Abstract
Exfoliation of plasma membrane components is a directed process that consumes energy and requires active cell metabolism. Proteins involved in regulating the survival and proliferation of eukaryotic cells are released on exfoliated vesicles. We examine here whether the Fas receptor and its cognate ligand (FasL) are present on vesicles shed from high metastatic potential CX-1 cells and low metastatic potential MIP-101 cells and from HuT 78 cells, respectively. Rates of exfoliation at 2 hours and cumulative levels of extracellular vesicles in serum-free medium conditioned by CX-1 cells are increased by 1.8-fold and 1.6-fold, respectively, relative to that in medium conditioned by MIP-101 cells. Although vesicles shed from both cancer cell lines contain Fas antigen, the amount of Fas per vesicle and the percentage of vesicles containing Fas are increased for vesicles isolated from MIP-101 cells, relative to those from CX-1 cells, as determined by immunogold particle labeling and electron microscopy and by immunofluorescence microscopy and flow cytometry. Results of metabolic labeling with 35S-methionine indicate that Fas biosynthesis is reduced by up to 3.3-fold for CX-1 cells, relative to that of MIP-101 cells, consistent with the finding of decreased Fas on vesicles shed from the plasma membrane of CX-1 cells. Although mRNA for soluble Fas receptor is detectable in both cell lines, depletion of shed vesicles from serum-free medium by ultracentrifugation removes all detectable biological activity. FasL is detected on vesicles exfoliated from HuT 78 cells by immunoelectron microscopy and Western blot analysis. FasL-bearing vesicles induce apoptosis of Fas-expressing cancer cells at the same level as observed by treatment with monoclonal anti-Fas antibody. Furthermore, Fas-bearing extracellular vesicles from MIP-101 but not from CX-1 cells protect the CX-1 cell line from FasL-induced and anti-Fas–mediated apoptosis, indicating that Fas present on shed vesicles is biologically active. We conclude that the Fas antigen and its cognate ligand are exfoliated from the cell surface in a bioactive configuration. Exfoliation may provide a mechanism for long-range signal-directed apoptosis while maintaining Fas/FasL on a membrane surface.
FAS ANTIGEN (Fas; CD95 receptor), a cysteine-rich type I transmembrane glycoprotein, is a member of the tumor necrosis factor (TNF) family. Its molecular mass ranges from 45 to 52 kD.1-3 In common with other members of the TNF receptor family, Fas spans the plasma membrane once; its intracellular domain is distinct from that of any other member of the family. Many cell types, including lymphoid, myeloid, hematopoietic progenitor, epithelial, and endothelial cells, express Fas on the cell surface and undergo apoptosis when treated with anti-Fas antibody4,5 or the cognate ligand for Fas, Fas ligand (FasL; CD95L).6 In addition to normal cells, many malignant cells of hematologic and nonhematologic origin express Fas, which can be triggered to signal apoptotic cell death after ligation of Fas at the plasma membrane by FasL or by functional antibodies to Fas.7 8
FasL is a type II transmembrane protein with an apparent molecular mass varying between 36 and 43 kD.9,10 Recently, a 30-kD variant of FasL has been immunoprecipitated from extracts of plasma membranes.11 It, too, belongs to the TNF family of cytokines.11,12 However, in contrast to the ubiquitous expression Fas, FasL is predominantly expressed on the surface of activated T lymphocytes, natural killer (NK) cells, and Sertoli cells. Recent studies have shown that colon13 and liver14 carcinomas, as well as melanoma15 cells also express FasL that may trigger apoptosis of activated T cells, a process that may enhance tumor cell survival.14-16 Recent evidence suggests that the interaction of (membrane bound) FasL with Fas involves oligomerization of three FasL molecules on the surface of effector cells that subsequently bind Fas molecules (also present as trimeric complexes) on the surface of target cells.17 In this model, each ligand subunit interacts with two of the three Fas molecules, resulting in formation of a receptor complex.18
An increased concentration of soluble Fas has been detected in serum of patients with solid tumors,19 T-cell leukemias, or B-cell leukemias.20 Moreover, soluble Fas released from COS cells transfected with cDNA encoding for Fas protein lacking a membrane-spanning domain inhibits cell death induced by anti-Fas antibody.21 Together, these findings suggest that reduced tumor cell apoptosis may occur as soluble Fas competes with tumor cell-surface Fas for T-cell/NK cell-surface FasL.22Similarly, increased serum levels of soluble FasL have been detected in patients with large granular lymphocytic leukemia and NK cell lymphoma.23 In these patients, conversion of cell-surface bound FasL to its soluble form was attributed to the action of a matrix metalloproteinase.23
Components of the plasma membrane of both normal and malignant cells are continually exfoliated from the surface as plasma membrane-derived vesicles.24,25 Exfoliation may play a role in malignant cell survival by permitting tumor-specific cell surface antigens to be shed from the plasma membrane, thus avoiding recognition by host immune defence mechanisms.26 Several reports have suggested that, in addition to cloaking themselves from the host's immune system, tumor cells actively participate in downregulating the function of antigen-presenting cells by releasing plasma membrane component-bearing vesicles, thereby increasing their chance of survival.26-28Recently, Dolo et al29 demonstrated that transforming growth factor-β–bearing vesicles released from human breast carcinoma cells inhibit the proliferation of specific target lymphocytes that recognize cell surface tumor antigens.
These findings, coupled with accruing evidence for the participation of Fas/FasL interaction in the maintenance of tissue homeostasis, prompted us to investigate whether Fas and/or FasL are shed on plasma membranes-derived vesicles from high (CX-1) and low (MIP-101) metastatic potential human colorectal carcinoma cell lines30 and from the cell-surface of HuT 78 cells, an activated human T-cell line. Our results show that (1) Fas and FasL are released on shed vesicles, (2) Fas-bearing vesicles efficiently inhibit FasL-and anti-Fas antibody-mediated apoptosis, and (3) FasL-bearing vesicles induce cell death. These findings indicate that Fas and FasL are present on vesicles in a bioactive conformation. Furthermore, our finding that Fas-bearing vesicles inhibit FasL-bearing vesicle-mediated cell death suggests that Fas/FasL recognition takes place when these proteins are presented to each other as components of extracellular vesicles.
MATERIALS AND METHODS
Cell cultures.
Human colorectal adenocarcinoma cell lines, CX-1 and MIP-101, were a generous gift from Dr P. Thomas (Deaconess Hospital, Harvard Medical School, Boston, MA). The HuT 78 cell line (American Type Culture Collection, Rockville, MD) is an activated T-cell line obtained from a patient with Sezary syndrome that is known to express FasL. Cell lines were established in T225 cell culture flasks (Costar Corp, Cambridge, MA) and regularly maintained, as described previously.30Briefly, cells were cultured in RPMI-1640 (RPMI) medium (GIBCO BRL, Grand Island, NY) supplemented with 10% fetal calf serum (FCS) and containing 100 U/mL of penicillin and 100 mg/mL streptomycin (P/S) (GIBCO BRL) in a humidified atmosphere containing 5% CO2at 37°C. Before harvesting vesicles, cells were permitted to grow under low serum conditions as follows: cells in confluent flasks were washed three times with phosphate-buffered saline, pH 7.2 (PBS), and gently detached with 15 mL PBS containing 15 mmol/L sodium citrate, pH 7.2. After centrifugation at 800g for 5 minutes, the citrate solution was aspirated and the cells were resuspended in 20 mL RPMI containing 8% FCS and P/S. Two milliliters of the cell suspension was introduced into T225 culture flasks containing 50 mL of RPMI supplemented with 8% FCS and P/S. Cells were allowed to reach confluency and passaged once again in a serum concentration that was further reduced by 2%. This process was repeated until the cells were maintained in media containing 2% FCS and P/S. When cells were 60% to 70% confluent, media was aspirated, cells were washed three times with PBS, and 200 mL of fresh, serum-free RPMI was added to each flask. Flasks were returned into the incubator and cells allowed to grow for 16 to 18 hours. Typically, greater than 95% of cells were capable of excluding trypan blue.
Cell surface labeling.
Eighteen hours before cell surface labeling, media in tissue culture flasks was aspirated and the cells were washed three times with 30 mL PBS. CX-1 and MIP-101 cells were incubated overnight in 100 mL serum-free RPMI medium. On the day of the experiment, medium was aspirated and the cells were washed three times with 30 mL PBS and detached with 25 mL citrate solution. Cells were harvested by centrifugation, resuspended in 2 mL PBS, and filtered through a 30-μm nylon mesh (Falcon; Becton Dickinson, Franklin Lakes, NJ) to remove clumps. The volume was adjusted to 3.5 mL with PBS and 1 × 107 cells were surface labeled by incubation with two Iodo-Beads (Pierce, Rockford, IL) and 2 mCi 125I (Amersham Life Science, Oakville, Ontario, Canada). The cells were gently mixed by slowly inverting the 5-mL tube for 1 minute, and the reaction was quenched by removing the beads. The cells were pelleted by centrifugation for 5 minutes at 700g and washed three times with 5 mL PBS. Radiolabeled cells were then cultured in serum-free RPMI under the conditions described above.
Vesicle preparation.
Serum-free conditioned medium was decanted from flasks and centrifuged at 2,000g for 10 minutes to remove cells and debris, as described previously31. The supernatant was subjected to ultracentrifugation at 100,000g for 12 hours at 8°C. Supernatant was discarded and the vesicles were washed twice by resuspending the pellet in PBS, followed by ultracentrifugation at 100,000g for 12 hours at 8°C. Vesicles were used immediately or stored at −80°C until needed. Vesicles collected from 125I-labeled cells were resuspended in 5 mL scintillation cocktail (Dupont, Kingston, Ontario, Canada) and the amount of radioactivity (cpm) was determined by scintillation counting.
Viability assay.
Cell viability was correlated to the capacity of cells to reduce 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide to formazan, as described by the manufacturer of the MTT assay (Sigma Chemical Co, St Louis, MO). Briefly, 2 × 105 cells were cultured in wells of a 96-well microtiter plate (Becton Dickinson) for 24 hours under the conditions described above. After the incubation period, media was removed and replaced with 200 μL fresh RPMI medium containing 10% FCS, cycloheximide (10 μg/mL), and 100 ng isotype-matched antibody (IgM) as a control (Calbiochem Corp, LaJolla, CA) or 100 ng murine monoclonal antihuman Fas antibody, CH. 11 (Kamiya Biomedical Co, Thousand Oaks, CA). Twenty microliters of vesicles (40 μg total protein) prepared from CX-1 or MIP-101 cells and resuspended in PBS was added to appropriate wells. Cells were incubated for 24 hours, after which MTT reagent was added. The cells were incubated for an additional 4 hours, and levels of formazan were quantified with an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm.
Relative resistance of CX-1 and MIP-101 cell lines to anti-Fas–induced and FasL-induced cell death was determined in a similar fashion, with the exception that RPMI medium was not replaced after the initial 24-hour incubation period; cycloheximide and antibodies were added to the conditioned medium, as described above.
Fas biosynthesis.
Biosynthesis of Fas was measured using 35S-methionine, as described previously.32 Briefly, CX-1 and MIP-101 cells maintained in RPMI medium supplemented with 10% FCS were gently detached by treatment with 15 mmol/L citrate-PBS solution and harvested by centrifugation at 800g for 5 minutes. Cells (1.5 × 107) were washed twice with PBS and resuspended in methionine-free RPMI-1640 medium supplemented with 10% FCS at a density of 5 × 105 cells/mL, and 300 μCi35S-methionine (Amersham Life Science) was added. Cells were incubated for 2 or 16 hours at 37°C in a humidified atmosphere with 5% CO2. Trypan blue staining showed that, at the time of harvest, greater than 95% of the cells excluded dye.
Preparation of RNA.
Ten million MIP-101 or CX-1 cells grown under serum-free conditions were detached from T225 flasks and harvested by centrifugation at 800g for 5 minutes. The cell pellet was solubilized in 1 mL Trizol reagent (GIBCO-BRL) and 0.2 mL chloroform (Fisher Scientific, Montreal, Quebec, Canada) and then centrifuged at 12,000g for 15 minutes at 4°C, as instructed by the manufacturer. The aqueous phase was transferred to a new tube and 0.5 mL isopropanol was added. After 10 minutes of incubation, the tube was centrifuged at 12,000g for 10 minutes at 4°C. The RNA precipitate was washed with 1 mL 75% ethanol and centrifuged one more time, and the pellet was allowed to dry for 15 minutes at room temperature. Isolated total RNA was dissolved in 30 μL diethyl pyrocarbonate (DEPC)-treated water and quantified by UV spectroscopy using an Ultrospec 3000 (Pharmacia Biotech, Baie D'Urfe, Quebec, Canada).
Reverse transcriptase-polymerase chain reaction (RT-PCR).
First-strand cDNA synthesis was accomplished by using 1 μg total RNA and random hexamer primer as substrates for Moloney-murine leukemia virus (M-MuLV) reverse transcriptase (MBI-Fermentas). A 3 μL aliquot of cDNA was used as template for PCR amplification with the following primer pair, 5′-GGA GCT GCC TCT TCT TCC-3′ and 5′-ACA CTA ATT GCA TAT ACT CAG AACTG-3′, corresponding to the complete CD95 (Fas antigen) coding region.33
Immunoprecipitation.
35S-methionine–labeled CX-1 and MIP-101 cells (1 × 107) were collected by centrifugation at 800g for 5 minutes. Pellets were dissolved in 1 mL 50 mmol/L Tris-HCl buffer, pH 8.0, containing 0.5% Triton X-100 (Sigma Chemical Co), 10 mmol/L EDTA, and 150 mmol/L NaCl. Subsequently, suspensions were mixed by inversion while incubating at 4°C for 30 minutes. After solubilization, lysates were dialyzed against 0.05% Triton X-100 in Tris-HCl buffer at 4°C for 16 hours. The radiolabeled samples were cleared with goat IgG (Becton Dickinson) immobilized on protein G beads (Pierce), and Fas was immunoprecipitated by incubating the mixture with goat anti-Fas (provided by Dr L. Owen-Schaub, University of Texas, MD Anderson Cancer Center, Houston, TX) bound to protein G beads at 4°C for 2 hours. Beads were washed with 0.05% Triton X-100 in Tris-HCl buffer three times and resuspended in 75 μL of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (0.25 mol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.1% bromophenol blue). Samples were placed for 5 minutes in a water bath at 100°C and centrifuged in a minifuge for 10 seconds, and the pelleted beads were discarded. A 15-μL aliquot of each sample was retained to determine the amount (cpm) of 35S-methionine incorporated in immunoprecipitated Fas. The remainder of each sample was electrophoresed in a 7.5% polyacrylamide gel, and reactive bands were visualized by autoradiography.
Protein assay.
Protein levels were determined using micro BCA assay (Pierce), according to the protocol suggested by the manufacturer.
Electron microscopy.
Exfoliated vesicle samples were placed on a formvar-carbon coated 200 mesh copper grid that was glow-discharged just before application. Samples were allowed to adhere for 1 minute and then wicked off with filter paper and allowed to air dry. The grids were blocked with 2% BSA in PBS for 30 minutes and then incubated for 1 hour with mouse anti-Fas IgM (10 μg/mL). After three rinses in PBS and another block for 10 minutes, the grids were incubated for 2.5 hours at 4°C on drops of goat antimouse IgM labeled with 10-nm gold particles. The grids were rinsed three times in PBS and then rinsed three times with distilled water, stained for 1 minute with 2% ammonium molybdate, dried, and examined and photographed in a Philips CM10 transmission electron microscope (JOEL Ltd, Tokyo, Japan) at 60 kV.
Western analysis.
Expression of Fas and FasL was determined by Western blot analysis. Solubilized plasma membranes or plasma membrane-derived vesicles were immunoprecipitated and electrophoresed in SDS-7.5% polyacrylamide. Resolved protein bands were transferred to nitrocellulose, blocked overnight with Tris-buffered saline (20 mmol/L Tris buffer, 137 mmol/L NaCI, pH 8.0) supplemented with 0.1% Tween-20 (TBS-T), and incubated for 1 hour at room temperature with either murine polyclonal anti-FasL antibody (BMS 140; Cedarlane Laboratories, Hornby, Ontario, Canada) or rabbit polyclonal anti-FasL antibody (Cedarlane Laboratories) that was diluted 1,000-fold. The nitrocellulose membrane was washed three times with 50 mL TBS-T. Lanes treated with murine anti-Fas antibody were incubated for 1 hour with rabbit antimouse IgG (H + L) (Jackson ImmunoResearch Laboratories, Mississauga, Ontario, Canada) diluted 1,000-fold in TBS-T and then washed three times with TBS-T. Subsequently, all lanes were treated for 30 minutes with horseradish peroxidase-conjugated goat antirabbit antibody diluted 5,000-fold in TBS-T. Blots were developed with chemiluminescence substrate (ECL; Amersham Life Science).
Fas antigen was deglycosylated as follows. To 200 μL of plasma membranes suspended in PBS was added sufficient SDS to give a final concentration of 0.15%. The sample was boiled for 5 minutes and the pH was adjusted to 7.8. After the addition of 3 U N-glyconase (Boehringer Mannheim, Laval, Quebec, Canada) to the aliquot, the sample was incubated at 37°C for 18 hours. Subsequently, an additional 3 U of enzyme was introduced into the aliquot, and the sample was incubated at 37°C for 18 hours. Deglycosylated proteins were solubilized, immunoprecipitated, and subjected to Western blot analysis, as described above.
Immunofluorescent microscopy and flow cytometry.
MIP-101 and CX-1 cells were cultured in T-75 flasks (Falcon; Becton Dickinson), as described above. Cells were washed three times with PBS and detached with 10 mL PBS-citrate solution. The cell suspension was centrifuged at 300g for 5 minutes, and the pellet was then resuspended in at 2 × 106 cells/mL PBS containing 1% FCS (PBS-FCS). One hundred microliters (2 × 105cells) of the cell suspension was transferred to plastic tubes (Falcon; 11.5 × 75 mm) and 200 ng of isotype-matched antibody (IgM) as a control (Calbiochem Corp) or 200 ng murine monoclonal antihuman Fas antibody (CH. 11) was added. The cells were mixed gently and placed on ice for 20 minutes. After the incubation period, the cells were washed twice with 2 mL PBS-FCS (centrifuged at 300g for 5 minutes), and 100 μL (50 μg/mL) of goat antimouse IgG/IgM, (H + L)-fluorescein conjugated antibody (Pierce) was added. The cells were mixed gently and the tubes were placed on ice for 20 minutes. The cells were washed twice with PBS-FCS and harvested by centrifugation at 300g for 5 minutes. The cell pellet was resuspended in 50 μL mounting fluid (1 part PBS and 9 parts glycerol), mounted on slides under coverslips (Fisher, Montreal, Quebec, Canada), and sealed with nail varnish. Cells were examined under fluorescence illumination using an Optiphot-2 microscope (Nikkon, Mississauga, Ontario, Canada) equipped with a 40× magnification objective. Photomicrographs were obtained using a Nikon camera (model FX-35DX; Nikon, Mississuaga, Ontario, Canada). Samples studied by flow cytometry were prepared in identical fashion, except that, after the final wash, the cell pellet was resuspended in 1 mL PBS before analysis.
Vesicles were harvested from serum-free medium conditioned by CX-1 or MIP-101 cells and enumerated on a FACS VANTAGE sorter (Becton Dickinson, Mountain View, CA) at the flow rate of 100 μL/min, side scatter of 400 V, and FL1 of 700 V. Briefly, 5 × 106vesicles were resuspended in 2 mL PBS-FCS, to which 100 ng mouse antihuman Fas IgM was added. Samples were incubated at 4°C for 30 minutes. Vesicles were washed as follows. The sample volume was increased to 30 mL with PBS-FCS and vesicles were pelleted by centrifugation at 100,000g at 4°C for 4 hours. Pellets were washed, resuspended in a final volume of 2 mL, and incubated at 4°C for 30 minutes in the presence of rabbit dichloro triazinyl amino fluorescein (DTAF)-conjugated antimouse IgM (20 μg/mL). Vesicles were washed, resuspended, and analyzed by flow cytometry.
Statistical analysis.
Unless otherwise stated, means were compared using the Student'st-test as calculated by Graphpad Prism version 2.0 statistical software (Graphpad Prism Inc, San Diego, CA).
RESULTS
Quantitative exfoliation from high (CX-1) and low (MIP-101) metastatic potential cell lines.
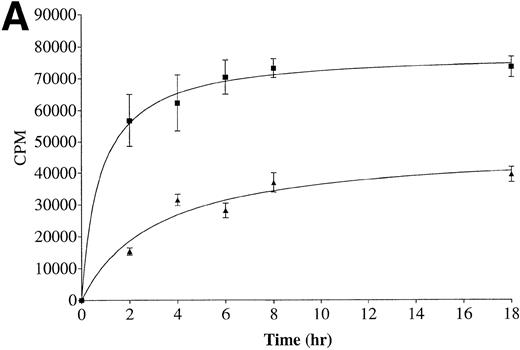
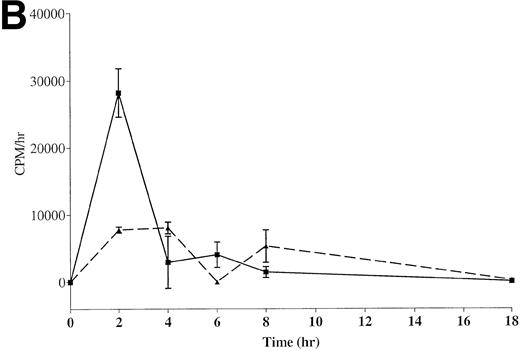
The kinetics of exfoliation from well-differentiated and high metastatic potential CX-1 cells was compared with that of poorly differentiated and low metastatic potential MIP-101 cells. As evident from Fig 1A, CX-1 cells release more125I-labeled protein in association with shed vesicles over time (0 to 18 hours) than do MIP-101 cells. Cumulative radioactivity shed after 18 hours is 1.8-fold greater for CX-1 cells than for MIP-101 cells. When rates of exfoliation are compared (Fig 1B), both cell lines exhibit the largest release of radioactivity (cpm) per hour after 2 hours of incubation, consistent with our results of kinetic studies for shedding from normal human B cells and continuously maintained cell lines.31 The rate of shedding from CX-1 cells at 2 hours was 3.6-fold greater than that of shedding from MIP-101 cells.
Kinetics of exfoliation from CX-1 and MIP-101 human colorectal adeno-carcinoma cells. (A) Cumulative radioactivity (cpm) released on shed vesicles collected from 1 × 107surface-125I-labeled CX-1 (▪) and MIP-101 (▴) cells after indicated incubation periods was quantified by scintillation counting. (B) Rates of shedding were calculated by dividing total radioactivity (cpm) released by the incubation time. Shown are the means ± SD of three separate experiments. Note that shedding from CX-1 cells is quantitatively increased relative to shedding from MIP-101 cells.
Kinetics of exfoliation from CX-1 and MIP-101 human colorectal adeno-carcinoma cells. (A) Cumulative radioactivity (cpm) released on shed vesicles collected from 1 × 107surface-125I-labeled CX-1 (▪) and MIP-101 (▴) cells after indicated incubation periods was quantified by scintillation counting. (B) Rates of shedding were calculated by dividing total radioactivity (cpm) released by the incubation time. Shown are the means ± SD of three separate experiments. Note that shedding from CX-1 cells is quantitatively increased relative to shedding from MIP-101 cells.
Visualization of Fas on shed vesicles.
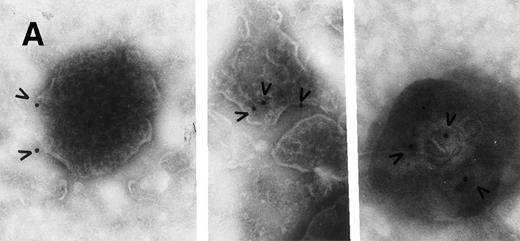
Plasma membrane-derived vesicles isolated from medium conditioned by CX-1 or MIP-101 cells were visualized by transmission electron microscopy, and Fas antigen was detected on their surface by immunogold particle labeling. Vesicle size and texture were heterogeneous, findings typical for shed vesicles.24 As shown in Figs 2 and3, vesicles from both cell lines are heterogeneous in shape and size (ranging from 0.05 to 0.5 μm) as well as in distribution of Fas (arrowheads) on the shed vesicle surface. No difference in vesicles released from MIP-101 versus CX-1 cells was apparent by electron microscopy. However, vesicles derived from CX-1 cells (Fig 3) show relatively less immunogold particle labeling than do vesicles shed from MIP-101 cells (Fig 2). The distribution of Fas on vesicles derived from plasma membranes of MIP-101 cells was typical for all fields that were examined. In contrast, vesicles shed from CX-1 cells failed to show the presence of Fas in the majority of fields that were examined.
Electron micrograph of immunogold-labeled Fas-bearing vesicles shed from MIP-101 cells. Fas (arrowheads) was detected with gold particle-conjugated antimouse anti-Fas antibody. The micrograph is at 57,000× magnification and the inset is at 68,000× magnification; scale bar = 0.2 μm.
Electron micrograph of immunogold-labeled Fas-bearing vesicles shed from MIP-101 cells. Fas (arrowheads) was detected with gold particle-conjugated antimouse anti-Fas antibody. The micrograph is at 57,000× magnification and the inset is at 68,000× magnification; scale bar = 0.2 μm.
Electron micrographs of vesicles shed from CX-1 cells. Large arrowheads point to gold particles in association with Fas antigen on plasma membrane-derived vesicles. In (A), a few gold particles (small arrowheads) not associated with vesicles can be seen and apparently represent nonspecific background. Both micrographs are at 92,000× magnification. Scale bar = 0.2 μm.
Electron micrographs of vesicles shed from CX-1 cells. Large arrowheads point to gold particles in association with Fas antigen on plasma membrane-derived vesicles. In (A), a few gold particles (small arrowheads) not associated with vesicles can be seen and apparently represent nonspecific background. Both micrographs are at 92,000× magnification. Scale bar = 0.2 μm.
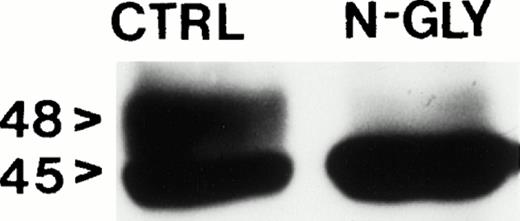
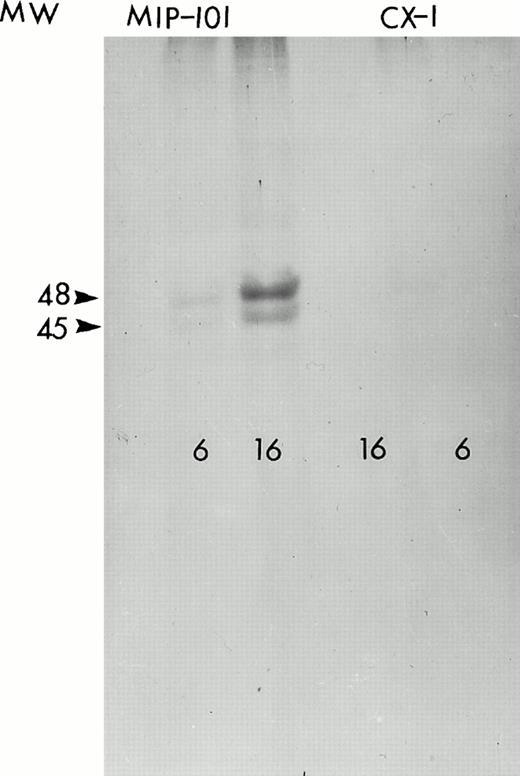
Western blot analysis of detergent solubilized MIP-101–derived shed vesicles and immunoprecipitated with anti-Fas antibody show two bands with apparent molecular weights of 45 and 48 kD corresponding to differentially glycosylated Fas protein (Fig 4). Treatment of the vesicle extract with N-glyconase before SDS-PAGE results in the disappearance of the higher molecular weight band, indicating that, in fact, the proteins immunoprecipitated with anti-Fas antibody are not unrelated proteins but rather are glycoslyated isoforms of the same protein recognized by the antibody.
Western blot analysis of solubilized extracellular vesicles harvested from MIP-101 conditioned medium. Vesicles extracted with n-octyl β-D-glucopyranoside and treated with N-glyconase before SDS-PAGE produce a single band with an apparent molecular weight of 45 kD when probed with anti-Fas antibody (N-gly). Vesicles extracted with detergent and subjected to SDS-PAGE without N-glyconase treatment show two bands with apparent molecular weights of 45 and 48 kD when probed with anti-Fas antibody (C).
Western blot analysis of solubilized extracellular vesicles harvested from MIP-101 conditioned medium. Vesicles extracted with n-octyl β-D-glucopyranoside and treated with N-glyconase before SDS-PAGE produce a single band with an apparent molecular weight of 45 kD when probed with anti-Fas antibody (N-gly). Vesicles extracted with detergent and subjected to SDS-PAGE without N-glyconase treatment show two bands with apparent molecular weights of 45 and 48 kD when probed with anti-Fas antibody (C).
Biosynthesis and cell-surface expression of Fas.
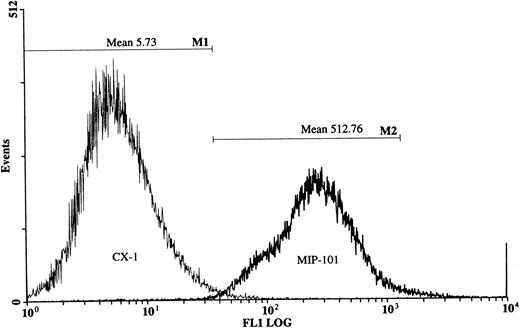
The relative distribution of Fas on the cell-surface of CX-1 and MIP-101 cells was reevaluated and quantified using fluorescence microscopy. A discontinuous ring of fluorescence was observed on the surface of CX-1 cells (Fig 5A), compared with an intense fluorescence that was evident on MIP-101 cells (Fig5B). Consistent with our findings in Fig 5A and B, flow analysis of CX-1 and MIP-101 cells stained with IgM anti-Fas antibody and anti-IgG FITC-conjugated antibody shows a 90-fold decrease (mean channel, 5.7 for CX-1 cells v 512.8 for MIP-101) in Fas antigen expression at the cell surface of CX-1 cells relative to MIP-101 cells (Fig 6). These results confirm our initial observation that Fas may be downregulated in CX-1 cells, relative to MIP-101 cells.
Immunofluorescence microscopy indicates that CX-1 cells (A) express lower quantities of cell-surface Fas than MIP-101 cells (B). Cells were labeled with mouse anti-Fas antibody and then treated with antimouse FITC-conjugated antibody. Note that CX-1 cells (A) show a discontinuous ring of fluorescence on their cell surface; in contrast, MIP-101 cells (B) stain very intensely with secondary antibody.
Immunofluorescence microscopy indicates that CX-1 cells (A) express lower quantities of cell-surface Fas than MIP-101 cells (B). Cells were labeled with mouse anti-Fas antibody and then treated with antimouse FITC-conjugated antibody. Note that CX-1 cells (A) show a discontinuous ring of fluorescence on their cell surface; in contrast, MIP-101 cells (B) stain very intensely with secondary antibody.
Flow cytometry of MIP-101 and CX-1 human colorectal carcinoma cells. MIP-101 (2 × 103) or CX-1 (2 × 105 ) were treated with murine anti-Fas antibody and then stained with fluorescein-conjugated antimouse antibody as described in the Materials and Methods. MIP-101 (MIP-101) cells display greater fluorescence (mean channel, 512.76) than CX-1 (CX-1) cells (mean channel, 5.73), indicating greater expression of Fas antigen on the latter cell line.
Flow cytometry of MIP-101 and CX-1 human colorectal carcinoma cells. MIP-101 (2 × 103) or CX-1 (2 × 105 ) were treated with murine anti-Fas antibody and then stained with fluorescein-conjugated antimouse antibody as described in the Materials and Methods. MIP-101 (MIP-101) cells display greater fluorescence (mean channel, 512.76) than CX-1 (CX-1) cells (mean channel, 5.73), indicating greater expression of Fas antigen on the latter cell line.
To assess whether Fas is released at a high density on vesicles shed from the surface of CX-1 cells (relative to that from MIP-101 cells), extracellular vesicles were harvested, labeled with anti-Fas antibody plus FITC-conjugated second antibody, and assessed by flow cytometry. In general, labeling of vesicles shed from CX-1 cells was more homogeneous than that of vesicles shed from MIP-101 cells. In two separate studies, the mean ± SD percentage of labeled extracellular vesicles from CX-1 cells was 1.95% ± 0.78%, whereas that for vesicles from MIP-101 cells was 20.0% ± 3.1% (P < .01). Together with electron micrographic results showing the presence of Fas on very few vesicles shed from CX-1 cells (Fig 3), these observations suggest that exfoliation plays a limited role in reducing the level of cell surface Fas. This possibility prompted us to investigate rates of Fas biosynthesis in CX-1 and MIP-101 cells that were labeled with35S-methionine.
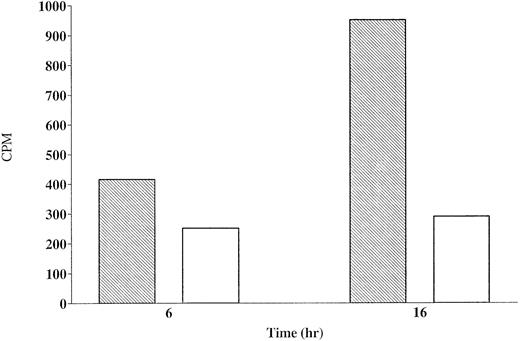
Figure 7 shows the amount of radiolabeled-Fas specifically (cpm bound by anti-Fas minus cpm bound by control antibody) precipitated from lysates obtained from CX-1 or MIP-101 cells. After 6 hours, CX-1 cells synthesize 1.6-fold less Fas than do MIP-101 cells. After 16 hours of incubation, CX-1 cells synthesize 3.3-fold less Fas, relative to MIP-101 cells. These results are supported by autoradiographs of 35S-labeled cells showing the presence of molecular weight 45,000 and 48,000 proteins (corresponding to the molecular masses of differentially glycosylated Fas antigen) immunoprecipitated with anti-Fas antibody and subjected to SDS-PAGE (Fig 8). Together, the results suggest that reduced levels of Fas on extracellular vesicles may be the result of diminished Fas synthesis and consequently, decreased amounts of Fas on plasma membranes from which vesicles are shed.
Synthesis of Fas by CX-1 and MIP-101 cells. CX-1 (□) and MIP-101 (▧) cells were cultured in methionine-free medium for 6 or 16 hours with 300 μCi 35S-methionine. Cells were lysed with Triton X-100 and the lysates were immunoprecipitated sequentially with goat IgG and goat anti-Fas IgG antibody. Radioactivity in aliquots (one fifth of the total sample) of each immunoprecipitated sample was determined by scintillation. Note that Fas synthesis is greater for MIP-101 cells than for CX-1 cells (P < .05). Shown are mean values of a single experiment. Similar results were obtained in two additional studies.
Synthesis of Fas by CX-1 and MIP-101 cells. CX-1 (□) and MIP-101 (▧) cells were cultured in methionine-free medium for 6 or 16 hours with 300 μCi 35S-methionine. Cells were lysed with Triton X-100 and the lysates were immunoprecipitated sequentially with goat IgG and goat anti-Fas IgG antibody. Radioactivity in aliquots (one fifth of the total sample) of each immunoprecipitated sample was determined by scintillation. Note that Fas synthesis is greater for MIP-101 cells than for CX-1 cells (P < .05). Shown are mean values of a single experiment. Similar results were obtained in two additional studies.
Autoradiogram of 35S-labeled, immunoprecipitated protein. Fas antigen immunoprecipitated from lysates of MIP-101 (left) and CX-1 (right) cells incubated for 6 or 16 hours with 300 μCi 35S-methionine were resolved on a 7.5% SDS-polyacrylamide gel by electrophoresis. The autoradiogram was obtained by exposing an x-ray film to the dried gel for 5 days at −80°C.
Autoradiogram of 35S-labeled, immunoprecipitated protein. Fas antigen immunoprecipitated from lysates of MIP-101 (left) and CX-1 (right) cells incubated for 6 or 16 hours with 300 μCi 35S-methionine were resolved on a 7.5% SDS-polyacrylamide gel by electrophoresis. The autoradiogram was obtained by exposing an x-ray film to the dried gel for 5 days at −80°C.
Soluble receptor for Fas is produced by CX-1 and MIP-101 cells.
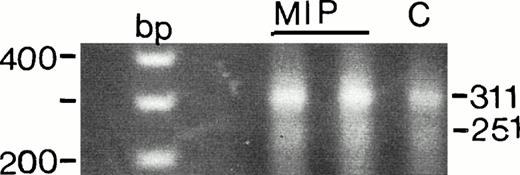
A soluble form of the receptor for Fas that lacks a transmembrane domain may be released from tumor cells via the process of secretion.21,22,34 Release of soluble Fas may provide a mechanism by which malignant cells escape immunodestruction.35 To assess whether MIP-101 or CX-1 cells produce soluble Fas, both cell lines were examined for mRNA encoding a soluble form of the receptor for Fas. Amplification of the entire coding region showed two transcripts, corresponding to the membrane-bound and soluble forms of Fas antigen (Fig 9). RT-PCR showed that, although the major transcript appears to be 311 bp in size (membrane-bound form), a minor transcript of 251 bp (soluble form) is also present in both cell lines.33 No other bands were detected. It is noteworthy that mRNA for both forms of Fas appears to be less abundant in CX-1 cells, relative to that detected in MIP-101 cells, a finding that is similar to the observation that CX-1 cells produce a lower level of membrane-associated Fas receptor than do MIP-101 cells (Figs 5-8).
Detection of Fas antigen splice variants in MIP-101 (MIP) and CX-1 (C) cells by RT-PCR. Two transcripts of 311 and 251 bp corresponding to the membrane-bound form and soluble form of Fas antigen, respectively, are amplified after PCR of first-strand cDNA. PCR products were electrophoresed in 2% agarose and visualized under UV light after staining with ethidium bromide. Note that the intensity of both bands is lower for CX-1 relative to MIP-101 cells, suggesting decreased presence of FAS mRNA in the latter cell line. Also note that the 251-bp band is less intense then the 311-bp band, suggesting that the membrane-bound form of Fas predominates in CX-1 and MIP-101 cells.
Detection of Fas antigen splice variants in MIP-101 (MIP) and CX-1 (C) cells by RT-PCR. Two transcripts of 311 and 251 bp corresponding to the membrane-bound form and soluble form of Fas antigen, respectively, are amplified after PCR of first-strand cDNA. PCR products were electrophoresed in 2% agarose and visualized under UV light after staining with ethidium bromide. Note that the intensity of both bands is lower for CX-1 relative to MIP-101 cells, suggesting decreased presence of FAS mRNA in the latter cell line. Also note that the 251-bp band is less intense then the 311-bp band, suggesting that the membrane-bound form of Fas predominates in CX-1 and MIP-101 cells.
Biological effects of vesicles shed from CX-1 and MIP-101 on anti-Fas–mediated cytotoxicity.
In previous experiments, we observed that CX-1 cells treated with anti-Fas antibody are more sensitive to anti-Fas–mediated cell death than are MIP-101 cells, as assessed by an MTT assay.36Therefore, experiments were performed to determine whether vesicles shed from MIP-101 and CX-1 cells alter sensitivity to cell death induced by anti-Fas antibody. The finding of reversal of anti-Fas antibody-induced apoptosis would suggest that Fas present on shed vesicles is biologically active, analogous to the bioactivity of other molecules expressed on the surface of shed vesicles.37-40
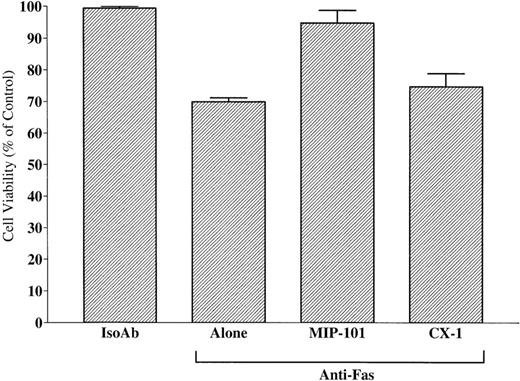
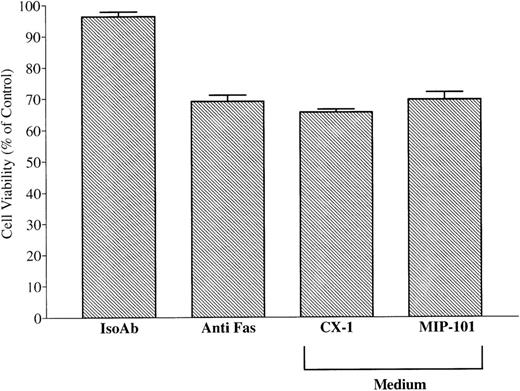
As shown in Fig 10, the viability of CX-1 cells after treatment with anti-Fas IgM alone (69.9% ± 0.7%) was enhanced (P < .01) when these cells were treated with anti-Fas in the presence of vesicles shed from MIP-101 cells (94.9% ± 2.3%). In contrast, addition of anti-Fas IgM to CX-1 cells in the presence of vesicles from medium conditioned by CX-1 cells did not increase cell viability, compared with treatment of CX-1 cells with anti-Fas alone (74.2% ± 2.5% v 69.9% ± 0.7%, respectively; P > .05). These results, together with immunoelectron and immunofluorescence microscopy results showing high Fas antigen density on vesicles derived from MIP-101 cells, indicate that Fas shed on the surface of exfoliated vesicles is biologically active. Moreover, the amount of activity present on shed vesicles correlates with the amount of antigen that can be determined by immunoelectron and immunoflourscence microscopy and by flow cytometry.
Shed vesicles block anti-Fas–mediated CX-1 cell death. Negative and positive controls were established by treating 2 × 105 CX-1 cells with 100 ng noncytotoxic, isotype-matched antibody (IsoAb), or antihuman Fas IgM (Anti-Fas Alone), respectively. Viability of CX-1 cells was assessed in anti-Fas IgM-containing cultures prepared with 40 μg total membrane protein from vesicles shed from MIP-101 or CX-1 cells, as indicated. Note that, although Fas-mediated apoptosis is abolished in the presence of vesicles shed from MIP-101 cells (relative to anti-Fas alone, P < .01), apoptosis is unaffected (P > .10) by the addition of vesicles shed from CX-1 cells. Shown are the mean ± SD values in three separate experiments.
Shed vesicles block anti-Fas–mediated CX-1 cell death. Negative and positive controls were established by treating 2 × 105 CX-1 cells with 100 ng noncytotoxic, isotype-matched antibody (IsoAb), or antihuman Fas IgM (Anti-Fas Alone), respectively. Viability of CX-1 cells was assessed in anti-Fas IgM-containing cultures prepared with 40 μg total membrane protein from vesicles shed from MIP-101 or CX-1 cells, as indicated. Note that, although Fas-mediated apoptosis is abolished in the presence of vesicles shed from MIP-101 cells (relative to anti-Fas alone, P < .01), apoptosis is unaffected (P > .10) by the addition of vesicles shed from CX-1 cells. Shown are the mean ± SD values in three separate experiments.
Bioactivity is undetectable in vesicle-free medium conditioned by CX-1 or MIP-101 cells.
Because CX-1 and MIP-101 cells produce the soluble form of Fas (Fig 9), it is possible that protection from anti-Fas–mediated apoptosis is due to the release of soluble Fas receptor rather than to vesicle-associated Fas. Therefore, serum-free medium conditioned by CX-1 or MIP-100 cells was prepared and centrifuged under conditions that deplete extracellular vesicles but that do not remove soluble proteins of less than 100 kD.38 Vesicle-free medium conditioned by either CX-1 or MIP-101 did not increase the viability of CX-1 cells that were treated with anti-Fas IgM (65.6% ± 1.0% and 69.6% ± 2.0%, respectively), relative to cells treated with anti-Fas IgM plus fresh RPMI medium (65.8% ± 3.4%; Fig 11A). Although these results suggest that physical presentation of Fas in association with a membrane surface is important for expression of biological activity, they do not exclude the possibility that small amounts of bioactive soluble Fas are released from CX-1 and MIP-101 cells that are undetectable in the MTT assay.33
Conditioned medium from MIP-101 and CX-1 cells or HuT 78 fails to protect against anti-Fas–mediated apoptosis or induce apoptosis, respectively. Serum-free medium conditioned by MIP-101, CX-1, or HuT 78 cells was depleted of extracellular vesicles by sequential centrifugation at 800g for 30 minutes and at 100,000g at 8°C for 12 hours. Negative and positive controls were established by treating 2 × 105 CX-1 cells with 100 ng noncytotoxic, isotype-matched IgM (IsoAb), or antihuman Fas IgM (anti-Fas), respectively. (A) CX-1 cells were treated with 100 ng antihuman Fas Ab and 100 μL medium conditioned by CX-1 (CX-1) or MIP-101 (MIP-101) cells, respectively. Note that CX-1 and MIP-101 cell-conditioned medium fails to protect CX-1 cells from anti-Fas–induced cell death. (B) Positive and negative controls were established, as described above. CX-1 cells were treated 100 μL HuT 78 conditioned medium (HuT 78). Note that HuT 78 cell-conditioned medium fails to induce apoptosis of CX-1 cells. Shown are the mean ± SD values in three separate experiments.
Conditioned medium from MIP-101 and CX-1 cells or HuT 78 fails to protect against anti-Fas–mediated apoptosis or induce apoptosis, respectively. Serum-free medium conditioned by MIP-101, CX-1, or HuT 78 cells was depleted of extracellular vesicles by sequential centrifugation at 800g for 30 minutes and at 100,000g at 8°C for 12 hours. Negative and positive controls were established by treating 2 × 105 CX-1 cells with 100 ng noncytotoxic, isotype-matched IgM (IsoAb), or antihuman Fas IgM (anti-Fas), respectively. (A) CX-1 cells were treated with 100 ng antihuman Fas Ab and 100 μL medium conditioned by CX-1 (CX-1) or MIP-101 (MIP-101) cells, respectively. Note that CX-1 and MIP-101 cell-conditioned medium fails to protect CX-1 cells from anti-Fas–induced cell death. (B) Positive and negative controls were established, as described above. CX-1 cells were treated 100 μL HuT 78 conditioned medium (HuT 78). Note that HuT 78 cell-conditioned medium fails to induce apoptosis of CX-1 cells. Shown are the mean ± SD values in three separate experiments.
Visualization of FasL on shed vesicles.
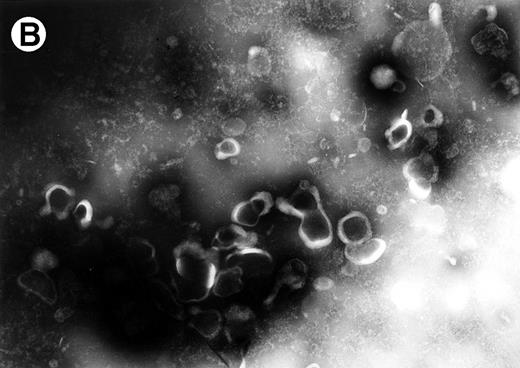
To determine whether FasL is also released on shed vesicles, we examined extracellular vesicles from HuT 78 cells which are known to express high levels of FasL at their cell surface. Vesicles harvested from HuT 78 cell-conditioned medium were labeled with rabbit anti-FasL antibody and antirabbit immunogold-conjugated antibody and examined by electron microscopy. As with vesicles derived from MIP-101 and CX-1 cells, vesicles isolated from HuT 78 cells are heterogenous in shape and texture. As shown in Fig 12A, vesicles labeled with immunogold particles (arrowheads) range in size from 0.2 to 0.4 μm. In control experiments, no labeling was observed for vesicles treated with immunogold-conjugated antibody alone (Fig12B).
Electron micrographs of vesicles shed from HuT 78 cells. Arrowheads point to gold particles in association with FasL on plasma membrane-derived vesicles (A). No labeling is observed if vesicles are treated with immunogold particle-conjugated antibody alone (B). Micrograph (A) is 102,000× magnification and micrograph (B) is at 63,000× magnification.
Electron micrographs of vesicles shed from HuT 78 cells. Arrowheads point to gold particles in association with FasL on plasma membrane-derived vesicles (A). No labeling is observed if vesicles are treated with immunogold particle-conjugated antibody alone (B). Micrograph (A) is 102,000× magnification and micrograph (B) is at 63,000× magnification.
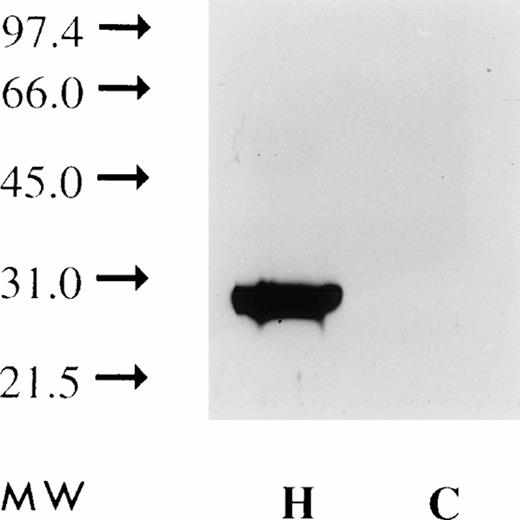
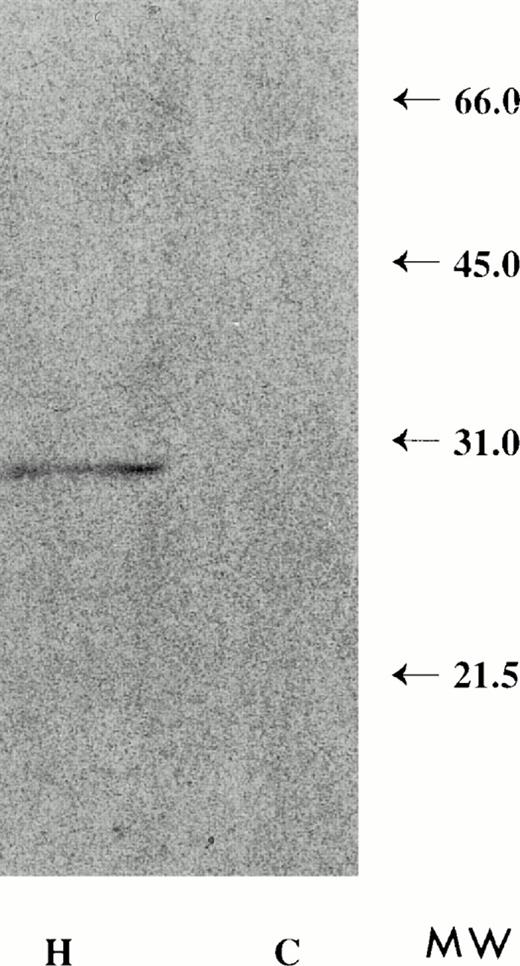
Detergent solubilized plasma membranes or vesicles derived from HuT 78 cells, immunoprecipitated with anti-FasL antibody and subjected to Western blot analysis, show a 30-kD band (Figs 13 and14). The apparent molecular mass of this protein is similar to that of FasL,11 consistent with the notion that FasL protein on shed vesicles and on the plasma membrane are similar in size. In contrast, FasL is not immunoprecipitated from plasma membranes or vesicles derived from the CX-1 colorectal cell line.
Plasma membranes extracted with detergent and subjected to Western blot analysis using anti-FasL antibody (as described in the Materials and Methods) show a single band with an apparent molecular weight of 30 kD (H). Similar analysis of CX-1 plasma membranes shows no reactivity (C).
Plasma membranes extracted with detergent and subjected to Western blot analysis using anti-FasL antibody (as described in the Materials and Methods) show a single band with an apparent molecular weight of 30 kD (H). Similar analysis of CX-1 plasma membranes shows no reactivity (C).
Detergent extracts of HuT 78 plasma membrane-derived extracellular vesicles, immunoprecipitated with anti-FasL antibody and analyzed by Western blot, produce a 30-kD molecular weight band (H). Detergent extracts of vesicles collected from CX-1 conditioned medium fail to show the 30-kD molecular weight band (C).
Detergent extracts of HuT 78 plasma membrane-derived extracellular vesicles, immunoprecipitated with anti-FasL antibody and analyzed by Western blot, produce a 30-kD molecular weight band (H). Detergent extracts of vesicles collected from CX-1 conditioned medium fail to show the 30-kD molecular weight band (C).
Effects of Fas ligand-expressing extracellular vesicles on cell viability.
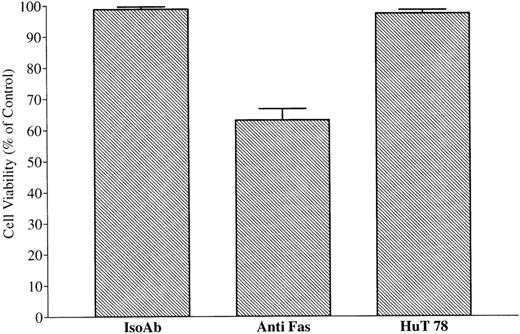
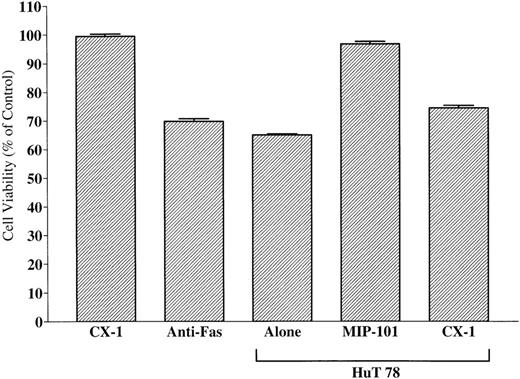
To test whether FasL-bearing vesicles derived from HuT 78 cells display biological activity, CX-1 cells were incubated with FasL-associated vesicles, and their viability was measured 24 hours later, using the MTT assay. As shown in Fig 15, viability of CX-1 cells is decreased by incubation with FasL-bearing vesicles or monoclonal anti-Fas antibody (positive control; P < .01). Cells treated with either FasL-bearing vesicles or monoclonal anti-Fas antibody induce comparable decreases of the percentage of cell viability (69.4% ± 0.6% and 64.4% ± 0.6%, respectively;P > .05). By contrast, viability is unchanged (98.83% ± 0.69%; P > .05) when CX-1 cells are incubated with CX-1–derived extracellular vesicles (negative control). Vesicles shed from the surface of MIP-101 cells (but not those from CX-1 cells) restore nearly full viability (97.5% ± 0.8% v93.9% ± 0.6%, for control CX-1 cells incubated with vesicles shed from CX-1 cells v test CX-1 cells incubated with mixtures of vesicles shed from Hut 78 cells and MIP-101 cells, respectively). Furthermore, depletion of shed vesicles from serum-free medium conditioned by HuT-78 cells results in removal of the biological activity of FasL (Fig 11 B), suggesting that soluble FasL is either inactive or present in an undetectable quantity. Together, these results provide strong evidence that FasL on shed vesicles is biologically active and that FasL interacts with its cognate receptor, Fas, when the two proteins are presented together as components of mixed populations of extracellular vesicles.
Effect of FasL-bearing extracellular vesicles on CX-1 cell viability. Negative and positive controls were established by incubating 2 × 105 CX-1 cells with 40 μg total membrane protein prepared from vesicles shed from CX-1 cells (CX-1) or 100 ng antihuman Fas IgM (Anti-Fas), respectively. Vesicles shed from Hut 78 cells suppress viability by nearly 40% (Hut 78 Alone). Whereas the addition of 40 μg total membrane protein of vesicles shed from MIP-101 cells restores nearly full viability (Hut 78 + MIP-101), the addition of 40 μg total membrane protein of vesicles shed from CX-1 cells has a minimal effect (Hut 78 + CX-1). Note that the viability of cells incubated with mixtures of vesicles prepared from Hut 78 cells and MIP-101 cells is increased, relative to that of cells incubated with mixtures of vesicles prepared from Hut 78 cells and CX-1 cells (P < .01). Shown are the mean ± SD values in three separate experiments.
Effect of FasL-bearing extracellular vesicles on CX-1 cell viability. Negative and positive controls were established by incubating 2 × 105 CX-1 cells with 40 μg total membrane protein prepared from vesicles shed from CX-1 cells (CX-1) or 100 ng antihuman Fas IgM (Anti-Fas), respectively. Vesicles shed from Hut 78 cells suppress viability by nearly 40% (Hut 78 Alone). Whereas the addition of 40 μg total membrane protein of vesicles shed from MIP-101 cells restores nearly full viability (Hut 78 + MIP-101), the addition of 40 μg total membrane protein of vesicles shed from CX-1 cells has a minimal effect (Hut 78 + CX-1). Note that the viability of cells incubated with mixtures of vesicles prepared from Hut 78 cells and MIP-101 cells is increased, relative to that of cells incubated with mixtures of vesicles prepared from Hut 78 cells and CX-1 cells (P < .01). Shown are the mean ± SD values in three separate experiments.
DISCUSSION
Survival and differentiation signals are transmitted among eukaryotic cells via interactions of growth factors released by effector cells in the form of (1) soluble cytokines and/or (2) shed vesicle-bound growth factors, with cognate receptors expressed on the target cell surface.24,41 Alternatively, membrane-bound growth regulators are presented to cognate receptors via direct physical interactions at the cell surface, a process referred to as juxtacrine communication.42 Comparably, apoptosis signals are transmitted by soluble factors (eg, TNF-α), by perforin-containing vesicles, or by direct physical interaction of Fas ligand expressed on cytotoxic T lymphocytes and NK cells with Fas receptor present on target cells.43-45
Our finding that bioactive Fas and FasL are shed on vesicles derived from the plasma-membrane of MIP-101 cells and HuT 78 cells, respectively, is in accord with the notion that membrane-bound growth factors, such as mBPA, macrophage–colony-stimulating factor (M-CSF), and flt3-flk2, are released on extracellular vesicles where they express biological activity.46-49 The release of cell-surface molecules on shed vesicles may provide a mechanism for communication among cells that are not necessarily adjacent to each other but in which long-lasting stimulation can be achieved. Proximal interactions mediated by signals carried on extracellular vesicles may be analogous to juxtacrine communication between effector cells and target cells. Ligand-bearing extracellular vesicles may permit long-range interactions while still maintaining effector molecules concentrated on a membrane surface, thereby restraining their dilution in the pericellular environment.
We determined here that vesicles shed from the surface of viable MIP-101, CX-1, and HuT-78 cells display Fas of FasL. Because it is possible that extracellular vesicles are themselves released from potentially rare cells undergoing apoptosis during short-term culture, it was important to distinguish shed vesicles from apoptotic bodies. Unlike shed vesicles, apoptotic bodies are plasma membrane bound structures that contain nuclear fragments and well-preserved organelles such as mitochondria and endoplasmic reticulum.50,51 In virtually all fields examined by electron microscopy, no evidence was found for the presence of either nuclear material or organelles (intact or partially degraded) within extracellular vesicles (Figs 2, 3, and12). Accordingly, vesicles released from MIP-101, CX-1, and HuT-78 cells are morphologically identical to vesicles shed from normal human lymphocytes, which, in previous studies, we have found to contain no cytoplasmic markers.38 47
The release of proteins on exfoliated membrane vesicles is dependent on intracellular processes, including mRNA synthesis and translation, as well as posttranslational modification.31 Because shedding from the cell surface requires energy and active cell metabolism and occurs from distinct regions of the plasma membrane, exfoliation is a directed (rather than random) process.46,47 Although the mechanism of exfoliation is not completely understood, we have observed that contractile proteins participate in the exfoliation of cytokines in association with shed vesicles.52 Furthermore, the observations that nonmetastatic cells shed less then their metastatic counterparts,53 coupled with the findings that highly metastatic cells exhibit fewer associations between actin and vinculin and the plasma membrane,54 suggest that disruption of cytoskeletal elements may be a prerequisite for extracellular vesicle formation. Calpain, an intracellular calcium dependent protease, demonstrates high specificity for anchor cytoskeletal elements,55 and its activation has been directly implicated with increased shedding of plasma membrane proteins.56Phospholipases may also play a role in the release of vesicles from the cell-surface. Before the release of plasma membrane-derived vesicles, fusion must take place of closely approximated membranes at the base of the vesicle. Phospolipases may mediate the fusion of adjacent regions of the membrane by generating detergentlike molecules (lysophosopholipids) that enhance interactions among membrane structures.57
Exfoliation provides a mechanism of cell-cell signal trafficking for not only cytokines but also other molecules, including the transferrin receptor,58 C3 component of the complement system, fragment crystallizable (Fc) domain of antibodies,59 as well as class I and class II major histocompatibility (MHC) antigens.40,60 It has been suggested that survival and metastatic propensity of many tumor cell types are enhanced by downregulation of cell-surface tumor antigens, thereby permitting escape from immune recognition and destruction.57,61Studies showing that, in some cases, high metastatic potential cell lines shed greater amounts of plasma membrane-derived vesicles than do their low metastatic potential counterparts have strengthened this concept.62 63 The shedding patterns we observed for high (CX-1) versus low (MIP-101) metastatic potential cells (Fig 1) are consistent with this notion. Whether the increased level of shedding from CX-1 cells protects against imunne recognition and/or facilitates metastasis remains to be investigated.
The physiologic significance of circulation soluble Fas in sera from patients with malignant tumors is unclear. One hypothesis is that its presence impairs apoptotic death of cancer cells initiated by T and NK cells. For example, Hughes and Crispe64 showed that a soluble isoform of murine Fas (generated by alternative splicing of Fas mRNA) physiologically limits apoptosis that is induced by Fas-Fas ligand association. Nevertheless, the source of soluble Fas is not always clear. Knipping et al20 reported that soluble Fas harvested from medium conditioned by B-lymphoblastoid cells migrates to the same position as the membrane-bound form of Fas when electrophoresed in polyacrylamide. These investigators concluded that soluble Fas is not generated by simple proteolytic cleavage from the cell surface, but rather that soluble Fas is secreted into the medium, possibly because it may lack a transmembrane domain.20Whereas mRNA for soluble Fas is present in CX-1 and MIP-101 cells (Fig9), results of vesicle-depletion experiments suggest that the soluble form of the receptor may be biologically inactive, using the MTT assay (Fig 11). On the other hand, it is well known that vesicles expressing immune regulatory molecules are shed in vivo.65,66 Our results showing that Fas is present on vesicles shed from the surface of MIP-101 cells (Fig 2) raise the possibility that soluble Fas found in human serum may be associated with circulating shed vesicles. Indeed, methods used by Knipping et al20 to isolate soluble Fas are nearly identical to those we have used for isolation of vesicles from conditioned medium in this study and from sera of leukemia patients.38,67 Moreover, vesicle-associated Fas is similar in molecular mass (45 and 48 kD; Fig4) to soluble Fas found in human serum (48 to 52 kD20).
We observed that CX-1 cells express a lower quantity of cell-surface Fas when compared with MIP-101 cells. This finding was confirmed by immunofluorescence studies (Figs 5 and 6). We further show that CX-1 cells synthesize Fas at a lower rate than do MIP-101 cells (Fig 7). However, low metastatic potential MIP-101 cells are more resistant to anti-Fas antibody-mediated apoptosis than are CX-1 cells. This paradox may be explained by our finding that MIP-101 cells release plasma membrane-derived vesicles that contain a greater level of Fas than that present on vesicles shed from the surface of CX-1 cells (Fig 3), even though shedding rates are lower for MIP-101 cells (Fig 1). Thus, MIP-101 cells may release vesicles bearing high levels of Fas that effectively compete with cell surface Fas for binding of anti-Fas antibody, thereby blocking Fas-mediated cell death. In such a scenario, anti-Fas antibody added to a culture of MIP-101 cells is engaged by vesicle-associated Fas and, despite elevated expression of cell surface Fas (relative to CX-1 cells), MIP-101 cells escape anti-Fas antibody-mediated apoptosis. This model predicts that extracellular vesicles released from CX-1 cells having low levels of Fas will not effectively protect against anti-Fas antibody-induced apoptosis, a result that was observed in our studies (Fig 10). Moreover, vesicles derived from MIP-101 cells (but not those from CX-1 cells) inhibit CX-1 cell death induced by FasL-bearing vesicles derived from HuT 78 cells (Fig 15), activated T cells whose surface expresses FasL (Fig 13). These results suggest that, like Fas expressed on MIP-101 cells, FasL expressed on HuT 78 cells is released on extracellular vesicles and is present in a bioactive conformation. Accordingly, vesicle-bound FasL appears to be capable of interacting with vesicle-bound Fas or cell-surface Fas.
Recently, Sato et al68 have shown that lymphoma cells express and release soluble FasL into the circulation in vivo, an event that may be associated with development of liver damage and pancytopenia. Their results are consistent with an earlier publication by Tanaka et al23 demonstrating that individuals with large granular lymphocytic leukemia or NK cell lymphoma are susceptible to systematic tissue damage resulting from the action of a metalloprotease that cleaves cell-surface FasL and releases a soluble variant of the ligand into the circulation of these patients. Our findings indicate that FasL is also released from the cell-surface on exfoliated vesicles (Figs 12, 13, and 14). Whether release of FasL on vesicles shed from malignant cells occurs in vivo remains unknown.
In summary, our results provide evidence for an alternative mechanism for the release of Fas and its natural ligand from the surface of cells derived from human cancers. We show that a colorectal carcinoma cell line (MIP-101) releases Fas on plasma membrane-derived vesicles that are shed in vitro and that are capable of neutralizing apoptosis induced by anti-Fas antibody or FasL. Furthermore, FasL is also released on exfoliated vesicles in a bioactive conformation. These findings add to the growing body of evidence suggesting that Fas released from the cell-surface of tumor cells can potentially act to evade detection by immune cells. The results support the notion that exfoliation facilitates the presentation of signals for cell survival/death on the membrane surface of effector cells.
Supported in part by the Bayer/Canadian Red Cross Society Research and Development Fund and by Grant No. 00014-94-0049 issued to Georgetown University from the Office of Naval Research in support of the International Consortium for Research on the Health Effects of Radiation.
Address reprint requests to Nicholas Dainiak, MD, Department of Medicine, Bridgeport Hospital, 267 Grant St, Bridgeport, CT 06610.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal