Abstract
Low-intensity fluorescence of rhodamine-123 (Rh-123) discriminates a quiescent hematopoietic stem cell (HSC) population in mouse bone marrow, which provides stable, long-term hematopoiesis after transplantation. Rh-123 labels mitochondria with increasing intensity proportional to cellular activation, however the intensity of staining also correlates with the multidrug resistance (MDR) phenotype, as Rh-123 is a substrate for P-glycoprotein (P-gp). To address the mechanisms of long-term repopulating HSC discrimination by Rh-123, mouse bone marrow stem and progenitor cells were isolated based on surface antigen expression and subsequently separated into subsets using various fluorescent probes sensitive to mitochondrial characteristics and/or MDR function. We determined the cell cycle status of the separated populations and tested for HSC function using transplantation assays. Based on blocking studies using MDR modulators, we observed little efflux of Rh-123 from HSC obtained from young (3- to 4-week-old) mice, but significant efflux from HSC derived from older animals. A fluorescent MDR substrate (Bodipy-verapamil, BodVer) and Rh-123 both segregated quiescent cells into a dim-staining population, however Rh-123–based separations resulted in better enrichment of HSC function. Similar experiments using two other fluorescent probes with specificity for either mitochondrial mass or membrane potential indicated that mitochondrial activation is more important than either mitochondrial mass or MDR function in defining HSC in young mice. This conclusion was supported by morphologic studies of cell subsets separated by Rh-123 staining.
HEMATOPOIESIS PROCEEDS throughout life. To maintain normal numbers of blood cells, millions of mature cells are generated daily. This rate of production requires a progenitor compartment that proliferates extensively. Hematopoietic stem cells (HSC) produce progenitors for all hematopoietic lineages and are the source of the cellular elements of the blood over the life span of the organism.
In certain strains of mice, the engrafting HSCs can be entirely localized within a population of cells characterized by low-level expression of the T-lymphocyte antigen Thy-1.1, low or no detectable expression of a panel of markers specific for differentiated hematopoietic lineages, and high-level expression of the Ly-6A/E (Sca-1) antigen.1 Staining these cells with the vital mitochondrial dye rhodamine-123 (Rh-123) can resolve two functionally distinct subsets.2 The Rh-123high population only transiently repopulates hematopoietic cell lineages, while the Rh-123low stem cell population permanently reconstitutes hematopoiesis.3-5
Two mechanisms have been proposed for the ability of Rh-123 to discriminate functional subsets of HSC. Because Rh-123 stains mitochondria with increasing intensity as cells become activated,6 the probe may detect a reduced mitochondrial activation state in quiescent long-term repopulating cells.7 Decreased intracellular accumulation of Rh-123 also results from efflux of the dye, mediated by multidrug resistance (MDR) genes such as P-glycoprotein (P-gp).8 Rh-123 is a known substrate for P-gp and has been used extensively as an indicator for P-gp activity.
P-gp is a family of plasma membrane glycoproteins encoded by three genes in the mouse. Two genes, mdr1a and mdr1b, encode proteins that function as energy-dependent efflux pumps9; the mdr2 gene product may function as a phospholipid transporter important in normal hepatobiliary function.10 The mouse mdr1a and mdr1b isoforms and their human homologue MDR1 transport structurally diverse molecules out of cells and have similar substrate specificities and sensitivities to pharmacologic modulators.11
A second multidrug transporter, the multidrug-resistance associated protein (MRP), has recently been characterized.12 MRP differs from P-gp in substrate specificity and susceptibility to pharmacologic modulators. Rh-123 has been shown to be a relatively specific substrate for P-gp, but not MRP,13 even though expression of MRP confers resistance to Rh-123 toxicity.14
Verapamil is an efficient inhibitor of MDR-mediated drug efflux. A green fluorescent derivative of this drug, Bodipy-verapamil (BodVer) has been shown to function as an MDR substrate without significant MDR inhibition.15 BodVer preferentially accumulates in the lysosomes of normal, drug-sensitive NIH 3T3 cells, but is rapidly transported out of MDR cells. Therefore, separation of HSC based on accumulation of BodVer should reflect only MDR function and lysosomal content without being affected by mitochondrial characteristics.
Other fluorescent probes are potential candidates for assessing the roles of MDR function versus mitochondrial membrane potentials in defining repopulating HSC. Nonyl acridine orange (NAO), a probe that stains mitochondria independently of their energetic state, is an indicator of mitochondrial mass.16 Because NAO has also been shown to be an MDR substrate,17 separations of HSC using this probe would reflect mitochondrial mass and MDR function, but not mitochondrial activation. JC-1 is a cationic, dual emission, membrane potential-sensitive mitochondrial probe.18 The green fluorescent monomer forms red fluorescent “J-aggregates” at high concentrations. At appropriate dye concentrations, the emission wavelength of JC-1 fluorescence in mitochondria is an indicator of mitochondrial membrane potential.19 It has not been previously reported that JC-1 is an MDR substrate.
To address the relationship between MDR function, mitochondrial membrane potential, and functional heterogeneity in the HSC compartment, we isolated stem and progenitor cells from normal mouse bone marrow and separated subsets of these cells using fluorescent probes, which are sensitive to MDR function alone (BodVer) or mitochondrial characteristics and MDR function (Rh-123, JC-1, and NAO). To further address the role of MDR activity in defining repopulating HSC, we performed separations in the presence and absence of MDR blockers. We measured the cell cycle status of each separated cell population and tested for HSC function in transplantation assays. The results suggest that in young mice, mitochondrial characteristics play a greater role in defining primitive HSC than does MDR function.
MATERIALS AND METHODS
Animals.
C57BL-Thy-1.1/Ly-5.1 double congenic mice, C57BL/6J (B/6; Thy-1.2/Ly-5.2/Ly-1.2), and C57BL/6-Alpha-17 (Alpha; Thy-1.2/Ly-5.2/Ly-1.1) mice were bred and maintained in the University of Utah Animal Resource Center. All animals were maintained on acidified (pH 2.5) drinking water and autoclaved chow (Purina Mills Inc, St Louis, MO) ad libitum.
Cell preparation.
Cells were prepared using Hanks' Balanced Salt Solution (HBSS) containing 5% fetal calf serum. Bone marrow cells were prepared from young C57BL-Thy-1.1/Ly-5.1 mice (3 to 4 weeks old except as indicated) by crushing femora and tibia in HBSS using a mortar and pestle. Lymph node cells were prepared from pooled inguinal, axillary, brachial, and cervical lymph nodes by gentle teasing with forceps. Both samples were subjected to repeated pipetting and filtered through nylon mesh (85 μm, Small Parts Inc, Miami Lakes, FL) to remove connective tissue and debris.
Fluorescent dye uptake assay.
A leukemic cell line (K562) overexpressing the human P-gp isoform MDR1 was kindly provided by Dr Igor Roninson, University of Illinois, Chicago, IL. The parental K562 cells, which have no detectable P-gp mRNA,20 were obtained from the American Type Culture Collection (Rockville, MD). Cells were maintained in phenol red-free RPMI 1640 medium containing 10% fetal calf serum. To evaluate whether probes were MDR1 substrates, 0.5 × 106 cells/mL K562 or K562-MDR cells were stained in the presence and absence of the MDR blockers verapamil (50 μg/mL) or cyclosporin A (20 μg/mL). All fluorescent probes were obtained from Molecular Probes, Eugene, OR and used at the following concentrations: Bodipy-verapamil (BodVer), 100 nmol/L; JC-1, 5 μmol/L; NAO, 10 nmol/L; Rh-123, 250 nmol/L. Cells were maintained at 37°C in the presence of the individual probes, and 1-mL samples were withdrawn every 20 minutes for 3 hours. Samples were mixed with 2 mL cold phosphate-buffered saline (PBS) containing 50 μg/mL verapamil to stop efflux of probe and were centrifuged at 0°C for 5 minutes at 1,200 rpm. Cells were resuspended in 1 mL cold PBS containing 50 μg/mL verapamil before analysis for mean fluorescence intensity using a FACScan instrument (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Hematopoietic stem and progenitor cell enrichments.
Bone marrow cells were magnetically lineage-depleted and subsequently stained with fluorescein-conjugated anti–Thy-1.1, phycoerythrin-conjugated anti–Sca-1, and biotin-conjugated anti–Sca-2, followed after a wash with streptavidin-Red 613 (GIBCO-BRL; Life Technologies, Inc, Grand Island, NY) as previously described.21 A FACS-Vantage instrument (Becton Dickinson Immunocytometry Systems) was used for sorting Thy-1.1lowSca-1+Sca-2− stem and progenitor cells from the lineage-depleted bone marrow population. Sorted cells were collected by centrifugation, and a small sample (10% of the total yield) was removed for evaluation of sort purity (usually >90%).
Staining and efflux of Rh-123, BodVer, NAO, and JC-1.
The sorted stem and progenitor cells were resuspended in 37°C HBSS containing 200 nmol/L Rh-123, 500 nmol/L BodVer, 2 nmol/L NAO, or 400 nmol/L JC-1. After a 20-minute incubation at 37°C, the cells were collected by centrifugation, resuspended, and incubated at 37°C for 20 minutes in HBSS. The cells were then centrifuged, resuspended in 0.5 mL HBSS, and held at 4°C during the second fluorescence-activated cell sorting (FACS). When included in the experiment, MDR modulators were added to each phase of the staining and efflux at concentrations predetermined to block Rh-123 uptake by Chinese hamster ovary cell lines overexpressing mouse mdr1a or mdr1b isoforms (verapamil, 25 μg/mL; reserpine, 10 μg/mL; cyclosporin A, 10 μg/mL; cell lines were generously provided by Dr Philippe Gros, McGill University, Montreal, Quebec, Canada).11 The modulators were also confirmed to be nontoxic to bone marrow cells in methylcellulose colony assays when used at the above concentrations.
Second FACS sort.
The sorted stem and progenitor cells were sorted a second time, selecting for the dullest and brightest cells in the Rh-123, BodVer, NAO, or JC-1 staining distribution. Reanalysis of the sorted cells showed a high level of purity (>97%). Rh-123low/Rh-123high, BodVerlow/BodVerhigh, NAOlow/NAOhigh, and JC-1low/JC-1high cells were sorted as populations into glass tubes, and dilutions were made based on the electronic count of the cell sorter to obtain the desired number of cells. In some experiments, an automatic cell deposition unit (Becton Dickinson Immunocytometry Systems) was used to deposit the required number of cells into the wells of a 96-well microtiter plate, with each well also containing 105 normal B6-Thy-1.2/Ly-5.2 bone marrow cells in 200 μL HBSS. The contents of the wells were then collected in 1-cc insulin syringes (Becton Dickinson, no. 9410) and quantitatively transferred into recipient mice.
Irradiations and reconstitutions.
Bone marrow recipient animals (B/6 or Alpha) were exposed to 13 Gy of radiation from a 137Cs source (Mark I gamma irradiator; J.L. Sheperd and Associates, Glendale, CA) at a dose rate of 0.5 Gy/min. The dose was delivered in two equal fractions separated by a 3-hour rest as previously described.22
Analysis of transplant recipients.
At various times after transplantation, peripheral blood samples were collected for immunofluorescent staining. Donor- and host-derived cells were distinguished with monoclonal antibody (MoAb) specific for the two alleles of Ly-5.23 Dual-color immunofluorescence, using fluorescein-conjugated anti–Ly-5 reagents specific for the donor alleles and biotin conjugates of antibodies specific for T-cell, B-cell, and myeloid lineages, was used to phenotype donor-derived cells as previously described.24
Marrow repopulating assay (MRA).
Approximately 2,000 cells of each of the Rh-123, JC-1, or NAO-separated populations were transplanted into lethally irradiated B/6 or Alpha mice. Bone marrow from these animals or untransplanted controls was harvested 12 to 13 days later, and cellular reconstitution was evaluated by determining the number of cells per femur. These cells were then transplanted into a second set of irradiated recipients for determination of the frequency of day 13 spleen colony-forming units (CFU-S) per femur of each recipient group as previously described.25
Cell cycle analysis.
Sorted cell suspensions were fixed in methanol and stained in a PBS-EDTA solution containing 1% Triton X-100, 100 U/mL RNAse, and 5 mg/mL propidium iodide before analysis for DNA content by FACScan.
Cell imaging.
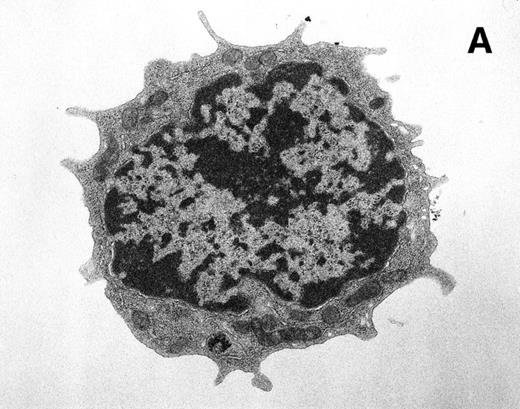
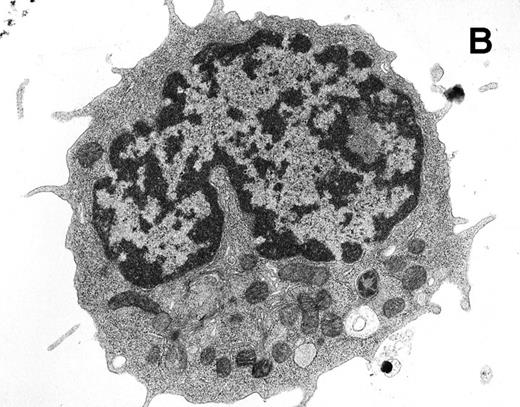
For transmission electron microscopy, Rh-123low and Rh-123high cell subsets were sorted into 1.5-mL microfuge tubes containing 2.5% glutaraldehyde/4% paraformaldehyde in 0.1 mol/L sodium phosphate buffer (pH 7.2) containing 0.1 mol/L sucrose. After a 1-hour fixation, cells were stained with OsO4 and uranyl acetate, embedded in Spurr's resin, and sectioned. Silver-gold sections were collected on naked 300 mesh copper grids and poststained with uranyl acetate and lead heavy metal stains. Examination and photography used a Hitachi HU11-E-1 electron microscope (Hitachi, Ltd, Tokyo, Japan) at 75 kV using Kodak SO-163 electron image film (Eastman Kodak, Rochester, NY). Random images of Rh-123low and Rh-123high cells were evaluated visually for mitochondrial distribution and activation, based on mitochondrial staining intensity and morphology. The photomicrographs shown (see Fig 8) are representative of 17 images of Rh-123low cells and 32 images of Rh-123highcells.
Ultrastructural analysis of isolated Rh-123low (A) and Rh-123high (B) HSC. The original magnification was 8,200X in each case. Pronounced mitochondrial clustering is apparent in the nuclear cleft region of the Rh-123high cell (B), correlating with the bipolar perinuclear localization of Rh-123 staining seen in Fig 7B. Activated mitochondria in (B) can be identified by increased electron density due to thickening of cristae and by the characteristic structural collapse and “wagon wheel” appearance of actively respiring mitochondria; these features were rarely noted in micrographs of Rh-123low cells (A). The micrographs are representative of 17 images of Rh-123low cells and 32 images of Rh-123high cells.
Ultrastructural analysis of isolated Rh-123low (A) and Rh-123high (B) HSC. The original magnification was 8,200X in each case. Pronounced mitochondrial clustering is apparent in the nuclear cleft region of the Rh-123high cell (B), correlating with the bipolar perinuclear localization of Rh-123 staining seen in Fig 7B. Activated mitochondria in (B) can be identified by increased electron density due to thickening of cristae and by the characteristic structural collapse and “wagon wheel” appearance of actively respiring mitochondria; these features were rarely noted in micrographs of Rh-123low cells (A). The micrographs are representative of 17 images of Rh-123low cells and 32 images of Rh-123high cells.
For confocal microscopic analysis, isolated viable Rh-123low and Rh-123high cells were wet-mounted in 5 μL of HBSS and visualized using a Bio-Rad MRC 1000 confocal laser scanning system (Bio-Rad Laboratories, Hercules, CA) coupled to a Zeiss Axiovert 135 microscope (Carl Zeiss, Inc, Thornwood, NY). Excitation used an argon laser tuned to 488 nm and fluorescence emissions were collected through a 525-nm bandpass filter. Fluorescent and visible images were collected simultaneously through a 63X objective and later analyzed using NIH Image software (National Institutes of Health, Bethesda, MD).
RESULTS
Fluorescent labeling of Thy-1.1lowSca-1+Lin− HSC with various probes.
To address the mechanism that allows Rh-123low staining to identify the subset of multipotent HSC providing long-term engraftment after transplantation,3 we tested the ability of other fluorescent probes to segregate functionally distinct HSC subsets. Probes were selected for their ability to function as indicators of mitochondrial characteristics and/or MDR function. Each probe produced a spectrum of fluorescence intensity when used to label Thy-1.1lowSca-1+Lin− stem and progenitor cells obtained from 3- to 4-week-old mice, with the range of dullest to brightest staining (gating on the upper and lower 5% of total cells) being 40-fold (JC-1), 30-fold (Rh-123), 20-fold (NAO), and 7-fold (BodVer) (Fig 1). Only JC-1 labeled HSC at a duller intensity than unseparated bone marrow when equivalent concentrations of dye were used; this indicates the absence of preferential efflux of Rh-123, BodVer, and NAO from HSC relative to unseparated bone marrow. The staining intensity of JC-1 in HSC reverted to the levels seen in unseparated bone marrow samples when the staining was performed in the presence of the MDR blockers, suggesting preferential efflux of JC-1 by an MDR mechanism in HSC. Staining of HSC with BodVer and NAO resulted in relatively unimodal staining distributions with intermediate levels of fluorescence compared with unseparated bone marrow. Rh-123 stained HSC more brightly than bone marrow and included a discernable shoulder at the high end of the staining distribution (Fig 1).
Fluorescence staining intensity observed after reacting the indicated fluorescent probes with normal bone marrow cells (shaded histograms) or Thy-1.1lowSca-1+Lin− HSC sorted from 3 to 4 week-old donor animals (open histograms). Staining and efflux were performed as described in Materials and Methods.
Fluorescence staining intensity observed after reacting the indicated fluorescent probes with normal bone marrow cells (shaded histograms) or Thy-1.1lowSca-1+Lin− HSC sorted from 3 to 4 week-old donor animals (open histograms). Staining and efflux were performed as described in Materials and Methods.
Effect of donor age on Rh-123 efflux.
To address discrepancies of Rh-123 staining patterns in stem and progenitor populations reported by different laboratories,3,26 we compared bone marrow and Thy-1.1lowSca-1+Lin− HSC staining profiles from mice of various ages. A striking effect of donor age on efflux of Rh-123 was apparent, as shown in Fig 2A. In all cases, the distinct Rh-123low population observed in Thy-1.1lowSca-1+Lin− HSC obtained from older animals could be reversed by MDR modulators (Fig2B, and additional data not shown). MDR modulators consistently increased the fluorescence intensity of Rh-123low cells, but not Rh-123high cells, suggesting that the Rh-123low subset is defined by efflux of the Rh-123 probe as previously reported.8 However, MDR modulators had little effect on the Rh-123 staining profile of Thy-1.1lowSca-1+Lin− HSC obtained from 3- to 4-week-old mice, or on unseparated bone marrow cells from any age mouse (Fig 2B). These results show a specific and pronounced effect of mouse age on Rh-123 efflux from Thy-1.1lowSca-1+Lin− HSC, similar to previous reports of upregulated P-gp function in mouse27 and human28 T lymphocytes derived from aged donors.
Effect of bone marrow donor age on efflux of Rh-123. Thy-1.1lowSca-1+Lin− HSC and normal unfractionated bone marrow isolated from groups of mice of the indicated ages were stained with Rh-123 as described in Materials and Methods. (A) Shaded profiles indicate Rh-123 fluorescence in normal bone marrow cells, while solid profiles show fluorescence of Thy-1.1lowSca-1+Lin− HSC isolated from the same bone marrow preparation. (B) Solid lines indicate the Rh-123 fluorescence intensity of Thy-1.1lowSca-1+Lin− HSC isolated from 10-month or 3-week-old mice, except in the indicated panel where the solid line indicates total bone marrow cells. Stippled profiles indicate the same cells stained with Rh-123 in the presence of 20 μg/mL cyclosporin A; similar results were obtained using verapamil as an MDR modulator (data not shown).
Effect of bone marrow donor age on efflux of Rh-123. Thy-1.1lowSca-1+Lin− HSC and normal unfractionated bone marrow isolated from groups of mice of the indicated ages were stained with Rh-123 as described in Materials and Methods. (A) Shaded profiles indicate Rh-123 fluorescence in normal bone marrow cells, while solid profiles show fluorescence of Thy-1.1lowSca-1+Lin− HSC isolated from the same bone marrow preparation. (B) Solid lines indicate the Rh-123 fluorescence intensity of Thy-1.1lowSca-1+Lin− HSC isolated from 10-month or 3-week-old mice, except in the indicated panel where the solid line indicates total bone marrow cells. Stippled profiles indicate the same cells stained with Rh-123 in the presence of 20 μg/mL cyclosporin A; similar results were obtained using verapamil as an MDR modulator (data not shown).
Evaluation of MDR-mediated efflux of fluorescent probes.
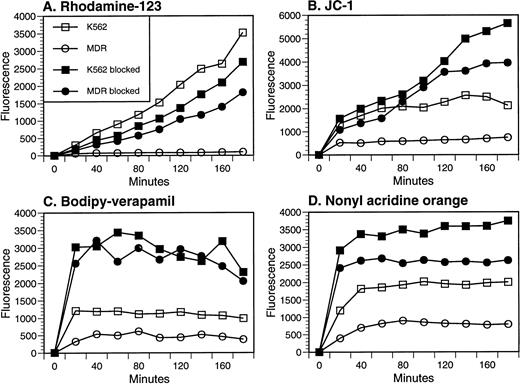
To test and compare the ability of probes to act as MDR substrates, we measured the dye loading kinetics in a pair of cell lines, the human chronic myelogenous leukemia line K562 and a sister clone overexpressing human MDR1. To do this, we suspended the cell lines in equal concentrations of dye and measured the increase in fluorescence intensity with time. The activity of MDR1 in the transduced cell line could be visualized as a decreased rate of loading relative to the parental line, particularly when Rh-123 or JC-1 were used as substrates (Fig 3A and B). Rh-123 and JC-1 efflux could be partially reversed with verapamil or cyclosporin A, but even in the presence of the blockers, there was a differential between the parental and transfectant cell lines, suggesting that inhibition of efflux was incomplete. An efflux mechanism endogenous to K562 cells was detected when NAO and BodVer were used as substrates, as dye loading into both parental and MDR1 transfectants was enhanced in the presence of verapamil or cyclosporin A (Fig 3C and D). This effect was also noted late in the time course of JC-1 loading (Fig 3B). K562 cells are known to express a newly described MDR protein, the multidrug resistance-associated protein (MRP).29 The substrate specificity of MRP (low specificity for Rh-123 as a substrate,13 and sensitivity to modulation by verapamil and cyclosporin A30) fits with the interpretation that MRP is the endogenous pump responsible for efflux of JC-1, BodVer, and NAO seen in Fig 3B through D. Despite this endogenous efflux activity, the differential loading of the two cell lines in the absence of modulators confirms that both NAO and BodVer are P-gp substrates15,16and establishes that JC-1 is also an MDR substrate. In addition, these experiments confirm multiple efflux mechanisms to which the four probes are differentially sensitive.13
Determination of P-gp–mediated efflux of four fluorescent probes. Each probe was incubated with the parental K562 or MDR1-transfected cell line at 37°C, and mean fluorescence intensity was measured as a function of time. Verapamil (50 μg/mL) was added to each sample at the time of washing to prevent efflux before fluorescence measurement. When included as a modulator of efflux, either cyclosporin A (20 μg/mL) or verapamil (50 μg/mL) was present during the incubation period, as well as during analysis. Symbols in each panel represent parental K562 cells (□, ▪) or MDR1 transfected K-562 cells (○, •); open symbols represent uptake in the absence of modulators, while closed symbols indicate that either cyclosporin A (B and C) or verapamil (A and D) was present during probe loading.
Determination of P-gp–mediated efflux of four fluorescent probes. Each probe was incubated with the parental K562 or MDR1-transfected cell line at 37°C, and mean fluorescence intensity was measured as a function of time. Verapamil (50 μg/mL) was added to each sample at the time of washing to prevent efflux before fluorescence measurement. When included as a modulator of efflux, either cyclosporin A (20 μg/mL) or verapamil (50 μg/mL) was present during the incubation period, as well as during analysis. Symbols in each panel represent parental K562 cells (□, ▪) or MDR1 transfected K-562 cells (○, •); open symbols represent uptake in the absence of modulators, while closed symbols indicate that either cyclosporin A (B and C) or verapamil (A and D) was present during probe loading.
Functional analysis of HSC separated by fluorescent mitochondrial probes.
Thy-1.1lowSca-1+Lin− HSC were isolated from 3- to 4-week-old mice and stained with mitochondrial dyes JC-1, NAO, or Rh-123. JC-1 and Rh-123 preferentially stain activated mitochondria,31 while NAO stains mitochondria independently of their energetic state.16 The brightest and dullest 30% of cells in each staining distribution (Fig 1) were selected by cell sorting, and 2,000 cells were transplanted into lethally irradiated recipient mice. Twelve to 13 days later, bone marrow was harvested from the primary recipients and transplanted into secondary recipients for determination of MRA based on the content of CFU-S–13 in marrow isolated from the primary recipients. As shown in Table 1, both Rh-123 and JC-1 segregated MRA activity into the dullest staining population. In contrast, NAO did not segregate MRA activity between dull- and bright-staining cells. Table 1 illustrates the use of MRA for detecting primitive stem and progenitor cells in a relatively short-term assay25(compare the low/high ratios for marrow cellularity with those for CFU-S/femur). Because all three probes are substrates for MDR pumps (Fig 3), these results support the hypothesis that the ability of Rh-123 and JC-1 to separate two classes of HSC is dependent on their ability to discriminate mitochondrial membrane potentials rather than MDR-mediated efflux.
MRA of HSC Subsets Isolated by Fluorescent Staining With Rh-123, JC-1, or NAO
| Cells Transplanted . | Cells/Femur . | Low/High Ratio . | CFU-S/Femur . | Low/High Ratio . |
|---|---|---|---|---|
| Experiment No. 1 | ||||
| None | 3.1 × 105 | 4.7 ± 1.1 | ||
| Rh-123low | 5.3 × 106 | 115 ± 78-150 | ||
| Rh-123high | 2.2 × 106 | 2.41 | 4.4 ± 2.4 | 26.1 |
| JC-1low | 1.6 × 107 | 206 ± 66 | ||
| JC-1high | 2.8 × 106 | 5.71 | 0.6 ± 1.3 | 343 |
| Experiment No. 2 | ||||
| None | 4.7 × 105 | 3.4 ± 0.3 | ||
| Rh-123low | 1.3 × 107 | 425 ± 202 | ||
| Rh-123high | 3.3 × 106 | 3.94 | 3.8 ± 2.5 | 112 |
| NAOlow | 4.6 × 106 | 49 ± 8.5 | ||
| NAOhigh | 4.8 × 106 | 0.96 | 75 ± 18-151 | 0.65 |
| Cells Transplanted . | Cells/Femur . | Low/High Ratio . | CFU-S/Femur . | Low/High Ratio . |
|---|---|---|---|---|
| Experiment No. 1 | ||||
| None | 3.1 × 105 | 4.7 ± 1.1 | ||
| Rh-123low | 5.3 × 106 | 115 ± 78-150 | ||
| Rh-123high | 2.2 × 106 | 2.41 | 4.4 ± 2.4 | 26.1 |
| JC-1low | 1.6 × 107 | 206 ± 66 | ||
| JC-1high | 2.8 × 106 | 5.71 | 0.6 ± 1.3 | 343 |
| Experiment No. 2 | ||||
| None | 4.7 × 105 | 3.4 ± 0.3 | ||
| Rh-123low | 1.3 × 107 | 425 ± 202 | ||
| Rh-123high | 3.3 × 106 | 3.94 | 3.8 ± 2.5 | 112 |
| NAOlow | 4.6 × 106 | 49 ± 8.5 | ||
| NAOhigh | 4.8 × 106 | 0.96 | 75 ± 18-151 | 0.65 |
Mouse HSC isolated by the Thy-1.1lowSca-1+Lin− phenotype were further separated based on levels of JC-1, NAO, or Rh-123 staining. Cells in the upper 30% and lower 30% of staining intensity were isolated in each case, and approximately 2,000 cells of each population were transplanted into two lethally irradiated syngeneic mice. Twelve to 13 days later the cellularity of the femurs was determined; the numbers indicate the cell count derived from four pooled femurs. CFU-S/femur indicates the content of day 13 CFU-S per femur in the primary transplant recipients (mean ± SD), as measured in groups of four secondary recipient mice except as noted.
Eight secondary recipients analyzed.
Three secondary recipients analyzed.
Selection of HSC subsets using an MDR probe.
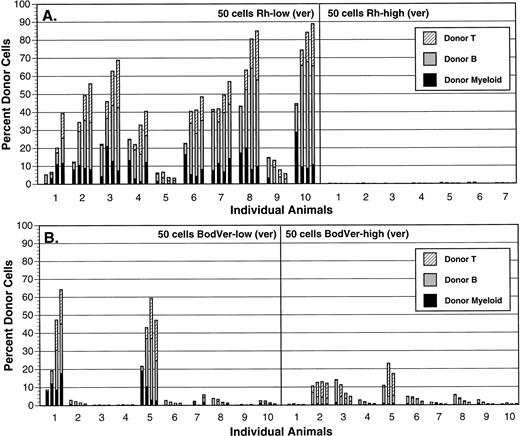
To further address the relative roles played by MDR function and mitochondrial activation in defining primitive HSC, we used BodVer to isolate subsets of HSC based on MDR function in the absence of a mitochondrial specificity. In parallel, we separated the same cell preparations using Rh-123. To establish relative repopulating abilities of HSC stained at low and high levels with each probe, we performed competitive repopulation assays with each population. As shown in Fig 4, long-term repopulating activity was concentrated in the Rh-123low population, as 100% of animals transplanted with 50 of these cells were strongly reconstituted (>30% of circulating blood cells) with donor-derived peripheral blood cells of T, B, and myeloid lineages 12 weeks posttransplant. No recipients of 50 Rh-123high cells were reconstituted with donor-derived cells (not shown), while only one of eight recipients of 200 Rh-123high cells was reconstituted in multiple hematopoietic lineages with donor-derived cells (Fig 4A). Transplantation of 50 BodVerlow cell cells resulted in 20% long-term repopulating efficiency (two of 10 mice were strongly reconstituted in T, B, and myeloid lineages), while the BodVerhigh cell population showed little or no long-term repopulating activity after transplantation of 200 cells (Fig 4B). Therefore, although some segregation of repopulating activity was observed between BodVerlow and BodVerhighpopulations, selection of HSC based on efflux of BodVer did not concentrate HSC function in the low-staining fraction to the same extent as did selection with Rh-123.
Comparison of long-term repopulating activity in Thy-1.1lowSca-1+Lin− HSC separated using Rh-123 (A) or BodVer (B). The brightest and dullest 15% of cells in each staining distribution were isolated from Ly-5.1 bone marrow, and the indicated numbers of each population were transplanted into lethally irradiated Ly-5.2 recipient animals in the presence of 105 normal Ly-5.2 bone marrow cells. Each animal (represented by each set of bars in the graphs) was bled 4, 6, 8, and 12 weeks posttransplant (time points are represented by individual bars within each set of four bars in the graphs), and Ly-5.1 peripheral blood cells were identified and phenotyped by flow cytometry.
Comparison of long-term repopulating activity in Thy-1.1lowSca-1+Lin− HSC separated using Rh-123 (A) or BodVer (B). The brightest and dullest 15% of cells in each staining distribution were isolated from Ly-5.1 bone marrow, and the indicated numbers of each population were transplanted into lethally irradiated Ly-5.2 recipient animals in the presence of 105 normal Ly-5.2 bone marrow cells. Each animal (represented by each set of bars in the graphs) was bled 4, 6, 8, and 12 weeks posttransplant (time points are represented by individual bars within each set of four bars in the graphs), and Ly-5.1 peripheral blood cells were identified and phenotyped by flow cytometry.
Inhibition of MDR function in HSC using MDR blockers.
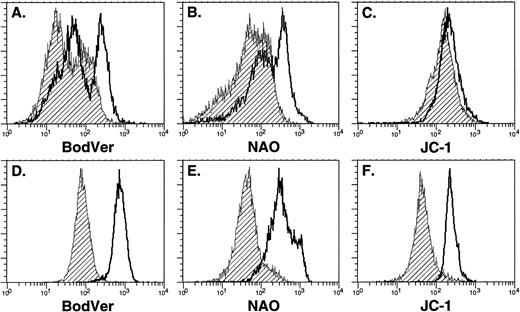
We used three functionally distinct MDR blockers to address the role of active dye efflux in determining the functional distribution of Thy-1.1lowSca-1+Lin− HSC after staining with the fluorescent probes. Little effect was observed when normal bone marrow cells were incubated with the individual probes alone compared with incubations in the presence of MDR blockers (Fig 5A through C). A very slight increase in fluorescent intensity was observed when JC-1 staining was performed in the presence of blockers, similar to that observed with Rh-123 (Fig2). A more profound shift was noted in BodVer or NAO intensity in the presence of blockers (Fig 5A and B). This observation is consistent with the data shown in Fig 3, which indicates that BodVer and NAO are significant substrates for a non–P-gp MDR mechanism compared with Rh-123 and JC-1. When parallel experiments were performed to evaluate the sensitivity of purified Thy-1.1lowSca-1+Lin− HSC to modulation of staining intensity by MDR blockers, we observed that incubation of the probes in the presence of MDR blockers always resulted in a shift of the staining intensity of the entire population of Thy-1.1lowSca-1+Lin− HSC (Fig 5D through F), in marked contrast to the results obtained with Rh-123 (Fig 2). These findings suggest that a non–P-gp efflux mechanism is selectively expressed in Thy-1.1lowSca-1+Lin− HSC compared with the total bone marrow population. BodVer, NAO, and JC-1 are substrates for both efflux mechanisms, while Rh-123 is relatively selective for P-gp.13 Alternatively, the modulators may selectively alter the subcellular distribution of the probes in Thy-1.1lowSca-1+Lin−HSC.32 However, the results shown in Fig 5 are similar to the data shown in Fig 3, where MDR blockers enhanced uptake of BodVer into both parental and MDR1-transfected cell lines. This result is consistent with the interpretation that a membrane pump distinct from P-gp is selectively active in bone marrow stem and progenitor cells.
Retention of fluorescent probes by normal bone marrow cells (A through C) and Thy-1.1lowSca-1+Lin− HSC (D through F) in the presence or absence of pharmacologic MDR modulators. All plots were derived from a single experiment using 7-week-old donor animals. Shaded histograms indicate staining with probe alone, while open histograms indicate staining in the presence of cyclosporin A as an MDR modulator to block dye efflux. In all cases, cells were incubated with the probe for 20 minutes at 37°C, washed, and incubated in the absence of probe for an additional 20 minutes at 37°C to allow efflux. Cyclosporin A, when used, was present during both stages of the incubation; similar results were obtained using verapamil or reserpine as MDR modulators. Cells subsets were isolated for subsequent analysis (Table 2, Fig 6) by gating on and sorting the dullest and brightest 15% of cells in each profile.
Retention of fluorescent probes by normal bone marrow cells (A through C) and Thy-1.1lowSca-1+Lin− HSC (D through F) in the presence or absence of pharmacologic MDR modulators. All plots were derived from a single experiment using 7-week-old donor animals. Shaded histograms indicate staining with probe alone, while open histograms indicate staining in the presence of cyclosporin A as an MDR modulator to block dye efflux. In all cases, cells were incubated with the probe for 20 minutes at 37°C, washed, and incubated in the absence of probe for an additional 20 minutes at 37°C to allow efflux. Cyclosporin A, when used, was present during both stages of the incubation; similar results were obtained using verapamil or reserpine as MDR modulators. Cells subsets were isolated for subsequent analysis (Table 2, Fig 6) by gating on and sorting the dullest and brightest 15% of cells in each profile.
Effect of MDR inhibition on the cell cycle status of HSC subsets.
To address the effects of MDR blockers on the selection of HSC subsets using Rh-123 and BodVer, we analyzed the cell cycle status of dull and bright cell populations isolated with both dyes. Cell populations selected as either Rh-123low or BodVerlow were comprised predominantly of quiescent cells (≈ 3% S+G2/M) while the Rh-123high and BodVerhigh populations contained high numbers of cycling cells (≈ 30% in S+G2/M; Table 2). Blockade of efflux by verapamil had little effect on the cell cycle distribution observed in these subsets (Table 2), despite the major difference in fluorescence intensity observed when verapamil was used to block BodVer staining (Fig 5D). The frequency of S+G2/M cells in the bright-staining fractions was somewhat variable between experi-ments, but within individual experiments the values were always very similar in the presence or absence of verapamil. In contrast, cyclosporin A and reserpine had little effect on the frequency of cycling cells in the dull-staining populations, but had a pronounced effect on this parameter in bright-staining cells (Table 2). Because of this effect and other potential effects of cyclosporin A,32 we used verapamil to inhibit MDR function in subsequent cell enrichments.
Percentage of Cycling Cells in Rh-123 and BodVer Subsets of HSC
| . | Rh-123low . | Rh-123high . | BodVerlow . | BodVerhigh . |
|---|---|---|---|---|
| No blocker | 3.2 ± 3.5 | 29.9 ± 4.5 | 3.0 ± 1.2 | 30.2 ± 7.7 |
| Verapamil | 3.4 ± 1.6 | 29.1 ± 0.5 | 4.5* | 32.3* |
| Reserpine | 2.8 ± 0.7 | 46.0 ± 14.3 | 2.2 ± 0.4 | 54.3 ± 7.4 |
| Cyclosporin A | 3.7 ± 1.7 | 46.9 ± 5.7 | 3.2 ± 1.4 | 49.9 ± 3.2 |
| . | Rh-123low . | Rh-123high . | BodVerlow . | BodVerhigh . |
|---|---|---|---|---|
| No blocker | 3.2 ± 3.5 | 29.9 ± 4.5 | 3.0 ± 1.2 | 30.2 ± 7.7 |
| Verapamil | 3.4 ± 1.6 | 29.1 ± 0.5 | 4.5* | 32.3* |
| Reserpine | 2.8 ± 0.7 | 46.0 ± 14.3 | 2.2 ± 0.4 | 54.3 ± 7.4 |
| Cyclosporin A | 3.7 ± 1.7 | 46.9 ± 5.7 | 3.2 ± 1.4 | 49.9 ± 3.2 |
Thy-1.1lowSca-1+Lin− cells were stained and separated based on levels of Rh-123 and BodVer staining in the absence or presence of the indicated P-gp blockers. In each case, the brightest 15% (Rh-123low and BodVerlow) of total cells were sorted, and cell cycle analysis was performed for each cell population. Each number represents the percentage of cells in the S/G2/M regions of the DNA histogram (mean ± SD) as determined in three or four separate sorting experiments except as noted.
Average values from two experiments.
Verapamil blockade does not markedly change long-term engraftment by HSC selected using either Rh-123 or BodVer.
To further investigate the role of MDR function in the selection of HSC subsets using Rh-123 and BodVer, we performed another competitive repopulation experiment. Rh-123low cells, isolated in the presence of verapamil, mediated long-term repopulation in eight of 10 animals, and all positive animals were reconstituted in T, B, and myeloid lineages at a level equal to or greater than 40% of the total peripheral blood cells (Fig 6A). This result is comparable to results from cell selections performed in the absence of verapamil, where 10 of 10 animals were reconstituted (Fig4A). Addition of verapamil to the selection protocol did not influence the failure of Rhhigh cells to mediate reconstitution. The recovery of long-term repopulating cells after selections using BodVer was also not influenced by verapamil, as two of 10 animals transplanted with 50 BodVerlow cells engrafted regardless of the presence of verapamil (Fig 6B, compare with Fig 4B). BodVerhigh cells isolated in the presence of verapamil provided only marginal reconstituting activity at a dose of 50 cells (Fig 6B).
Comparison of long-term repopulating activity in Thy-1.1lowSca-1+Lin− stem cells separated using Rh-123 (A) or BodVer (B) in the presence of verapamil as an MDR inhibitor. The brightest and dullest 15% of cells in each staining distribution was isolated from Ly-5.1 bone marrow and transplanted into lethally irradiated Ly-5.2 recipient animals in the presence of 105 normal Ly-5.2 bone marrow cells. Each animal (represented by each set of bars in the graphs) was bled 4, 6, 8, and 12 weeks posttransplant (time points are represented by each individual bar in the graphs), and Ly-5.1 peripheral blood cells were identified and phenotyped by flow cytometry.
Comparison of long-term repopulating activity in Thy-1.1lowSca-1+Lin− stem cells separated using Rh-123 (A) or BodVer (B) in the presence of verapamil as an MDR inhibitor. The brightest and dullest 15% of cells in each staining distribution was isolated from Ly-5.1 bone marrow and transplanted into lethally irradiated Ly-5.2 recipient animals in the presence of 105 normal Ly-5.2 bone marrow cells. Each animal (represented by each set of bars in the graphs) was bled 4, 6, 8, and 12 weeks posttransplant (time points are represented by each individual bar in the graphs), and Ly-5.1 peripheral blood cells were identified and phenotyped by flow cytometry.
Morphologic analysis of HSC subsets isolated by differential Rh-123 staining.
We used laser-scanning confocal analysis and electron microscopy to characterize the morphology of cell populations recovered after selection by Rh-123 staining of Thy-1.1lowSca-1+Lin− HSC. Confocal microscopy showed the intracellular distribution of Rh-123 to be rather uniform within Rh-123low cell populations (Fig 7A). Of 38 Rh-123low cells observed, 33 exhibited little or no evidence of localized accumulation of the dye as shown in Fig 7A, while five of 38 cells showed evidence of bipolar, perinuclear dye accumulation. In contrast, the Rh-123high cells consistently exhibited accumulation of Rh-123 in a bipolar fashion, within the nuclear cleft and on the opposite side of the nucleus (Fig 7B). Optical sections at 3-μm intervals showed the bipolar staining pattern in 28 of 28 Rh-123high cells, with the maximal staining intensity often occurring at different section intervals for the two sites of dye accumulation (Fig 7B and data not shown). Ultrastructural analysis of the two subsets of HSC (Fig 8) showed pronounced clustering of activated mitochondria of Rh-123high cells in 27 of 32 sections evaluated, while little activation was evident in sections of Rh-123lowcells (clustered or activated mitochondria evident in four of 17 sections evaluated). The mitochondrial distribution evident in the ultrastructural analysis shown by representative micrographs in Fig 8is consistent with previously published micrographs of HSC derived from bone marrow33 and peripheral blood34 and is also consistent with the interpretation that the distribution of Rh-123 fluorescence observed in the confocal images of these cells (Fig 7) corresponds to mitochondrial accumulation of the dye.
Laser-scanning confocal microscopy of isolated Rh-123low (A) and Rh-123high (B) HSC. In each pair of images, the top frame was captured from transmitted light, while the bottom frame was a simultaneous fluorescent image. Identical magnifications (63X oil objective), illumination, and signal processing conditions were used to image the two cell types, assuring that the images are representative of the differential size and fluorescence intensity of the cells. Fluorescence images were inverted and pseudocolored so that increasing fluorescence intensity is indicated from blue to red. Note the relatively homogeneous cytoplasmic staining in the Rh-123low cells compared with the intense bipolar perinuclear staining seen in Rh-123high cells. The images are representative of 38 Rh-123low cells and 28 Rh-123high cells evaluated in two separate experiments.
Laser-scanning confocal microscopy of isolated Rh-123low (A) and Rh-123high (B) HSC. In each pair of images, the top frame was captured from transmitted light, while the bottom frame was a simultaneous fluorescent image. Identical magnifications (63X oil objective), illumination, and signal processing conditions were used to image the two cell types, assuring that the images are representative of the differential size and fluorescence intensity of the cells. Fluorescence images were inverted and pseudocolored so that increasing fluorescence intensity is indicated from blue to red. Note the relatively homogeneous cytoplasmic staining in the Rh-123low cells compared with the intense bipolar perinuclear staining seen in Rh-123high cells. The images are representative of 38 Rh-123low cells and 28 Rh-123high cells evaluated in two separate experiments.
DISCUSSION
Rh-123, a cationic fluorescent dye, accumulates selectively in the mitochondria of eukaryotic cells.6 The localization of Rh-123 to mitochondria is thought to depend on the negatively charged membrane potential across the inner mitochondrial membrane. Rh-123 is also a substrate for P-gp, and recent studies have suggested a prominent P-gp activity in primitive HSC based on dye-efflux experiments.8,35 36 We designed experiments to address the relative importance of these two mechanisms in defining HSC.
We observed that efflux of Rh-123 from Thy-1.1lowSca-1+Lin− HSC increases as a function of age (Fig 2). Very little efflux was noted from cells obtained from 3- to 4-week-old mice, indicating that P-gp activity is minimal in young mice. Nevertheless, Rh-123 separation of cell subsets from these animals, in the absence (Fig 4) or the presence (Fig 6) of MDR blockers, resulted in a profound enrichment of long-term repopulating cells in the Rh-123low population. To further characterize the respective roles of mitochondrial retention versus P-gp–mediated efflux of Rh-123 in identifying primitive HSC, we used a panel of fluorescent probes to isolate subsets of Thy-1.1lowSca-1+Lin− cells for subsequent analysis of HSC activity. Ideally, one would use probes selective for either mitochondrial membrane potential or MDR-mediated efflux. However, every mitochondrial probe we have evaluated to date is also a substrate for MDR efflux (Fig 3 and additional data not shown). Of those tested in this study, only the probes selective for mitochondrial membrane potential (Rh-123 and JC-1) effectively separated and enriched primitive HSC from the Thy-1.1lowSca-1+Lin− cells. Selections based on retention of the MDR substrate BodVer did not enrich long-term repopulating HSC to the same extent as those based on Rh-123 (Fig 4). While JC-1 exhibited efflux properties similar to those of BodVer and NAO when evaluated in HSC (Fig 5D through F), only JC-1 was able to mimic the ability of Rh-123 to enrich primitive HSC (Table1).
Very little change in the fluorescence intensity of HSC was observed when Thy-1.1lowSca-1+Lin−cells from 3- to 4-week-old mice were stained with Rh-123 in the presence of verapamil or cyclosporin A (Fig 2), in contrast to the major shift in the retention of BodVer (Fig 5). This observation suggests the expression of a non–P-gp efflux pump in mouse HSC. Interestingly, while the blockers reversed MDR-mediated efflux of BodVer, there was no effect on the frequency of long-term repopulating HSC when Thy-1.1lowSca-1+Lin−cells were fractionated under these conditions (Fig 6). This result may be due to an incomplete blockade of efflux, or to selective accumulation of BodVer in the lysosomal compartment15rather than specific efflux of the probe. Verapamil also failed to block the ability of Rh-123 to localize long-term repopulating HSC in the dull-staining population. It is possible that the verapamil blockade was incomplete, allowing residual P-gp–mediated efflux of the probe. However, because P-gp activity in cells from young mice is minimal and because the Rh-123low population was completely shifted into the major staining peak in the presence of verapamil (Fig2), the evidence supporting this interpretation is not compelling. Overall, the most conservative interpretation of our data is that in young mice, P-gp activity is minimal in HSC and functional segregation of long-term repopulating cells by Rh-123 is due to selective staining of activated mitochondria.
A number of studies have shown that functional heterogeneity is associated with the cell cycle status of murine HSC, and that HSCs in the G0/G1 phase of the cell cycle are more enriched for radioprotection and long-term reconstitution activities compared with cells in S/G2/M phase.37 To evaluate this correlation between cell cycle status and long-term repopulating activity, we measured the cycling status of HSC populations isolated with Rh-123 or BodVer in the presence or absence of MDR blockers. Cells isolated by BodVerlow or Rh-123low staining were predominantly in the G0/G1 phase, in either the presence or absence of MDR modulators (Table 2). In addition, verapamil did not influence the frequency of cycling cells in the BodVerhigh and Rh-123high populations, while both cyclosporin A and reserpine enhanced the staining of cycling cells with either Rh-123 or BodVer. The engraftment results shown in Figs 4 and 6 suggest that the G0/G1 cell cycle state by itself is a poor marker for the subset of Thy-1.1lowSca-1+Lin− HSC that mediates long-term engraftment because equivalent enrichments for G0/G1 cells were obtained with either probe (Table 2).
Analysis of transmission electron micrographs has suggested a selective increase in mitochondrial size, but not number, in Rh-123high HSC when compared with Rh-123lowcells.33 The reported twofold increase in mitochondrial size would not by itself be sufficient to account for the 30-fold increase in fluorescence intensity that is apparent between Rh-123low and Rh-123high HSC (Fig 1). To further evaluate the mitochondrial localization of Rh-123 in Thy-1.1lowSca-1+Lin− HSC, we used laser scanning confocal microscopy to visualize the intracellular distribution of the probe and correlated these observations with transmission electron micrographs of parallel populations of cells (Figs 7 and 8). These results confirm cellular localization of the probe, which is coincident with mitochondrial distribution in both cell populations and show a marked bipolar perinuclear clustering of activated mitochondria in Rh-123high cells. This observation may indicate segregation of mitochondria before cell division, or may be a result of cellular activation. In contrast, the distribution of fluorescence in the Rh-123low cells was rather uniform in the majority of cells analyzed. If P-gp–mediated efflux of Rh-123 was the sole mechanism by which these cell subsets are resolved, one would expect to detect quantitatively distinct, but morphologically equivalent, distribution of fluorescence in cells separated by Rh-123 staining; this clearly was not the case (Fig 7).
Collectively, the results reported here support the hypothesis that mitochondrial membrane charge potentials play a major mechanistic role in the ability of Rh-123 to discriminate the quiescent long-term repopulating HSC population from activated progenitor cells. Active efflux of fluorescent probes by MDR mechanisms seems to exaggerate the differential intensity between low and high Rh-123 staining, particularly in older animals, but does not absolutely correlate with long-term repopulating HSC function. Any influence of MDR in defining primitive HSC would seem to be specific to the P-gp efflux pump, as pump substrates with a broader substrate specificity range (BodVer and NAO) do not replicate the ability of Rh-123 to identify primitive HSC in young animals. This conclusion is strengthened by recent studies of mice genetically deficient in both mdr1a and mdr1b38; efflux of Rh-123 from bone marrow progenitor cells derived from the double knockout animals is completely absent, but partially present in either single knockout, proving a specific role for both of the mdr1-type P-gps in determining the Rh-123 staining intensity of mouse hematopoietic cells. However, no functional analysis of cell subsets separated on the basis of Rh-123 staining intensities was reported, as the mixed genetic background of these animals precludes transplantation experiments. If mitochondrial activation plays a major role in the mechanism of Rh-123 staining in HSC as suggested here, one would predict that Rh-123 still will be capable of segregating functional subsets of primitive hematopoietic cells in the double mdr knockouts. This and other studies of the mechanism(s) underlying the ability of Rh-123 to identify primitive HSC will help guide future efforts to identify more specific probes for this population of cells, particularly in situations where P-gp expression is coincident with HSC function.8,26,35,39 40
The significance of MDR function in the HSC compartment remains to be determined. The mdr1 double knockout animals display no physiologic abnormalities in any parameter measured, including hematologic and immunologic phenotype and function.38 However, vital functions of these proteins may be supplemented by other membrane pumps in their absence. Monoclonal antibodies against P-gp have been shown to inhibit cytokine secretion from PHA-activated lymphocytes,41,42 suggesting that a possible physiologic role of P-gp could be considered in hematopoiesis via hematopoietic growth factor secretion or regulation. Upregulation of P-gp function in older animals may suggest a role in maintaining the stem cell pool with age, a proposal that is supported by studies in which overexpression of P-gp in transplanted bone marrow was associated with extended serial transplantation potential.43 Although the results reported in this study do not support a major role for MDR function in the HSC compartment of young animals, further studies aimed at characterizing this function during the course of aging are clearly warranted.
ACKNOWLEDGMENT
The authors thank Ann Cline and Diane Brooks for excellent technical assistance in flow cytometry. Dr Wayne Green provided advice and assistance in the cell cycle analysis studies. We also thank Dr Preet Chaundry for helpful discussions, suggesting the experiments to test efflux of fluorescent probes using MDR transfectant cell lines, and reviewing the manuscript.
Supported by Grants No. RO1 HL56857 and P50 DK49219 from the National Institutes of Health and the Huntsman Cancer Institute at the University of Utah. The Flow Cytometry, Cell Imaging, and Irradiation core facilities of the Huntsman Cancer Institute, supported by National Cancer Institute Cancer Center Support Grant No. P30 CA42014, were used for these studies.
Address reprint requests to Gerald J. Spangrude, PhD, Department of Pathology, Room 5C 130 SOM, University of Utah, Salt Lake City, UT 84132.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal