Abstract
Nitric oxide (NO), an important effector molecule involved in immune regulation and host defense, was shown to induce apoptosis in lymphoma cells. In the present report the NO donor glycerol trinitrate was found to induce apoptosis in Jurkat cells that are sensitive to CD95-mediated kill. In contrast, a CD95-resistant Jurkat subclone showed substantial protection from apoptosis after exposure to NO. NO induced mRNA expression of CD95 (APO-1/Fas) and TRAIL/APO-2 ligands. Moreover, NO triggered apoptosis in freshly isolated human leukemic lymphocytes which were also sensitive to anti-CD95 treatment. The ability of NO to induce apoptosis was completely blocked by a broad-spectrum ICE (interleukin-1β converting enzyme)-protease/caspase inhibitor and correlated with FLICE/caspase-8 activation. This activation was abrogated in some neoplastic lymphoid cells but not in others by the inhibitor of protein synthesis cycloheximide. Our results were confirmed using an in vitro experimental model of coculture of human lymphoid target cells with activated bovine endothelial cells generating NO as effectors. Furthermore, the inhibition of endogenous NO production with the inducible NO synthase inhibitor NG-monomethyl-L-arginine caused a complete abrogation of the apoptotic effect. Our data provide evidence that NO-induced apoptosis in human neoplastic lymphoid cells strictly requires activation of caspases, in particular FLICE, the most CD95 receptor-proximal caspase. Depending on the cell line tested this activation required or was independent of the CD95 receptor/ligand system.
NITRIC OXIDE (NO) is an important pleiotropic molecule involved in neurotransmission, regulation of vascular homeostasis, and effector functions of macrophages and endothelial cells.1,2 NO is derived from the oxidation of L-arginine catalyzed by constitutive or inducible NO synthase (iNOS). Large amounts of NO produced by iNOS in macrophages and endothelial cells after challenge with lipopolysaccharide and cytokines, eg, interferons, interleukin-1, and tumor necrosis factor-α (TNF-α), were effective against various microbes and tumor cells.3,4Cytokine-activated macrophages and vascular endothelial cells were reported to kill tumor cells in vitro, and NO produced by these cells was shown to play a major role in this antitumor cytotoxicity.5-7 The regression of murine sarcoma liver metastasis correlated with the expression of iNOS after treatment with a macrophage activator.8 The cytotoxic effect of NO was found to be associated with apoptosis (programmed cell death) in normal9-12 and tumor cells.13-15 Furthermore, the transfection of highly metastatic murine melanoma cells with iNOS caused partial apoptosis of these cells associated with suppression of their tumorigenicity and metastatic potential.16Collectively, these data show that NO production represents an important host-defense mechanism against tumor cells.
Apoptosis is an active process of cellular self destruction with unique morphologic and molecular characteristics. It can be induced by external stimuli or by withdrawal of survival factors such as growth factors or cytokines. Activation of a cascade of caspases (ICE-like proteases) seems to be a common intracellular effector pathway to which different apoptosis signals are connected.17 Members of the TNF receptor superfamily of cell-surface molecules play a critical role in apoptosis of lymphoid cells.18 CD95L (APO-1L/FasL) and TNF-α induce apoptosis by binding to their respective death domain–containing receptors, CD95 and TNFR-1.19 Binding of CD95L or agonistic antibodies to CD95 leads to a signal transduction cascade initiated by death receptor–associated molecules such as FADD/MORT120-22 and FADD-associated FLICE/MACH (caspase-8), a chimeric molecule containing a death effector domain and a proteolytic ICE-like domain.23,24 This leads to proteolytic processing of caspases into active proteases. FLICE is the first in a cascade of caspases activated by CD95.25
The CD95 system is critical for growth control of T cells. Elimination of peripheral T cells involves the induction of a suicide or fratricide mechanism triggered by CD95 ligand/receptor interaction.26-28 Triggering of CD95 ligand/receptor interaction in lymphoid and nonlymphoid cells may also be caused by cellular stress-inducing agents, eg, by cytotoxic drugs.29Another member of the TNF superfamily, named TRAIL/APO-2L, has recently been isolated30-32 and, like CD95L, induces rapid apoptosis in a variety of transformed cell lines.30-32 Human receptors for TRAIL, named death receptor-4 and 5 (DR-4, DR-5), have recently been identified.33-35
We have previously shown that activated bovine endothelial cells (BEC) induced apoptosis in murine metastatic lymphoma cells. This effect was mediated to a large extent by NO.14 Here we studied the mechanism of NO-dependent apoptotic death using well-defined human T- and B-cell lines as well as freshly isolated leukemic lymphocytes, and investigated in particular the involvement of death receptors in this process. Our results suggest that production of endogenous NO by endothelial cells or exposure to exogenous NO triggers apoptosis in human neoplastic lymphoid cells through activation of caspases, including FLICE, the most CD95-upstream caspase. Interestingly, NO is able to induce activation of FLICE not only via CD95/CD95L interaction but also independently of death receptors via direct caspase activation.
MATERIALS AND METHODS
Cell lines.
Human T- and B-lymphoid cell lines (Jurkat APO-S and APO-R, CEM, BJAB, BL60, and K50) were maintained at 37°C in RPMI 1640 medium (GIBCO-BRL, Eggenstein, Germany) containing 5% fetal calf serum. CD95-resistant (APO-R) Jurkat cells were derived from the parental CD95-sensitive (APO-S) clone by long-term culture in the presence of a lethal dose of activating CD95 antibody.36Northern and Western blot analysis showed that APO-R Jurkat cells expressed CD95 mRNA but failed to express CD95 protein. Both cell clones (APO-S and APO-R) were equally sensitive to CD95-independent apoptosis and expressed the same cell-surface markers.36BJAB B cells were stably transfected with a dominant negative FADD (FADD-DN) deletion mutant (kindly provided by Dr V.M. Dixit, Ann Arbor, MI), which contains only the death domain.20 Western blot analysis showed about 30-fold higher expression of the FADD mutant protein compared with wild-type FADD in the BJAB FADD-DN transfectants (data not shown). CEM is a T-cell leukemic cell line. The BL60 Burkitt lymphoma cell line expresses low amounts of CD95 and fails to express CD95L mRNA upon treatment with different apoptotic stimuli.36 K50 is a stable transfectant of BL60 that expresses CD95 on the cell surface. Peripheral blood mononuclear cells (PBMC) from a patient with T-cell acute lymphoblastic leukemia (T-ALL) were freshly isolated using density gradient with Lymphoprep (Nycomed Pharma, Oslo, Norway), and directly after isolation the cells were used for further experiments. To evaluate apoptotic effects of NO, all above-mentioned human lymphoid cells were treated with glycerol trinitrate (GTN; Merck, Darmstadt, Germany), which is known to constitutively produce NO in the incubation medium.
Bovine endothelial cells (BEC), kindly provided by B. Liliensiek and Dr P. Nawroth (University of Heidelberg, Heidelberg, Germany), were maintained in culture medium under standardized conditions.37 The activation of these cells to produce NO and coculture conditions of BEC with lymphoid cells have been described elsewhere.14 Briefly, BEC were treated with human TNF-α (Knoll AG, Ludwigshafen, Germany) at a concentration of 1 nmol/L for 16 hours. Inhibitor of inducible NO synthase (iNOS) NG-monomethyl-L-arginine (NMMA; Calbiochem, La Jolla, CA) was added to the culture medium at a concentration of 0.5 mmol/L simultaneously with TNF to block NO synthesis in stimulated BEC. An effector/target ratio of 25:1 was used.
Antibodies and inhibitors.
Purified anti-CD95 (anti–APO-1) monoclonal antibody (MoAb)38 was used for induction of apoptosis and for cell-surface expression studies. F(ab′)2 anti-CD95 blocking antibody was used as described elsewhere.27zVAD-fmk (Bachem Biochemica, Heidelberg, Germany) was used for inhibition of caspase activity at a final concentration of 20 μmol/L in culture medium. Cycloheximide (CHX; Boehringer Mannheim, Mannheim, Germany) was used for blocking of protein synthesis at concentrations ranging from 0.1 to 1 μg/mL.
Apoptosis and cytotoxicity assays.
Apoptosis was assessed by determining DNA fragmentation (DNA-release assay) after lysing the cells in a hypotonic solution (0.1% sodium citrate, 0.1% Triton X-100 [BioRad, Munich, Germany]) containing 50 μg/mL propidium iodide as described39 and analyzed by flow cytometry using a FACScan analyzer with CELLQuest software (Becton Dickinson, Heidelberg, Germany). Cytotoxicity was evaluated after treatment of live cells with 1 μg/mL propidium iodide (Molecular Probes Inc, Eugene, OR) for 5 minutes before FACS analysis. Ten thousand cells per sample were analyzed by flow cytometry (FACScan; Becton Dickinson) using CELLQuest software.
Polymerase chain reaction (PCR) and Northern blot analysis.
Total RNA was isolated from APO-S Jurkat T cells at different time points after addition of 0.2 mmol/L GTN using guanidium isothiocyanate. Semi-quantitative PCR analysis of the CD95L expression was performed using CD95L-specific primers and β-actin primers as described.36 The PCR products were electrophoresed through a 1.5% agarose gel, then blotted and hybridized with a32P-labeled human CD95L fragment27 and as a control with a β-actin probe. The same PCR conditions were used to amplify the TRAIL/APO-2 ligand using the following specific primers: sense primer, CCC AAT GAC GAA GAG AGT ATG AA; and antisense primer, ACC ATT TGT TTG TCG TTC TTT GTG together with the β-actin specific primers. The expected size of the amplified TRAIL-specific product was 422 bp. Northern blot analysis using CD95-specific fragments was performed as described elsewhere.40 Briefly, mRNA was isolated from Jurkat cells at different time points after addition of GTN according to Vennström and Bishop.41 mRNA (24 μg per lane) was electrophoresed through a 1% agarose-formaldehyde gel and blotted onto a nylon membrane. Hybridization was performed with the same probes as described for PCR.
Densitometric quantification.
Levels of CD95L and TRAIL mRNA expression were quantified by densitometry of autoradiograms using the Adobe Photoshop and the SCAN Analysis programs for Macintosh (Munich, Germany). Each relative expression value represents the ratio between the densities of specific mRNA transcripts to β-actin transcripts.
Western blotting.
Western blot detection of FLICE was performed as described.42 Postnuclear supernatants equivalent to 1 × 106 cells were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis all samples were transferred to Hybond nitrocellulose membrane (Amersham Buchler, Braunschweig, Germany), blocked with 2% bovine serum albumin in phosphate-buffered saline (PBS)/Tween (PBS + 0.05% Tween-20) for at least 1 hour, washed with PBS/Tween, and incubated with the MoAb C15 (directed against the p18 subunit of FLICE) in PBS/Tween for 16 hours at 4°C. The blots were washed with PBS/Tween and incubated with goat anti-mouse IgG2b (1:20,000) for 1 hour at room temperature. After washing with PBS/Tween the blots were developed with the chemiluminescence method (ECL) following the manufacturer's protocol (Amersham Buchler, Braunschweig, Germany).
RESULTS
NO induces higher apoptotic cell death in APO-S than in APO-R Jurkat cells.
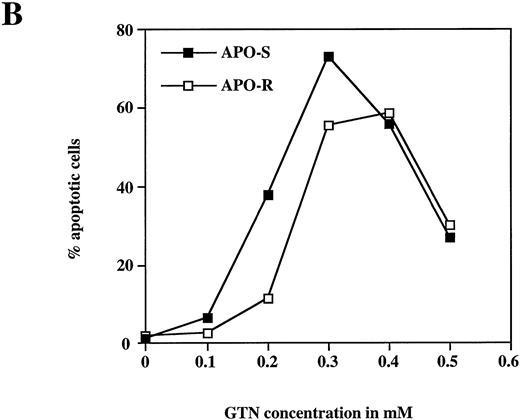
To examine the potential role of the CD95/CD95L system in NO-dependent cell death, two Jurkat clones with different sensitivity to CD95 agonistic antibodies were used as targets. The APO-S Jurkat cells express CD95 on the cell surface and are very sensitive to anti-CD95–induced kill, while the APO-R clone, lacking expression of CD95 on the cell surface, is resistant to treatment with anti-CD95. After exposure to the NO donor glycerol trinitrate (GTN) for 24 hours (at a concentration of 0.2 mmol/L), an increased percentage of apoptotic cells (up to 35%) was observed in the APO-S Jurkat cells. In contrast, the apoptotic fraction in the APO-R clone under the same conditions was only 11% (Fig 1A). NO induced apoptosis in a time-dependent manner. Treatment of APO-S cells with GTN for 48 hours induced more than 50% apoptotic cells as evidenced by DNA-release from the accumulation of subdiploid nuclei in the treated cells (Fig 1A). The apoptotic effect of NO was elevated with increasing GTN concentrations up to 0.3 mmol/L (Fig 1B). A cytotoxicity test performed in parallel using 0.2 mmol/L GTN showed the same level of APO-S Jurkat cell death as the above DNA-release assay (36% and 34% dead cells, respectively), indicating that the observed NO-mediated cytotoxicity was caused by apoptosis. Concentrations of GTN higher than 0.3 mmol/L led to a reduction in the percentage of apoptotic cells (Fig 1B) and to an increase in the percentage of dead cells as shown by cytotoxicity tests (data not shown). Thus, APO-S Jurkat cells showed higher NO-mediated apoptosis than the APO-R subclone. This difference was more pronounced at lower NO doses (eg, 0.2 mmol/L, Fig 1B). The sensitivity to CD95-triggering correlated with the sensitivity of cells to NO, and the loss of CD95 cell-surface expression correlated with higher resistance to NO.
NO induces higher apoptotic cell death in APO-S than in APO-R Jurkat cells. (A) Time-dependent apoptosis induced by NO donor GTN (0.2 mmol/L) in APO-S and APO-R Jurkat cells up to a time period of 48 hours. (B) Concentration-dependent apoptosis stimulated by GTN in APO-S and APO-R Jurkat cells after 24 hours of incubation. Percentage of apoptotic cells was measured by the DNA-release assay using treatment with a hypotonic propidium iodide solution at 4°C overnight in the dark followed by flow cytometric analysis. Experiments were performed in triplicates. SD was less than 10%.
NO induces higher apoptotic cell death in APO-S than in APO-R Jurkat cells. (A) Time-dependent apoptosis induced by NO donor GTN (0.2 mmol/L) in APO-S and APO-R Jurkat cells up to a time period of 48 hours. (B) Concentration-dependent apoptosis stimulated by GTN in APO-S and APO-R Jurkat cells after 24 hours of incubation. Percentage of apoptotic cells was measured by the DNA-release assay using treatment with a hypotonic propidium iodide solution at 4°C overnight in the dark followed by flow cytometric analysis. Experiments were performed in triplicates. SD was less than 10%.
To test whether the presence of NO is needed for the whole period of incubation to achieve a maximal apoptotic effect, APO-S Jurkat cells were treated with 0.2 mmol/L GTN for 2, 4, 8, and 12 hours. Then the cells were washed and fresh medium was added for the rest of the incubation time until 24 hours. Incubation for at least 8 hours and subsequent withdrawal of the NO donor for 16 hours resulted in the same percentage of apoptotic cells as that observed in cells after 24 hours of incubation in the presence of NO for the whole period (40% and 37% apoptotic cells, respectively).
NO induces CD95L and TRAIL/APO-2L mRNA expression.
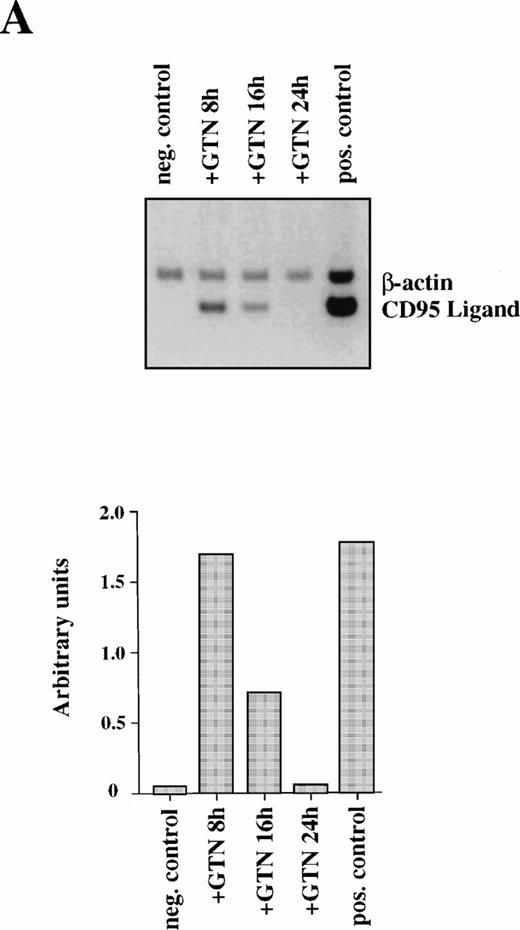
Because CD95L binding to the receptor triggers a whole cascade of events leading to the characteristic features of apoptosis, we studied the ability of NO to regulate CD95L mRNA expression. Semi-quantitative PCR analysis of APO-S Jurkat cells showed NO-mediated upregulation of CD95L mRNA. The highest increase of the ligand expression was reached after 8 hours of incubation with GTN (Fig2A). The NO-dependent induction of CD95L mRNA was transient and decreased upon prolonged incubation with the NO donor GTN (Fig 2A). The PCR data were confirmed by Northern blot analysis using specific probes for CD95L and β-actin mRNA (data not shown). In contrast, NO was not able to enhance expression of the CD95 receptor on the cell surface as shown by FACS analysis using specific anti-CD95 antibodies (data not shown).
Induction of CD95L (A) and TRAIL/APO-2L (B) mRNA expression by GTN in APO-S Jurkat cells. Cells were treated for different periods of time with 0.2 mmol/L GTN, and total RNA was isolated. After semi-quantitative RT-PCR with specific primers for CD95L or TRAIL/APO-2L, PCR products were run on a 1.5% agarose gel. APO-S cells stimulated with PMA (20 ng/mL; Sigma) and ionomycin (1 μmol/L; Calbiochem) for 6 hours were used as positive control. Both pictures were scanned using the Adobe Photoshop and the Scan Analysis programs. The ratio between the densities of the specific band to the β-actin band is shown in the histograms on the lower panel (indicated in arbitrary units). (A) The amplified products for CD95L (447 bp) and β-actin (661 bp) are labeled. The gel was blotted and hybridized with CD95L and β-actin–specific probes. (B) The amplified products specific for TRAIL (422 bp) and β-actin (661 bp) are presented.
Induction of CD95L (A) and TRAIL/APO-2L (B) mRNA expression by GTN in APO-S Jurkat cells. Cells were treated for different periods of time with 0.2 mmol/L GTN, and total RNA was isolated. After semi-quantitative RT-PCR with specific primers for CD95L or TRAIL/APO-2L, PCR products were run on a 1.5% agarose gel. APO-S cells stimulated with PMA (20 ng/mL; Sigma) and ionomycin (1 μmol/L; Calbiochem) for 6 hours were used as positive control. Both pictures were scanned using the Adobe Photoshop and the Scan Analysis programs. The ratio between the densities of the specific band to the β-actin band is shown in the histograms on the lower panel (indicated in arbitrary units). (A) The amplified products for CD95L (447 bp) and β-actin (661 bp) are labeled. The gel was blotted and hybridized with CD95L and β-actin–specific probes. (B) The amplified products specific for TRAIL (422 bp) and β-actin (661 bp) are presented.
Furthermore, production of NO in culture medium could also stimulate expression of another recently identified death cytokine, TRAIL/APO-2L. Maximal level of TRAIL mRNA expression was also achieved after 8 hours of incubation with GTN in APO-S Jurkat cells, but in contrast to CD95L it remained stable up to 24 hours (Fig 2B).
Thus, we have shown that NO induces expression of mRNA for two death cytokines, CD95L and TRAIL. The data suggest that CD95L and TRAIL participate in the NO-dependent apoptotic cell death.
NO-induced apoptosis in freshly isolated human leukemic lymphocytes requires caspase activity and involves the CD95 pathway.
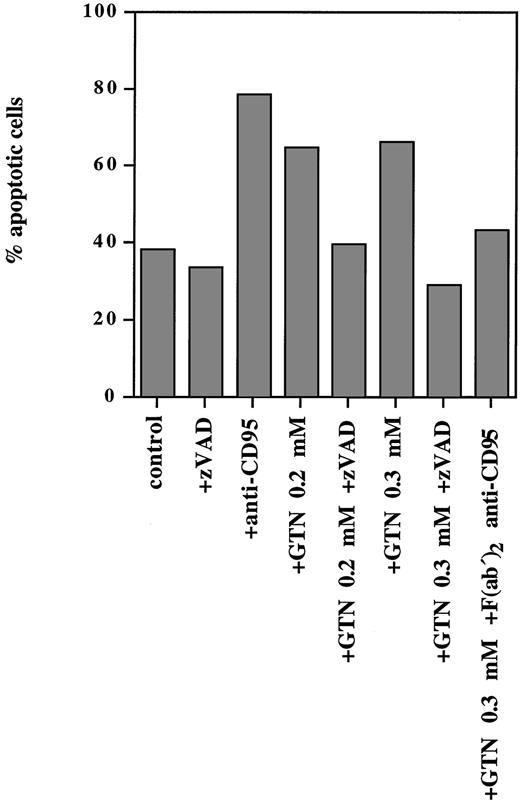
NO was able to induce programmed cell death not only in lymphoid cell lines but also in PBMC isolated from a patient with T-ALL (Fig 3). Exposure of freshly isolated leukemic cells to anti-CD95 MoAbs for 24 hours resulted in 79% apoptotic cells, whereas treatment of these cells with 0.2 mmol/L and 0.3 mmol/L GTN induced 65% and 67% apoptotic cells, respectively (spontaneous apoptosis was 38%). Pretreatment with the F(ab′)2 anti-CD95 blocking antibody (that specifically blocks the interaction of CD95/CD95L) significantly inhibited the observed NO-dependent apoptotic effect (Fig 3).
NO-induced apoptosis in freshly isolated human leukemic cells requires caspase activity and involves the CD95 pathway. PBMC from a patient with T-ALL isolated by Lymphoprep separation were plated at a density of 5 × 105 cells/mL and incubated for 24 hours with anti-CD95 (10 μg/mL), or broad-spectrum caspase-specific inhibitor zVAD-fmk (zVAD; 20 μmol/L) or with F(ab′)2 anti-CD95 blocking antibody (1 μg/mL) and/or GTN at the concentrations indicated. Percentage of apoptotic cells was measured by the DNA-release assay.
NO-induced apoptosis in freshly isolated human leukemic cells requires caspase activity and involves the CD95 pathway. PBMC from a patient with T-ALL isolated by Lymphoprep separation were plated at a density of 5 × 105 cells/mL and incubated for 24 hours with anti-CD95 (10 μg/mL), or broad-spectrum caspase-specific inhibitor zVAD-fmk (zVAD; 20 μmol/L) or with F(ab′)2 anti-CD95 blocking antibody (1 μg/mL) and/or GTN at the concentrations indicated. Percentage of apoptotic cells was measured by the DNA-release assay.
It is well documented that CD95- and TRAIL-signaling leads to activation of caspases.43,44 Because the zVAD-fmk broad-spectrum caspase inhibitor can completely block both signaling pathways,43 44 we examined whether it could abrogate NO-dependent apoptosis in human leukemic lymphocytes. It was shown that this inhibitor completely blocked apoptosis triggered by NO as shown in Fig 3. Thus, NO-mediated apoptosis in freshly isolated human leukemic cells strictly required caspase activity and involved the CD95 system.
NO-dependent apoptosis is blocked by a broad-spectrum caspase inhibitor and induces activation of FLICE.
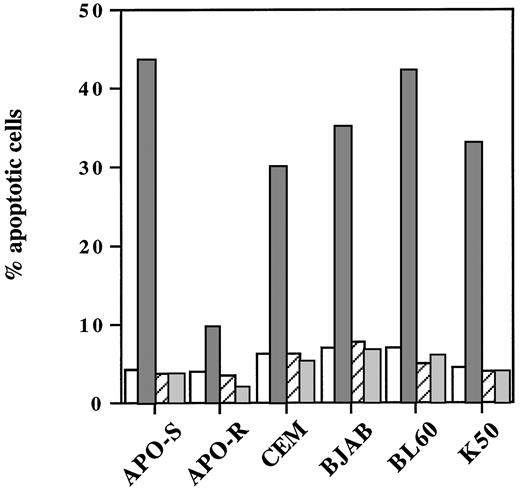
We next examined whether the caspase inhibitor zVAD-fmk could abrogate NO-induced cell death not only in freshly isolated human leukemic lymphocytes, but also in different neoplastic lymphoid cell lines. As shown in Fig 4, pretreatment with this caspase inhibitor caused a complete abrogation of NO-mediated cell death in these cells. Interestingly, zVAD-fmk inhibited also the low level of apoptosis induced by NO in APO-R Jurkat cells. In conclusion, we showed that NO-mediated apoptosis in human T- and B-cell lines strictly required caspase activity.
Blocking of NO-induced apoptosis in different neoplastic lymphoid cells by the caspase inhibitor zVAD-fmk. Cells were plated at a density of 3 × 105 cells/mL and incubated with 0.2 mmol/L GTN (APO-S and APO-R) or 0.25 mmol/L GTN (CEM, BJAB, BL60, and K50) and/or with 20 μmol/L zVAD-fmk (zVAD). Percentage of apoptotic cells was measured by the DNA-release assay. Data shown are representative of three individual experiments. SD was less than 10%. (□), Control; (▧), +GTN; (▨), +GTN +zVAD; (▧), +zVAD.
Blocking of NO-induced apoptosis in different neoplastic lymphoid cells by the caspase inhibitor zVAD-fmk. Cells were plated at a density of 3 × 105 cells/mL and incubated with 0.2 mmol/L GTN (APO-S and APO-R) or 0.25 mmol/L GTN (CEM, BJAB, BL60, and K50) and/or with 20 μmol/L zVAD-fmk (zVAD). Percentage of apoptotic cells was measured by the DNA-release assay. Data shown are representative of three individual experiments. SD was less than 10%. (□), Control; (▧), +GTN; (▨), +GTN +zVAD; (▧), +zVAD.
Because FLICE is the first in a cascade of caspases activated by CD95,25 we tested whether NO would be able to induce FLICE cleavage into active subunits. It has been previously shown that FLICE is activated upon triggering of CD95 leading to the release of the active subunits p18 and p10 into the cytosol.25 42Treatment of the APO-S cells with the NO-donor GTN for longer than 8 hours (up to 24 hours) resulted in accumulation of the active FLICE subunit p18 (Fig 5A). Moreover, NO-dependent activation of FLICE could also be observed in the freshly isolated leukemic cells after incubation with the NO donor GTN (data not shown). Furthermore, increasing concentrations of the protein synthesis inhibitor cycloheximide (CHX) could almost completely inhibit the NO-induced activation of FLICE when APO-S cells were treated for 10 hours with GTN (Fig 5B). This correlated with a significant reduction in the number of apoptotic cells (31.4% apoptotic cells in the presence of GTN v 4.1% in the presence of GTN and 1.0 μg/mL CHX for 24 hours). Therefore, protein synthesis is required for the NO-dependent activation of FLICE during apoptosis in Jurkat cells.
NO-induced activation of FLICE in lymphoid cell lines. (A) Time-dependent effect of NO in APO-S and BL60 cells. (B) Inhibition of FLICE activation upon treatment with protein synthesis inhibitor cycloheximide (CHX) in APO-S but not in BL60 cells. Cells were treated with 0.2 mmol/L GTN for the time period indicated and/or with CHX at different concentrations. Western blot analysis with anti-FLICE MoAb was performed. The full-length FLICE and the p18 active cleavage product are presented.
NO-induced activation of FLICE in lymphoid cell lines. (A) Time-dependent effect of NO in APO-S and BL60 cells. (B) Inhibition of FLICE activation upon treatment with protein synthesis inhibitor cycloheximide (CHX) in APO-S but not in BL60 cells. Cells were treated with 0.2 mmol/L GTN for the time period indicated and/or with CHX at different concentrations. Western blot analysis with anti-FLICE MoAb was performed. The full-length FLICE and the p18 active cleavage product are presented.
NO was also able to induce apoptosis and FLICE activation in BL60 and K50 cells (Figs 4 and 5A). Both cells failed to express CD95L mRNA and to stimulate expression of TRAIL/APO-2L mRNA upon treatment with GTN (data not shown). Significant accumulation of the active FLICE subunit p18 could be detected as early as 8 hours after exposure to GTN in both cell lines (Fig 5A and data not shown). Interestingly, in contrast to Jurkat cells, pretreatment of BL60 cells with CHX did not inhibit NO-dependent activation of FLICE (Fig 5B). Our results provide evidence that NO-mediated FLICE activation in Jurkat cells requires protein synthesis consistent with upregulation of CD95L and TRAIL and the involvement of the CD95 system. In contrast, the mechanism of NO-mediated cell death in BL60 cells is independent of death ligand production. Therefore, this apoptosis could not be blocked by CHX.
CD95-resistant lymphoid cells can be sensitive to NO-induced kill.
Because different human lymphoid cell lines differ in sensitivity toward induction of apoptosis by CD95L and TRAIL,43 45 we compared the sensitivity of different cells to anti-CD95 MoAb and to NO treatment. Lymphoid cell lines which were resistant to CD95-induced cell death (APO-R Jurkat cells and BJAB FADD-DN transfectants expressing a dominant negative FADD mutant) were found to also be resistant to NO-induced apoptosis, in contrast to the parental cells, which were sensitive to both stimuli (Table1). In different cell lines NO could induce apoptosis either via CD95 or it could be independent of death receptors. The CD95-dependent cell lines APO-S Jurkat, CEM, T-ALL, and BJAB, which undergo cell death upon treatment with anti-CD95, were also NO-sensitive. The CD95-independent BL60 cell line, which is resistant to anti-CD95 treatment and which is unable to produce CD95L, was nevertheless NO sensitive (Table 1).
Sensitivity of Different Human Lymphoid Cell Lines Toward Apoptotic Cell Death Mediated by Anti-CD95 MoAb and NO
| NO-Sensitive | NO-Resistant | |||||||
| CD95-Dependent | CD95-Independent | |||||||
| Cell Lines | % Apoptosis | Cell Lines | % Apoptosis | Cell Lines | % Apoptosis | |||
| +Anti-CD95 | +GTN | +Anti-CD95 | +GTN | +Anti-CD95 | +GTN | |||
| APO-S Jurkat | 92.3 | 32.7 | BL60 | 0.5 | 42.4 | APO-R Jurkat | 0.5 | 7.8 |
| CEM | 57.9 | 29.0 | K50 | 35.1 | 33.2 | DN FADD BJAB | 11.2 | 2.5 |
| T-ALL | 40.4 | 26.5 | ||||||
| BJAB | 83.1 | 29.2 | ||||||
| NO-Sensitive | NO-Resistant | |||||||
| CD95-Dependent | CD95-Independent | |||||||
| Cell Lines | % Apoptosis | Cell Lines | % Apoptosis | Cell Lines | % Apoptosis | |||
| +Anti-CD95 | +GTN | +Anti-CD95 | +GTN | +Anti-CD95 | +GTN | |||
| APO-S Jurkat | 92.3 | 32.7 | BL60 | 0.5 | 42.4 | APO-R Jurkat | 0.5 | 7.8 |
| CEM | 57.9 | 29.0 | K50 | 35.1 | 33.2 | DN FADD BJAB | 11.2 | 2.5 |
| T-ALL | 40.4 | 26.5 | ||||||
| BJAB | 83.1 | 29.2 | ||||||
The different cell lines were plated at a density of 3 × 105 cells/mL and incubated for 16 hours in the presence or absence of 100 ng/mL agonistic anti-CD95 MoAb. Cells were incubated under the same conditions with 0.2 mmol/L GTN (APO-S, APO-R, and freshly isolated PBMC from a patient with T-ALL or 0.25 mmol/L GTN (CEM, BJAB, BJAB FADD-DN, BL60, and K50) for 24 hours. The percentage of apoptotic cells was determined by the DNA-release assay. Data shown are representative of three independent experiments (except freshly isolated leukemic lymphocytes). SD was less than 10%.
These data suggest that NO-mediated active cell death involves CD95-dependent as well as independent apoptotic pathways.
NO produced by activated BEC induces apoptosis in CD95-sensitive B- and T-cell lines.
Previously we have shown that TNF-α–activated BEC produced NO which could induce apoptosis in murine lymphoma cells.14 This in vitro experimental system is based on the direct contact of tumor target cells with BEC effectors and thus resembles pathophysiological conditions in vivo. When we co-incubated APO-S or BJAB human lymphoid cells for 8 hours with activated BEC we detected significant apoptosis (Table 2). A specific inhibitor of iNOS, NMMA, could decrease the apoptosis observed in malignant lymphoid cells down to the background levels seen with nonactivated BEC. Therefore, the observed apoptosis is completely caused by NO secretion by BEC and not by the secretion of other cytotoxic molecules. It is also obvious from this experimental model that APO-R Jurkat cells were less sensitive to NO than the parental APO-S clone. Similarly, BJAB FADD-DN cells showed protection from cell death initiated by NO, in contrast to the parental nontransfected clone BJAB, which showed sensitivity to NO (Table 2).
NO Produced by Activated BEC Induces Apoptosis in CD95-Sensitive Human Lymphoid Cell Lines
| Targets . | % Apoptosis . | |||||
|---|---|---|---|---|---|---|
| BEC . | BEC + TNF . | BEC + TNF + NMMA . | BEC + TNF + CHX . | BEC + TNF + zVAD . | BEC + TNF + F(ab′)2 Anti-CD95 . | |
| APO-S Jurkat | 3.6/1.7 | 28.7/30.8 | 1.5/1.4 | 14.5/16.9 | 4.4/1.4 | 16.6/16.2 |
| APO-R Jurkat | 1.7/1.7 | 10.2/10.2 | 1.2/3.3 | — | — | — |
| BJAB | 2.3/1.2 | 23.8/23.1 | 2.3/3.2 | 1.4/3.4 | 3.7/4.8 | 13.3/13.8 |
| BJAB FADD-DN | 1.5/1.1 | 6.5/4.1 | 1.7/2.7 | — | — | — |
| Targets . | % Apoptosis . | |||||
|---|---|---|---|---|---|---|
| BEC . | BEC + TNF . | BEC + TNF + NMMA . | BEC + TNF + CHX . | BEC + TNF + zVAD . | BEC + TNF + F(ab′)2 Anti-CD95 . | |
| APO-S Jurkat | 3.6/1.7 | 28.7/30.8 | 1.5/1.4 | 14.5/16.9 | 4.4/1.4 | 16.6/16.2 |
| APO-R Jurkat | 1.7/1.7 | 10.2/10.2 | 1.2/3.3 | — | — | — |
| BJAB | 2.3/1.2 | 23.8/23.1 | 2.3/3.2 | 1.4/3.4 | 3.7/4.8 | 13.3/13.8 |
| BJAB FADD-DN | 1.5/1.1 | 6.5/4.1 | 1.7/2.7 | — | — | — |
BEC were treated with human TNF-α (1 nmol/L final concentration) or TNF-α and NMMA (0.5 mmol/L final concentration), then washed twice with medium and cocultured for 8 hours with neoplastic lymphoid cells (effector/target ratio, 25:1). The latter cells were then collected and DNA release assay was performed. For inhibition experiments, lymphoid cells were treated for the whole period of coculture with 2 μg/mL CHX, or 20 μmol/L zVAD-fmk (zVAD) or 1 μg/mL F(ab′)2anti-CD95 blocking antibody. Percentage of apoptotic cells induced by these inhibitors alone was in all the cases less than 5%. Also, the percentage of nonspecific apoptosis in the control cells (not coculture with BEC) was in all the cases less than 7%. Data from two independent experiments are presented.
The apoptotic effect mediated by activated BEC in the CD95-sensitive cells could be completely blocked by the caspase inhibitor zVAD-fmk (Table 2). These data are consistent with the results presented in Fig4 using exogenous NO. Treatment of the APO-S Jurkat T cells with the protein synthesis inhibitor CHX or with the F(ab′)2anti-CD95 blocking antibody, which prevents binding of CD95L to the receptor, could partially (approximately 40% in both cases) inhibit NO-mediated active cell death (Table 2). The data indicate that protein synthesis is required and that binding of CD95L to the receptor is, at least in part, involved in the NO-induced apoptosis in these cells. When BJAB cells were tested in the same way, GTN-induced apoptosis could be blocked completely by zVAD-fmk or by CHX and only partially (approximately 58%) by F(ab′)2 anti-CD95.
Therefore, using a physiological test system in vitro—blood vessel endothelium interacting with lymphoid cells— we could show that endogenously produced NO induced apoptotic cell death in human neoplastic lymphoid cells. This involved CD95/CD95L interaction and was entirely dependent on caspase activation.
DISCUSSION
Apart from its many regulatory and effector functions NO, produced by activated macrophages and endothelial cells, is an important cytotoxic molecule responsible for lysis of tumor cells.6,7,46,47Recently it was found that endogenously produced or exogenously added NO could induce cytotoxicity through apoptosis in murine thymocytes,10 peritoneal macrophages,9splenocytes,11 and tumor cells.13-15 However, the underlying mechanism of the NO-induced apoptotic effect has not yet been clearly elucidated.
In the present study we examined the involvement of the CD95/CD95L system and signal transduction pathway in NO-induced apoptosis in human neoplastic lymphoid cells. To address this question we compared CD95-sensitive Jurkat cells and their CD95-resistant subclone, characterized by lack of the CD95 receptor on the cell surface. We found that APO-S cells were more susceptible to exogenous NO-induced apoptosis than APO-R cells. A significant degree of cell death was observed after exposure of APO-S cells to 0.3 mmol/L of the NO donor GTN for 24 hours. Lower doses of GTN retarded induction of apoptosis. Treatment of the cells for 8 hours with GTN and its subsequent withdrawal for 16 hours was enough to achieve maximal apoptosis. It thus seems that NO is required to initiate an apoptotic program and then it is not needed thereafter.
Interestingly, NO-dependent apoptosis could be blocked in APO-S and BJAB cells by the protein synthesis inhibitor CHX. This finding suggests that protein synthesis is required (at least in part) and correlates with the fact that NO has to be present in the medium for 8 hours to induce a maximal apoptotic effect.
These data are also in agreement with the observation that NO treatment induces gene expression of CD95L and TRAIL/APO-2L and that the highest increase in the mRNA expression of CD95L and TRAIL is reached after treatment of the APO-S cells for 8 hours with the NO donor GTN. Incubation of APO-S cells with GTN resulted in a strong increase in the expression of CD95L and TRAIL mRNA. Although CD95L mRNA expression was transient and reduced after prolonged (up to 24 hours) incubation of the cells with NO, TRAIL expression was stable for the same time period. Unlike CD95L, whose transcripts are predominantly restricted to stimulated T cells and sites of immune privilege, TRAIL expression is detected in many normal human tissues, suggesting that TRAIL should not be cytotoxic to most tissues in vivo.30-32 Jeremias et al45 proposed that TRAIL-mediated apoptosis may play a role in antileukemic growth control in APO-S Jurkat cells, although TRAIL had only 25% of the activity of CD95L in inducing apoptosis. Such TRAIL-mediated apoptosis could account to some extent for the NO-induced apoptosis in lymphoid cells, and in particular in the CD95-independent cell death observed in the APO-R Jurkat clone.
Because CD95 receptor expression remained unaltered after NO treatment, it seems that NO triggers the death receptor systems by regulating the expression of ligands involved in apoptosis like CD95L and TRAIL/APO-2L. Signaling through CD95 involves the death domain–associated molecule FADD20-22 and the upstream caspase FLICE/MACH.23,24 Since NO failed to induce significant apoptosis in the CD95-resistant BJAB cells expressing a dominant negative FADD mutant and since the NO-induced apoptotic cell death could be partially inhibited by a blocking antibody that specifically inhibits the interaction of the CD95L with its receptor, we conclude that CD95-mediated signaling can be involved in NO-dependent death, at least to some extent. It is known that the CD95 system mediates activation-induced apoptotic cell death in T cells26-28 as well as apoptosis induced by drugs.29 It also plays a critical role in apoptosis induced by viral proteins.40 Based on our observations, NO has to be added to the list of inducers of apoptosis in which CD95 pathway is involved.
Activation of the cascade of caspases seems to be a common intracellular effector pathway to which different apoptosis signals are connected.17 Caspases have also been implicated in programmed cell death triggered by CD95L and TRAIL/APO-2L.43,44 The tripeptide zVAD-fmk, a broad-spectrum caspase inhibitor, is known to inhibit caspase cleavage.48 We here showed complete inhibition of NO-mediated apoptotic cell death by zVAD-fmk in different human lymphoid cell lines and in freshly isolated leukemic cells. It has recently been shown that proteolytic activity of caspases could be stimulated also in HeLa cells and in macrophages upon treatment with NO donors49 (Boggs S, personal communication, May 1997). Our data also provide evidence that NO induces FLICE activation in human neoplastic lymphoid cells. FLICE is the most upstream caspase in the CD95 signaling pathway and is activated upon triggering of CD95 leading to the release of the active subunits p18 and p10 into the cytosol, where they can activate other caspases.25 NO induced a clear accumulation of the p18 subunit of FLICE. Because the protein synthesis inhibitor CHX could almost completely abrogate NO-mediated activation of FLICE and because the kinetics of p18 accumulation correlated with the kinetics of CD95L and TRAIL mRNA expression, we conclude that NO-mediated activation of FLICE involves synthesis of CD95L and/or TRAIL in APO-S Jurkat cells. It seems that NO leads to activation of caspases through activation of CD95/CD95L and probably TRAIL death receptor systems.
Data on NO-induced apoptosis in different human malignant lymphoid cell lines were also extended to PBMC from a patient with T-ALL. Freshly isolated leukemic cells were sensitive both to anti-CD95 MoAb and to GTN treatment. Addition of the F(ab′)2 anti-CD95 blocking antibody decreased significantly the level of apoptosis triggered by NO. Moreover, NO donor GTN caused FLICE activation, and caspase inhibitor zVAD-fmk was able to block NO-mediated apoptosis. This suggests that caspase activation is strictly required and CD95 pathway is also involved in the NO-dependent death of ex vivo isolated human leukemic lymphocytes.
Interestingly, some lymphoid cells resistant to CD95-mediated apoptosis (eg, BL60) were nevertheless sensitive to treatment with NO. Caspases were shown to also mediate apoptosis in this case. It seems that NO can use an alternative pathway of FLICE activation which does not require CD95L or TRAIL/APO-2L protein synthesis because pretreatment of BL60 and K50 cells with CHX did not block the NO-induced FLICE activation. Moreover, both cell lines failed to induce expression of CD95L and TRAIL/APO-2L mRNA after GTN treatment. We suggest that this death receptor–independent pathway of FLICE activation might involve mitochondria that have recently been implicated in the apoptotic process and in the activation of caspases.50 Significant reduction of mitochondrial membrane potential through opening of permeability transition pores, resulting in hyperproduction of reactive oxygen species and caspase activation, have been postulated as a crucial step in apoptosis.50,51Mitochondrial alterations might be involved in NO-mediated activation of caspases as it has been shown for the cytotoxic drug betulinic acid, which may trigger CD95-independent apoptosis via activation of caspases.52 To our knowledge NO is the only apoptotic stimulus known thus far capable of inducing both CD95-dependent and independent activation of ICE proteases.
Our findings on apoptosis induced by NO donor GTN could be reproduced using an in vitro experimental system mimicking a relevant in vivo situation. Our model was based on coculture of lymphoid target cells with TNF-α–activated blood vessel endothelial cells as effector cells secreting NO. A similar system for murine and human lymphoma cells has been established by us and other groups.6,7,14,15A significant level of lymphoid cell apoptosis was observed which was abrogated completely in the presence of the iNOS inhibitor NMMA. The apoptotic fraction of the human lymphoid cell lines tested corresponded with the cytotoxicity induced in K562 cells using similar coculture conditions assessed by 51Cr release.15Additionally, the caspase inhibitor zVAD-fmk was found to block the observed programmed cell death completely. The protein synthesis inhibitor CHX as well as a blocking antibody that inhibits binding of CD95L to the receptor could partially abrogate this apoptotic effect. Therefore, we conclude that apoptosis observed in our model system was entirely caused by endogenously produced NO and that the activity of caspases was strictly required. More studies will be necessary to determine whether the mechanism of NO-mediated apoptosis is general or restricted to certain cell types.
Taken together, our results show that the production of endogenous NO by activated endothelial cells or exposure to exogenous NO exerts strong apoptotic effects in human neoplastic lymphoid cells. The observed cell death strictly requires caspase activity, in particular FLICE, the most CD95 receptor-proximal caspase, and involves activation of the CD95/CD95L system. Therefore, the cytotoxic effector molecule NO triggers apoptosis using known signaling pathways and can thereby play a critical role in host antitumor responses. Moreover, NO can induce caspase activation and apoptosis using alternative pathways that are independent of death receptor systems. This insight into the mechanism of NO-mediated cell death may have clinical relevance because similar data were also obtained with freshly isolated human leukemic lymphocytes. Thus, stimulation of the endogenous NO production could be of importance for effective treatment of leukemia patients and NO donors might be promising new agents in therapeutic protocols.
ACKNOWLEDGMENT
We thank B. Liliensiek and Dr P. Nawroth for providing the BEC, Dr V.M. Dixit for providing the dominant negative FADD mutant, and Dr K. Khazaie for critical reading of the manuscript.
Supported in part by the Deutsche Forschungsgemeinschaft (K.C. and M.E.P.) and by Mildred Sheel Stiftung (V.U. and M.R.).
Address reprint requests to Victor Umansky, PhD, Division of Cellular Immunology, Tumor Immunology Program, German Cancer Research Center, D-69120 Heidelberg, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal