
DAF staining was performed after culture in methylcellulose for 7 days.
Enforced expression of SCL did not influence cell death.
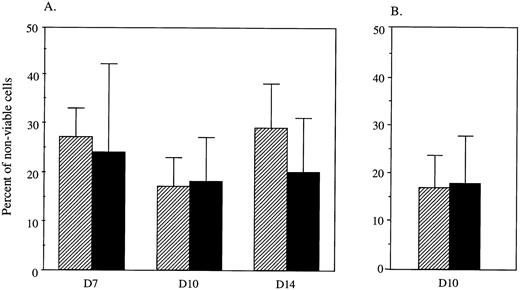
Although controversial,33 it has been suggested that SCL may play a role in protecting cells against apoptosis.16,34 56 It was therefore possible that SCL increased the size of erythroid colonies by decreasing the degree of cell death within these colonies. However, the proportion of dying cells within the erythroid colonies was unaltered after transduction with LNL6 and LNC(SCL) at days 7, 10, and 14 (Fig 4A). A similar result was obtained when the proportion of dying cells was examined by staining DNA with PI (Fig 4B). Erythroid colonies were selected using the same criteria as that used for documenting the size of erythroid colonies. Although this did not allow direct identification of apoptotic cells, it did indicate cells that were dying, irrespective of the mechanism. This suggested that an SCL-induced decrease in cell death was not the explanation for the increase in colony size and that enforced expression of SCL did not alter the proportion or the rate of hematopoietic cell death.
Proportion of nonviable cells per G418R erythroid colony. CD34+ cells were retrovirally infected with LNL6 (▨) or LNC(SCL) (▪) and placed into agar cultures stimulated for erythroid colonies for 7, 10, and 14 days. For each experiment, 40 G418R colonies were pooled in 150 μL PBS and analyzed as outlined below. The results were divided by the number of colonies pooled. (A) The percentage of dying cells was determined by comparing the number of cells that stained with eosin to the total number of cells present. Data points represent the mean ± SD percentage of nonviable cells per erythroid colony at days 7 (n = 3 experiments), 10 (n = 6 experiments), and 14 (n = 6 experiments). (B) The number of dying cells was determined by staining with PI. Fluorescently stained nonviable cells were quantitated using UV microscopy and compared with the total number of cells observed under phase contrast to determine the percentage of nonviable cells per G418R erythroid colony at day 10 (n = 5 experiments).
Proportion of nonviable cells per G418R erythroid colony. CD34+ cells were retrovirally infected with LNL6 (▨) or LNC(SCL) (▪) and placed into agar cultures stimulated for erythroid colonies for 7, 10, and 14 days. For each experiment, 40 G418R colonies were pooled in 150 μL PBS and analyzed as outlined below. The results were divided by the number of colonies pooled. (A) The percentage of dying cells was determined by comparing the number of cells that stained with eosin to the total number of cells present. Data points represent the mean ± SD percentage of nonviable cells per erythroid colony at days 7 (n = 3 experiments), 10 (n = 6 experiments), and 14 (n = 6 experiments). (B) The number of dying cells was determined by staining with PI. Fluorescently stained nonviable cells were quantitated using UV microscopy and compared with the total number of cells observed under phase contrast to determine the percentage of nonviable cells per G418R erythroid colony at day 10 (n = 5 experiments).
Enforced expression of SCL did not decrease the number of GM colonies.
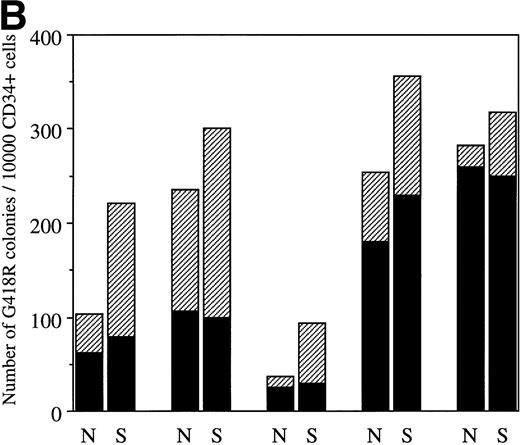
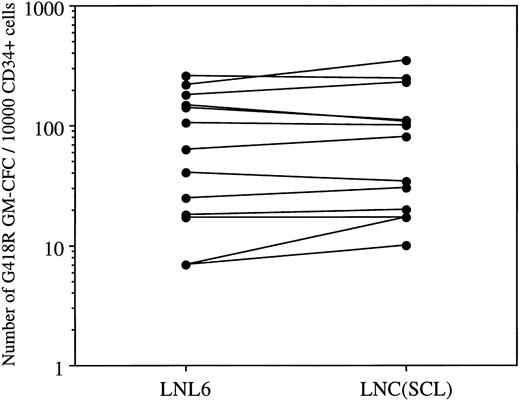
Although LNC(SCL)-transduced cells generated an increase in the total number of colonies, this was primarily accounted for by an increase in erythroid colonies. However, as shown in Fig 2B, there was the suggestion that, in some experiments, enforced SCL expression also increased the number of GM colonies. To directly examine whether SCL could influence the development of GM colonies, cultures were established under conditions in which no erythroid colony formation could result. Given the evidence implicating SCL in macrophage differentiation, an action on GM colony formation in such circumstances might be expected. However, enforced expression of SCL in human CD34+ cells did not consistently alter the number of GM or of eosinophil colonies. Figure5 shows the number of G418R GM colonies for each patient sample; the median number of G418R GM colonies/10,000 CD34+ cells after cocultivation with LNL6 and LNC(SCL) was 63 (range, 7 to 260) and 80 (range, 10 to 350), respectively (n = 13 experiments). Although this difference was not statistically significant (P = .43, paired t-test), in 6 experiments there was a modest increase in the number of GM colonies. Furthermore, enforced expression of SCL did not influence the type of myeloid colonies observed (Table 2); 29% were pure eosinophil colonies and there was no difference in the number of granulocyte and/or macrophage colonies containing eosinophils. Thus, in this system, enforced expression of SCL did not appear to alter myeloid differentiation.
Number of G418R GM colonies after transduction of CD34+ cells with LNL6 or LNC(SCL). Cultures were established in the presence of G418 (1.5 mg/mL) using 1 × 103 to 5 × 104 CD34+ cells. Each point shows the mean from 9-replicate cultures for an individual patient sample.
Number of G418R GM colonies after transduction of CD34+ cells with LNL6 or LNC(SCL). Cultures were established in the presence of G418 (1.5 mg/mL) using 1 × 103 to 5 × 104 CD34+ cells. Each point shows the mean from 9-replicate cultures for an individual patient sample.
Morphology of Myeloid Colonies From 4 Patient Samples
| Patient No. . | Colony Type (%) . | No. of Colonies Examined . | ||||||
|---|---|---|---|---|---|---|---|---|
| GM . | Eo . | GM/Eo . | ||||||
| LNL6 . | SCL . | LNL6 . | SCL . | LNL6 . | SCL . | LNL6 . | SCL . | |
| 1 | 50 | 52 | 35 | 26 | 15 | 22 | 48 | 66 |
| 2 | 60 | 66 | 23 | 17 | 17 | 17 | 187 | 172 |
| 3 | 70 | 53 | 24 | 36 | 6 | 11 | 163 | 237 |
| 4 | 56 | 58 | 32 | 35 | 12 | 7 | 65 | 85 |
| Mean | 59 ± 8 | 57 ± 6 | 29 ± 6 | 29 ± 9 | 12 ± 5 | 14 ± 7 | ||
| Patient No. . | Colony Type (%) . | No. of Colonies Examined . | ||||||
|---|---|---|---|---|---|---|---|---|
| GM . | Eo . | GM/Eo . | ||||||
| LNL6 . | SCL . | LNL6 . | SCL . | LNL6 . | SCL . | LNL6 . | SCL . | |
| 1 | 50 | 52 | 35 | 26 | 15 | 22 | 48 | 66 |
| 2 | 60 | 66 | 23 | 17 | 17 | 17 | 187 | 172 |
| 3 | 70 | 53 | 24 | 36 | 6 | 11 | 163 | 237 |
| 4 | 56 | 58 | 32 | 35 | 12 | 7 | 65 | 85 |
| Mean | 59 ± 8 | 57 ± 6 | 29 ± 6 | 29 ± 9 | 12 ± 5 | 14 ± 7 | ||
BM or PBPC CD34+ cells were transduced with either LNL6 or LNC(SCL) and placed into agar culture in the presence of purified, recombinant human GM-CSF (100 ng/mL), G-CSF (500 U/mL), and SCF (100 ng/mL). Agar cultures were fixed and then stained with Luxol-Fast blue. Colonies were examined under light microscope (×100) and characterized as granulocyte/macrophage (GM), eosinophil (Eo), or a mixture of both cell types (GM/Eo).
Enforced expression of SCL did not alter the frequency of clonogenic cells in erythroid and GM colonies.
To examine the self-generative capacity of progenitor cells infected with the LNC(SCL) retrovirus, individual erythroid and GM colonies were isolated at day 14 and placed into secondary agar cultures. Thirty-two percent of both LNL6- and SCL-erythroid colonies contained clonogenic cells, as did 28% and 29% of GM colonies derived from cells transduced with LNL6 and LNC(SCL), respectively. These results demonstrated that enforced expression of SCL did not alter the self-renewal capacity of freshly isolated human progenitor cells. Consistent with these results, attempts to generate cell lines from retrovirally transduced CD34+ cells were unsuccessful, despite the use of multiple growth factor combinations (data not shown).
Enforced expression of SCL increased the number of megakaryocyte colonies.
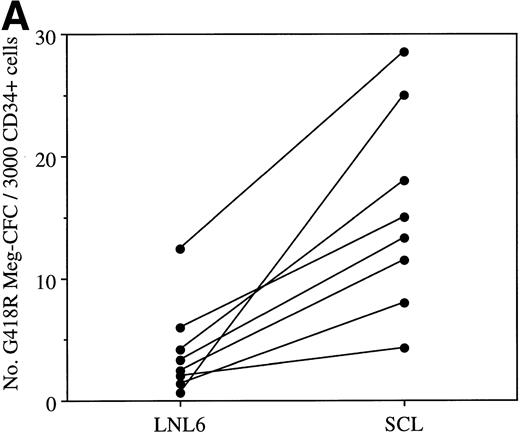
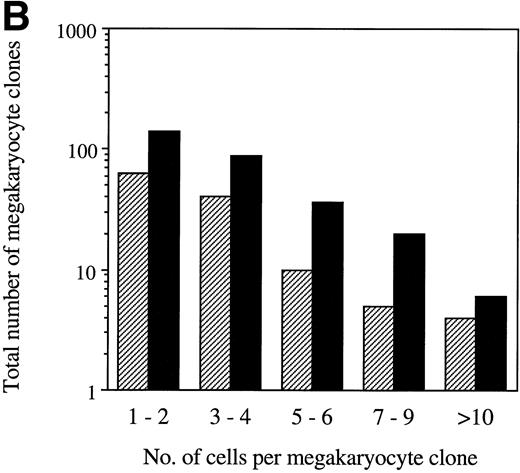
The normal expression pattern of SCL suggests a possible role for SCL in MK differentiation. MK colony formation was therefore investigated. As with erythroid colony formation, there was wide interpatient variation. However, in every case, enforced expression of SCL in CD34+ cells resulted in an increase in the number of MK colonies. There was a median increase of fourfold (range, 2- to 42-fold) compared with control cultures (P < .005; Fig 6A). Based on the erythroid colony data presented, we anticipated that SCL would also act to increase the size of MK colonies. However, this was not the case. Figure 6B shows data from 4 patient samples in which the number of cells per retrovirally transduced MK clone was quantitated. In all 4 samples, despite an increase in the number of MK clones after transduction with LNC(SCL), there was no difference in the clone size. The size of the MK colonies (≥3 cells) after transduction of cells with LNL6 was 5.5 ± 1.4 cells (mean ± SD, n = 80 colonies examined) versus 5.2 ± 0.8 cells (n = 187 colonies examined) for LNC(SCL). Similarly, there was no difference in the size of MK cells (data not shown). Thus, enforced expression of SCL increased the number of clonogenic MK progenitor cells but did not alter their proliferative potential.
(A) Number of G418R MK colonies after transduction of CD34+ cells with LNL6 or LNC(SCL). Cultures were established using 3,000 CD34+ cells in the presence of 1.5 mg/mL G418. Each data point represents an individual patient sample. Equivalent titers between LNL6 and LNC(SCL) (2% to 13% G418R GM-CFC) were confirmed within each experiment. (B) The number of cells per G418R MK clone after transduction of CD34+ cells with LNL6 (▨) or LNC(SCL) (▪). The total number of retrovirally transduced MK clones is shown. Clones from a total of 9 agar culture plates, representing 4 individual patient samples, are shown. Equivalent titers were observed for the LNL6 and LNC(SCL) retroviruses, as assessed by determining the percentage of G418R GM-CFC for each of the 4 patient samples. MK colonies contained 3 or more cells. The number of cells per MK clone was counted using an inverted microscope at ×100 magnification.
(A) Number of G418R MK colonies after transduction of CD34+ cells with LNL6 or LNC(SCL). Cultures were established using 3,000 CD34+ cells in the presence of 1.5 mg/mL G418. Each data point represents an individual patient sample. Equivalent titers between LNL6 and LNC(SCL) (2% to 13% G418R GM-CFC) were confirmed within each experiment. (B) The number of cells per G418R MK clone after transduction of CD34+ cells with LNL6 (▨) or LNC(SCL) (▪). The total number of retrovirally transduced MK clones is shown. Clones from a total of 9 agar culture plates, representing 4 individual patient samples, are shown. Equivalent titers were observed for the LNL6 and LNC(SCL) retroviruses, as assessed by determining the percentage of G418R GM-CFC for each of the 4 patient samples. MK colonies contained 3 or more cells. The number of cells per MK clone was counted using an inverted microscope at ×100 magnification.
DISCUSSION
In this report, we demonstrate that enforced expression of SCL in human CD34+ cells enhanced both erythroid and MK development, thereby providing further evidence of a role for SCL as a master regulator of hematopoiesis. The enhanced colony growth and colony size observed with enforced expression of SCL, a transcription factor, is typically associated with cytokine stimulation of hematopoietic progenitor cells.57-60 It is conceivable that SCL may be responsible for mediating some of the effects, eg, of SCF on erythroid progenitor cells, because an increase in SCL protein levels has been documented after the culture of human erythroid precursors in SCF.28 However, the results presented in the current report directly demonstrate the importance of the bHLH protein, SCL, in hematopoietic development and in the human system.
This is the first description of a direct functional role for SCL in MK development. Enforced expression of SCL enhanced the clonogenic potential of MK cells but did not influence the proliferative ability of these cells. This action is in contrast to, eg, thrombopoietin, in which the extracellular growth factor can increase both clonogenicity, colony size, and MK ploidy.59 In a previous study, SCL was overexpressed in the early myeloid murine cell line, 416B,61 and, consistent with the present study, there was no effect of SCL to stimulate proliferation of committed MK progenitors. However, an action of SCL to enhance clonogenicity of uncommitted progenitors was not evaluated. Moreover, both studies failed to demonstrate an effect of SCL on the differentiation and subsequent morphologic changes of mature MK, although this was seen with GATA-1 in the 416B cells. SCL can now be added to the short list of transcription factors known to be capable of influencing MK differentiation and development, with the other proteins being NF-E2,62 HOXA10,63 and members of the GATA family.61 64
The SCL-induced erythroid development is consistent with some of the known actions of SCL previously documented using erythroid cell lines. The experiments presented here demonstrated that, in an optimal environment (eg, the presence of EPO), SCL per se regulated erythropoiesis. This was evidenced in terms of enhanced clonogenicity, increased proliferation, and hastening of hemoglobinization. There are several potential mechanisms by which SCL might exert these effects on erythroid (and MK) progenitor cells. It is possible that SCL enhanced the survival of committed progenitor cells during the 3-day coculture period; however, this effect alone would be insufficient to explain the increased size and differentiation of the erythroid colonies (which implied an action during the agar culture phase). Furthermore, similar results were obtained using CD34+ cells derived from both adult and fetal sources despite differences in the transduction protocols, suggesting that the SCL-mediated effects were independent of conditions during the transduction period. Whereas there was no evidence for increased self-renewal or alteration in hematopoietic cell death within colonies, there was an increase in the size of erythroid colonies. The increased proliferative capacity of erythroid cells (as evidenced by increased colony size) may therefore be due to a shortened cell cycle time within SCL-erythroid colonies. This would be consistent with previous studies that demonstrated a role for SCL in proliferation of K562 cells.65 The enhancement of clonogenic capacity of both erythroid and MK progenitors raises the possibility that SCL may play a role in a bipotent erythroid (Ery)/MK progenitor cell or direct cellular differentiation towards such a bipotent progenitor. Studies of SCL expression have also suggested that endogenous SCL is likely to be important within these cells.33 66 In addition to these effects, SCL hastened erythroid differentiation suggesting further action on postmitotic erythroid cells.
Enforced expression of SCL did not perturb myeloid development. These findings were unexpected given previous studies, using cell lines, that have implied a role for SCL during myeloid differentiation. Based on the reported action of SCL in cells from myeloid progenitor cell lines,31-34 one might predict a decrease in GM colonies proportional to the increase in erythroid and MK precursors. Such a finding was reported after enforced expression of HOXA10 in murine hematopoietic progenitor cells,63 in which an increase in MK progenitor cells was reported with concomitant decreases in the granulocyte and macrophage compartments. In the present study, RT-PCR analysis confirmed the presence of both endogenous SCL and increased levels of retroviral SCL mRNA in GM colonies. However, given that the levels of transcription factor can influence hematopoietic differentiation,67 the absolute abundance of SCL protein may be important in myeloid differentiation. However, the absence of a consistent alteration in GM precursors does further support the idea that the effects of enforced expression of SCL may be manifested in a progenitor cell such as the Ery/MK progenitor. It is also consistent with the action of growth factors in which, eg, there is no evidence that MK differentiation induced by thrombopoietin occurs at the expense of other cell lineages.59,68 69
This lack of expected action of SCL on the cells of the GM lineage may also be a consequence of the use of multiple cytokines in this study (viz, GM-CSF, G-CSF, and SCF). It is possible that this combination of cytokines was able to elicit GM differentiation via intracellular signaling mechanisms that were not perturbed by the enforced expression of SCL. In contrast, the inhibitory action of SCL on myeloid differentiation using cell lines was seen only when single growth factors were used and then, eg, with leukemia-inhibitory factor (LIF) but not with IL-6.31 32
The findings of this study highlight the advantage of using primary human cells to examine the events involved in hematopoiesis. By definition, immmortalized cell lines inherently harbor unknown genetic mutations and, although these cell lines represent important hematologic tools, they cannot truly reflect normal cells. This may be demonstrated in the present study, eg, by the differences observed in the effect of SCL on GM differentiation and also by the unaltered self-renewal capacity of LNC(SCL)-transduced progenitor cells; this was in contrast to the increased self-renewal capacity observed with enforced expression of SCL in M1 myeloid progenitor cells,31 and the decrease in self-renewal capacity observed with antisense SCL in K562 cells.65
In summary, we have shown that SCL can increase megakaryocytopoiesis and direct the development of erythroid precursors derived from human CD34+ cells. Such an action of SCL is in keeping with the role of other bHLH proteins to regulate the differentiation of various cell types and arguably justifies the description of SCL as a master regulator of hematopoiesis.70
ACKNOWLEDGMENT
The authors gratefully acknowledge the assistance of Rachel Mansfield and the staff of the Centre for the Development of Cancer Therapeutics, Melbourne for the supply of patient samples and Amgen for the supply of cytokines. We also thank Dr Andrew Elefanty for helpful discussions and advice.
Supported in part by the National Health and Medical Research Council (Canberra, Australia), the Medical Research Council of Canada, and the National Cancer Institute of Canada with funds from the Canadian Cancer Society. D.S.P is a Terry Fox Research Fellow of the National Cancer Institute of Canada, supported with funds provided by the Terry Fox Run.
Address reprint requests to Ngaire J. Elwood, PhD, Rotary Bone Marrow Research Laboratories, Post Office, Royal Melbourne Hospital, Parkville, Victoria, 3050, Australia; e-mail: elwood@wehi.edu.au.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal