Abstract
Integrins respond to “inside-out” signals, which enable them to bind adhesive ligands, and ligand binding initiates “outside-in” signals that mediate anchorage-dependent cellular responses. RhoA is a GTPase that regulates certain actin rearrangements and transcriptional events. It has also been implicated in integrin signaling, but the exact relationship is not understood. To examine this further, platelets were incubated with C3 exoenzyme to adenine diphosphate (ADP)-ribosylate and inactivate RhoA, and the function of integrin αIIbβ3 was studied. Despite inactivation of ≥ 90% of RhoA, platelets exhibited normal inside-out signaling, as monitored by agonist-induced binding of a fibrinogen-mimetic anti-αIIbβ3 antibody and normal fibrinogen-dependent aggregation. On the other hand, RhoA inactivation decreased the adhesion of agonist-stimulated platelets to fibrinogen (P < .04) and the formation of vinculin-rich focal adhesions in platelets that did adhere (P < .001). These effects were selective because fibrin clot retraction, a response also dependent on αIIbβ3 and actin contractility, was unaffected by C3, as was the content of F-actin in resting or agonist-stimulated platelets. Similar results were obtained in a Chinese hamster ovary (CHO) cell model system of αIIbβ3: C3 exoenzyme (or overexpression of dominant-negative N19RhoA) failed to influence integrin activation state, but it blocked the formation of focal adhesions in cells spread on fibrinogen. These studies establish that RhoA plays a highly selective role in αIIbβ3 signaling, and they identify a subset of responses to integrin ligation that may be uniquely dependent on the actin rearrangements regulated by this GTPase.
INTEGRINS ARE αβ heterodimeric receptors that mediate cell-cell and cell-extracellular matrix interactions. Their function is tightly controlled through cellular regulation of ligand availability and receptor number and function. Of these variables, regulation of receptor function is responsible for acute changes in platelet adhesiveness during hemostasis, leukocyte transendothelial migration during inflammation, and vascular cell migration during wound healing.1-3 Such rapid integrin regulation, referred to as inside-out signaling, can be due to modulation of receptor conformation and affinity or modulation of receptor avidity. The latter may be promoted by integrin lateral diffusion and clustering and involves the actin cytoskeleton.1,4 5 The relative contributions of affinity and avidity modulation to the regulation of ligand binding appear to vary with the integrin and the cell type.
Ligand binding to integrins triggers outside-in signaling, a coordinated biochemical and mechanical response that affects anchorage-dependent cell growth, differentiation, survival, and motility.6-8 Recently, members of the Rho family of GTPases, among them Cdc42, Rac, and Rho, have also been shown to regulate cytoskeletal organization and gene transcription.9-11 Accordingly, there has been great interest in how the functions of integrins and Rho-GTPases are interrelated. Circumstantial evidence has implicated the Rho family of GTPases, and Rho in particular, in inside-out and outside-in signaling through integrins.
For example, treatment of leukocytes with C3 exoenzyme Clostridial toxin, which specifically adenine diphosphate (ADP)-ribosylates and inactivates Rho,12 blocks agonist-induced cell aggregation and adhesion, events that require activation of and ligand binding to β1 or β2 integrins.13,14Furthermore, in fibroblasts, overexpression of constitutively-active variants of Cdc42, Rac, and Rho or activation of the wild-type proteins by guanine nucleotide exchange factors triggers assembly of “integrin complexes” visible by immunofluorescence microscopy. In contrast, overexpression of dominant-negative variants or microinjection of Rho-inactivating toxins inhibits integrin complex assembly in response to growth factors.15 16 These results have suggested that Rho family GTPases regulate integrin affinity and/or avidity.
The integrin complexes regulated by Rho family members in fibroblasts and certain other cell types are present within discrete actin-based protrusive and adhesive structures: filopodia in the case of Cdc42, lamellipodia in the case of Rac, and focal adhesions in the case of Rho.10,17 The clustering of integrins into these structures may be essential for some of the cellular responses to integrin ligation. For example, inhibitors of actin polymerization or actin-myosin contractility block not only the formation of Rho-mediated stress fibers and focal adhesions, but also integrin-dependent tyrosine phosphorylation of FAK and paxillin.18 19 Thus, Rho and related GTPases may modulate signaling responses triggered through integrins.
The present studies were performed to systematically evaluate whether and how RhoA affects integrin signaling in a primary cell, the blood platelet. This system was chosen for several reasons. RhoA is the sole substrate for C3 exoenzyme in platelets,20 and these cells have been shown to contain a Rho guanine nucleotide dissociation inhibitor, guanine nucleotide exhange factors, and Rho effectors.9,21 Furthermore, affinity modulation of the platelet-specific integrin, αIIbβ3, is required for fibrinogen binding and platelet aggregation,1and C3 exoenzyme has been reported to partially inhibit thrombin-induced platelet aggregation.22 In addition, platelet aggregation is associated with tyrosine phosphorylation of FAK and translocation of RhoA to the Triton-insoluble cytoskeleton.23-25 Finally, platelet spreading on a fibrinogen matrix is dependent on αIIbβ3and is associated with the formation of focal adhesions and actin cables, structures characteristically regulated by Rho.26 27
Here we have monitored multiple facets of αIIbβ3 function using an experimental system in which the vast majority of RhoA in intact platelets has been inactivated by C3 exoenzyme. Parallel studies were conducted in a Chinese hamster ovary (CHO) cell model system that was developed to study αIIbβ3 function. The results establish that RhoA is not involved in regulating the ligand binding function of αIIbβ3. Rather, RhoA is involved in a subset of outside-in signaling responses that may be uniquely dependent on the specific actin rearrangements controlled by this GTPase.
MATERIALS AND METHODS
Reagents.
ADP, phorbol myristate acetate (PMA),1 prostaglandin E1 (PGE1), apyrase, and hirudin were from Sigma Chemical Co (St Louis, MO). α-Thrombin was from Organon Teknika (Durham, NC). Thrombin receptor-activating peptide (SFFLRN, referred to here as TRAP) was from Peninsula Laboratories (Belmont, CA). (32P)NAD was from Amersham Life Science, Inc (Arlington Heights, IL). Integrilin, a function-blocking cyclic peptide selective for αIIbβ3, was a gift from Dr David Phillips (Cor Therapeutics, South San Francisco, CA). Monoclonal antivinculin antibody was from Sigma, monoclonal anti-RhoA antibody was from Santa Cruz Biotechnology, Inc (Santa Cruz, CA), fluorescein isothiocyanate (FITC) goat antimouse IgG was from Biosource International (Camarillo, CA), and rhodamine-phalloidin, phallacidin, bodipy-phallacidin, and BCECF, AM were from Molecular Probes, Inc (Eugene, OR).
Dr Mark Ginsberg (Scripps Research Institute) provided CHO cell lines expressing αIIbβ3, mammalian expression vectors encoding Tac- α5 or constitutively-active V12H-Ras, and anti-LIBS6, an αIIbβ3activating antibody. Mammalian expression vectors encoding wild-type RhoA and dominant-negative N19RhoA and a pGEX vector encoding glutathione S-transferase (GST)/C3 exoenzyme were provided by Drs Gary Bokoch, Mark Renshaw, and Martin Schwartz (Scripps).28 Recombinant C3 exoenzyme was cleaved from GST with α-thrombin and sequentially gel-purified over glutathione-agarose, p-amino benzamidine-agarose, and Q-Sepharose (Sigma). After extensive dialysis against C3 “vehicle buffer” (150 mmol/L NaCl, 20 mmol/L HEPES, pH 7.35), C3 exoenzyme was concentrated to 1 mg/mL and stored at −70°C.
Platelet preparation and ADP-ribosylation of RhoA by C3 exoenzyme.
Fresh acid-citrate-dextrose (ACD)-anticoagulated blood was obtained from drug-free normal donors, and platelet-rich plasma was obtained.29 For some experiments, an additional aliquot of blood was anticoagulated with 0.38% sodium citrate to prepare citrated platelet-poor plasma. Platelet-rich plasma was supplemented with 1 μmol/L PGE1 and 1 U/mL apyrase and centrifuged for 15 minutes at 1,600 rpm at room temperature in a Sorvall GLC-2B centrifuge (Wilmington, DE). After gentle resuspension in a buffer containing 145 mmol/L NaCl; 20 mmol/L PIPES, pH 6.5; 1 μmol/L PGE1; and 1 U/mL apyrase; the platelets were sedimented again and resuspended in an “incubation buffer” containing 137 mmol/L NaCl; 2.7 mmol/L KCl; 1 mmol/L MgCl2; 5.6 mmol/L glucose; 1 mg/mL bovine serum albumin (BSA); 3.3 mmol/L NaH2PO4; and 20 mmol/L HEPES, pH 7.4. Platelets were then incubated at 1.4 × 109/mL for up to 4 hours at 37°C in the presence of 10 U/mL apyrase, 10 U/mL hirudin, and C3 exoenzyme or vehicle buffer. Preliminary experiments established that hirudin had no adverse effect on platelet function, but its presence was necessary to inactivate traces of thrombin variably contaminating C3 exoenzyme preparations.
ADP-ribosylation of Rho was determined as described by Morii et al,22 except that platelets were lysed at 4°C in radioimmunoprecipitation (RIPA) buffer (1% Triton X-100; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate (SDS); 158 mmol/L NaCl; 10 mmol/L Tris, pH 7.4; 1 mmol/L Na2EGTA; 0.5 mmol/L leupeptin; 0.25 mg/mL 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride; 5 μg/mL aprotinin). To exclude significant carry-over of C3 exoenzyme from the initial incubation, control platelets were supplemented with an appropriate amount of C3 immediately before washing and lysis.
Measurements of affinity modulation of αIIbβ3 and platelet aggregation.
After the incubation with C3 exoenzyme or vehicle buffer, platelets were diluted to 1.4 × 108/mL with incubation buffer and incubated for a further 15 minutes at room temperature with an agonist (ADP, PMA, or TRAP) and 40 μg/mL FITC-PAC1. PAC1 is a fibrinogen-mimetic, αIIbβ3-specific monoclonal antibody that binds to platelets in an activation-dependent and Arg-Gly-Asp (RGD)-inhibitable manner.30PAC1 binding to platelets was quantitated in a FACSCalibur flow cytometer (Becton-Dickinson Immunocytometry Systems, San Jose, CA).29 To study platelet aggregation responses to agonists, C3-treated or control platelets were diluted to 1.4 × 108/mL in citrated platelet-poor plasma as a source of fibrinogen and then stirred at 1,000 rpm for 2 to 3 minutes in an aggregometer (Chrono-Log, Broomall, PA). In some experiments, aggregation was also evaluated by counting the remaining single platelets in an electronic particle counter (Coulter Z1, Coulter Corp, Hialeah, FL).31
Measurement of αIIbβ3-dependent platelet adhesion.
After 3.5 hours incubation of platelets with C3 exoenzyme or vehicle buffer, platelets were labeled with 12 μmol/L BCECF, AM in dimethyl sulfoxide (DMSO) (0.4% final volume) for 30 minutes at 37°C. They were diluted to 4 × 108 cells/mL in incubation buffer, and platelet adhesion was initiated by adding 75-μL aliquots to fibrinogen-coated microtiter wells in the presence or absence of 30 or 100 nmol/L PMA. After 30 minutes at room temperature, nonadherent platelets were removed by aspiration, wells were washed twice with phosphate-buffered saline (PBS), and adherent platelets were quantitated in a fluorescence microplate reader at 485/530 nm. Adhesion was expressed as a percentage of input platelets and calculated from a standard curve derived by adding known numbers of labeled platelets to microtiter wells and measuring fluorescence.
Measurements of platelet F-actin content.
F-actin content was analyzed by flow cytometry with bodipy-phallacidin.32 Specifically, after an initial 30-second incubation of unstirred platelets at 37°C in the presence of 0.1 μmol/L PGE1 or 10 or 100 μmol/L TRAP, platelets were fixed with 4 volumes of 2.6% glutaraldehyde in 5.3 mmol/L EDTA for 2 hours at 37°C. After washing twice with PBS, the platelets were resuspended to half their initial volume and incubated at 37°C either with 3.3 μmol/L bodipy-phallacidin or bodipy-phallacidin in the presence of a 300-fold molar excess of unlabeled phallacidin. After 30 minutes, the platelets were washed twice with PBS and platelet fluorescence was analyzed in the FL1 channel of the flow cytometer. F-actin content was expressed in terms of specific bodipy-phallacidin fluorescence, which was defined as that competed for by the excess of unlabeled phallacidin, and it ranged from 88% to 91% of total fluorescence.
Clot retraction was measured essentially as described by Schoenwaelder et al.33 After incubation with C3 exoenzyme or vehicle buffer, varying numbers of platelets were added to a constant volume of citrated platelet-rich plasma in siliconized glass tubes. Clotting was initiated at 37°C by addition of 2 mmol/L CaCl2 and 8 U/mL α-thrombin, and the extent of clot retraction was assessed 90 minutes later by measuring the volume of fluid not incorporated into the clot.
Immunofluorescence microscopy of platelets.
Glass coverslips were coated with 100 μg/mL fibrinogen for 2 hours at 37°C and then washed with phosphate-buffered saline. After incubation with C3 exoenzyme or vehicle buffer, 1.4 × 107platelets in 0.5 mL of incubation buffer were added to the fibrinogen-coated coverslips for 60 minutes at 37°C. Nonadherent platelets were washed away and adherent cells were stained with monoclonal antivinculin antibody, FITC antimouse IgG, and rhodamine-phalloidin.27 Platelet spreading, vinculin-rich focal adhesions, and F-actin were analyzed with an MRC 1024 Bio-Rad laser scanning confocal imaging system attached to a Leitz Diaplan microscope (Leitz, Wetzlar, Germany).
Integrin signaling in CHO cells.
The effects of C3 exoenzyme on αIIbβ3function were studied in CHO cells stably-expressing either wild-type αIIbβ3 (A5 cells) or a constitutively-active αIIbβ3 chimera (αIIbα6A/β3β1), which contains the extracellular and transmembrane domains of αIIb and β3 and the cytoplasmic domains of α6A and β1.34 Cells were cultured in the presence of vehicle buffer or 5 to 20 μg/mL of C3 exoenzyme. After 24 hours, the cultures were supplemented again with C3, and 24 hours later they were processed for studies of PAC1 binding and fluorescence microscopy.34,35 In other experiments, αIIbα6A/β3β1CHO cells were transfected with hemaglutinin-tagged plasmid constructs encoding either dominant-negative N19RhoA or constitutively-active V12H-Ras along with a plasmid encoding a marker protein, Tac-α5. Forty-eight hours later, recombinant protein expression was assessed by Western blotting and αIIbβ3 affinity was measured in transfectants by flow cytometry using PAC1.35 As described previously,35 PAC1 binding to CHO cells was expressed as an activation index, where nonspecific binding in the presence of 10 μmol/L Integrilin was assigned a value of zero, and maximal specific binding in the presence of a saturating concentration of activating antibody anti-LIBS6 was assigned a value of 100.
RESULTS
ADP-ribosylation of RhoA in intact platelets by C3 exoenzyme.
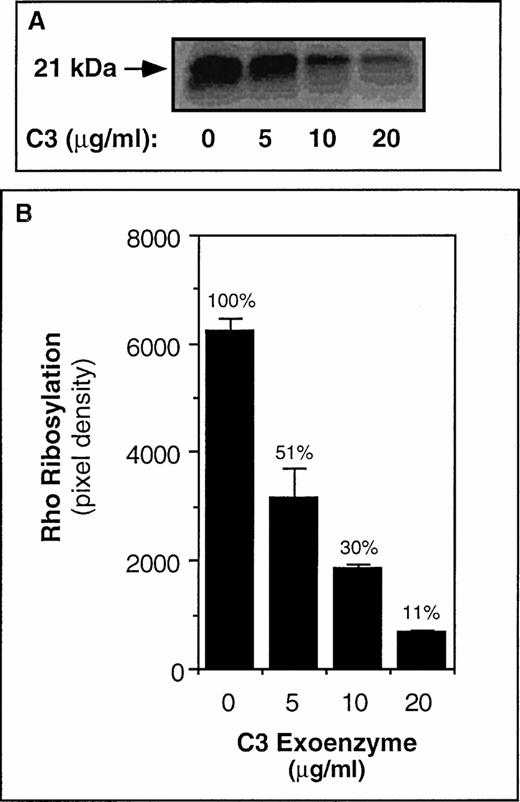
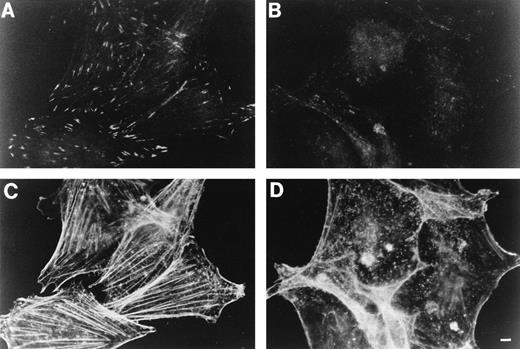
C3 exoenzyme was selected as a potentially useful tool to study RhoA-αIIbβ3 relationships because it specifically inactivates Rho12 and its sole substrate in platelets is RhoA.20 To determine whether the bacterially-expressed preparation of recombinant C3 exoenzyme was functional, studies were performed first with CHO cells that stably express αIIbβ3 (A5 cells).34Incubation of these cells for 48 hours with C3 exoenzyme resulted in a dose-dependent ADP-ribosylation of Rho, as determined by the subsequent inability of C3 exoenzyme to (32P)ADP-ribosylate Rho in cell lysates. Approximately one half of the Rho had been ADP-ribosylated and inactivated by 5 μg/mL of C3 exoenzyme and 90% by 20 μg/mL of C3 (Fig 1). Cells cultured with C3 and then allowed to spread on a fibrinogen matrix exhibited a marked reduction in focal adhesions and actin stress fibers (Fig 2).
ADP-ribosylation of Rho in A5 CHO cells by C3 exoenzyme. In (A) A5 CHO cells were cultured for 24 hours in the presence of vehicle buffer or the indicated amounts of C3 exoenzyme. The cultures were then supplemented again with the same amounts of C3, and 24 hours later the cells were washed and lysates were subjected to α(32P)ADP-ribosylation assay as described in Materials and Methods. The arrow points to the 32P-labeled Rho band. Note that the cells that had been cultured with C3 showed a subsequent decrease in incorporation of 32P, indicating that the Rho in these cells had become ADP-ribosylated during culture. In (B) the solid bars represent the means ± SEM of three independent experiments. The number above each bar represents the ribosylation response relative to that observed in the “No C3” control samples, which was arbitrarily assigned a value of 100%.
ADP-ribosylation of Rho in A5 CHO cells by C3 exoenzyme. In (A) A5 CHO cells were cultured for 24 hours in the presence of vehicle buffer or the indicated amounts of C3 exoenzyme. The cultures were then supplemented again with the same amounts of C3, and 24 hours later the cells were washed and lysates were subjected to α(32P)ADP-ribosylation assay as described in Materials and Methods. The arrow points to the 32P-labeled Rho band. Note that the cells that had been cultured with C3 showed a subsequent decrease in incorporation of 32P, indicating that the Rho in these cells had become ADP-ribosylated during culture. In (B) the solid bars represent the means ± SEM of three independent experiments. The number above each bar represents the ribosylation response relative to that observed in the “No C3” control samples, which was arbitrarily assigned a value of 100%.
Effect of C3 exoenzyme on focal adhesions and stress fibers in A5 CHO cells adherent to fibrinogen. A5 CHO cells were cultured as in Fig 1 in the presence of vehicle buffer (A and C) or 20 μg/mL of C3 exoenzyme (B and D). The cells were then resuspended in Dulbecco's modified Essential medium and incubated over fibrinogen-coated coverslips for 60 minutes at 37°C. After washing away nonadherent cells, adherent cells were fixed, permeabilized, and stained for vinculin (A and B) or F-actin (C and D). Vinculin-positive focal adhesions and actin stress fibers were plentiful in the control cells, but not in the cells treated with C3 exoenzyme. The results are representative of three experiments. Bar = 5 μm.
Effect of C3 exoenzyme on focal adhesions and stress fibers in A5 CHO cells adherent to fibrinogen. A5 CHO cells were cultured as in Fig 1 in the presence of vehicle buffer (A and C) or 20 μg/mL of C3 exoenzyme (B and D). The cells were then resuspended in Dulbecco's modified Essential medium and incubated over fibrinogen-coated coverslips for 60 minutes at 37°C. After washing away nonadherent cells, adherent cells were fixed, permeabilized, and stained for vinculin (A and B) or F-actin (C and D). Vinculin-positive focal adhesions and actin stress fibers were plentiful in the control cells, but not in the cells treated with C3 exoenzyme. The results are representative of three experiments. Bar = 5 μm.
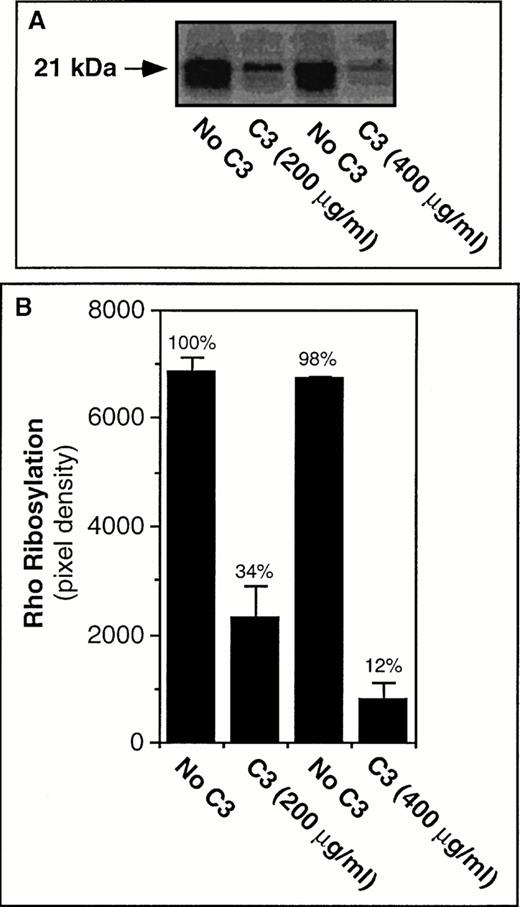
Next, to establish whether C3 exoenzyme would enter platelets and ADP-ribosylate RhoA in an experimentally useful time frame, washed platelets were incubated at 37°C for up to 4 hours in the presence of C3 or an equivalent volume of vehicle buffer. Preliminary studies indicated that 4 hours was the longest time that platelets could be incubated in this buffer system without an unacceptable loss of responsiveness. Incubation of platelets with C3 exoenzyme caused a time- and dose-dependent ADP-ribosylation of RhoA such that at 400 μg/mL of the toxin, approximately 90% of the GTPase had become inactivated (Fig 3). On the other hand, C3 treatment did not affect the platelet content of RhoA, as determined by immunoprecipitation and immunoblot analysis (not shown). These results indicate that efficient RhoA blockade can be achieved in platelets and CHO cells under the appropriate experimental conditions.
ADP-ribosylation of RhoA in platelets by C3 exoenzyme. In (A) washed platelets were incubated for 4 hours at 37°C in the presence of vehicle buffer (“No C3”) or C3 exoenzyme. The platelets were then washed and subjected to α(32P)ADP-ribosylation assay as described in Materials and Methods. In (B) the solid bars represent the means ± SEM of three experiments.
ADP-ribosylation of RhoA in platelets by C3 exoenzyme. In (A) washed platelets were incubated for 4 hours at 37°C in the presence of vehicle buffer (“No C3”) or C3 exoenzyme. The platelets were then washed and subjected to α(32P)ADP-ribosylation assay as described in Materials and Methods. In (B) the solid bars represent the means ± SEM of three experiments.
Effect of RhoA inactivation on the ligand binding function of αIIbβ3.
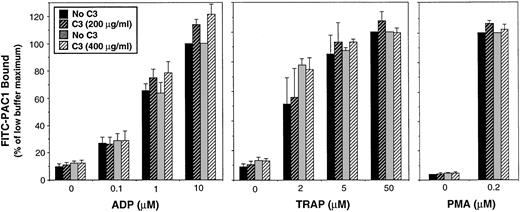
A fibrinogen-mimetic anti-αIIbβ3 monoclonal antibody (PAC1) was used to determine the effect of C3 exoenzyme on the activation state of αIIbβ3. After 4 hours of incubation with the vehicle buffer as a control, platelets exhibited the expected marked increase in PAC1 binding in response to ADP or TRAP, agonists that engage G protein-linked receptors and initiate inside-out signaling (Fig4).36 37 In three separate experiments, a 4-hour incubation with 200 or 400 μg/mL of C3 exoenzyme caused ADP ribosylation of 66.0% ± 10.3% (standard error of mean [SEM]) and 88.0% ± 5.1% of the RhoA, respectively. However, this treatment had no effect on PAC1 binding induced by either submaximal or maximal concentrations of ADP or TRAP (Fig 4). Moreover, C3 had no effect on PAC1 binding induced by PMA, which initiates activation of αIIbβ3 at the level of protein kinase C (Fig 4).
Effect of RhoA inactivation by C3 exoenzyme on agonist-induced affinity modulation of platelet αIIbβ3. Washed platelets were incubated with vehicle buffer (“No C3”) or C3 exoenzyme as in Fig 3. Cells were then diluted with incubation buffer and the affinity state of αIIbβ3 was assessed by flow cytometry using FITC-PAC1. PAC1 binding was expressed as a percentage, 100% being arbitrarily assigned to the maximal response for the “No C3” control sample that had been paired with the experimental sample containing 200 μg/mL of C3 exoenzyme. Data represent the means ± SEM of three experiments.
Effect of RhoA inactivation by C3 exoenzyme on agonist-induced affinity modulation of platelet αIIbβ3. Washed platelets were incubated with vehicle buffer (“No C3”) or C3 exoenzyme as in Fig 3. Cells were then diluted with incubation buffer and the affinity state of αIIbβ3 was assessed by flow cytometry using FITC-PAC1. PAC1 binding was expressed as a percentage, 100% being arbitrarily assigned to the maximal response for the “No C3” control sample that had been paired with the experimental sample containing 200 μg/mL of C3 exoenzyme. Data represent the means ± SEM of three experiments.
To determine if the inability of C3 exoenzyme to influence the αIIbβ3 activation process was unique to the cellular context of the platelet, the effect of C3 on αIIbβ3 in CHO cells was examined. In the A5 CHO cell line, αIIbβ3 is normally in a low-affinity state. In contrast, in CHO cells expressing the chimera, αIIbα6A/β3β1, the integrin is in constitutive, energy-dependent high-affinity state.34 Integrin affinity was monitored by the binding of PAC1, which was expressed as an activation index (see Materials and Methods). In two separate experiments, incubation of either cell line for 48 hours with 20 μg/mL of C3 exoenzyme had no effect on PAC1 binding, despite the fact that 90% of the RhoA had been inactivated (activation indices: A5 cells without C3 exoenzyme, 18; A5 cells with C3, 14; αIIbα6A/β3β1cells without C3, 42; αIIbα6A/β3β1cells with C3, 42). To validate this finding in another way, αIIbα6A/β3β1cells were transiently-transfected with dominant-negative N19RhoA. Western blotting indicated that N19RhoA was expressed to levels about fivefold greater than endogenous RhoA, but it had no effect on PAC1 binding to these cells. In contrast, and as reported previously,38 a constitutively-active H-Ras mutant (V12H-Ras) inhibited PAC1 binding to these cells (not shown). Taken together with the platelet experiments, these results strongly suggest that RhoA is dispensable for inside-out signaling reactions that modulate integrin affinity.
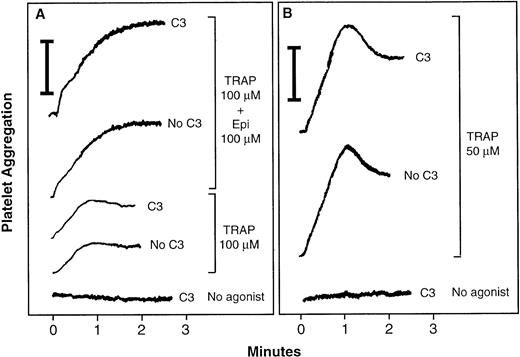
Because activation of αIIbβ3 leads to ligand binding and platelet aggregation, the effect of C3 exoenzyme on fibrinogen-dependent platelet aggregation was studied. After incubation with 400 μg/mL of C3 exoenzyme, platelets were diluted with citrated plasma as a source of fibrinogen and then stirred in an aggregometer. Platelets that had been incubated for 4 hours with vehicle buffer underwent normal primary aggregation in response to TRAP or a combination of TRAP and epinephrine (Fig5). However, secondary aggregation was impaired due to the prolonged incubation. C3 exoenzyme had no effect on primary aggregation, despite ADP-ribosylation of an average of 92.6% ± 0.7% of the RhoA (Fig5). C3 also had no effect when aggregation was monitored by a single platelet counting method. Thus, RhoA is not required for ligand binding to αIIbβ3 or for postligand binding events responsible for primary platelet aggregation.
Effect of RhoA inactivation by C3 exoenzyme on platelet aggregation. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3 (“C3”). Then citrated platelet-poor plasma was added back, and platelet aggregation was measured in stirred samples by aggregometry. In this system, light transmittance is assigned a value of 0% in nonaggregated, platelet-replete samples and 100% in platelet-free samples. Vertical bars indicate a 20% aggregation response. With this technique, primary aggregation is generally < 50% and secondary aggregation > 50%. (A and B) Show tracings from two separate experiments representative of four experiments.
Effect of RhoA inactivation by C3 exoenzyme on platelet aggregation. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3 (“C3”). Then citrated platelet-poor plasma was added back, and platelet aggregation was measured in stirred samples by aggregometry. In this system, light transmittance is assigned a value of 0% in nonaggregated, platelet-replete samples and 100% in platelet-free samples. Vertical bars indicate a 20% aggregation response. With this technique, primary aggregation is generally < 50% and secondary aggregation > 50%. (A and B) Show tracings from two separate experiments representative of four experiments.
Effect of RhoA inactivation on outside-in signaling through αIIbβ3.
C3-treated platelets were tested for their ability to undergo outside-in signaling responses. Although the initial adhesion of platelets to immobilized fibrinogen does not require platelet activation, the subsequent cytoskeletal reorganization and platelet spreading do.26,39 Some of these activation signals are probably triggered through αIIbβ3, although additional signals emanating from agonist receptors are required for full platelet spreading and adhesion.26 40 Therefore, the effect of C3 exoenzyme on platelet adhesion to fibrinogen was studied, both in the absence and presence of an exogenous agonist, PMA. In this series of experiments, 400 μg/mL of C3 exoenzyme caused ADP-ribosylation of 64.2% ± 5.6% of the RhoA. However, it had no effect on the adhesion of unstimulated platelets (Fig 6). As expected, stimulation with 30 or 100 nmol/L PMA increased the number of adherent platelets (Fig 6). Microscopic inspection showed that this was due to an increase in adhesion of single platelets in four of five experiments; however, in one experiment, small platelet aggregates were observed around adherent platelets. C3 exoenzyme caused a modest 25% decrease in the adhesion of PMA-stimulated platelets, and this decrease was statistically significant (P < .04) (Fig 6). These results indicate that RhoA is not required for the adhesion of unstimulated platelets to fibrinogen, but it may facilitate the adhesion of activated platelets to this substrate.
Effect of RhoA inactivation by C3 exoenzyme on platelet adhesion to immobilized fibrinogen. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3. Then platelets were labeled with BCECF as a fluorescent marker, washed, incubated in fibrinogen-coated microtitre wells for 30 minutes at room temperature, and adhesion was quantitated as described in Materials and Methods. Adhesion is expressed as the percentage of added platelets that adhered. Data represent the means ± SEM of five experiments.
Effect of RhoA inactivation by C3 exoenzyme on platelet adhesion to immobilized fibrinogen. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3. Then platelets were labeled with BCECF as a fluorescent marker, washed, incubated in fibrinogen-coated microtitre wells for 30 minutes at room temperature, and adhesion was quantitated as described in Materials and Methods. Adhesion is expressed as the percentage of added platelets that adhered. Data represent the means ± SEM of five experiments.
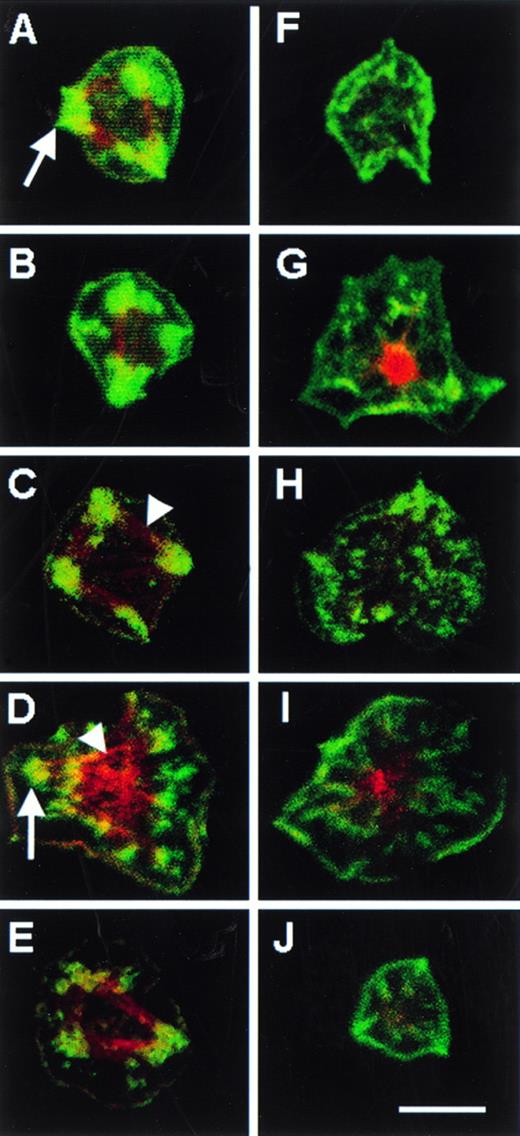
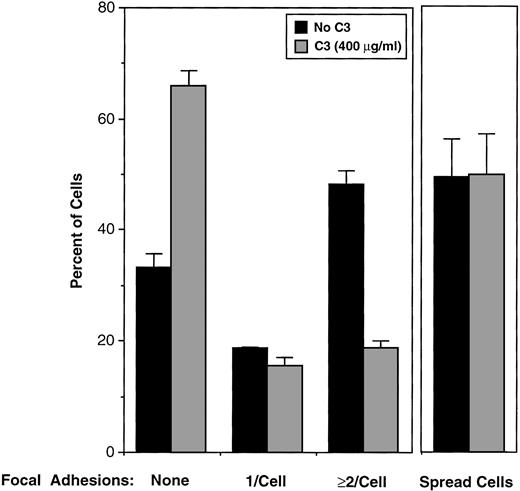
Platelets that spread on fibrinogen display membrane-based, ventral structures reminiscent of focal adhesions, which can be identified by their staining with antibodies to vinculin and by their intersection with F-actin cables stained with rhodamine-phalloidin.27 To assess the role of RhoA in the formation of these structures, C3-treated and control platelets were allowed to attach to fibrinogen-coated cover slips for 60 minutes in the presence of 100 nmol/L PMA, the latter added to enhance spreading. Although about one half of the adherent platelets became fully-spread in each case, there were marked differences between the C3-treated and control platelets in the number of focal adhesions and the extent of actin cables (Figs 7 and8). For example, 48.0% of spread control platelets contained ≥ 2 focal adhesions, and 33.0% contained no focal adhesions. In contrast, only 18.6% of C3-treated spread platelets contained ≥ 2 focal adhesions, and 66.0% contained no focal adhesions (Fig 8). These differences between control and C3-treated platelets were statistically significant (P < .001), and they establish that RhoA is involved in the integrin-dependent formation or stabilization of focal adhesions in platelets.
Focal adhesions and actin cables in fibrinogen-adherent platelets. Washed platelets were incubated for 4 hours at 37°C in the absence (A through E) or presence (F through J) of 400 μg/mL C3 exoenzyme. They were then diluted in incubation buffer and placed over fibrinogen-coated coverslips for 60 minutes at 37°C in the presence of 100 nmol/L PMA to enhance spreading. Vinculin was stained with a specific monoclonal antibody and FITC antimouse IgG and F-actin was stained with rhodamine phalloidin. Single views of individual spread platelets were obtained by confocal microscopy. The five platelet pairs shown (eg, A and F, B and G, etc) are from five separate platelet donors and are representative of predominant morphologies. Arrows point to some of the green-staining focal adhesions, which were scored positive on the basis of heavy focal vinculin staining, as reported previously.27 Arrowheads point to some of the red-staining actin cables often seen to connect the focal adhesions. Note that focal adhesions and actin cables were prominent in control platelets, but not in platelets treated with C3 exoenzyme. Bar = 5 μm.
Focal adhesions and actin cables in fibrinogen-adherent platelets. Washed platelets were incubated for 4 hours at 37°C in the absence (A through E) or presence (F through J) of 400 μg/mL C3 exoenzyme. They were then diluted in incubation buffer and placed over fibrinogen-coated coverslips for 60 minutes at 37°C in the presence of 100 nmol/L PMA to enhance spreading. Vinculin was stained with a specific monoclonal antibody and FITC antimouse IgG and F-actin was stained with rhodamine phalloidin. Single views of individual spread platelets were obtained by confocal microscopy. The five platelet pairs shown (eg, A and F, B and G, etc) are from five separate platelet donors and are representative of predominant morphologies. Arrows point to some of the green-staining focal adhesions, which were scored positive on the basis of heavy focal vinculin staining, as reported previously.27 Arrowheads point to some of the red-staining actin cables often seen to connect the focal adhesions. Note that focal adhesions and actin cables were prominent in control platelets, but not in platelets treated with C3 exoenzyme. Bar = 5 μm.
Effect of RhoA inactivation by C3 exoenzyme on focal adhesion formation in fibrinogen-adherent platelets. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3. Cells were incubated over fibrinogen-coated coverslips and adherent cells processed for fluorescence microscopy as in Fig 7. Five hundred control and C3-treated platelets were scored as being spread or unspread, the latter defined as rounded and ≤ 5 μm in diameter. Also, 500 spread platelets in each sample were scored for focal adhesions as illustrated in Fig 7. Data represent means ± SEM of four experiments. C3 caused ADP-ribosylation of 64.4% ± 8.0% of the RhoA in this series of experiments.
Effect of RhoA inactivation by C3 exoenzyme on focal adhesion formation in fibrinogen-adherent platelets. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3. Cells were incubated over fibrinogen-coated coverslips and adherent cells processed for fluorescence microscopy as in Fig 7. Five hundred control and C3-treated platelets were scored as being spread or unspread, the latter defined as rounded and ≤ 5 μm in diameter. Also, 500 spread platelets in each sample were scored for focal adhesions as illustrated in Fig 7. Data represent means ± SEM of four experiments. C3 caused ADP-ribosylation of 64.4% ± 8.0% of the RhoA in this series of experiments.
Effect of RhoA inactivation on clot retraction and platelet F-actin content.
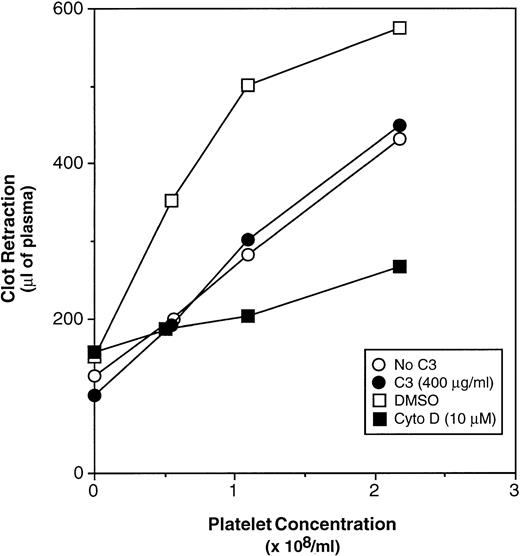
Clot retraction is dependent on the interaction of fibrin with αIIbβ3 and platelet contractility.41,42 Because RhoA has been implicated in the contractility of actin stress fibers, it is possible that these structures function in clot retraction.18 19 However, there was no difference in the extent of thrombin-induced clot retraction between control platelets (Fig 9, ○) and C3-treated platelets (Fig 9, •), despite the fact that the C3 caused ADP-ribosylation of 93.7% ± 2.0% of the RhoA in this series of experiments. In contrast, cytochalasin D, an inhibitor of actin polymerization, markedly inhibited clot retraction (Fig 9, ▪v □). These results indicate that the platelet events required for clot retraction are independent of RhoA.
Effect of RhoA inactivation by C3 exoenzyme on fibrin clot retraction. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3. Alternatively, platelets were incubated for 3 hours, 50 minutes without additive, and then with DMSO or 10 μmol/L cytochalasin D (Cyto D) for 10 minutes. Then platelets were added to citrated plasma in siliconized glass tubes, and clotting was initiated with 8 U/mL of thrombin and 2 mmol/L CaCl2. After 90 minutes at 37°C, clot retraction was quantitated as described in Materials and Methods. Data represent the means of three experiments. Error bars have been omitted for clarity.
Effect of RhoA inactivation by C3 exoenzyme on fibrin clot retraction. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3. Alternatively, platelets were incubated for 3 hours, 50 minutes without additive, and then with DMSO or 10 μmol/L cytochalasin D (Cyto D) for 10 minutes. Then platelets were added to citrated plasma in siliconized glass tubes, and clotting was initiated with 8 U/mL of thrombin and 2 mmol/L CaCl2. After 90 minutes at 37°C, clot retraction was quantitated as described in Materials and Methods. Data represent the means of three experiments. Error bars have been omitted for clarity.
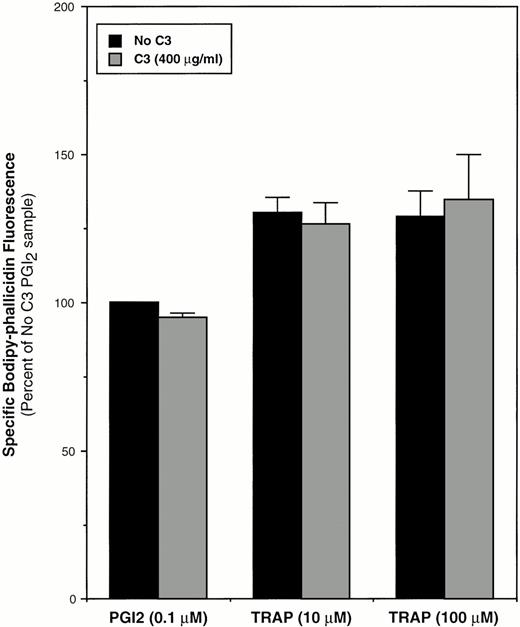
Recently, Hartwig et al43 showed that Rac, but not RhoA, is involved in stimulus-induced actin polymerization in suspended, permeabilized platelets. Consistent with those results, but using intact platelets and bodipy-phallacidin to evaluate F-actin content by flow cytometry, we found that inactivation of 68.5% ± 8.4% of the RhoA by C3 exoenzyme had no effect on basal F-actin content or on the increase in F-actin stimulated by TRAP (Fig 10).
Effect of RhoA inactivation by C3 exoenzyme on platelet F-actin content. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3. Platelets were then diluted with incubation buffer and stirred for 30 seconds at 37°C in the presence of PGI2 or TRAP, after which they were fixed and stained with bodipy-phallacidin for quantitation of F-actin as described in Materials and Methods. F-actin content was taken as the mean specific bodipy-phallacidin fluorescence expressed as a percentage of the “No C3” control sample incubated with PGI2. Data represent means ± SEM of three experiments.
Effect of RhoA inactivation by C3 exoenzyme on platelet F-actin content. Washed platelets were incubated for 4 hours at 37°C with vehicle buffer (“No C3”) or 400 μg/mL of C3. Platelets were then diluted with incubation buffer and stirred for 30 seconds at 37°C in the presence of PGI2 or TRAP, after which they were fixed and stained with bodipy-phallacidin for quantitation of F-actin as described in Materials and Methods. F-actin content was taken as the mean specific bodipy-phallacidin fluorescence expressed as a percentage of the “No C3” control sample incubated with PGI2. Data represent means ± SEM of three experiments.
DISCUSSION
Potential relationships between RhoA and integrins were evaluated here using the platelet as a model system, the adhesive and signaling functions of integrin αIIbβ3 as endpoints, and the inactivation of RhoA by C3 exoenzyme as an experimental tool. When washed, intact platelets were incubated with 400 μg/mL of C3 exoenzyme for 4 hours, the vast majority of the RhoA in the cells became ADP-ribosylated and inactivated. Although prolonged platelet incubation itself caused a loss of the labile secondary aggregation response, this system enabled us to systematically examine many of the adhesive and signaling functions of αIIbβ3. The major conclusions from this work are that: (1) more than 90% of the RhoA in platelets is dispensable for inside-out signaling reactions that are responsible for affinity modulation of αIIbβ3 and for initiation of platelet aggregation; (2) on the other hand, RhoA is involved in discrete outside-in signaling responses in fibrinogen-adherent platelets, most prominently the formation of focal adhesions; (3) however, in suspended platelets, RhoA does not appear to be a major regulator of F-actin content or of thrombin-induced clot retraction, and in fibrinogen-adherent platelets, RhoA does not regulate αIIbβ3-dependent cell spreading. Taken together, the present studies establish that RhoA plays a highly selective role in αIIbβ3 function, implying that integrin- and RhoA-regulated signaling pathways intersect, but they are not one and the same.
RhoA and inside-out signaling in platelets.
Morii et al22 showed previously that incubation of platelets for 2 hours with 5 to 20 μg/mL of C3 exoenzyme inhibited thrombin-induced platelet aggregation, despite the fact that less than 25% of the RhoA in the platelets had become ADP-ribosylated. They speculated that the ribosylated RhoA might function as a dominant-negative inhibitor of inside-out signaling. In the present studies, we were able to inactivate ≥ 90% of the RhoA by incubating the platelets with higher concentrations of C3 for 4 hours. However, RhoA inactivation had no effect on either agonist-induced affinity modulation of αIIbβ3 or primary platelet aggregation. These results were supported by experiments in CHO cells, which showed that PAC1 binding to an energy-dependent, constitutively-active αIIbβ3 chimera was unaffected by C3 exoenzyme or by overexpression of a dominant-negative Rho mutant. In contrast, PAC1 binding to this chimera was inhibited by constitutively-active V12H-Ras, as reported previously.38Based on the entire collection of data, we conclude that RhoA is not involved in affinity modulation of αIIbβ3. This “negative” conclusion has important implications for models of inside-out signaling in platelets and perhaps other cells.
Fibrinogen (or PAC1) binding to platelets is initiated primarily by changes in αIIbβ3 conformation that increase receptor affinity for ligands.44,45 Subsequent changes in the platelet cytoskeleton may increase receptor avidity so as to promote irreversible ligand binding and perhaps secondary platelet aggregation.1,45,46 Given its demonstrated effects on the cytoskeleton, it is conceivable that RhoA may help to regulate the formation of large, irreversible platelet aggregates, perhaps accounting for the previously observed effect of C3 exoenzyme on platelet aggregation.22 On the other hand, the former studies were potentially confounded by the use of Tris-containing buffers, which can be detrimental to platelet function. In the present study, we could not evaluate secondary platelet aggregation because this response was degraded by the long incubation times required to adequately incorporate C3 into the cells. Therefore, further studies will be required to determine the exact role of RhoA in secondary platelet aggregation.
C3 exoenzyme has been shown to inhibit β1 and β2 integrin-dependent adhesion events in PMA-stimulated leukocytes: aggregation of JY T-cells,13 adhesion of L1/2 B cells to immobilized VCAM-1,14 and adhesion of neutrophils to fibrinogen.14 These findings are consistent with Rho involvement either in leukocyte integrin affinity modulation, avidity modulation, or other postligand binding events. However, even in leukocytes, it is not necessary to postulate an effect of Rho on integrin affinity. In contrast to platelets, where a change in receptor affinity is the dominant mode of integrin activation, integrin clustering and avidity modulation may be major mechanisms in leukocytes.4,47 Because Rho is involved in reorganizing F-actin and promoting integrin clustering,16,18 it is not too surprising, therefore, to find that C3 exoenzyme impairs the initial aggregation of leukocytes, but not platelets. In agonist-stimulated platelets, the initial reversible phase of fibrinogen or PAC1 binding is relatively insensitive to inhibition by cytochalasins, providing additional evidence that initial activation of αIIbβ3 does not require major cytoskeletal changes.48-50
RhoA and outside-in signaling in platelets.
A number of platelet responses are presumed to be dependent, at least in part, on signaling events triggered by ligand binding to αIIbβ3. These include actin rearrangements to form filopodia and focal adhesions,27,51 aggregation, spreading on vascular matrices,52 clot retraction,33,53 and with some agonists, exocytotic secretion and vesiculation.54 Full responses generally require collaboration between signals generated through integrins and more traditional agonist receptors. Identification of specific roles for RhoA downstream of αIIbβ3 is complicated because RhoA may also function downstream of platelet agonists,24 and agonist and integrin pathways may converge at several levels. Nonetheless, the present study shows that RhoA inactivation partially inhibited the adhesion of PMA-stimulated platelets to fibrinogen. Thus, cytoskeletal reorganization controlled by RhoA may be required for αIIbβ3clustering or other postligand binding events so that adherent platelets can withstand the shear forces to which they are exposed in vitro during washing and possibly in vivo during encounters with the walls of damaged blood vessels.55 56
The most dramatic effect of C3 exoenzyme in fibrinogen-adherent platelets was on the number of focal adhesions (Fig 8). In addition to organization of stress fibers and focal adhesions, Rho has been implicated in cytokinesis and actin polymerization beneath certain plasma membranes. Each of these responses may be mediated by a specific subset of Rho effectors.9,57,58 In fibroblasts, stress fibers and focal adhesions are thought to be regulated by ROCK-1 and ROCK-2, serine-threonine kinases that induce actin-myosin contractility by phosphorylating myosin light chain59,60 and myosin phosphatase.61 Integrin clustering within these focal adhesions may actually be secondary to the formation and contraction of stress fibers.18 19Because platelets contain many of the same Rho effectors and downstream targets, the same may be true for these cells.
In fibroblasts, experiments with bacterial toxins that either inactivate or stimulate Rho family GTPases have established a causative link between Rho and tyrosine phosphorylation of the focal adhesion proteins, pp125FAK, paxillin, and p130Cas.62-65 In platelets, FAK activation requires both integrin ligation and actin polymerization and it occurs concomitantly with full aggregation or spreading23,40conditions in which actin rearrangements by RhoA have already occurred. Although tyrosine phosphorylation was not studied in C3-treated platelets, one function of RhoA in these cells may be to promote an appropriate actin-based microenvironment for FAK activation and certain other biochemical events in outside-in signaling. One such event may be activation of a subpopulation of phosphatidylinositol 3-kinase.25,66 67
C3 exoenzyme did not influence the content of F-actin in unstimulated or TRAP-stimulated platelets maintained in suspension, as determined by specific binding of bodipy-phallacidin (Fig 10). It is possible that the amount of RhoA inactivated by C3 in these particular experiments (68%) was insufficient to obtain an effect or that RhoA plays a more prominent role in actin polymerization in adherent platelets. Alternatively, regulation of actin filament content in platelets may be largely independent of RhoA. Indeed, Hartwig et al43 used detergent-permeabilized platelets to show that Rac, but not Rho, regulates uncapping and assembly of actin filaments in platelets stimulated by TRAP. Another prominent cytoskeletal process that was unaffected by C3 exoenzyme was fibrin clot retraction, even though 94% of the RhoA had become inactivated. The molecular events that regulate clot retraction are poorly understood, but binding of fibrinogen/fibrin to αIIbβ3 and platelet contractility are clearly required, as evidenced by the effects of αIIbβ3 blockade or inhibition of actin polymerization by cytochalasin D (Fig 9). Electron micrographic analyses of fibrin clots retracted under isometric conditions have shown platelet pseudopods containing actin filaments oriented along fibrin strands, which in turn, are oriented in the direction of tension.41 42 Apparently, Rho is not required for these particular protrusive and force-generating events.
Studies of cultured fibroblasts indicate that outside-in signaling through integrins may help to regulate Rho. For example, cell adhesion via integrins stimulates the production of phosphatidylinositol 4,5-bisphosphate, as does the stimulation of cell lysates by GTP-Rho.68 Also, cell adhesion to fibronectin leads to a slow increase in actin stress fibers and focal adhesions, and this Rho-dependent response can be accelerated by addition of an RGD peptide.65 Thus, cell adhesion through integrins may stimulate GTP-loading of Rho or inhibit Rho-GTP hydrolysis in a manner that complements these same responses to other plasma membrane receptors.9 10 Whether integrin αIIbβ3 regulates RhoA in platelets remains to be determined.
ACKNOWLEDGMENT
The authors are grateful to Drs Gary Bokoch, Mark Ginsberg, David Phillips, Mark Renshaw, and Martin Schwartz for providing DNA constructs, cell lines, and reagents; and to Zheng Luo for technical assistance.
Supported by Grants No. HL56595 and HL57900 from the National Institutes of Health, Bethesda, MD, and by a fellowship to H.K. from the Banyu-Merck Foundation (Tokyo, Japan).
L.L. and H.K. contributed equally to this work.
Address reprint requests to Sanford J. Shattil, MD, Department of Vascular Biology, The Scripps Research Institute, 10550 N Torrey Pines Rd, VB-5, La Jolla, CA 92037.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal