Abstract
NF-κB/Rel designates a widely distributed family of transcription factors involved in immune and acute phase responses. Here, the expression and function of NF-κB factors in erythroid proliferation and differentiation were explored. In an erythroleukemia cell line, TF-1, high levels of p105/p50, p100/p52, p65, and IκBα were detected 24 hours after growth factor deprivation. In response to granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulation, significant induction of p52 expression was observed. GM-CSF also induced nuclear translocation of both p52 and p65. No induction of NF-κB factors was observed with erythropoietin stimulation of TF-1 cells. Overexpression of p52 and p65 in TF-1 cells by transient transfection resulted in significant induction of a κB-TATA-luciferase reporter plasmid, showing that these factors are functional in vivo in erythroid cells. To determine whether NF-κB factors may play a role in normal erythropoiesis, levels of these factors were determined in burst-forming unit-erythroid (BFU-E)–derived cells at different stages of differentiation. The NF-κB factors p105/p50, p100/p52, and p65 were highly expressed in early BFU-E–derived precursors, which are rapidly proliferating, and declined during maturation. Furthermore, nuclear levels of NF-κB factors p50, p52, and p65 were higher in less mature precursors (day 10 BFU-E–derived cells) compared with more differentiated (day 14) erythroblasts. In nuclear extracts from day 10 BFU-E–derived cells, p50, p52, and p65 were able to form complexes, which bound to κB sites in the promoters of both the c-myb and c-mycgenes, suggesting that c-myb and c-myc may be among the κB-containing genes regulated by NF-κB factors in normal erythroid cells. Taken together, these data show that NF-κB factors are modulated by GM-CSF and suggest they function to regulate specific κB containing genes involved in erythropoiesis.
NF-κB/REL designates a widely distributed family of transcription factors involved in regulation of immune and acute phase responses and in the response to signals for rapid gene expression.1-3 NF-κB responsive genes include those for specific cytokines and growth factors, immunoreceptors, adhesion molecules, viruses, and growth-regulatory factors, such asc-myc.1-4 The NF-κB/Rel proteins share a highly conserved amino-terminal sequence called the Rel Homology Region (RHR), which includes the DNA binding and dimerization domain, as well as a nuclear localization signal.1 3 This family includes p50 and its precursor p105, p52 and its precursor p100, p65 (RelA), c-Rel, and RelB. P50 and p52 form functional Rel dimers with other family members, whereas dimers containing the unprocessed proteins (p105 and p100) remain sequestered in the cytoplasm. In contrast, p65, c-Rel, and RelB are not synthesized as precursors and also possess transcriptional activation domains.
The activity of Rel proteins is regulated through cytoplasmic retention by physical interactions with cytoplasmic inhibitors termed IκB (IκBα, IκBβ, IκBγ).1-4 Cytoplasmic complexes are composed of either Rel homo- or heterodimers bound to a member of the IκB family or heterodimers between a mature Rel protein and p105 or p100.1-4 Various cellular activating signals induce the phosphorylation of IκB, which in turn triggers its proteolysis through the proteosome pathway. Concurrently, the liberated NF-κB dimers are translocated to the nucleus, where they bind to target enhancer elements present in the promoters of regulated genes.1-6 The proteolytic processing of the precursor proteins, p100 and p105, similarly results in the release and nuclear translocation of NF-κB to the nucleus.
A number of cytokines have been shown to activate NF-κB including tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-2, as well as mitogens including phorbol myristate acetate.1-11Involvement of NF-κB in T-lymphocyte activation,7 B-cell development,8,12 monocyte/macrophage,8,10 and neutrophil activation13 has been shown. One mechanism through which NF-κB may directly influence cell growth is through regulation of the c-myc promoter.7 14 However, in contrast to activation of the immune system, the role of NF-κB transcription factors in the signal transduction pathways of cytokine receptor superfamily members regulating hematopoietic proliferation/differentiation has not been well described.
In this study, the expression and function of NF-κB in erythroid proliferation and differentiation was investigated using both TF-1 cells, an erythroleukemia cell line, and normal human progenitor-derived erythroblasts. Proliferation of TF-1 cells is dependent on hematopoietic growth factors including granulocyte-macrophage colony-stimulating factor (GM-CSF), erythropoietin (Epo), and IL-3.15 TF-1 cells deprived of growth factor for 24 hours expressed substantial levels of Rel proteins, including p105/p50, p100/p52, and p65. Stimulation of TF-1 proliferation with GM-CSF resulted in dramatic induction of p52 and nuclear translocation of p52 and p65. No significant induction of other NF-κB factors was observed. Transient-transfection studies with TF-1 cells showed that overexpression of both p52 and p65 lead to significant transactivation of a luciferase reporter gene driven by a κB enhancer, thus suggesting that p52 can bind NF-κB sites and modulate expression of regulated genes in vivo in TF-1 cells. Because GM-CSF regulates p52 expression and may subsequently influence gene expression in TF-1 cells, NF-κB proteins were examined in normal erythropoiesis using burst-forming unit-erythroid (BFU-E)–derived erythroblasts at day 7, 10, and 14 of differentiation.16The active Rel proteins p50, p52, and p65 and their precursors p105 and p100 had the highest expression in day 7 cells, which are rapidly proliferating, and declined as these cells terminally differentiated. Greater quantities of p50, p52, and p65 were detected in the nucleus of day 10 erythroblasts compared with the nucleus of more mature day 14 cells. Electrophoretic mobility shift assays (EMSA) using nuclear extracts from BFU-E–derived cells showed that nuclear complexes of p50, p52, and p65 bound to κB sites in the c-myb andc-myc promoters, suggesting that c-myb and c-myc may be among the κB-containing genes regulated by NF-κB factors in erythropoiesis.
MATERIALS AND METHODS
Culture of BFU-E–derived erythroblasts and TF-1 cells.
Peripheral blood was obtained from normal volunteer donors at The Milton S. Hershey Medical Center under protocols approved by the Institution's Clinical Investigation Committee. BFU-E–derived erythroblasts were cultured as described previously.16Briefly, peripheral blood mononuclear cells were separated on Ficoll-Paque (Pharmacia, Piscataway, NJ), and cultured in 0.9% methylcellulose media containing 30% fetal calf serum, 9.0 mg/mL deionized bovine serum albumin (Cohn fraction V; Sigma Chemical Co, St Louis, MO), 1.4 × 10−4 mol/L β-mercaptoethanol and 2 U/mL Epo (recombinant Epo > 100,000 U/mg; Amgen, Thousand Oaks, CA). Cells from maturing BFU-E–derived colonies were plucked from culture on days 7, 10, and 14 to study a well-defined population of normal human cells at distinct stages of maturation. Day 7 cells are poorly hemoglobinized blasts with a large proliferative capacity, day 10 cells are partially hemoglobinized proerythroblasts with decreased proliferative capacity, and day 14 cells are terminally differentiating polychromatophilic and orthochromatic normoblasts.16 Cytocentrifuge preparations of aliquots of BFU-E–derived cells routinely identified >99% as erythroid precursors. One hundred to 1,000 (day 7) BFU-E–derived colonies were plucked and pooled on each day.
TF-1 cells, a human erythroleukemia cell line,15 were cultured in RPMI 1640 medium containing 10% fetal calf serum and 1 to 2 ng/mL human recombinant GM-CSF (R & D Systems, Minneapolis, MN). To examine NF-κB transcription factor expression and induction, TF-1 cells were removed from growth factor for 24 hours and then stimulated with 2 ng/mL GM-CSF or 5 U/mL recombinant Epo. Samples were collected at intervals over 0 to 24 hours.
Cell lysate preparation and nuclear/cytoplasmic fractionation.
Whole cell lysates were prepared by suspending 1 × 106 TF-1 cells or BFU-E–derived erythroblasts in cell lysate buffer (50 mmol/L Tris HCl, pH 8.0; 150 mmol/L NaCl; 0.05% NP40; 100 mmol/L NaF; 1 mmol/L EDTA; 1 mmol/L EGTA; 0.08 mmol/L phenylmethylsulfonyl fluoride [PMSF]; 0.01 mg/mL of leupeptin; 0.01 mg/mL aprotinin). The suspension was vortexed and centrifuged at 10,000 rpm for 10 minutes. The supernatant was saved for Western blotting. Nuclear and cytoplasmic fractions were prepared as previously described by Schreiber et al.17 A total of 1 × 107 TF-1 cells or 1.5 × 107BFU-E–derived erythroblasts harvested at day 10 or 14 were washed twice with cold phosphate-buffered saline (PBS). The cell pellet was resuspended in 100 μL of cold buffer (10 mmol/L Hepes, pH 7.9; 10 mmol/L KCl; 0.1 mmol/L EDTA; 0.4% NP40; 1 mmol/L dithiothreitol [DTT]; 0.5 mmol/L PMSF; 1% volume protease inhibitor cocktail) and pipetted several times. The lysates were spun, and the supernatant was used for the cytosol preparation. The nuclear pellet was extracted with 50 μL of ice cold buffer (20 mmol/L Hepes, pH 7.9; 0.4 mol/L NaCl; 1 mmol/L EDTA; 1 mmol/L DTT; 1 mmol/L PMSF) and vigorously shaken for 15 minutes at 4°C. After centrifugation, the nuclear extract was collected and kept at −70°C.
Immunoblotting.
The whole cell lysate, nuclear, or cytoplasmic preparations were boiled for 5 minutes in protein sample buffer. Supernatants containing the protein content of 4 × 105 TF-1 cells or 2 × 105 BFU-E–derived erythroblasts were loaded on each lane of a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoresis was performed. Alternatively, 15 μg of nuclear or cytoplasmic extract from TF-1 cells or 20 μg nuclear or 15 μg cytoplasmic extract from BFU-E–derived cells was loaded in each lane. Proteins were electroblotted onto Hybond − ECL nitrocellulose membrane (Amersham Life Sciences, Bucks, England) according to recommended procedures of the manufacturer. After blocking in 5% dry milk in Tris-buffered saline-Tween (TBST) buffer, membranes then were incubated with anti-p50, which also recognizes p105, anti-p52, which also recognizes p100, anti-p65 (from Dr Warner Greene, Gladstone Institute of Virology and Immunology, University of California, San Francisco) or anti-IκBα antibodies.18 Donkey antirabbit antibody (1:2,000 dilution) was used as the secondary antibody and membranes were detected with the ECL-Western blotting system (Amersham).
TF-1 transfection and luciferase assay.
The κB-TATA-luciferase reporter plasmid was generated by transferring the insert, containing the human immunodeficiency virus (HIV)-1 κB enhancer and TATA box, from the κB-TATA-CAT into the pGL2 plasmid 5′ of the luciferase gene (Promega, Madison, WI).19,20 The tk-luc control plasmid contained the TATA box, but not the κB enhancers. The cDNAs encoding p65, p50, and p52 have been described previously.21-23 These cDNAs were cloned into the expression plasmid pCMV4 as described.20 24 TF-1 cells were transfected at a density of 1 × 106 cells/mL with Tfx-20 reagent (Promega) at 3:1 ratio Tfx-20 to DNA in the presence of GM-CSF. The quantity of reporter gene and effector protein plasmids used is presented in the Results. After 48 hours of culture, the transfectants were collected and suspended in a lysis buffer (Reporter lysis buffer; Promega). Cell extracts were normalized for protein recovery (Bio-Rad), and then subjected to luciferase assay (Promega). Luciferase activity was quantitated using a single photon channel of a scintillation counter (Beckman, Columbia, MD).
EMSA.
EMSA was performed by the method described previously.25Double-stranded oligonucleotides covering κB sites present in the promoters of the human c-myb (S.-C. Sun, et al, manuscript in preparation) or c-myc genes14 were labeled as described by Ganchi et al.22 A total of 4 μL (6 μg) of nuclear extracts was incubated with 0.5 to 2 μL of NF-κB (anti-p50, anti-p52, and anti-p65; from Dr Warner Greene) or anti–Bcl-3 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies in 12 μL of the binding buffer (0.5 μL of 1 μg/μL polydI-dC; 1 μL of 0.1 mol/L DTT; 3 μL of KCl-Dialysis buffer lacking KCl26; 12 μL H20) for 10 minutes at room temperature. Following this, 1 μL of 32P-radiolabeledc-myb–κB or c-myc–κB probe (1 × 105 cpm) was added and incubated for another 25 minutes. The DNA protein complexes were resolved on 5% native polyacrylamide gels.
For competition assays, the indicated amounts of unlabeled double-stranded oligonucleotides covering different types of κB sites were mixed with the EMSA binding buffer and nuclear extracts from day 10 BFU-E–derived cells, followed by adding 0.15 pmol (≈1 × 105 cpm) of a 32P-radiolabeled high-affinity κB palindromic probe (κB-pd).22
RESULTS
Expression of NF-κB transcription factors in TF-1 cells and induction by GM-CSF.
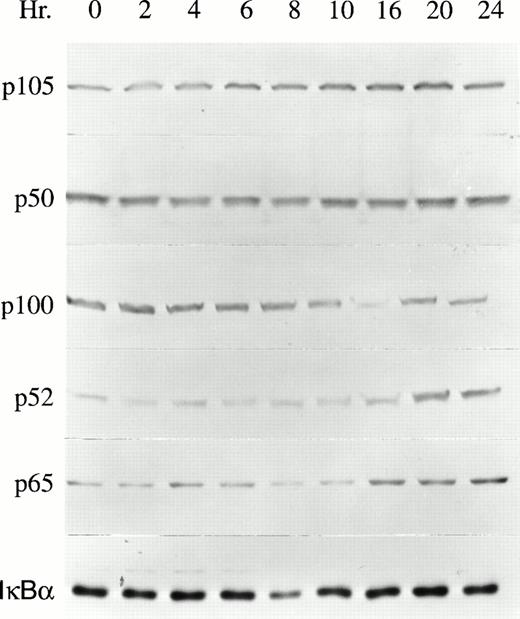
The proliferation of TF-1 cells is dependent on growth factors including GM-CSF, Epo, and IL-3.15 To determine whether NF-κB transcription factors are involved in growth factor–mediated TF-1 proliferation, the expression of NF-κB transcription factors was examined by Western blot analysis. After growth factor deprivation for 24 hours, TF-1 cells were induced to proliferate by culturing in the continuous presence of 2 ng/mL GM-CSF. TF-1 cells were collected at time intervals from 0 to 24 hours of GM-CSF stimulation. Results of five experiments are shown in Table 1 and a representative blot is shown in Fig 1. The NF-κB transcription factors p105, p50, p100, p52, and p65 were all expressed at detectable levels, even in growth factor–deprived cells. After GM-CSF stimulation, the major induction observed in whole cell lysates was significant enhancement of p52 protein levels (Table 1, Fig1, P < .05). The increase in p52 reached statistical significance at 16 hours and remained elevated for at least 24 hours post stimulation. Induction of p65 was also observed, but did not reach statistical significance. IκBα expression did not change in response to TF-1 stimulation, as shown previously.27 In contrast to these results, no significant induction of NF-κB factors was observed after Epo stimulation of TF-1 cells (data not shown). However, Epo sustains only the short-term growth of TF-1 cells.15 TF-1 cells, which express a truncated Epo receptor, also show impaired activation of STAT5 by Epo.28
NF-κB Expression in Response to GM-CSF Stimulation of TF-1 Cells
| . | Time (h) . | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 . | 2 . | 4 . | 8 . | 10 . | 16 . | 20 . | 24 . | |
| p105 | 0.51 ± 0.17 | 0.49 ± 0.37 | 0.36 ± 0.15 | 0.26 ± 0.07 | 0.29 ± 0.08 | 0.55 ± 0.19 | 0.41 ± 0.07 | 0.44 ± 0.16 |
| p50 | 0.47 ± 0.14 | 0.46 ± 0.23 | 0.35 ± 0.10 | 0.28 ± 0.09 | 0.35 ± 0.09 | 0.53 ± 0.17 | 0.50 ± 0.12 | 0.61 ± 0.23 |
| p100 | 0.70 ± 0.26 | 0.88 ± 0.32 | 0.72 ± 0.27 | 0.65 ± 0.22 | 0.62 ± 0.22 | 0.60 ± 0.25 | 0.64 ± 0.24 | 0.73 ± 0.31 |
| p52 | 0.60 ± 0.21 | 0.62 ± 0.27 | 0.53 ± 0.20 | 0.52 ± 0.22 | 0.53 ± 0.18 | 0.94 ± 0.29-150 | 1.05 ± 0.24-150 | 1.04 ± 0.22-150 |
| p65 | 0.98 ± 0.42 | 1.62 ± 0.78 | 1.32 ± 0.47 | 1.12 ± 0.41 | 1.25 ± 0.47 | 1.93 ± 0.70 | 1.63 ± 0.62 | 1.48 ± 0.70 |
| IκBα | 0.66 ± 0.27 | 0.80 ± 0.30 | 0.52 ± 0.25 | 0.25 ± 0.10 | 0.43 ± 0.20 | 0.60 ± 0.24 | 0.56 ± 0.29 | 0.61 ± 0.24 |
| . | Time (h) . | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 . | 2 . | 4 . | 8 . | 10 . | 16 . | 20 . | 24 . | |
| p105 | 0.51 ± 0.17 | 0.49 ± 0.37 | 0.36 ± 0.15 | 0.26 ± 0.07 | 0.29 ± 0.08 | 0.55 ± 0.19 | 0.41 ± 0.07 | 0.44 ± 0.16 |
| p50 | 0.47 ± 0.14 | 0.46 ± 0.23 | 0.35 ± 0.10 | 0.28 ± 0.09 | 0.35 ± 0.09 | 0.53 ± 0.17 | 0.50 ± 0.12 | 0.61 ± 0.23 |
| p100 | 0.70 ± 0.26 | 0.88 ± 0.32 | 0.72 ± 0.27 | 0.65 ± 0.22 | 0.62 ± 0.22 | 0.60 ± 0.25 | 0.64 ± 0.24 | 0.73 ± 0.31 |
| p52 | 0.60 ± 0.21 | 0.62 ± 0.27 | 0.53 ± 0.20 | 0.52 ± 0.22 | 0.53 ± 0.18 | 0.94 ± 0.29-150 | 1.05 ± 0.24-150 | 1.04 ± 0.22-150 |
| p65 | 0.98 ± 0.42 | 1.62 ± 0.78 | 1.32 ± 0.47 | 1.12 ± 0.41 | 1.25 ± 0.47 | 1.93 ± 0.70 | 1.63 ± 0.62 | 1.48 ± 0.70 |
| IκBα | 0.66 ± 0.27 | 0.80 ± 0.30 | 0.52 ± 0.25 | 0.25 ± 0.10 | 0.43 ± 0.20 | 0.60 ± 0.24 | 0.56 ± 0.29 | 0.61 ± 0.24 |
TF-1 cells were deprived of growth factor for 24 hours and then stimulated with 2 ng/mL GM-CSF for 0 to 24 hours. Whole cell lysates were prepared from five experiments and protein from an equivalent cell number was loaded on each lane. Immunoblotting was performed as described in Materials and Methods. The densities of bands were measured with a Molecular Dynamics Densitometer and Quantity One Software, and the mean ± SEM is shown.
Significant increase in response to growth factor stimulation (P < .05).
NF-κB expression in GM-CSF–stimulated TF-1 cells. Whole cell lysates were prepared from growth factor–deprived TF-1 cells stimulated with GM-CSF for 0 to 24 hours. The protein content of 4 × 105 TF-1 cells was loaded in each lane of a 10% polyacrylamide gel. The membranes were blotted with anti-p50, anti-p52, anti-p65, or anti-IκBα antibodies and detected with ECL. A representative blot from one of five independent experiments is shown.
NF-κB expression in GM-CSF–stimulated TF-1 cells. Whole cell lysates were prepared from growth factor–deprived TF-1 cells stimulated with GM-CSF for 0 to 24 hours. The protein content of 4 × 105 TF-1 cells was loaded in each lane of a 10% polyacrylamide gel. The membranes were blotted with anti-p50, anti-p52, anti-p65, or anti-IκBα antibodies and detected with ECL. A representative blot from one of five independent experiments is shown.
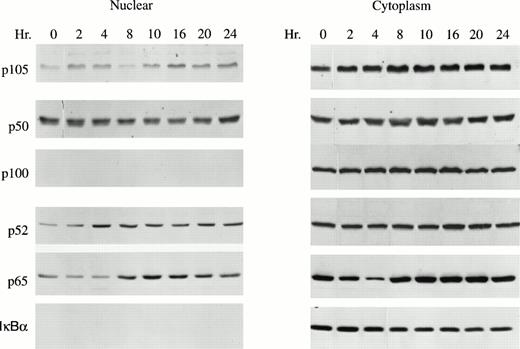
Nuclear and cytoplasmic extracts were isolated from GM-CSF–stimulated TF-1 cells to determine whether GM-CSF induced translocation of NF-κB transcription factors (p50, p52, p65). As expected, the inhibitory proteins p105, p100, and IκBα were detected primarily in cytoplasm. However, substantial quantities of the active factors p50, p52, and p65 were present in the nucleus (Fig 2). Nuclear levels of p52 and p65 significantly increased in response to GM-CSF stimulation (Fig 2, P < .05). These data suggest that NF-κB transcription factors may be involved in GM-CSF stimulation of TF-1 cell growth.
GM-CSF enhances p52 nuclear translocation in TF-1 cells. A total of 1 × 107 TF-1 cells stimulated with GM-CSF for 0 to 24 hours were used for nuclear or cytoplasmic fractionation. A total of 15 μg of nuclear or cytosolic protein was loaded on each lane of a 10% polyacrylamide gel and subjected to Western blotting with anti-p50, anti-p52, anti-p65, or anti-IκBα antibodies and ECL. A representative blot of four independent experiments is shown.
GM-CSF enhances p52 nuclear translocation in TF-1 cells. A total of 1 × 107 TF-1 cells stimulated with GM-CSF for 0 to 24 hours were used for nuclear or cytoplasmic fractionation. A total of 15 μg of nuclear or cytosolic protein was loaded on each lane of a 10% polyacrylamide gel and subjected to Western blotting with anti-p50, anti-p52, anti-p65, or anti-IκBα antibodies and ECL. A representative blot of four independent experiments is shown.
Overexpression of p52 and p65 in TF-1 cells activates a κB enhancer.
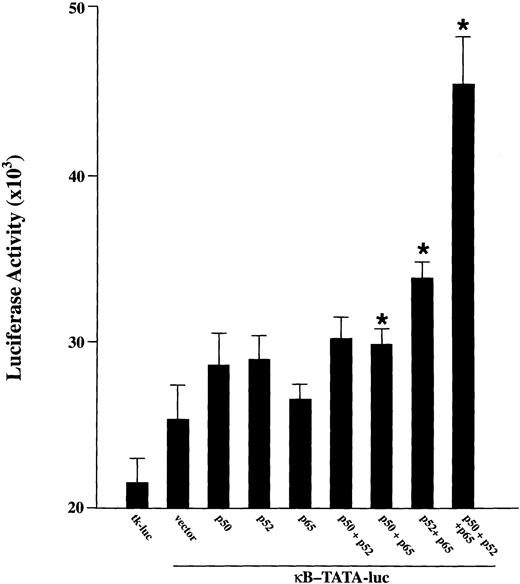
To examine whether p52 and p65 have a biologic role in TF-1 cells, functional reporter gene assays were performed with a κB-TATA-luciferase reporter plasmid.20 24 This plasmid was cotransfected into proliferating TF-1 cells (cultured in the presence of GM-CSF) along with cDNA for p50, p52, p65 alone or together. The tk-luc plasmid was transfected as a control. As shown in Fig 3, a low but significant level of κB-TATA-luc was expressed when this reporter plasmid was transfected alone. This result is consistent with our finding that endogenous NF-κB factors are induced by the growth factor GM-CSF. Cotransfection of the reporter plasmid with p50, p52, or p65 resulted in a modest enhancement of the basal luciferase expression. A significant induction of luciferase was induced in TF-1 cells by cotransfection of the κB-TATA reporter plasmid with p65 together with p52 or p50 (P≤ .05). A more dramatic luciferase stimulation was detected when all three major NF-κB subunits were coexpressed in the cells. These results show that both the endogenously induced and transfected NF-κB species are capable of inducing gene expression from the κB enhancer in TF-1 cells.
Activation of a κB-TATA-luciferase reporter plasmid after overexpression of NF-κB factors in TF-1 cells. A total of 0.5 μg of plasmids expressing p50, p52, or p65 was cotransfected with 0.5 μg of κB-TATA-luciferase reporter plasmid into TF-1 cells separately or in combination. A tκ-TATA-luciferase reporter lacking κB enhancer (tκ-luc, 0.5 μg) was used as negative control. The total amount of transfected DNA was kept constant by adding appropriate amounts of expression vector without insert. At 48 hours after transfection, cells were collected for luciferase assay. Results are expressed as mean ± standard error of mean (SEM) (× 103 cpm) for nine samples from three experiments. *Indicates a significant increase above the κB-TATA reporter plasmid (P ≤ .05).
Activation of a κB-TATA-luciferase reporter plasmid after overexpression of NF-κB factors in TF-1 cells. A total of 0.5 μg of plasmids expressing p50, p52, or p65 was cotransfected with 0.5 μg of κB-TATA-luciferase reporter plasmid into TF-1 cells separately or in combination. A tκ-TATA-luciferase reporter lacking κB enhancer (tκ-luc, 0.5 μg) was used as negative control. The total amount of transfected DNA was kept constant by adding appropriate amounts of expression vector without insert. At 48 hours after transfection, cells were collected for luciferase assay. Results are expressed as mean ± standard error of mean (SEM) (× 103 cpm) for nine samples from three experiments. *Indicates a significant increase above the κB-TATA reporter plasmid (P ≤ .05).
In the experiments shown in Fig 3 (see legend), 0.5 μg of plasmids expressing p50, p52, or p65 were cotransfected with 0.5 μg of κB-TATA-luciferase reporter plasmid into TF-1 cells separately or in combination. The total amount of transfected DNA (2.0 μg) was kept constant by adding appropriate amounts of expression vector without insert. In control experiments not shown here, 1.5 μg of plasmids expressing NF-κB factors p50 or p52 were cotransfected with 0.5 μg of κB-TATA-luciferase reporter plasmid in TF-1 cells. The luciferase activity was not significantly different from cotransfection with the κB-TATA-luciferase reporter plasmid alone or from transfection of 0.5 μg of the reporter plasmid with 0.5 μg of expression plasmid for the specific NF-κB factor.
NF-κB expression and nuclear translocation decline during normal erythroid differentiation.
The function of NF-κB was then investigated in normal erythroid differentiation. Human BFU-E–derived erythroid precursors were removed from culture on day 7, 10, and 14 of maturation.16 Western blotting was performed using whole cell lysates from day 7, 10, and 14 cells and results are shown in Fig 4. Expression of NF-κB/Rel transcription factors was greatest in early precursors (day 7) and declined as erythroid precursors terminally differentiated. IκBα, a major cytoplasmic inhibitor of NF-κB, was still present in substantial quantities at day 14.
NF-κB expression in day 7, 10, and 14 BFU-E–derived erythroblasts. Normal human BFU-E–derived erythroblasts were harvested at different days of differentiation and the whole cell lysate from 2 × 105 cells was loaded on each lane of a 10% polyacrylamide gel. Western blotting was performed with anti-p50, anti-p52, anti-p65, or anti-IκBα antibodies and detection with ECL. Five independent experiments were performed and a representative blot is shown.
NF-κB expression in day 7, 10, and 14 BFU-E–derived erythroblasts. Normal human BFU-E–derived erythroblasts were harvested at different days of differentiation and the whole cell lysate from 2 × 105 cells was loaded on each lane of a 10% polyacrylamide gel. Western blotting was performed with anti-p50, anti-p52, anti-p65, or anti-IκBα antibodies and detection with ECL. Five independent experiments were performed and a representative blot is shown.
Transcriptional activation of NF-κB/Rel is associated with nuclear translocation of p50, p52, and p65. To evaluate the subcellular localization of NF-κB transcription factors during normal erythropoiesis, nuclear and cytoplasmic extracts were isolated from BFU-E–derived cells at day 10 and 14 of differentiation and subjected to Western blotting analysis (Fig 5). Colony size at day 7 was too small to harvest sufficient numbers of cells for subcellular fractionation. In both day 10 and day 14 BFU-E–derived cells, the majority of NF-κB/Rel proteins was found in the cytoplasm. However, a significant amount of p50, p52, and p65 were present in the nucleus of day 10 cells, suggesting that they may function to modulate genes with specific κB sites (Fig 5). In day 14 cells, nuclear levels of these factors were reduced.
NF-κB/Rel and IκBα in nuclear and cytoplasmic extracts of day 10 and 14 BFU-E–derived cells. Nuclear and cytoplasmic extracts were prepared from 1.5 × 107 day 10 or 14 BFU-E–derived cells. A total of 20 μg of nuclear or 15 μg of cytoplasmic extract was loaded on each lane of a 10% polyacrylamide gel and Western blotting performed with anti-p50, anti-p52, anti-p65, and anti-IκBα antibodies and ECL. Four experiments were performed with similar results.
NF-κB/Rel and IκBα in nuclear and cytoplasmic extracts of day 10 and 14 BFU-E–derived cells. Nuclear and cytoplasmic extracts were prepared from 1.5 × 107 day 10 or 14 BFU-E–derived cells. A total of 20 μg of nuclear or 15 μg of cytoplasmic extract was loaded on each lane of a 10% polyacrylamide gel and Western blotting performed with anti-p50, anti-p52, anti-p65, and anti-IκBα antibodies and ECL. Four experiments were performed with similar results.
Experiments with nuclear and cytoplasmic extracts of day 10 BFU-E–derived cells (Fig 5) suggested that a higher molecular weight isoform of p52 predominated in the nucleus compared with the cytoplasm, where both higher and lower molecular weight bands were observed. The molecular nature of the upper band remains to be further investigated, but it was not sensitive to calf intestine phosphatase (results not shown).
Nuclear NF-κB factors bind to κB sites in the c-myband c-myc promoters.
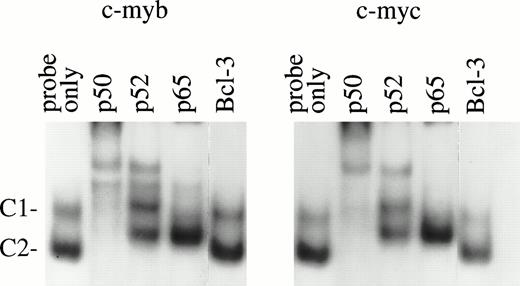
To identify potential target genes of NF-κB/Rel proteins found in normal erythroid cells, EMSA was performed using NF-κB binding sites that recently were identified in the promoter regions of the humanc-myb (S.-C. Sun, et al, manuscript in preparation) and c-mycgenes.14 These sites were chosen because the importance ofc-myb and c-myc in erythroid proliferation is well-established.29-33 In normal day 10 BFU-E–derived cells, two major protein/DNA complexes (C1 and C2) were detected withc-myb and c-myc κB sites (Fig 6). Antibody supershift assays showed that both the C1 and C2 complexes immunoreacted with anti-p50 and anti-p52, although anti-p52 could not shift these two complexes completely. Anti-p65 (RelA) supershifted the C1 and partially shifted C2 complex. These results suggest that p50, p52, and p65 bind to these NF-κB sites as complexes of heterodimers. Similar bands were generated with both c-myb and c-myc probes even though the two oligos have certain sequence variations at the NF-κB binding sites. Our previous work suggested that anti–Bcl-3 antibody could inhibit p52 binding to a c-myb NF-κB site in GM-CSF–induced TF-1 cells.27 However, in normal day 10 BFU-E–derived cells, such inhibition was not observed. This is most likely due to the low expression of Bcl-3 protein in cells at this stage of differentiation.27
EMSA of nuclear extracts from day 10 BFU-E–derived cells. A total of 6 μg of nuclear extracts from day 10 BFU-E–derived cells was incubated with different NF-κB antibodies for 10 minutes before adding 32P-labeled c-myb or c-mycκB binding oligonucleotide probes. Two DNA protein complexes were generated. Complex 1 and 2 (C1 and C2) were supershifted by anti-p50 and anti-p52; C1 was supershifted by anti-p65. Anti–Bcl-3 antibody had a minimal effect on these complexes. Three experiments were performed with similar results.
EMSA of nuclear extracts from day 10 BFU-E–derived cells. A total of 6 μg of nuclear extracts from day 10 BFU-E–derived cells was incubated with different NF-κB antibodies for 10 minutes before adding 32P-labeled c-myb or c-mycκB binding oligonucleotide probes. Two DNA protein complexes were generated. Complex 1 and 2 (C1 and C2) were supershifted by anti-p50 and anti-p52; C1 was supershifted by anti-p65. Anti–Bcl-3 antibody had a minimal effect on these complexes. Three experiments were performed with similar results.
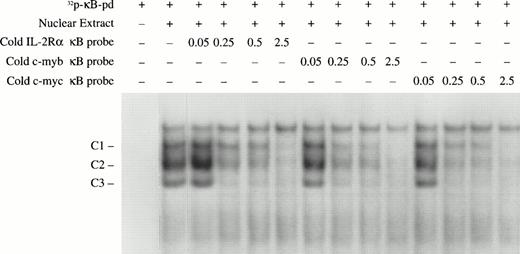
Competition assays were performed to compare the binding affinity of the c-myb and c-myc κB sites with that of classical κB enhancers. Different amounts of unlabeled double-stranded oligonucleotides covering the IL-2 receptor alpha gene κB, the c-myb κB, and c-myc κB, were used to compete for binding NF-κB with a 32P-radiolabeled κB probe (κB-pd).22 As shown in Fig 7, all of these κB sequences were able to completely compete for NF-κB binding with the κB-pd at 2.5 picomoles (approximately 18-fold of the labeled probe). Competition was found with three complexes detected with the κB-pd probe. Compared with the classical IL-2Rα κB, the κB sites from thec-myb and c-myc genes exhibited slightly higher affinity. Together, these data provide evidence that the NF-κB transcription factors p50, p52, and p65 in early erythroid precursors bind to κB-specific sites in the promoters of c-myb andc-myc and may participate in regulation of expression of these genes.
Nucleotide competition assays to compare the NF-κB binding affinity of different κB sites. The indicated amounts (in picomoles) of unlabeled double-stranded oligonucleotides covering the κB sites from the IL-2Rα, c-myb, and c-mycgenes were mixed with the EMSA-reaction buffer. Nuclear extracts from day 10 BFU-E–derived cells were also added to the EMSA reaction buffer followed by the addition of 0.15 pmole (1 × 105 cpm) of the 32P-labeled κB-pd probe. The NF-κB/DNA complexes are indicated.
Nucleotide competition assays to compare the NF-κB binding affinity of different κB sites. The indicated amounts (in picomoles) of unlabeled double-stranded oligonucleotides covering the κB sites from the IL-2Rα, c-myb, and c-mycgenes were mixed with the EMSA-reaction buffer. Nuclear extracts from day 10 BFU-E–derived cells were also added to the EMSA reaction buffer followed by the addition of 0.15 pmole (1 × 105 cpm) of the 32P-labeled κB-pd probe. The NF-κB/DNA complexes are indicated.
DISCUSSION
The role of NF-κB and related proteins in control of cell growth has been recognized. NF-κB participates in control of inducible expression of certain genes encoding hematopoietic growth factors including M-CSF, G-CSF, GM-CSF, IL-2, and IL-6.1,8 NF-κB is also involved in the response to T- and B-cell activating signals, leading to proliferation and differentiation.1,8 NF-κB transcription factors are widely expressed. However, their involvement in the response of hematopoietic precursors to cytokine stimulation has not been well characterized. Transcription factors shown to be specifically involved in regulation of erythroid proliferation and differentiation include GATA-1,34 SCL (or TAL1), and other basic helix-loop-helix (bHLH) transcription factors,16,35NF-E236 and RBTN2.37 Here, we have determined that high levels of active NF-κB transcription factors are present in TF-1 cells and that GM-CSF induces a significant increase in expression of p52. Furthermore, GM-CSF stimulation of TF-1 results in a significant increase in nuclear translocation of both p52 and p65. Coexpression of p52 and p65 in TF-1 cells resulted in induction of a κB-TATA-luciferase reporter gene and showed that enhancement of levels of p52, p65, and p50 has a functional role in gene activation in vivo in TF-1 cells. Together, these data support the conclusion that the NF-κB family of transcription factors is involved in regulation of gene expression in erythroid cells in response to GM-CSF stimulation.
Several experimental approaches suggest that NF-κB factors are also involved in normal erythropoiesis. The NF-κB factors p105, p50, p100, p52, and p65 are present in early normal erythroid precursors and decline during differentiation, showing a dynamic expression of these components. p50, p52, and p65 are all present in the nuclei of day 10 erythroblasts, suggesting that they have a functional role, as nuclear expression of NK-κB factors has been shown to have specificity.38 The ability of p50, p52, and p65 in nuclear extracts of normal progenitor-derived erythroblasts to form complexes with κB elements in the promotors of two oncogenes required in erythroid development, c-myb and c-myc, suggests two potential target genes through which NK-κB factors may modulate erythropoiesis. The κB sites found in the c-myb andc-myc promotors compete effectively for NF-κB factor binding with classical κB sites. The importance of c-myb in early erythroid proliferation29-32 and the association ofc-myb activation with progression of erythroid progenitors into the S phase of the cell cycle has been shown.31 The high level of NF-κB observed here in early erythroid precursors is consistent with the temporal pattern of c-myb expression previously observed in differentiating erythroid cells.30,31 Furthermore, in a human erythroleukemia cell line, TF-1, GM-CSF stimulated expression of NF-κB factors (shown here) and induced binding to the κB site in the c-mybpromoter along with induction of c-myb mRNA expression.27 NF-κB factors in extracts from erythroid cells were also shown here to interact with a κB site upstream of thec-myc promoter.14 Interactions at this site may also regulate erythroid growth, as c-myc is required in erythroid proliferation.32 33 Other target genes with important regulatory functions in hematopoietic cells, which have κB-specific sites, likely exist and need to be identified.
In TF-1 cells, GM-CSF induces p52 expression and nuclear translocation of p52 and p65. This may affect different κB-sites because the transcriptional activity of p52 homodimers or heterodimers has been shown to depend on nuclear levels of p52, on nuclear levels of active dimer molecules such as p65, and on the relative affinity for κB sites of homo- (p52/p52) or heterodimers (p52/p65).39 It is notable that maximal induction of the κB-TATA-luciferease reporter was observed after cotransfection with p50, p52, and p65, and these conditions are found in GM-CSF–stimulated TF-1 cells, which have high endogenous levels of p50 (Fig 2), as well as in early normal erythroid precursors. Bcl-3, an IκB-related protein, can form complexes with p52 or p50 homodimers, which may activate κB-specific gene expression.39-44 Bcl-3 can also antagonize DNA binding of p50 homodimers and serve as a κB-specific repressor.1,45We have recently shown that Bcl-3 expression and nuclear translocation are induced by GM-CSF in TF-1 cells and that in these cells, Bcl-3 appears to form a complex with p52 on a κB element present in the promoter of the c-myb gene.27 However, Bcl-3/p52 complexes could not be detected in day 10 normal BFU-E–derived cells with supershift assays with either the c-myb or c-mycprobes (Fig 6). This is consistent with our observation that Bcl-3 expression is low in day 10 BFU-E–derived cells.27 Bcl-3 and p52 may interact with c-myb or c-myc κB sites at an earlier stage of normal erythroid differentiation, where both are more highly expressed. Differential affinity of p52 homodimers (p52/p52) or heterodimers (p52/p65, p52/p52/Bcl-3) for various κB sequences, compared with those containing p50, may result in different activation potentials of these complexes and may provide some specificity of response to effector stimulation.39-45 For the human interferon-β promoter, NF-κB participates with at least two other transcriptional activator proteins and the structural protein HMG to form a complex, which requires a precise arrangement of binding sites on the DNA helix, and may modulate intrinsic enhancer architecture.3 46 In erythroid cells, NF-κB is likely to be part of a multifactor complex in which additional unidentified factors participate.
NF-κB/Rel knock-out mice have been produced to determine the function of each component. IκBα-deficient mice exhibited severe runting, skin defects, and extensive granulopoiesis.47 Although the increased granulopoiesis seen in IκBα−/−mice was attributed to elevated G-CSF production or activation of other genes,47 based on data presented here, we hypothesize that an alternative explanation might be that the GM-CSF signaling pathway is enhanced by removal of the cytoplasmic inhibitor IκBα. The observation that absence of p50 suppresses, but does not eliminate, the phenotype of IκBα−/− mice is consistent with the data presented here. Hematocrits were not increased in IκBα−/− mice. Granulocytes may be selectively increased because GM-CSF stimulates early and late myeloid progenitors/precursors, whereas GM-CSF stimulates the proliferation of early erythroid progenitors/precursors, but Epo regulates the proliferation and differentiation of late erythroid cells. The role of NF-κB in macrophage/monocyte production is unknown, unlike its involvement in macrophage/monocyte activation.8,10 Targeted disruption of the p50 subunit of NF-κB alone has not been reported to result in any defects in hematopoiesis other than multifocal defects in immune responses.48 However, a functional redundancy of p50 and p52 in hematopoietic cells may exist and has not yet been explored with knock-out mice. Mice lacking p65 (Rel A) show embryonic lethality and liver degeneration at 15 to 16 days of gestation,49 too early to definitively assess the effect on hematopoiesis. Additional knock-out experiments with multiple components will be required to determine the precise function of NF-κB factors in hematopoietic growth factor–regulated proliferation and differentiation.
Angiosarcoma. The scalp tumor of a 47-year-old woman was excised and found to be a malignant spindle cell tumor by light microscopy. By electron microscopy, the cells were diagnostic for endothelial cells. These polarized cells grew in small clusters and displayed numerous caveolae (inset) that were attached to borders with adjacent cells in areas that were devoid of basal lamina. The caveolae were also located along surfaces interfacing with the extracellular matrix and bound by basal lamina (arrows). Additional cytoplasmic contents included numerous filaments, mitochondria and small strands of nondilated rough endoplasmic reticulum. Note that single caveolae are attached to plasma membranes and are present in clusters attached to the plasma membranes (inset). Collections of caveolae such as these have been termed vesiculo-vacuolar organelles. Immunoperoxidase stains confirmed the endothelial origin for this tumor. (Original magnification ×31,000 [inset, ×72,000].) (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)
Angiosarcoma. The scalp tumor of a 47-year-old woman was excised and found to be a malignant spindle cell tumor by light microscopy. By electron microscopy, the cells were diagnostic for endothelial cells. These polarized cells grew in small clusters and displayed numerous caveolae (inset) that were attached to borders with adjacent cells in areas that were devoid of basal lamina. The caveolae were also located along surfaces interfacing with the extracellular matrix and bound by basal lamina (arrows). Additional cytoplasmic contents included numerous filaments, mitochondria and small strands of nondilated rough endoplasmic reticulum. Note that single caveolae are attached to plasma membranes and are present in clusters attached to the plasma membranes (inset). Collections of caveolae such as these have been termed vesiculo-vacuolar organelles. Immunoperoxidase stains confirmed the endothelial origin for this tumor. (Original magnification ×31,000 [inset, ×72,000].) (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)
ACKNOWLEDGMENT
The authors thank Dr Warner C. Greene for the antipeptide specific antisera for NF-κB/Rel proteins. We are grateful to Dr Toshio Kitamura for providing TF-1 cells. The authors would like to thank Tina Eberly and Maxine Gerberich for careful preparation of the manuscript.
Supported by Grants No. DK46778 (to B.A.M.), CA68471 (to S.-C.S.), and MO1 RR10732 (GCRC grant) from the National Institutes of Health, Bethesda, MD, and a grant from The Pennsylvania State University Cancer Center. S.-C.S. is a scholar of the American Society of Hematology. B.A.M. is the recipient of an American Cancer Society Faculty Award.
Address reprint requests to Barbara A. Miller, MD, Department of Pediatrics, The Milton S. Hershey Medical Center, PO Box 850, Hershey, PA 17033-0850.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



![Angiosarcoma. The scalp tumor of a 47-year-old woman was excised and found to be a malignant spindle cell tumor by light microscopy. By electron microscopy, the cells were diagnostic for endothelial cells. These polarized cells grew in small clusters and displayed numerous caveolae (inset) that were attached to borders with adjacent cells in areas that were devoid of basal lamina. The caveolae were also located along surfaces interfacing with the extracellular matrix and bound by basal lamina (arrows). Additional cytoplasmic contents included numerous filaments, mitochondria and small strands of nondilated rough endoplasmic reticulum. Note that single caveolae are attached to plasma membranes and are present in clusters attached to the plasma membranes (inset). Collections of caveolae such as these have been termed vesiculo-vacuolar organelles. Immunoperoxidase stains confirmed the endothelial origin for this tumor. (Original magnification ×31,000 [inset, ×72,000].) (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4136/4/m_blod41054001y.jpeg?Expires=1767894066&Signature=gvcQZ7fltVTfu66KGuMWDSk4UfWNRLwbU4f4kV8OYQ8utBzvHc0NCznRRIxgY6CtTJywPGBvEM29iQG0oJj2Ik54ZFPBEK4qd16NnmJeWFQI8wcG4nj3tZa2DwHAKfaL5Sql5CiUatk7ZFeZAt3faTz4QWTPM4fBmtJ~leqf3s85y6Wcyiks-nh4r4Q7vPLwYRElQ8uMIIW0nuNmhRQxS0Xfat0icf7uQeiFRQXwq1COuORkV03-2FXShqPAvYymkTTVq1R47RFA-ddO6696-ggJ9nJtsWqeU~r51y3D8uMUZAli2WA7k-bSJgA5WNlpiierVQYhX99pj0ldEp1RZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal