Abstract
Macrophages and dendritic cells derive from a hematopoietic stem cell and the existence of a common committed progenitor has been hypothesized. We have recently found in normal human marrow a subset of CD34+ cells that constitutively expresses HLA-DR and low levels of CD86, a natural ligand for the T cell costimulation receptor CD28. This CD34+ subset can elicit responses from allogeneic T cells. In this study, we show that CD34+/CD86+ cells can also present tetanus toxoid antigen to memory CD4+ T cells. CD86 is expressed at low levels in macrophages and high levels in dendritic cells. Therefore, we have tested the hypothesis that CD34+/CD86+ cells are the common precursors of both macrophages and dendritic cells. CD34+/CD86+ marrow cells cultured in granulocyte-macrophage colony-stimulating factor (GM-CSF)–generated macrophages. In contrast, CD34+/CD86− cells cultured in GM-CSF generated a predominant population of granulocytes. CD34+/CD86+ cells cultured in GM-CSF plus tumor necrosis factor-α (TNF-α) generated almost exclusively CD1a+/CD83+ dendritic cells. In contrast, CD34+/CD86− cells cultured in GM-CSF plus TNF-α generated a variety of cell types, including a small population of dendritic cells. In addition, CD34+/CD86+ cells cultured in granulocyte colony-stimulating factor failed to generate CD15+granulocytes. Therefore, CD34+/CD86+ cells are committed precursors of both macrophages and dendritic cells. The ontogeny of dendritic cells was recapitulated by stimulation of CD34+/CD86− cells with TNF-α that induced expression of CD86. Subsequent costimulation of CD86+cells with GM-CSF plus TNF-α lead to expression of CD83 and produced terminal dendritic cell differentiation. Thus, expression of CD86 on hematopoietic progenitor cells is regulated by TNF-α and denotes differentiation towards the macrophage or dendritic cell lineages.
B7-2 (CD86) IS THE MAJOR functional ligand for CD28 and CTLA-4, critical T-cell signaling molecules that determine whether antigen stimulation results in immunity or tolerance.1-3 CD86 is expressed on the surface of murine and human antigen-presenting cells (APC), constitutively at low levels on monocytes/macrophages and at high levels on dendritic cells. After cellular activation, CD86 is upregulated on macrophages and dendritic cells and induced on B cells.4-6 We have recently found that CD86 is also expressed constitutively on a small subset of CD34+ human marrow cells.7 Among other CD34+ hematopoietic cells, CD34+/CD86+ cells are unique in their ability to present alloantigen to T cells. These data have led us to formulate the hypothesis that CD86 expression on CD34+ cells is the first evidence of commitment to the macrophage and dendritic cell lineages.7 The existence of a common progenitor for macrophages and dendritic cells was first suggested by Reid et al,8 who observed that mixed colonies could be cultured from human marrow or blood mononuclear cells in the presence of lymphocyte-conditioned medium.9
The development of recombinant hematopoietic growth factors has allowed the identification of differentiation pathways for many cell types.10 Granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes the expansion of granulocytes and macrophages from committed myeloid precursors.10 The cooperation between GM-CSF and tumor necrosis factor-α (TNF-α) is crucial for the generation of human dendritic/Langerhans cells from CD34+hematopoietic progenitors.8,11,12 Stem cell factor (SCF) alone is without effect on colony formation, but it enhances both the number and size of macrophage and dendritic cell colonies generated in vitro by GM-CSF and TNF-α.13 Therefore, recent progress has allowed us to test the hypothesis that CD34+/CD86+ cells are progenitors committed to differentiate into macrophages and dendritic cells, but beyond the ability to differentiate into granulocytes.
In this report, we demonstrate first that CD34+/CD86+ marrow cells can present antigenic protein to CD4+ T cells and are therefore capable of APC function. We show second that CD34+/CD86+ cells differentiate into macrophages, but not granulocytes, upon exposure to GM-CSF. Third, we show that CD34+/CD86+ cells differentiate into CD1a+/CD83+ dendritic cells after exposure to GM-CSF plus TNF-α. Finally, we demonstrate that TNF-α induces expression of CD86 on CD34+/CD86− cells and promotes commitment of this population to the dendritic lineage. Thus, CD34+/CD86+ cells are immunocompetent APC and are committed precursors of macrophages and dendritic cells.
MATERIALS AND METHODS
Bone marrow samples.
Bone marrow samples were obtained at Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) after obtaining informed consent from healthy donors. All samples were processed immediately after being drawn. Bone marrow mononuclear cells were isolated by ficoll-hypaque density gradient centrifugation at 1,000g for 20 minutes.
Cell separation.
CD34+ marrow cells were purified by adherence of anti-CD34-biotin–labeled cells to an immunoaffinity column of avidin-coated beads (Ceprate LC kit; kindly provided by CellPro, Inc, Bothell, WA). Briefly, marrow mononuclear cells were obtained by density-gradient centrifugation on ficoll-hypaque, washed twice with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), resuspended at 108 cells/mL, incubated with biotinylated anti-CD34 monoclonal antibody (MoAb) 12.8 for 25 minutes at room temperature, washed again, resuspended in 5% BSA, and loaded onto the CellPro Ceprate column. Absorbed CD34+ cells were detached from the avidin-coated beads by manually squeezing the column. CD34+ cells were incubated with IgG1 anti-CD34 MoAb HPCA-2-fluorescein isothiocyanate (FITC) and with IgG2b anti-CD86 MoAb IT2.2-PE for 30 minutes at 4°C. CD34+/CD86+ and CD34+/CD86− cells were separated by fluorescence activated cell sorting (FACS) using a FACS Vantage (Becton Dickinson, San Jose, CA). Aliquots of sorted fractions were reanalyzed for light scatter and fluorescence to verify their purity. T cells were obtained from peripheral blood mononuclear cells of HLA-DR-typed normal adult volunteers and enriched by passing through a nylon wool column. For antigen presentation assays, CD4+ cells were purified by incubation of peripheral blood mononuclear cells with anti-CD4 MoAb SK3-PE followed by cell sorting using a FACS Vantage.
Culture media.
For expansion of monocytes and dendritic cells, purified CD34+/CD86+ and CD34+/CD86− cells were cultured in 24-well plates at 3 to 15 × 103 cells/mL in Iscove's media (FHCRC) containing 20% heat-inactivated fetal calf serum (FCS; Hyclone Lab, Logan, UT), 50 μg/mL gentamicin (Lyphomed, Deerfield, IL), and 7.3 × 10−5 mol/L monothioglycerol (Sigma Chemical Co, St Louis, MO). The following cytokines were added: 20 ng/mL SCF and 100 ng/mL human recombinant GM-CSF (provided by Dr Robert Andrews, FHCRC, Seattle, WA), 10 ng/mL human granulocyte colony-stimulating factor (G-CSF; Amgen Inc, Thousand Oaks, CA), and 10 to 50 ng/mL human recombinant TNF-α (R&D Systems, Minneapolis, MN).14 Cultured cells were harvested from the bottom of the well by scraping with the rubber-tipped plunger from a 1-mL syringe. Functional assays were established in RPMI-HEPES enriched with 15% pooled human serum (PHS), 100 U/mL penicillin-streptomycin, 100 U/mL L-glutamine, and 1 mmol/L Na-pyruvate (GIBCO BRL, Grand Island, NY).
Assays for antigen presentation.
Tetanus toxoid (1 μg/mL; Lift Biological Laboratories, Inc, Campbell, CA) was used to test for antigen-specific T-cell responses in preimmunized individuals. Purified CD34+/CD86+and CD34+/CD86− cells were tested for APC function. After FACS, APC were washed, resuspended in 10% FCS, and irradiated at 3,000 cGy. Proliferation assays were set-up in 96-well V-bottom plates with 2 × 104 FACS-sorted autologous CD4+ cells per well in RPMI-HEPES containing 10% FCS. On day 5, cells were harvested after exposure to 3H-thymidine (1 μCi/well) for 18 hours.
Primary mixed leukocyte culture (MLC).
Fresh or cultured CD34+/CD86+ and CD34+/CD86− cells were tested in MLC for stimulatory activity. Stimulator cells were suspended in 15% PHS and irradiated at 3,000 cGy, and serial dilutions were prepared beginning at 2 to 5 × 103 cells/well. Responder T cells were plated at 5 × 104 cells/well with stimulator cells in round-bottomed 96-well plates. Cultures were maintained in a humidified atmosphere at 37°C and 5% CO2. Cells were pulsed with 1 μCi/well 3H-thymidine for 18 hours before harvest on day 6 to measure proliferation.
Limiting dilution assay (LDA).
Purified stimulator cells were suspended in 15% PHS, irradiated (,3000 cGy), and dispensed in 24 replicates at 250, 125, 63, 32, 16, 8, and 4 cells/well in V-bottomed plates with responding T lymphocytes from normal HLA-DR–incompatible volunteers. Responders were plated at 1 × 104 cells/well with stimulator cells or with medium alone. Cultures were maintained in a humidified atmosphere at 37°C and 5% CO2. Cells were pulsed with 1 μCi/well3H-thymidine for the last 18 hours in culture and harvested on day 6 to measure proliferation. The LDA is a quantal dose-response assay in which an immune response is measured for individual cultures that vary in the number of cells tested.15 In our assay, the number of stimulator cells is varied with 24 replicates for each dose. Wells were considered positive if counts per minute (CPM) were greater than the mean plus 3 SD over the negative control given by responder cells cultured in medium without stimulator cells. The frequency of MLC-stimulating cells was calculated according to Taswell15 as the reciprocal of the number of stimulator cells resulting in 37% nonresponding wells. The χ2minimization method was used to assess the probability that data fit to a Poisson model, as described.15
Cell surface phenotyping, cytochemical staining, and microscopic analysis.
MoAbs used for immunofluorescence experiments in this study are listed in Table 1. Nonreactive MoAbs of the same isotype and subclass were used as controls. Incubation of MoAbs with cell populations were performed in 1% BSA in PBS at 4°C for 30 minutes. Stained cells were analyzed by cytofluorography on a FACScan (Becton Dickinson). Wright-Giemsa staining was performed on cytospins of cultured cells. Cells were suspended in 10% FCS at 1 × 105 cells/mL. Slides were photographed at 100× magnification with an oil immersion lens. Phase contrast photographs were taken at 20× amplification with standard lens.
MoAbs Used in This Study
| Specificity . | Clone . | Isotype . | Conjugate . | Source . |
|---|---|---|---|---|
| CD1a | OKT6 | IgG1 | FITC | Ortho Diagnostic System (Raritan, NJ) |
| CD4 | SK3 | IgG1 | PE | Becton Dickinson |
| CD14 | MoP9 | IgG2b | FITC, PE | Becton Dickinson |
| CD15 | V1MC6 | IgM | FITC | Caltag (Burlingame, CA) |
| CD34 | HPCA-2 | IgG1 | FITC, PE | Becton Dickinson |
| CD80 | L30T.4 | IgG1 | PE | Becton Dickinson |
| CD83-150 | HB-15a | IgG2b | None | Duke University Medical Center (Durham, NC) |
| CD86 | IT2.2 | IgG2b | PE | PharMingen (San Diego, CA) |
| HLA-DR | L243 | IgG2a | PerCP | Becton Dickinson |
| Specificity . | Clone . | Isotype . | Conjugate . | Source . |
|---|---|---|---|---|
| CD1a | OKT6 | IgG1 | FITC | Ortho Diagnostic System (Raritan, NJ) |
| CD4 | SK3 | IgG1 | PE | Becton Dickinson |
| CD14 | MoP9 | IgG2b | FITC, PE | Becton Dickinson |
| CD15 | V1MC6 | IgM | FITC | Caltag (Burlingame, CA) |
| CD34 | HPCA-2 | IgG1 | FITC, PE | Becton Dickinson |
| CD80 | L30T.4 | IgG1 | PE | Becton Dickinson |
| CD83-150 | HB-15a | IgG2b | None | Duke University Medical Center (Durham, NC) |
| CD86 | IT2.2 | IgG2b | PE | PharMingen (San Diego, CA) |
| HLA-DR | L243 | IgG2a | PerCP | Becton Dickinson |
Abbreviations: FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll protein.
Binding by unlabeled HB-15a was detected by PE-conjugated goat-antimouse IgG2b antibody (Southern Biotechnology Associates, Inc, Birmingham, AL).
Phagocytosis assays.
Nile Red imbedded latex particles (2-μm microspheres; Molecular Probes, Eugene, OR) were opsonized in 50% Ultraserum (Gemini Bio-Products, Inc, Calabasas, CA) by incubation at 37°C for 30 minutes, followed by washing with RPMI-HEPES. Cells (1 to 2 × 104) were incubated for 2 hours at 37°C with opsonized particles at 1:100 (cells:beads) in 10% FCS at a final volume of 200 μL. Cells were washed by resuspending cells in PBS, underlaying the suspension with 100% FCS, and centrifugation at 1,000 RPM for 10 minutes followed by several washes in PBS. Negative controls were cells not exposed to latex particles. Cells were analyzed by cytofluorography.
TNF-primed cells.
CD34+/CD86− cells (5 × 104 cells/mL) were cultured in media containing SCF and TNF-α for 6 days to induce expression of CD86. CD86+ and CD86− cells were separated by FACS and recultured in media containing SCF and GM-CSF, in the presence or absence of TNF-α, at 1 to 4 × 104 cells/mL for an additional 6 days.
RESULTS
Antigen presentation by CD34+/CD86+ marrow cells.
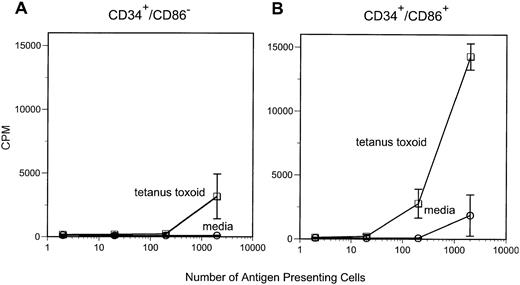
We have previously demonstrated that CD34+/CD86+ marrow cells are capable of stimulating proliferative responses of purified allogeneic T cells.7 In this study, we tested the ability of CD34+/CD86+ cells to present soluble antigen to memory T cells. Purified CD34+/CD86+ or CD34+/CD86− cells were irradiated (3,000 cGy) and cultured with autologous purified CD4+ T cells from tetanus toxoid-immunized donors in media or in the presence of tetanus toxoid protein. Marrow CD34+/CD86+cells were competent at presenting tetanus toxoid and inducing T-cell proliferation (Fig 1). In contrast, CD34+/CD86− cells had consistently less APC activity and, in some experiments, CD34+/CD86− cells completely lacked the ability to present tetanus toxoid antigen. These results indicate that CD34+/CD86+ marrow cells can capture and process antigenic protein, present peptides to memory CD4+T cells, and induce a proliferative response.
CD34+/CD86+ cells can present tetanus toxoid antigen to autologous CD4+ T cells. APC were irradiated (3,000 cGy) and cultured with purified autologous CD4+ T cells in either media alone or in the presence of 1 μg/mL tetanus toxoid. Proliferation was measured on day 5 after 18 hours of labeling with 3H-thymidine.
CD34+/CD86+ cells can present tetanus toxoid antigen to autologous CD4+ T cells. APC were irradiated (3,000 cGy) and cultured with purified autologous CD4+ T cells in either media alone or in the presence of 1 μg/mL tetanus toxoid. Proliferation was measured on day 5 after 18 hours of labeling with 3H-thymidine.
Generation of macrophages from CD34+/CD86+precursors.
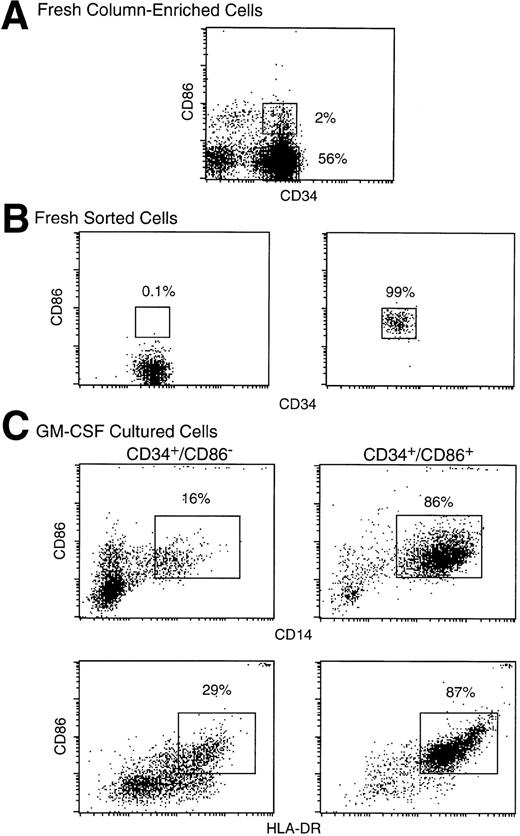
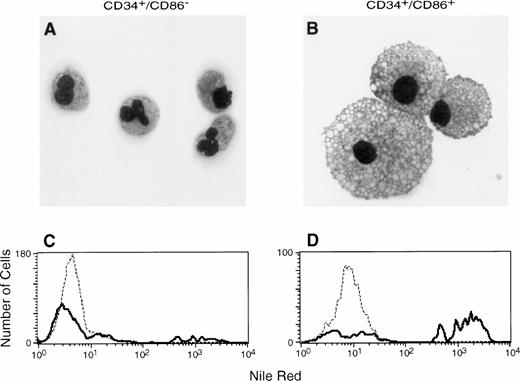
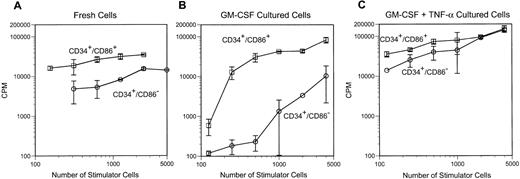
To determine if CD34+/CD86+ cells are committed to the macrophage lineage, CD34-enriched marrow cells (Fig 2A) were FACS-sorted and purified CD34+/CD86+ and CD34+/CD86− cells (Fig 2B) were cultured in SCF and GM-CSF. By day 9, CD34+/CD86+ cells expanded 14- ± 4-fold and CD34+/CD86−cells expanded 16- ± 4-fold (n = 3). GM-CSF–cultured CD34+/CD86+ cells generated a population of cells containing predominantly CD14+, HLA-DR+, and CD86 dimly positive macrophages (Fig 2C). Only a small proportion of the population (5% ± 4%, n = 6) expressed CD1a, and there were no cells expressing high levels of CD86 and HLA-DR, typical of cultured dendritic cells. In contrast, the progeny of CD34+/CD86− cells contained significantly fewer macrophages. Cultured CD34+/CD86+ and CD34+/CD86− cells were harvested, cytocentrifuged, and stained with Wright-Giemsa. The progeny of CD34+/CD86− cells contained granulocytes at various stages of differentiation, from promyelocytes to mature neutrophils (Fig 3A). In contrast, the majority of the CD34+/CD86+ cell progeny had the typical morphology of activated macrophages (Fig 3B). To test for phagocytosis, cells were incubated with opsonized latex particles for 2 hours at 37°C and the percentage of phagocytic cells was determined by flow microfluorimetry. More CD34+/CD86+-derived cells internalized latex particles compared with CD34+/CD86−-derived cells (34% ± 24% v 9% ± 3% [n = 3]; Fig 3C and D). GM-CSF–cultured cells were also tested for their ability to stimulate allogeneic T cells. Cells were harvested from cultures, irradiated, added in serial concentration to nylon wool-purified allogeneic T cells, and cultured for 6 days. CD34+/CD86+-derived cells maintained high levels of stimulatory activity compared with freshly isolated CD34+/CD86+ cells (Fig 4A and B). In contrast, cells derived from CD34+/CD86− progenitors cultured with GM-CSF had less stimulatory activity than fresh CD34+/CD86− cells. Therefore, morphological, phenotypical, and functional data demonstrated that monocyte/macrophages were generated in higher proportion from GM-CSF–cultured CD34+/CD86+ cells than CD34+/CD86− cells.
CD34+/CD86+ cells cultured in SCF plus GM-CSF acquire the surface phenotype of monocytes. Human marrow CD34+ cells were enriched by column immunoabsorption (A). CD34+/CD86+ and CD34+/CD86− cells were purified by FACS-sorting through the gates shown in (A) and cultured for 9 days in SCF and GM-CSF. Sorted fresh (B) or cultured (C) cells were stained and analyzed by three-color microfluorimetry.
CD34+/CD86+ cells cultured in SCF plus GM-CSF acquire the surface phenotype of monocytes. Human marrow CD34+ cells were enriched by column immunoabsorption (A). CD34+/CD86+ and CD34+/CD86− cells were purified by FACS-sorting through the gates shown in (A) and cultured for 9 days in SCF and GM-CSF. Sorted fresh (B) or cultured (C) cells were stained and analyzed by three-color microfluorimetry.
CD34+/CD86+ cells cultured in SCF plus GM-CSF acquire the morphology and phagocytic properties of macrophages. Cytospins were prepared after 9 days of culture in the presence of SCF and GM-CSF, followed by Wright-Giemsa staining and microscopic analysis. CD34+/CD86−precursors gave origin predominantly to granulocytes, in addition to a small number of monocytes and macrophages (A; 100× amplification using oil immersion). CD34+/CD86+precursors generated macrophages (B). In a phagocytosis assay, latex particles were internalized by 12% of CD34+/CD86− cell progeny stimulated by SCF plus GM-CSF (C) compared with 61% of CD34+/CD86+ cell progeny stimulated by SCF plus GM-CSF (D).
CD34+/CD86+ cells cultured in SCF plus GM-CSF acquire the morphology and phagocytic properties of macrophages. Cytospins were prepared after 9 days of culture in the presence of SCF and GM-CSF, followed by Wright-Giemsa staining and microscopic analysis. CD34+/CD86−precursors gave origin predominantly to granulocytes, in addition to a small number of monocytes and macrophages (A; 100× amplification using oil immersion). CD34+/CD86+precursors generated macrophages (B). In a phagocytosis assay, latex particles were internalized by 12% of CD34+/CD86− cell progeny stimulated by SCF plus GM-CSF (C) compared with 61% of CD34+/CD86+ cell progeny stimulated by SCF plus GM-CSF (D).
The progeny of GM-CSF and GM-CSF/TNF-α–cultured CD34+/CD86+ cells induce proliferative response of allogeneic CD4+ cells. CD34+/CD86+ and CD34+/CD86− cells were cultured for 9 days in GM-CSF plus SCF, in the absence (B) or presence (C) of TNF-α. Fresh (A) and cultured cells (B and C) were resuspended in 15% PHS. Cells were irradiated at 3,000 cGy, and serial dilutions were cultured with HLA-DR–mismatched CD4+ T cells at 5 × 104 responders/well in U-bottomed plates. Cells were harvested on day 6 after 18 hours of exposure to3H-thymidine.
The progeny of GM-CSF and GM-CSF/TNF-α–cultured CD34+/CD86+ cells induce proliferative response of allogeneic CD4+ cells. CD34+/CD86+ and CD34+/CD86− cells were cultured for 9 days in GM-CSF plus SCF, in the absence (B) or presence (C) of TNF-α. Fresh (A) and cultured cells (B and C) were resuspended in 15% PHS. Cells were irradiated at 3,000 cGy, and serial dilutions were cultured with HLA-DR–mismatched CD4+ T cells at 5 × 104 responders/well in U-bottomed plates. Cells were harvested on day 6 after 18 hours of exposure to3H-thymidine.
Generation of dendritic cells from CD34+/CD86+ precursors.
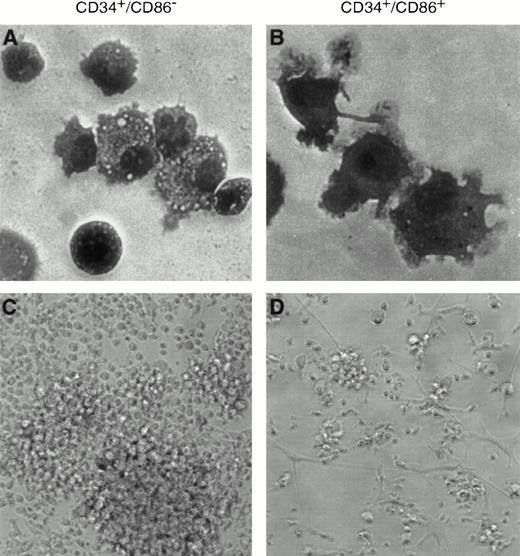
We wanted to determine if CD34+/CD86+ cells were also precursors of dendritic cells. When cultured in TNF-α, GM-CSF, and SCF for 9 to 14 days, CD34+/CD86− cells were expanded 212- ± 140-fold, whereas CD34+/CD86+ cells were expanded 15- ± 7-fold (n = 3). The cell surface phenotype of TNF-α, GM-CSF, and SCF-cultured CD34+/CD86+ cells was consistent with their dendritic morphology. Cells were 60% to 76% CD1a+ (n = 6), with the majority of the population being CD83+ and bright for both HLA-DR and CD86 (Fig 5). A smaller percentage of cells with the phenotype of dendritic cells were generated from CD34+/CD86− precursors: cells were 14% to 33% CD1a+, dim for HLA-DR and CD86, and negative for CD83. The progeny of CD34+/CD86+cells contained a predominant population of cells with prominent cytoplasmic projections typical of dendritic cells (Fig 6B and D). In contrast, CD34+/CD86− cells gave origin to a polymorphic population containing a variety of cell types (Fig 6A and C).
CD34+/CD86+ cells cultured in TNF-α, GM-CSF, and SCF acquire the surface phenotype of dendritic cells. After 14 days of culture in TNF-α, GM-CSF, and SCF, CD34+/CD86− and CD34+/CD86+-derived cells were stained and analyzed by three-color microfluorimetry.
CD34+/CD86+ cells cultured in TNF-α, GM-CSF, and SCF acquire the surface phenotype of dendritic cells. After 14 days of culture in TNF-α, GM-CSF, and SCF, CD34+/CD86− and CD34+/CD86+-derived cells were stained and analyzed by three-color microfluorimetry.
Dendritic cells are generated by stimulation of CD34+/CD86+ cells with TNF-α, GM-CSF, and SCF. CD34+/CD86− (A and C) and CD34+/CD86+ (B and D) cells were cultured in the presence of TNF-α, GM-CSF, and SCF for 9 days. Cells were either Wright-Giemsa–stained and photographed at 100× amplification using oil immersion (A and B) or cells were photographed at 20× amplification by phase contrast (C and D). CD34+/CD86− precursors generated a heterogeneous population of cells. In contrast, CD34+/CD86+ cells generated predominantly cells with dendritic morphology.
Dendritic cells are generated by stimulation of CD34+/CD86+ cells with TNF-α, GM-CSF, and SCF. CD34+/CD86− (A and C) and CD34+/CD86+ (B and D) cells were cultured in the presence of TNF-α, GM-CSF, and SCF for 9 days. Cells were either Wright-Giemsa–stained and photographed at 100× amplification using oil immersion (A and B) or cells were photographed at 20× amplification by phase contrast (C and D). CD34+/CD86− precursors generated a heterogeneous population of cells. In contrast, CD34+/CD86+ cells generated predominantly cells with dendritic morphology.
MLC was used to determine the antigen-presenting capacity of fresh CD34+/CD86+ and CD34+/CD86− cells and cells cultured in TNF-α, GM-CSF, and SCF. Purified CD34+/CD86+and CD34+/CD86− cells were set up in limited dilution cultures with nylon wool purified responder T cells from HLA-DR–incompatible donors. A single-hit Poisson model was used to estimate the frequency of immunocompetent APC from dose-response data.15 The frequency of cells able to elicit an alloimmune response was 3.55% ± 0.50% in fresh CD34+/CD86+ cells and 0.68% ± 0.04% in fresh CD34+/CD86− cells (n = 3; Fig 7A). Therefore, the CD34+/CD86+ population contained more cells able to elicit an alloimmune response. The alloantigen-presenting activity was also determined for cells cultured in growth factors for 12 days. The frequency of allostimulating cells was 12.8% ± 11.0% among CD34+/CD86+-derived cells and 1.89% ± 1.85% among CD34+/CD86−-derived cells (Fig 7B). A bulk MLC also demonstrated that, at low numbers of stimulator cells, the CD34+/CD86+ progeny was a more potent stimulator of allogeneic CD4+ T cells than the CD34+/CD86− progeny (Fig 4C). Therefore, morphology, surface phenotype, and APC function demonstrated that a higher proportion of dendritic cells were derived from CD34+/CD86+ cells than from CD34+/CD86− cells.
A high frequency of fresh CD34+/CD86+ and CD34+/CD86+-derived cells cultured in TNF-α, GM-CSF, and SCF stimulate the proliferation of allogeneic T cells. Fresh (A) and TNF-α, GM-CSF, and SCF-cultured (B) CD34+/CD86+ and CD34+/CD86− cells and were irradiated (3,000 cGy) and mixed with allogeneic, nylon wool-purified T cells at 10,000 responders/well. Stimulator cells were serially diluted and set up in 96-well V-bottomed plates in 24 replicates. Similar results were obtained in three experiments of identical design. For the experiment shown, the χ2 and frequency (f) of stimulating cells in each population were as follows: fresh CD34+/CD86− cells, χ2 = 2.0, f = 0.6% (95% confidence interval [CI], 0.5% to 0.8%); fresh CD34+/CD86+ cells, χ2=4.7, f = 3.1% (95% CI, 2.4% to 3.8%); cultured CD34+/CD86− cells, χ2 = 1.33, f = 4.0% (95% CI, 3.0% to 5.0%); and cultured CD34+/CD86+ cells, χ2 = 0.68, f = 25.0% (95% CI, 16.7% to 33.3%).
A high frequency of fresh CD34+/CD86+ and CD34+/CD86+-derived cells cultured in TNF-α, GM-CSF, and SCF stimulate the proliferation of allogeneic T cells. Fresh (A) and TNF-α, GM-CSF, and SCF-cultured (B) CD34+/CD86+ and CD34+/CD86− cells and were irradiated (3,000 cGy) and mixed with allogeneic, nylon wool-purified T cells at 10,000 responders/well. Stimulator cells were serially diluted and set up in 96-well V-bottomed plates in 24 replicates. Similar results were obtained in three experiments of identical design. For the experiment shown, the χ2 and frequency (f) of stimulating cells in each population were as follows: fresh CD34+/CD86− cells, χ2 = 2.0, f = 0.6% (95% confidence interval [CI], 0.5% to 0.8%); fresh CD34+/CD86+ cells, χ2=4.7, f = 3.1% (95% CI, 2.4% to 3.8%); cultured CD34+/CD86− cells, χ2 = 1.33, f = 4.0% (95% CI, 3.0% to 5.0%); and cultured CD34+/CD86+ cells, χ2 = 0.68, f = 25.0% (95% CI, 16.7% to 33.3%).
Failure to generate granulocytes from CD34+/CD86+ cells.
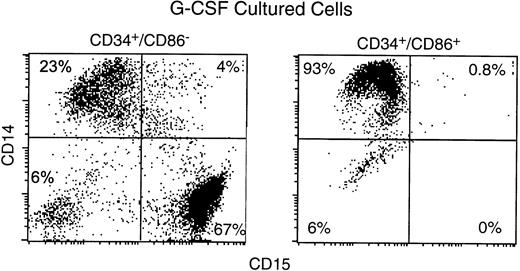
To determine the potential for their differentiation into granulocytes, CD34+/CD86− and CD34+/CD86+ cells were cultured with G-CSF (Fig 8). By day 11, cells positive for CD15 (a marker specific for granulocytes) were 73% ± 7% among the progeny of CD34+/CD86− cells, but only 0.3% ± 0.4% among the progeny of CD34+/CD86+ cells (n = 3). In contrast, CD14+ cells were 23% ± 7% among the progeny of CD34+/CD86− cells, compared with 92% ± 4% among CD34+/CD86+ cells. This experiment confirmed that CD34+/CD86+ cells contain precursors of monocytes/macrophages, but they do not contain precursors of granulocytes.
CD34+/CD86+ cells cultured in G-CSF do not generate granulocytes. Human marrow CD34+/CD86+ and CD34+/CD86− cells were purified by FACS-sorting and cultured for 9 to 11 days in G-CSF. Cultured cells were stained and analyzed by microfluorimetry.
CD34+/CD86+ cells cultured in G-CSF do not generate granulocytes. Human marrow CD34+/CD86+ and CD34+/CD86− cells were purified by FACS-sorting and cultured for 9 to 11 days in G-CSF. Cultured cells were stained and analyzed by microfluorimetry.
Induction of CD86 expression on CD34+/CD86− cells by TNF-α and induction of CD1a expression on CD86+ cells by GM-CSF.
CD34+/CD86− cells were cultured in TNF-α plus SCF to examine the effect of these growth factors on cell differentiation. TNF-α induced a time-dependent increase in CD86 expression, with 29% of the cells expressing CD86 by day 2 and 54% of the cells expressing CD86 by day 6. In contrast, GM-CSF or medium alone did not induce CD86 expression (not shown). To evaluate whether TNF-α–induced CD86 expression represents lineage differentiation, we primed CD34+/CD86− cells in TNF-α plus SCF for 6 days and then separated the CD86− and CD86+ subsets by FACS (Fig 9A). Exposure of TNF-α–primed CD86+ cells to GM-CSF in secondary culture for an additional 6 days generated a population which expressed CD1a on 60% to 81% (n = 5) of the cells (Fig 9A), whereas TNF-α–primed CD86− cells generated a population containing 8% to 31% (n = 4) CD1a+ cells (not shown). Continued exposure of TNF-α–primed CD86+ cells to TNF-α in the presence of GM-CSF allowed cells to mature into CD83+, CD1a− cells with high levels of HLA-DR, CD86 (Fig 9A), and CD80 expression (not shown). All progeny of TNF-α–primed CD86+ cells had the morphology of dendritic cells (Fig 9B and C). However, without continued exposure to TNF-α, dendritic cells had short projections (Fig 9B) and lacked expression of CD83, a marker of mature dendritic cells. In contrast, with continued exposure to TNF-α, dendritic cells acquired long projections (Fig 9C) and expressed CD83. Thus, CD34+ cells can be induced by TNF-α to express CD86 and become committed to the dendritic cell lineage. Terminal differentiation into dendritic cells requires stimulation with both TNF-α and GM-CSF. We tested the alloantigen presenting function of dendritic cells generated from TNF-α–primed CD86+ cells and found strong stimulation of allogeneic T cells (Fig 9D).
TNF-α induces expression of CD86 on CD34+/CD86− marrow cells and commits the CD86+ population to the dendritic lineage. FACS-sorted CD34+/CD86− marrow cells were cultured with TNF-α and SCF for 6 days. Day-6 CD86-bright cells were purified by FACS-sorting and cultured for an additional 6 days in GM-CSF and SCF, with or without continued exposure to TNF-α. Expression of cell surface markers was analyzed by microfluorimetry (A). Day-12 cell cultures were photographed at 20× amplification by phase contrast (B and C). In addition, cultured cells were resuspended in 15% PHS, irradiated at 3,000 cGy, and used as stimulators. Cells were plated at serial dilution with HLA-DR–mismatched CD4+ T cells at 5 × 104 responders/well. Cultures were harvested on day 6 after 18 hours of exposure to 3H-thymidine (D).
TNF-α induces expression of CD86 on CD34+/CD86− marrow cells and commits the CD86+ population to the dendritic lineage. FACS-sorted CD34+/CD86− marrow cells were cultured with TNF-α and SCF for 6 days. Day-6 CD86-bright cells were purified by FACS-sorting and cultured for an additional 6 days in GM-CSF and SCF, with or without continued exposure to TNF-α. Expression of cell surface markers was analyzed by microfluorimetry (A). Day-12 cell cultures were photographed at 20× amplification by phase contrast (B and C). In addition, cultured cells were resuspended in 15% PHS, irradiated at 3,000 cGy, and used as stimulators. Cells were plated at serial dilution with HLA-DR–mismatched CD4+ T cells at 5 × 104 responders/well. Cultures were harvested on day 6 after 18 hours of exposure to 3H-thymidine (D).
DISCUSSION
The CD34 antigen is expressed by 1% to 4% of normal human marrow cells.16-19 Analysis of the surface phenotype demonstrates that 90% to 95% of CD34+ cells coexpress antigens that indicate commitment to the myeloid, erythroid, or lymphoid lineages.17-19 We have found that 6% ± 3% of CD34+ cells coexpress CD86, the major functional ligand for T-cell costimulation receptors CD28 and CTLA-4.7CD34+/CD86+ cells are on average 1.2 times larger than CD34+/CD86− cells and express very high levels of the leukointegrin β chain CD18, an adhesion molecule present on committed progenitors that are devoid of long-term culture-initiating activity.7,20CD34+/CD86+ cells efficiently present alloantigen to T cells,7 and we show here that they can also present tetanus toxoid protein to CD4+ T cells (Fig1). Thus, expression of CD86 on differentiated CD34+ marrow cells is correlated with the acquisition of antigen-presenting function.
GM-CSF stimulates normal hematopoietic progenitor cells to differentiate in vitro and form colonies containing granulocytes and macrophages.21 Therefore, we used GM-CSF in a liquid culture system to test whether CD34+/CD86+marrow cells may differentiate preferentially towards the macrophage lineage as opposed to other lineages. We found that culture of purified CD34+/CD86+ cells in the presence of SCF and GM-CSF leads predominantly to a population of activated macrophages. In contrast, culture of CD34+/CD86− cells under the same conditions gives rise to a cell population containing predominately granulocytes. Not even after stimulation with G-CSF do CD34+/CD86+ cells differentiate into granulocytes. The experimental data presented here, including cell morphology by light microscopy, CD14+ surface phenotype, phagocytic function, and allostimulatory activity of GM-CSF–stimulated cells, support the model that CD34+/CD86+ cells are differentiated towards the macrophage lineage and likely derive from a CD34+/CD86− bipotential precursor of granulocytes and macrophages.22
The combination of GM-CSF and TNF-α induces human hematopoietic progenitors to differentiate into dendritic cells.8,11-13Dendritic cells are the most effective APC that initiate the sensitization of MHC-restricted T cells, the rejection of organ transplants, and the formation of T-dependent antibodies.23Dendritic cells acquire antigens in tissues and migrate to lymphoid organs, where they identify and activate antigen-specific T cells.24-28 Dendritic cells express high levels of antigen-presenting molecules HLA-A, B, C, DR, DQ, and DP, as well as accessory molecules CD40, CD50, CD54, CD58, CD80, and CD86 that mediate T-cell binding and costimulation.3,29,30 Fully mature dendritic cells also express the specific marker CD83.31Dendritic cells, macrophages, and granulocytes arise from a common progenitor in the bone marrow, but a bipotential CD34+precursor of dendritic cells and macrophages has not been defined.32,33 However, culture of CD34+ cells from human cord blood or marrow can generate a CD14+bipotential intermediate in the presence of SCF, GM-CSF, and TNF-α.34,35 This intermediate cell type develops along the dendritic cell pathway when stimulated by GM-CSF and TNF-α or along the macrophage pathway when stimulated with M-CSF or medium alone.34,35 Further evidence that macrophages and dendritic cells share a common lineage is provided by the observation that human CD14+ blood mononuclear cells can differentiate into CD83+ dendritic cells in the presence of GM-CSF and interleukin-4.36,37 Zhou and Tedder38 have shown that the transition from monocytes to dendritic cells is enhanced by the addition of TNF-α. Our data show that CD34+/CD86+ marrow cells differentiate into a population composed predominantly of macrophages (when stimulated by GM-CSF) or of dendritic cells (when stimulated by GM-CSF and TNF-α). In contrast, CD34+/CD86− cells, cultured in the presence of GM-CSF and TNF-α, generate a heterogeneous population. Therefore, CD34+/CD86+ cells are progenitors committed to the macrophage and the dendritic cell lineages. Differentiation into macrophages requires stimulation by GM-CSF, whereas differentiation into dendritic cells requires both GM-CSF and TNF-α.
TNF-α appears critical for commitment of hematopoietic progenitors to the dendritic lineage.11 Our data show that stimulation with TNF-α induces CD86 expression on CD34+/CD86− cells. This population of TNF-α–induced CD86+ cells differentiated into CD1a+ dendritic cells in response to GM-CSF, in contrast to CD34+ cells with constitutive expression of CD86+, which differentiated into macrophages in response to GM-CSF. Therefore, the effect of TNF-α on CD34+ cells is probably not limited to induction of CD86 expression, but may involve other events critical for lineage differentiation. In our study, TNF-α–primed CD86+ cells generated immature CD1a+ dendritic cells when cultured in GM-CSF and SCF. When TNF-α was included in the culture with GM-CSF and SCF, dendritic cells matured into CD83+ cells with high levels of HLA-DR, CD80, and CD86 expression and fully developed dendritic processes. Using MLC, we could not detect differences in the capacity of the two populations of dendritic cells to stimulate allogeneic CD4+T lymphocytes, possibly because both populations were extremely potent.
The precise role of TNF-α in dendritic cell differentiation might be defined by gene targeting experiments. This aim is complicated by the availability of at least three types of receptors that bind both TNF-α and lymphotoxin-α.39 However, mice deficient for both TNF-α and lymphotoxin-α have serious defects in the development, structure, and function of the immune system and are highly susceptible to Listeria monocytogenes infection. Further studies of mutant mice are likely to demonstrate whether the role of TNF-α in dendritic cell development is facultative or obligatory.40
Our data propose the existence of bipotential hematopoietic precursors committed towards the macrophage and the dendritic cell lineages. The identification of CD86 as a functional surface marker for such precursors will allow tracking studies in human tissues to fully disclose the development of mature progeny in vivo. Because the nature of the APC is critical for the development of immunity or tolerance, the availability of a marker for committed progenitors of dendritic cells will allow for their enrichment and use in studies of vaccination.41 On the other hand, tolerance may be induced by transfer of antigen-primed tolerogenic APC depleted of dendritic cell precursors.42
ACKNOWLEDGMENT
The authors thank Dr Robert Andrews, Dr Martin Cheever, Dr John Hansen, and Dr James Young for helpful discussions and for their critical review of the manuscript. We thank Dr Robert Andrews for providing us with SCF and GM-CSF, Dr Thomas Tedder for providing anti-CD83 MoAb HB-15a, and Linda O'Neal for her assistance with cytochemical staining. We are also grateful to CellPro, Inc for providing us with Ceprate kits.
Supported by Grants No. AI33484 and AI37678 from the National Institutes of Health (Bethesda, MD).
Address correspondence to Claudio Anasetti, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 7. A high frequency of fresh CD34+/CD86+ and CD34+/CD86+-derived cells cultured in TNF-α, GM-CSF, and SCF stimulate the proliferation of allogeneic T cells. Fresh (A) and TNF-α, GM-CSF, and SCF-cultured (B) CD34+/CD86+ and CD34+/CD86− cells and were irradiated (3,000 cGy) and mixed with allogeneic, nylon wool-purified T cells at 10,000 responders/well. Stimulator cells were serially diluted and set up in 96-well V-bottomed plates in 24 replicates. Similar results were obtained in three experiments of identical design. For the experiment shown, the χ2 and frequency (f) of stimulating cells in each population were as follows: fresh CD34+/CD86− cells, χ2 = 2.0, f = 0.6% (95% confidence interval [CI], 0.5% to 0.8%); fresh CD34+/CD86+ cells, χ2=4.7, f = 3.1% (95% CI, 2.4% to 3.8%); cultured CD34+/CD86− cells, χ2 = 1.33, f = 4.0% (95% CI, 3.0% to 5.0%); and cultured CD34+/CD86+ cells, χ2 = 0.68, f = 25.0% (95% CI, 16.7% to 33.3%).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/10/10.1182_blood.v91.10.3892/3/m_blod41016007x.jpeg?Expires=1769126913&Signature=jbnSzkNEfv6TrdQCsH-cLFCqyYLDe4l~9I-145dSk3ZlCgJko1tkAyGGx6L6LRvKjmHx3M11t5YtCGrl~UNs1b2jxy78gTYtJ47ehLQquA8wkfK~dM0ew42Mq7PjUUgqnu4hWBIGowAXBxR-fOteTG9kp~1~2~1hKwJy~17s5KzHAA98l1~FZUHCpqFJ6~n~G9pexU94aZg2r~tigg4Ep4QRyT68x9LxN~WszaQ1vUei34N~O2XWRw5cH4V7JU0Ykanv1QPgm4mO-btWXKVHHNNiWH67IuzDDU3Z22EcBAos3LFQ5DbJriVjJQ~kvIPXacSQpa9gTaDuokNBIJVKbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal