Abstract
Adult T-cell leukemia (ATL) is characterized by massive infiltration of circulating ATL cells into a variety of tissues, a finding often associated with poor prognosis. Leukocyte migration from circulation into tissue depends on integrin-mediated adhesion to endothelium, and integrins are tightly regulated by several stimuli, such as inflammatory chemokines. However, the exact mechanisms that enhance adherence of leukemic cells to the endothelium and infiltration into tissues remain to be fully understood. We investigated the mechanisms of extravasation of leukemic cells using ATL cells and report the following novel features of endogenous chemokine-induced adhesion of ATL cells to the endothelium. ATL cells spontaneously adhered to endothelial cells without exogenous stimulation. Integrin leukocyte function-associated antigen-1 (LFA-1) on ATL cells was spontaneously activated. ATL cells produced high amounts of chemokines, macrophage inflammatory protein-1α (MIP-1α), and MIP-1β. Adhesion of ATL cells to endothelial cells and the expression of activated form of LFA-1 were reduced by pretreatment with pertussis toxin, wortmannin, or anti–MIP-1α and MIP-1β antibodies or transfection with antisense of MIP-1α or MIP-1β. Spontaneous polymerization of cytoskeletal F-actin was observed in ATL cells, which was also inhibited by pertussis toxin and wortmannin. We propose that ATL cells adhere to endothelial cells through an adhesion cascade similar to normal leukocytes and that the chemokines produced by ATL cells are involved in triggering integrin LFA-1 through cytoskeletal rearrangement induced by G-protein–dependent activation of phosphoinositide 3-kinases in an autocrine manner. These events result in a strong adhesion of ATL cells to the endothelium and spontaneous transendothelial migration.
ADULT T-CELL LEUKEMIA (ATL) is a peripheral CD4+ T-cell malignancy caused by infection with human T-lymphotropic virus-1 (HTLV-1) and is associated with a marked increase of peripheral ATL cells with monoclonal growth during the acute phase. ATL is characterized by a rapid infiltration of circulating ATL cells into several tissues and secondary lymphoid organs, a process often associated with poor prognosis.1,2Extravasation and infiltration of hematologic malignant cells into tissues probably reflect the biologic properties of these cells, expression and function of relevant adhesion molecules on these cells, and their adhesive interaction with endothelial cells.3 In a series of studies from our laboratories, we have reported that one integrin, the leukocyte function-associated antigen-1 (LFA-1), plays a central role in ATL cell adhesion to its calcium-dependent ligand, intercellular adhesion molecule-1 (ICAM-1), a process involving heparan sulfate proteoglycan on the cell by binding heparin-binding chemokines.4-6
Leukocyte integrin LFA-1 and very late antigen-4 (VLA-4) mediate adhesion of circulating leukocytes to endothelial ligands.7The adhesive capacity of integrins expressed on peripheral leukocytes is tightly regulated. Although integrins expressed on resting cells do not mediate firm adhesion to endothelial ligands, stimulation of these cells results in a rapid increase in integrin function.8-10Thus, activation of integrin is essential for integrin-mediated adhesion in which a signal transduced to the leukocyte converts the functionally inactive integrin to an active adhesive configuration. In this regard, we have previously reported that the chemokine macrophage inflammatory protein-1β (MIP-1β) triggers integrin and induces adhesion of T-cell subsets to endothelial integrin-ligands.11-13 Several recent studies have supported the potential importance of chemokines in inflammatory responses, specifically that various chemokines, including MIP-1β, produced in large amounts at the site of inflammation activate integrins on leukocytes and result in their accumulation in the tissues. The mechanisms causing activation of integrin are thought to involve conformational changes of ectodomain of integrins and/or clustering of integrins on the cell membrane that may induce active, adhesive configuration of integrins, resulting from cytoskeletal actin-polymerization associated with endodomain of integrins.14-16
However, the exact mechanisms that enhance the adherence of circulating leukemic cells to the endothelium and their subsequent infiltration into the tissues or those regulating integrin adhesiveness to endothelium are not very clear at present. We investigated in the present study the mechanisms of integrin-mediated adhesion of leukemic cells to endothelial ligands and examined the signaling and cytoskeletal interaction of integrins using ATL cells. ATL is a unique model for the following reasons. (1) It is characterized by a malignant expansion of peripheral mature CD4+ T cells. (2) ATL cells result from monoclonal proliferation and form in each patient a phenotypically and functionally homogeneous population of cells. (3) There is a high tendency for malignant cells to infiltrate multiple organs. The present study was performed to investigate the relevance of integrin expression to its adhesive function and regulatory mechanisms, with special emphasis on signaling and rearrangement of the cytoskeleton upon adhesion to the endothelium and subsequent tissue infiltration of ATL cells.
MATERIALS AND METHODS
ATL cells and ATL cell lines.
We examined 20 patients with ATL, 1 control patient with chronic T-cell leukemia (T-CLL) which was not caused by infection with HTLV-1, 4 established HTLV-1–infected T-cell lines (MT-1, MT-2, HUT-102, and SALT-3; a kind gift from Prof K. Sagawa, Kurume University Medical School, Kurume, Japan), and 10 normal healthy volunteers. ATL was diagnosed based on clinical features, hematologic findings, serum antibodies against HTLV-1, and monoclonal integration of HTLV-1 proviral genome.1,17 Highly purified CD4+ T and ATL cells were prepared by exhaustive negative selection10from peripheral blood mononuclear cells of normal donors and ATL patients using magnetic beads (Dynal, Oslo, Norway) and a cocktail of a variety of antibodies, including CD19 monoclonal antibody (MoAb) FMC63, CD16 MoAb 3G8, CD11b MoAb NIH11b-1, CD14 MoAb 63D3, and CD8 MoAb B9.8.4.
Antibodies and reagents.
The following MoAbs were used as purified Ig in the preparation of T and ATL cells, staining and analysis of cell surface molecules, and blocking of cellular adhesion: activated LFA-1 MoAb NKI-L16,18 CD19 MoAb FMC63 (H. Zola, Bedford Park, Australia), CD8 MoAb B9.8.4 (B. Malissen, Marseille, France), CD11b MoAb NIH11b-1, CD49d (VLA-4) MoAb NIH49d-1, CD54 (ICAM-1) MoAb 84H10 (S. Shaw, Bethesda, MD), CD49d MoAb HP2/1 (F. Sanchez-Madrid, Madrid, Spain),19,20 CD16 MoAb 3G8 (D. Siegel, Bethesda, MD), CD69 MoAb FN50, phycoerythrin (PE)-conjugated CD45RO MoAb UCHL-1, CyChrom-conjugated CD4 MoAb Leu-3a (Fujisawa, Osaka, Japan), CD14 MoAb 63D3, CD11a (LFA-1α) MoAb TS1/22, major histocompatibility complex (MHC) class I MoAb W6/32, and control MoAb Thy1.2 (ATCC, Rockville, MD). ICAM-1 was purified by affinity column chromatography from the Reed-Sternberg cell line L428 as previously described.10 21Multiple inhibitors of intracytoplasmic signaling were applied to each assay system and all reagents were used at the indicated concentrations. At these concentrations, none of these inhibitors produced cytotoxic effects on ATL cells, as confirmed by trypan blue staining. We used wortmannin (Wako Pure Chemical, Osaka, Japan), a phosphoinositide 3 (PI 3)-kinase inhibitor; pertussis toxin, uncoupler of certain G-proteins from their complex; H88 and H89, A-kinase inhibitors; H7 and staurosporine (Seikagaku, Tokyo, Japan), C-kinase inhibitors; herbimycin A (Sigma, St Louis, MO) and genestein (Carbiochem, San Diego, CA), tyrosine kinase inhibitors; and cytochalasin B (Sigma) and cytoskeleton-disrupting reagent.
Preparation of sense and antisense oligonucleotide of MIP-1α and MIP-1β and their transfection into ATL cells.
Sense and antisense oligonucleotide sequences of MIP-1α and MIP-1β were 5′-CACCTGCTCAGAATCA, 5′-TGATTCTGAGCAGGTG, 5′-ATGAAGCTCTGCGTG, and 5′-CACGCAGAGCTTCAT, respectively. We synthesized 15-base deoxyribonucleotides on an automated solid-phase synthesizer (Sawady Technology, Tokyo, Japan). The oligomers were purified by affinity-gel chromatography embedded ether-toyopearl (Tosoh, Tokyo, Japan) carrying hydrophobic affinity and gel filtration effect (DNA stec-1000; ASTEC, Fukuoka, Japan), precipitated with ethanol, lyophilized to dryness, and dissolved in the culture medium. Oligonucleotides were introduced into ATL cells using a cationic liposome-mediated transfection method.22 Oligonucleotides dissolved in 100 μL of serum-free medium (OPTI-MEM; Life Technologies, Gaithersburg, MD) were mixed with 5 μL of Lipofectin reagent (LipofectAMINE; Life Technologies) in the same volume of OPTI-MEM and incubated for 10 minutes at room temperature. The oligonucleotide/liposome complex was added to ATL cells plated in a 6-well culture dish (3 × 105 cells/well), incubated for 6 hours in OPTI-MEM, and then replaced with 10% fetal calf serum (FCS) containing RPMI1640 (Nissui, Tokyo, Japan) for 24 hours. The concentration of oligonucleotides in the conditioned medium was 2.2 mmol/L.
Adhesion assay.
Adhesion assay of ATL cells, cell lines to human umbilical vein-derived endothelial cells (HUVECs), or purified ICAM-1 glycoproteins was performed as previously described.5,10 HUVECs were placed onto 48-well culture plates (Costar, Cambridge, MA) coated with 2% gelatine and cultured to confluence in Dulbecco's modified Eagle's medium (D-MEM; Nissui) containing 100 U/mL penicillin G, 100 U/mL streptomycin, 20% heat-inactivated FCS, endothelial mitogen 20 μg/mL (Biomedical Technologies, Stoughton, MA), and Heparin (10 U/mL). After washing with phosphate-buffered saline (PBS), HUVECs were stimulated with 1 ng/mL interleukin-1β (IL-1β; Otsuka, Tokyo, Japan) for 4 hours at 37°C. Purified ICAM-1 (50 ng/well) was applied to 48-well plates in Ca/Mg-free PBS at 4°C overnight. Binding sites were subsequently blocked with Ca/Mg-free PBS/3% human serum albumin (HSA; Green-Cross, Osaka, Japan) for 2 hours at 37°C to reduce nonspecific attachment. The plates were washed three times with PBS before the addition of T cells. A total of 2 × 105 T cells, ATL cells, and ATL cell lines were labeled with 51Cr (Dupont NEN, Wilmington, DE) in RPMI1640 with 1% HSA and were added to the culture with or without the relevant adhesion blocking MoAb in the presence or absence of phorbol myristate acetate (PMA; 10 ng/mL; Sigma), which is a pharmacologically relevant trigger of integrin adhesiveness. All MoAbs were used at a saturating concentration of 10 μg/mL, which was shown in previous studies to produce a maximum inhibition of the relevant adhesive interaction.10 After a settling phase of 30 minutes at 4°C, which also allowed MoAb binding, the plates were rapidly warmed to 37°C for 30 minutes and then gently washed twice with RPMI-1640 at room temperature to completely remove nonadherent T cells and ATL cells. The contents of each well containing adherent T cells or ATL cells were lysed with 250 μL of 1% Triton X-100 (Sigma), and the emission of the contents of each well was measured using a γ-counter. Data were expressed as the mean percentage of the binding of indicated cells from a representative experiment.
Flow microfluorometry.
Staining and flow cytometric analyses of freshly obtained ATL or normal T cells were performed using a FACScan (Becton Dickinson, Mountain View, CA) and standard procedures as described previously.6 10 Briefly, 2 × 105 cells were incubated with negative control MoAb thy1.2, LFA-1 (CD11a) MoAb TS1/22, VLA-4 (CD49d) MoAb NIH49d-1, activated LFA-1 MoAb NKI-L16, CD69 MoAb FN50 in fluorescence-activated cell sorting (FACS) media consisting of Hanks' balanced salt solution (HBSS; Nissui), 0.5% HSA, and 0.2% NaN3 (Sigma) for 30 minutes at 4°C. After washing the cells three times with FACS media, they were further incubated with fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG Ab for 30 minutes at 4°C. After washing and incubation with irrelevant MoAbs, the cells were further incubated with a mixture of PE-conjugated CD45RO MoAb UCHL-1 and CyChrom-conjugated CD4 MoAb Leu-3a for 30 minutes at 4°C. The staining of cells with MoAbs was detected by gating on CD4 and CD45RO double-positive cells using FACScan. Amplification of the MoAb-binding was provided by a three-decade logarithmic amplifier. Quantification of cell surface antigens on single cells was calculated using standard beads (QIFKIT; DAKO Japan, Kyoto, Japan).
Enzyme-linked immunosorbent assay (ELISA) of MIP-1β and MIP-1α in supernatant or cytosol of ATL cells.
Freshly isolated normal T cells and ATL cells (1 × 106) were washed in PBS and lysed with 250 μL of PBS containing 2% N-octyl-β-D-glucopyraide (OGP; Sigma). The culture supernatants were collected from normal T cells (1 × 106) and ATL cells (1 × 106) after 24 hours of incubation in RPMI1640 with 5% FCS at 37°C without any stimulation. The level of MIP-1β protein in each sample was measured by MIP-1β and MIP-1α ELISA system (R&D Systems, Minneapolis, MN). The sensitivity of the assay was 4.0 pg/mL of MIP-1β and MIP-1α and was not affected by the presence of OPG at the concentration used to lyse cells. Results were expressed in nanograms per milliliter per 1 × 105 cells.
F-actin polymerization assay.
For microscopic analysis, T cells, ATL cells, and cell lines were allowed to settle for 30 minutes at 4°C on fibronectin-coated slides. After incubation for 1 minute at 37°C, the cells were fixed with 1% formaldehyde. F-actin was stained with rhodamine-phalloidin (1 U/slide; Molecular Probes, Inc, Eugene, OR) and analyzed later with a confocal laser microscope system (model LSM 410UV, LD Achroplan 20 objective lens; Carl Zeiss, Juna, Germany).
Transmigration assay.
We assessed the transmigration of ATL cells in 3-mm pore, 12-well microchemotaxis chambers (Transwell; Costar) precoated with IL-1β–activated HUVECs for 48 hours at 37°C. ATL cells and ATL cell lines were labeled with 51Cr (1 × 106/well in RPMI-1640 with 10% FCS) and placed in the insert wells. After incubation for 2 hours, cells that migrated into each of the lower wells were retrieved and dissolved in 250 μL of 1% Triton X-100, and γ-emission of well contents was determined. Data were expressed as the mean percentage of transmigrated cells from a representative experiment.
RESULTS
ATL cells and cell lines spontaneously adhered to HUVECs.
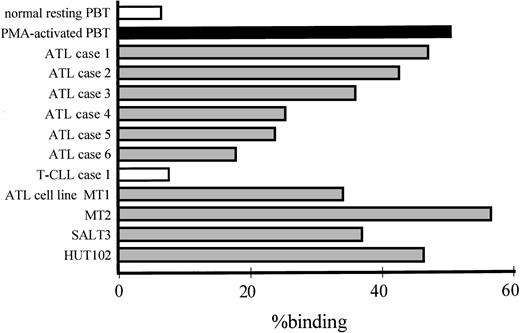
One of the most characteristic features of ATL cells is their marked infiltration into tissues during the acute phase.1,17,23Leukocyte migration from circulation into the tissue is mediated by adhesion of leukocyte integrins to their ligands, including ICAM-1, on endothelial cells. However, leukocyte integrins are normally inactive; hence, the regulation of integrin-dependent adhesion is critical to the migration of virtually all hematopoietic cells.8 10 As shown in Fig 1, ATL cells obtained from 6 representative ATL patients and 4 ATL cell lines spontaneously adhered to IL-1–activated HUVECs after 30 minutes of incubation, without any exogenous stimulus. In contrast, resting peripheral T cells did not adhere to HUVECs and T cells bound only after they were activated with PMA, a potent stimulator of integrins. CD4+ leukemic cells from a patient with HTLV-1–negative T-cell leukemia also did not bind to HUVECs.
Spontaneous adhesion of ATL cells to IL-1β–activated HUVECs. Adhesion of resting or PMA-activated peripheral normal CD4+ T cells, ATL cells freshly obtained from 6 representative ATL patients, CD4+ leukemic cells from a patient with HTLV-1–negative T-CLL, and 4 ATL cell lines (MT1, MT2, SALT-3, and HUT-102) that were labeled with 51Cr to IL-1β–activated HUVECs was assessed after 30 minutes of incubation at 37°C. γ-Emission of the lysate of only adherent cells was determined. Data are expressed as the mean percentage of binding of indicated cells from a representative experiment.
Spontaneous adhesion of ATL cells to IL-1β–activated HUVECs. Adhesion of resting or PMA-activated peripheral normal CD4+ T cells, ATL cells freshly obtained from 6 representative ATL patients, CD4+ leukemic cells from a patient with HTLV-1–negative T-CLL, and 4 ATL cell lines (MT1, MT2, SALT-3, and HUT-102) that were labeled with 51Cr to IL-1β–activated HUVECs was assessed after 30 minutes of incubation at 37°C. γ-Emission of the lysate of only adherent cells was determined. Data are expressed as the mean percentage of binding of indicated cells from a representative experiment.
ATL cells expressed an activated form of LFA-1.
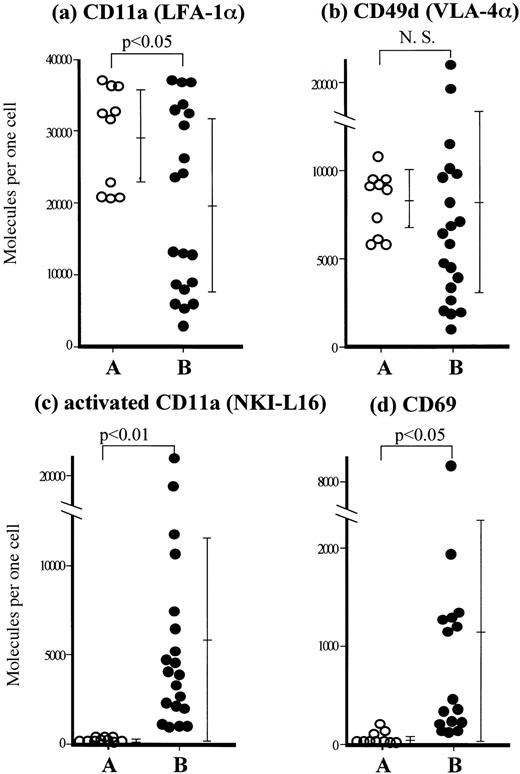
Using flow cytometry, we next examined whether the augmented adhesion of ATL cells to endothelial cells depends on increased expression of adhesion molecules or the expression of activated molecules. The expression of LFA-1 on ATL cells was significantly lower than on normal T cells (Fig 2a). In contrast, the expression of VLA-4 on ATL cells was similar to that on normal T cells (Fig 2b). LFA-1 requires an active configuration to bind to its ligand, a process that can be induced by a variety of stimuli,24-27and NKI-L16 MoAb reacts with a Ca2+-dependent activation epitope located on the ectodomain of α-chain of LFA-1.18 28 The expression of the activated form of LFA-1 as recognized by NKI-L16 MoAb was significantly higher on most ATL cells than on normal resting CD4+CD45RO+ T cells (Fig 2c). The majority of ATL cells also expressed CD69 (Fig 2d) as well as MHC class II antigens and CD25 (data not shown), both regarded as early activation markers. These results suggest that spontaneous adhesion of ATL cells to the endothelium depends on activated LFA-1 rather than on the amount of adhesion molecules expressed by ATL cells.
Phenotypic analysis of fresh ATL cells by flow cytometry. Staining and flow cytometric analyses of resting peripheral blood T cells from 10 normal volunteers (○) and ATL cells freshly obtained from peripheral blood of 20 ATL patients (•) were performed with (a) LFA-1α (CD11a) MoAb TS1/22, (b) VLA-4 α (CD49d) MoAb NIH49d-1, (c) an anti-activated form of LFA-1α MoAb NKI-L16, and (d) CD69 MoAb by gating on CD4 and CD45RO double-positive cells using FACScan. Each point represents the number of molecules expressed per cell, calculated using standard QIFKIT beads. Bars represent the mean ± SD of each group and statistical significance was determined by the Student's t-test.
Phenotypic analysis of fresh ATL cells by flow cytometry. Staining and flow cytometric analyses of resting peripheral blood T cells from 10 normal volunteers (○) and ATL cells freshly obtained from peripheral blood of 20 ATL patients (•) were performed with (a) LFA-1α (CD11a) MoAb TS1/22, (b) VLA-4 α (CD49d) MoAb NIH49d-1, (c) an anti-activated form of LFA-1α MoAb NKI-L16, and (d) CD69 MoAb by gating on CD4 and CD45RO double-positive cells using FACScan. Each point represents the number of molecules expressed per cell, calculated using standard QIFKIT beads. Bars represent the mean ± SD of each group and statistical significance was determined by the Student's t-test.
ATL cells spontaneously secreted chemokines MIP-1β and MIP-1α.
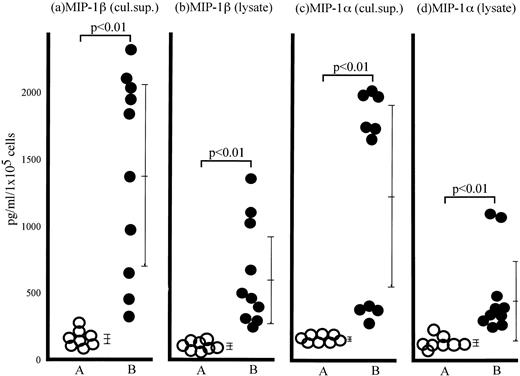
We and others have proposed that chemokines such as MIP-1β and MIP-1α functionally trigger T-lymphocyte integrins.11,29 30 ATL cells produced significant amounts of MIP-1α and MIP-1β protein in the culture supernatant as well as in the cytosol without any stimulation, whereas normal resting T cells did not produce any of these chemokines (Fig 3). These results suggest that MIP-1α and MIP-1β are spontaneously produced by ATL cells and might trigger integrin-mediated adhesion of ATL cells to endothelial cells in an autocrine manner.
Spontaneous MIP-1β and MIP-1α production from ATL cells. The cytokine levels in culture supernatants (a and c) collected from normal CD4+ T cells (group A) and the ATL cells (group B) after 24 hours of incubation at 37°C without any stimulation or cytosol (b and d) of normal CD4+ T cells (group A) and ATL cells freshly obtained from peripheral blood of ATL patients (group B) were determined by MIP-1β (a and b) and MIP-1α (c and d) ELISA system. Each point represents the concentration of MIP-1β and MIP-1α in the lysate or supernatant derived from 1 × 105 cells of individual subjects. Bars represent the mean ± SD of each group (Student's t-test).
Spontaneous MIP-1β and MIP-1α production from ATL cells. The cytokine levels in culture supernatants (a and c) collected from normal CD4+ T cells (group A) and the ATL cells (group B) after 24 hours of incubation at 37°C without any stimulation or cytosol (b and d) of normal CD4+ T cells (group A) and ATL cells freshly obtained from peripheral blood of ATL patients (group B) were determined by MIP-1β (a and b) and MIP-1α (c and d) ELISA system. Each point represents the concentration of MIP-1β and MIP-1α in the lysate or supernatant derived from 1 × 105 cells of individual subjects. Bars represent the mean ± SD of each group (Student's t-test).
Signaling pathways involved in increased integrin-dependent adhesion of ATL cells to ICAM-1 and HUVECs.
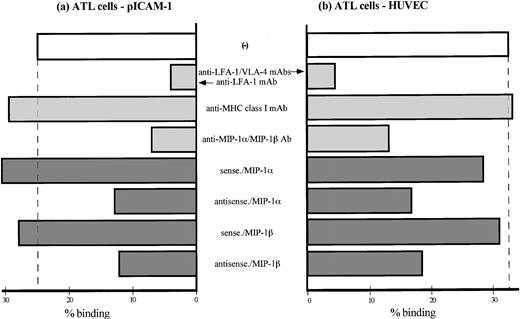
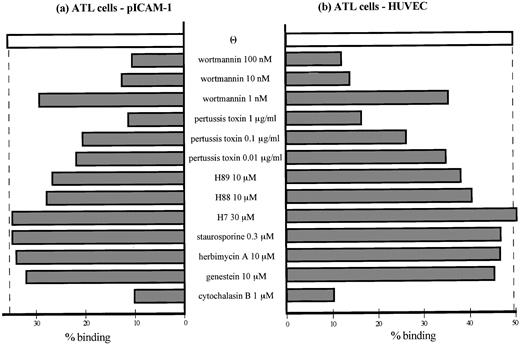
ATL cells spontaneously adhered to purified ICAM-1 (Fig 4a) as well as IL-1β–activated HUVEC (Fig 4b). MoAb blocking studies, in which ATL cell adhesion to activated HUVECs or ICAM-1 were inhibited by anti–LFA-1/VLA-4 MoAbs or by anti–LFA-1 MoAb alone, respectively, indicated that the adhesion was mediated by ATL cell integrins and their endothelial ligands. Chemokine receptors belong to the serpentine family of seven transmembrane G-protein–coupled receptors.31 To determine if G-proteins are involved in the induction of ATL cell adhesion to the endothelium, we also analyzed the ability of several signaling inhibitors to block adhesion. Pretreatment of cells with pertussis toxin (which uncouples certain G-proteins from their complex) reduced integrin-dependent increased adhesion of ATL cells to purified ICAM-1 and activated HUVECs in a concentration-dependent manner (Fig 5a and b). Adhesion also diminished by 10 to 100 nmol/L of wortmannin, a PI 3-kinase inhibitor. Two A-kinase inhibitors, H89 and H88, also slightly decreased the adhesion. However, H7 and staurosporin (C-kinase inhibitors) and herbimycin A and genestein (tyrosine kinase inhibitors) did not influence adhesion. Furthermore, the data in Fig 4 show that increased adhesion was also inhibited by pretreatment of ATL cells with a mixture of anti–MIP-1α and anti–MIP-1β Abs and was reduced by transfection of antisense oligonucleotides, but not sense oligonucleotides, of either MIP-1α or MIP-1β. Thus, these data indicate that, although the increased and spontaneous integrin-mediated adhesion of ATL cells to ICAM-1 and HUVECs is mediated by a number of signaling pathways, it mainly depends on activation of G-protein–sensitive PI 3-kinase that is stimulated by endogenous chemokines MIP-1α and MIP-1β.
Adhesion of peripheral ATL cells to IL-1–activated HUVECs and purified ICAM-1. A proportion of ATL cells were transfected with sense or antisense oligonucleotides of MIP-1β and MIP-1α and preincubated for 24 hours at 37°C. Another group of ATL cells were pretreated with a mixture of anti–MIP-1α and anti–MIP-1β Abs for 4 hours at 37°C. Adhesion assay of ATL cells to purified ICAM-1 (a) or IL-1–activated HUVECs (b) was performed in the presence or absence of indicated adhesion-blocking MoAbs (10 μg/mL). Data are expressed as the mean percentage of bound cells from a representative experiment of 4 patients.
Adhesion of peripheral ATL cells to IL-1–activated HUVECs and purified ICAM-1. A proportion of ATL cells were transfected with sense or antisense oligonucleotides of MIP-1β and MIP-1α and preincubated for 24 hours at 37°C. Another group of ATL cells were pretreated with a mixture of anti–MIP-1α and anti–MIP-1β Abs for 4 hours at 37°C. Adhesion assay of ATL cells to purified ICAM-1 (a) or IL-1–activated HUVECs (b) was performed in the presence or absence of indicated adhesion-blocking MoAbs (10 μg/mL). Data are expressed as the mean percentage of bound cells from a representative experiment of 4 patients.
Effects of multiple signaling inhibitors on adhesion of peripheral ATL cells to IL-1–activated HUVECs and purified ICAM-1. ATL cells were pretreated with or without indicated concentrations of multiple inhibitors of intracytoplasmic signaling to purified ICAM-1 (a) and IL-1–activated HUVECs (b). Adhesion assay was performed as described in the Materials and Methods. Data are expressed as the mean percentage of bound cells from a representative experiment of 4 patients.
Effects of multiple signaling inhibitors on adhesion of peripheral ATL cells to IL-1–activated HUVECs and purified ICAM-1. ATL cells were pretreated with or without indicated concentrations of multiple inhibitors of intracytoplasmic signaling to purified ICAM-1 (a) and IL-1–activated HUVECs (b). Adhesion assay was performed as described in the Materials and Methods. Data are expressed as the mean percentage of bound cells from a representative experiment of 4 patients.
Signaling pathways involved in expression of activated form LFA-1 on ATL cells.
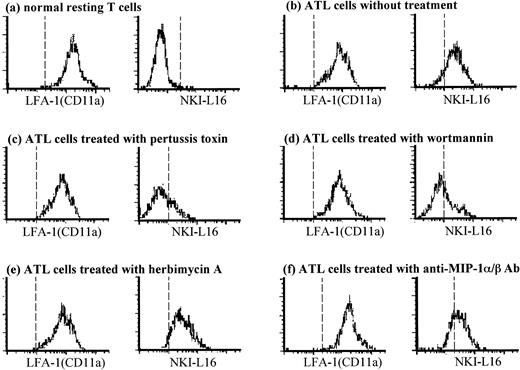
Figure 6 shows histograms of conventional LFA-1α chain (CD11a) and the activated form of LFA-1 recognized by NKI-L16 MoAb on normal resting T cells and ATL cells from a representative normal donor and from an ATL patient. The resting T cells (Fig 6a) and ATL cells (Fig 6b) expressed LFA-1 equally. However, the activated form of LFA-1 was highly expressed on ATL cells (Fig 6b), but not on resting T cells (Fig 6a). The spontaneous expression of activated LFA-1 on ATL cells diminished by pretreatment of these cells with pertussis toxin (Fig 6c) or wortmannin (Fig 6d), but not with herbimycin A (Fig 6e). In contrast, the expression of conventional LFA-1 did not change by these inhibitors (Fig 6c, d, and e). Furthermore, pretreatment of cells with a mixture of anti–MIP-1α and anti–MIP-1β Abs resulted in a decrease of the activated form of LFA-1 (Fig 6f), but not LFA-1 molecules (Fig 6f) on ATL cells (mean fluorescence intensity for NKI-L16 MoAb-staining decreased from 370 to 260; the number of molecules/cell bound with NKI-L16 MoAb decreased from 4,200 to 1,000; calculated by QIFKIT). These results suggest that the spontaneous expression of activated form of LFA-1 on ATL cells may depend on cytoskeletal rearrangement induced through G-protein–dependent activation of PI 3-kinase stimulated by endogenous chemokines.
Staining for the activated form of LFA-1α (CD11a) on peripheral ATL cells using NKI-L16 MoAb. Peripheral normal T cells (a) and ATL cells (b through f) were pretreated with or without 1 μg/mL pertussis toxin for 1 hour at 37°C (c), 100 nmol/L wortmannin (d), 10 μmol/L herbimycin A (e), or a mixture of anti–MIP-1α and anti–MIP-1β Abs (f) for 2 hours at 37°C. The cells were subsequently stained with CD11a MoAb TS1/22 or NKI-L16 MoAb. Cells in the CD4 and CD45RO gate (triple fluorescence) were analyzed by using a flow cytometer. Representative histograms from each one of the volunteers or patients are shown.
Staining for the activated form of LFA-1α (CD11a) on peripheral ATL cells using NKI-L16 MoAb. Peripheral normal T cells (a) and ATL cells (b through f) were pretreated with or without 1 μg/mL pertussis toxin for 1 hour at 37°C (c), 100 nmol/L wortmannin (d), 10 μmol/L herbimycin A (e), or a mixture of anti–MIP-1α and anti–MIP-1β Abs (f) for 2 hours at 37°C. The cells were subsequently stained with CD11a MoAb TS1/22 or NKI-L16 MoAb. Cells in the CD4 and CD45RO gate (triple fluorescence) were analyzed by using a flow cytometer. Representative histograms from each one of the volunteers or patients are shown.
Actin polymerization in ATL cells was inhibited by pertussis toxin and wortmannin.
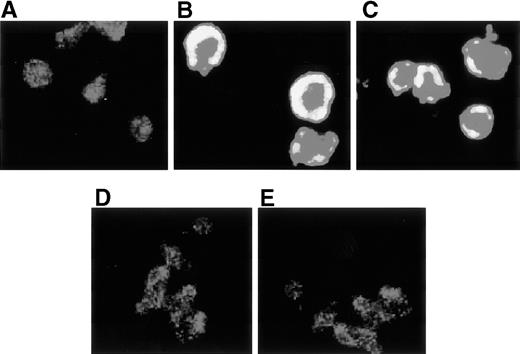
Actin polymerization is a dynamic process critical to cellular adhesion and functional LFA-1 is associated with polymerized F-actin.25 29 Resting T cells seeded on fibronectin did not spread and their F-actin content and distribution remained constant as observed by confocal microscopy (Fig 7A). In contrast, freshly obtained ATL cells (Fig 7B and C) showed increased expression of F-actin in the cell cortex, and marked spreading and polymerization of F-actin was observed without any stimulation. However, when ATL cells were pretreated with pertussis toxin or wortmannin, F-actin-polymerization was markedly reduced (Fig 7D and E). These results suggest that the spontaneous polymerization of F-actin in ATL cells may be induced by signaling through G-protein–dependent activation of PI 3-kinase.
Confocal microscopical analysis of polymerized F-actin on ATL cells. Resting normal T cells (A), ATL cells obtained from 2 representative ATL patients (B and C), and ATL cells pretreated with 1 μg/mL pertussis toxin (D) or 100 nmol/L wortmannin (E) for 2 hours at 37°C were incubated on fibronectin-coated slides for 1 minute, and the F-actin in these cells was stained with rhodamine-phalloidin and was observed by confocal microscopy (original magnification ×1,000).
Confocal microscopical analysis of polymerized F-actin on ATL cells. Resting normal T cells (A), ATL cells obtained from 2 representative ATL patients (B and C), and ATL cells pretreated with 1 μg/mL pertussis toxin (D) or 100 nmol/L wortmannin (E) for 2 hours at 37°C were incubated on fibronectin-coated slides for 1 minute, and the F-actin in these cells was stained with rhodamine-phalloidin and was observed by confocal microscopy (original magnification ×1,000).
Endogenous MIP-1α and MIP-1β were involved in spontaneous transmigration of ATL cells.
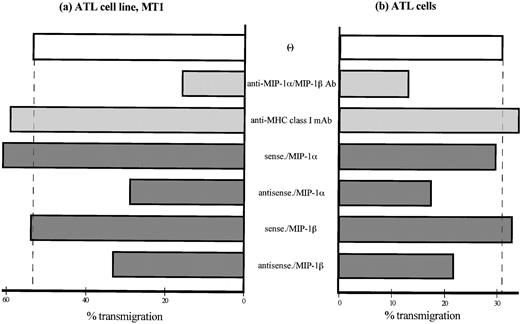
Finally, we assessed the effects of endogenous MIP-1α and MIP-1β on transendothelial migration. ATL cells and MT1 cell lines spontaneously transmigrated through IL-1–activated HUVECs without any exogenous stimuli after 2 hours of incubation (Fig 8a and b). The increased transendothelial migration was reduced by pretreatment of ATL cells with a mixture of anti–MIP-1α and anti–MIP-1β Abs as well as by transfection of antisense oligonucleotides, but not by sense oligonucleotides, of either MIP-1α or MIP-1β. Thus, spontaneous transendothelial migration of ATL cells as well as increased integrin-mediated adhesion of ATL cells to endothelial ligands is mediated by a number of signaling pathways that are likely to be mediated by endogenous chemokines MIP-1α and MIP-1β.
Transmigration of ATL cells through IL-1–activated HUVECs. Transmigration of ATL cells was assessed in 3-μm pore, 12-well microchemotaxis chambers precoated by IL-1β–activated HUVECs for 48 hours at 37°C. An ATL cell line MT1 (a) and ATL cells from patients (b), both labeled with 51Cr, were placed in the insert wells. After incubation for 2 hours at 37°C, cells that have migrated into each of the lower wells were retrieved and dissolved and γ-emissions of the well contents were determined. Data are expressed as the mean percentage of transmigrated cells from a representative experiment.
Transmigration of ATL cells through IL-1–activated HUVECs. Transmigration of ATL cells was assessed in 3-μm pore, 12-well microchemotaxis chambers precoated by IL-1β–activated HUVECs for 48 hours at 37°C. An ATL cell line MT1 (a) and ATL cells from patients (b), both labeled with 51Cr, were placed in the insert wells. After incubation for 2 hours at 37°C, cells that have migrated into each of the lower wells were retrieved and dissolved and γ-emissions of the well contents were determined. Data are expressed as the mean percentage of transmigrated cells from a representative experiment.
DISCUSSION
The process of leukocyte transmigration from circulation into the tissue involves a series of processes, including tethering, triggering of integrins, firm integrin-mediated adhesion, and diapedesis.8,15,32 Leukocyte integrins adhere firmly to their endothelial ligands that belong to the Ig superfamily, including ICAM-1 and VCAM-1. However, integrins on resting circulating leukocytes do not mediate such adhesion until activated by certain stimuli.7,9,10 In contrast, the present results showed that ATL cells of ATL patients spontaneously adhered to endothelial cells as well as purified ICAM-1 and subsequently transmigrated through endothelial cells without any exogenous stimulation. In addition, ATL cells spontaneously expressed the activated form of integrin LFA-1 as recognized by NKI-L16 MoAb that reacts with a Ca2+-dependent activation epitope located on the ectodomain of α-chain of LFA-1.18,28,33 Active configuration of LFA-1 is thought to be induced by a conformational change of LFA-1 and/or clustering of the LFA-1 molecules.24-27Several studies have suggested that F-actin polymerization and association with integrins are involved in activation of integrins.18,25,29,34 35 ATL cells showed a clear increase of F-actin in the cell cortex and marked spreading, polymerization, and rearrangement of F-actin without the addition of any stimulus, whereas the distribution of F-actin on resting T cells remained stable. Furthermore, the cytoskeleton-disrupting agent cytochalasin B reduced spontaneous adhesion of ATL cells to endothelial cells and ICAM-1. Based on these results, we suggest that cytoskeletal rearrangement may be involved in activation of integrin and subsequent induction of adhesion of ATL cells to endothelial cells in vitro.
The adhesive capacity of integrins is tightly regulated through intracellular signals, a process referred to as inside-out signaling.34 Recent studies suggest that activation of integrin can be induced by multiple signaling pathways that may depend on different integrin regulators, including G-proteins, tyrosine kinases, protein kinase C, cAMP pathway, and PI 3-kinases. This can result in actin polymerization and association of LFA-1 with cytoskeletal proteins.15,16,24,29,30,36-39 In the present study, pretreatment of ATL cells with either pertussis toxin or wortmannin reduced polymerization of F-actin, diminished the expression of activated LFA-1, and inhibited integrin-mediated adhesion of the cells to endothelial ligands. However, the effect of these signaling inhibitors was incomplete, indicating that other pathways might be involved as well. For instance, two A-kinase inhibitors, H89 and H88, also slightly decreased integrin-mediated adhesion of ATL cells to endothelial ligands. However, herbimycin A and genestein (tyrosine kinase inhibitors) and H7 and staurosporine (protein kinase C-inhibitors) did not change the spontaneous adhesion of ATL cells. G-protein–coupled receptors are known to activate PI 3-kinases and integrin adhesiveness through ligation of the receptor with fMLP and certain chemokines such as RANTES and monocyte chemotactic protein-1 (MCP-1).36 Furthermore, it is thought that PI 3-kinase is controlled by G-protein–coupled chemoattractant receptors and is involved in cytoskeletal changes associated with localized polymerization of actin filaments and highly cross-linked membrane-associated fibers.16 Based on these early findings, our results suggest that G-protein–dependent activation of PI 3-kinases is likely to be involved in the induction of active LFA-1 through cytoskeletal reorganization in ATL cytoplasm and subsequent amplification of adhesion of ATL cells, although the pathways downstream of PI 3-kinase as well as their role in these cytoskeletal changes are unknown at present.
We and others have previously proposed that chemokine MIP-1β and IL-8 induce integrin-mediated adhesion of T cells and neutrophils, respectively.11,40 ATL cells produced high quantities of both MIP-1α and MIP-1β in the culture supernatant as well as in the cytoplasmic fraction of ATL cells. We have reported that ATL cells produce a variety of cytokines, including IL-1, IL-6, and tumor necrosis factor-α (TNF-α).4,41 Among these cytokines, the spontaneous production of MIP-1α and MIP-1β might be a characteristic feature of ATL cells, because the HTLV-1–viral product tax induces both MIP-1α and MIP-1β.42,43 Furthermore, pretreatment of ATL cells with anti–MIP-1α and anti–MIP-1β Abs as well as transfection of antisense oligonucleotide of either MIP-1α or MIP-1β into ATL cells partially reduced the expression of activated LFA-1 and integrin-mediated adhesion of ATL cells to endothelial ligands and, subsequently, their transendothelial migration. Several chemokines, including fMLP, platelet activation factor (PAF), and leukotrien B4, are known to induce leukocyte adhesion through integrin activation and all these signals are via serpentine G-protein–coupled receptors on leukocytes.37,44,45 Our finding that pertussis toxin inhibited both the expression of the active form of LFA-1 and integrin-mediated adhesion of ATL cells suggests that MIP-1α and MIP-1β produced by ATL cells could transduce signaling through G-protein by binding to its serpentine receptor on the cells and induce continuous triggering of integrin-mediated adhesion of ATL cells by an autocrine mechanism. We have also reported that ATL cells characteristically express large amounts of heparan sulfate proteoglycan on their surface and heparan sulfate binds chemokines MIP-1α and MIP-1β, which are heparin-binding cytokines.5,11 Heparan sulfate proteoglycan is synthesized and binds any heparin-binding cytokine in cytoplasmic organelles such as Golgi bodies, which are then transferred to the cell surface holding the cytokines46 47 and chemokines. Thus, endogenous cytokines and chemokines accumulate on the cell surface and are presented to chemokine receptors in an autocrine manner.
Extravasation and infiltration of circulating hematologic malignant cells into tissues is likely to be a consequence of their biologic properties, expression and function of relevant adhesion molecules on these cells, and their adhesive interaction with endothelial cells.3 Circulating ATL cells first bind to the endothelium through a loose tethering of selectin to its ligand such as sialyl LewisX, because ATL cells express high number of sialyl LewisX.48 In the present study, we observed that polymerization of F-actin and effective triggering of LFA-1 on circulating ATL cells is essential for the binding of ATL cells to the endothelium. Our MoAb-blocking studies of ATL cell adhesion to the endothelium indicated that the firm adhesion was integrin-dependent. Based on these results, we propose that the concept of adhesion cascade involved in leukocyte adhesion to endothelium can be expanded to include leukemic cell migration and metastasis. Especially for ATL cells, spontaneous triggering of integrin on ATL cells induced by endogenous chemokines could explain the strong binding of ATL cells to the endothelium and their spontaneous transendothelial migration. It is noteworthy that not only resting T cells, but also other leukemic T cells such as CEM and Jurkat do not express the activated form of LFA-1, despite significant differences in LFA-1 expression.18 These results indicate that chemokine-dependent triggering of LFA-1 through G-protein–dependent activation of PI 3-kinases and subsequent induction of integrin-mediated adhesion may result in the aberrant behavior of ATL cells and clinical features marking the infiltration of cells into multiple organs during the acute phase. Furthermore, we have also demonstrated that ATL cells show marked clustering through the LFA-1/ICAM-1 pathway in vitro,4 18 suggesting that the high adhesiveness of ATL cells could be involved not only in their infiltration into tissues, but also in nodular proliferation in multiple tissues in vivo, both of which lead to poor prognosis. The findings using ATL cells in this study warrant further studies to unravel the mechanisms of integrin activation in other types of cells and the pathogenesis of leukemic cell infiltration and proliferation, which could lead to new pharmacologic approaches to control these diseases.
ACKNOWLEDGMENT
The authors thank T. Adachi for the excellent technical assistance. We also thank the following investigators for providing MoAbs and cell lines: Dr B. Malissen for B9.8.4 MoAb; Dr K. Sagawa for MT1, MT2, HUT-102, and SALT-3 cell lines; Dr F. Sanchez-Madrid for HP2/1 MoAb; Dr S. Shaw for 84H10, NIH11β-1, and NIH49d-1 MoAbs and L428 cell line; Dr U. Siebenlist for anti–MIP-1β Ab and anti–MIP-1α Ab; Dr D. Siegel for 3G8 MoAb; and H. Zola for FMC63 MoAb.
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Yoshiya Tanaka, MD, The First Department of Internal Medicine, University of Occupational and Environmental Health, Japan, School of Medicine, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal